Abstract
Background
Increasing popularity of Mathan Tailam (mattan tailam, pachai ennai) for the treatment of diabetic foot ulcer necessitated standardization and quality control of this medicated oil both in large scale production and quality check in marketed drug.
Objective
Present study aims to develop standard operating procedure for the preparation of Mathan Tailam as per Siddha Formulary of India and its standardization using suitable analytical techniques.
Materials and methods
Mathan Tailam was prepared as per Siddha Formulary of India. Physicochemical parameters and preliminary phytochemical screening were carried out using standard methods. The in-house prepared sample underwent physico-chemical analysis, qualitative phytochemical analysis, Gas chromatography-mass spectrum (GC-MS) analysis, High performance thin layer chromatographic (HPTLC) fingerprinting profile and inductively coupled plasma-optical emission spectroscopic (ICP-OES) analysis.
Results
Physico-chemical parameters of the prepared formulation were comparable to that of coconut oil. Aqueous methonolic extract of this drug was found to be positive for alkaloid, saponin, coumarin, steroid, triterpinoid, quinine and furan. The GC-MS values were comparable to that of the base used i.e., the coconut oil. HPTLC fingerprinting profile revealed the presence of phytochemicals in the medicated oil derived from both coconut oil and Datura metel. ICP-OES addressed the mineral portion of the formulation and its safety in heavy metal aspect.
Conclusion
All these parameters can be utilized for the overall quality check over its preparation and formulation.
Keywords: GC-MS, Mathan Tailam, Phytochemical, Physico-chemical, HPTLC, ICP-OES
Graphical abstract
1. Introduction
Mathan Tailam, a herbomineral classical Siddha formulation [1] is used as a remedy for healing suppurative wound and is very useful in healing diabetic ulcers [2]. Medicated oil is prepared by protracted boiling of base oil with juice of prescribed herbal drug and mineral drug till it is dehydrated or near dehydration. This process results in the transfer of some therapeutically active principles of the ingredients into the base oil [3]. Thus, traditional method of tailam preparation assures the enrichment of oil with active principles. Mathan Tailam is a herbomineral Siddha formulation prescribed widely for several conditions such as eczema, weeping eczema, itches, wounds, chronic ulcers, bed sores, anal fistula, ear infections, carbuncle ulcer of diabetes, per anal abscess, non-healing of external ulcers, folliculitis, alopecia and burn wound etc. [4]. Real standard of a drug should be of its therapeutic value, a biological parameter, which varies from individual to individual and species to species. The biological standards can be supplemented and complemented by enhancing the physico-chemical parameters already employed by making use of latest techniques. Information on quality control standards for Mathan Tailam described in CCRAS publication covers only physico-chemical parameters which address mostly the characters of base oil and not about the herbal and mineral portion of the drug [5]. There is a need that as and when more aspects on scientific knowledge are unfolded, new parameters can be included which will make the tests and standards more meaningful. Increasing popularity of this medicated oil for the treatment of diabetic foot ulcer necessitated the improved methods of standardization. Hence the present work was carried out to develop Standardized Operating Procedure (SOP) for the preparation of Mathan Tailam and standardization using suitable analytical techniques namely physico-chemical parameters, qualitative phytochemical parameters, Gas chromatography-mass spectrum (GC–MS) analysis, High performance thin layer chromatographic (HPTLC) and inductively coupled plasma-optical emission spectroscopic (ICP-OES) analysis.
2. Material and methods
2.1. Standardized operating procedure for preparation
Datura fastuosa L. (Datura metel L.) belongs to the family Solanaceae, Tamil name- Karuoomathai was collected from Chennai, Tamil Nadu. The plant was authenticated by Prof. P. Jayaraman, Director, Institute of Herbal Botany, Plant Anatomy and Research Centre, Chennai, based on the organoleptic, and macroscopic examination of fresh sample [6]. The specimen (voucher No: PARC/2015/3055) was deposited in his Institute for future reference. Edible quality oil of Cocos nucifera Linn. was procured from local market and its quality was checked by using physico-chemical parameters like iodine value, saponification value, acid value, peroxide value, refractive index, specific gravity and rancidity [7]. Required quantity of copper sulfate was procured from local market and purified by dissolving in water followed by subsequent recrystallization [8]. It was then tested for the presence of copper qualitatively by adding excess of ammonia to a small amount of solution prepared out of it. This first produced a bluish precipitate followed by a deep blue colored solution indicating the presence of copper while presence of sulfate was confirmed by treating the solution with barium chloride which resulted in formation of white precipitate [9]. Raw materials complying pharmacopoeia quality were further used for Mathan Tailam preparation. Fresh leaves of the plant were cleaned with running water and drained enough to remove excess water. Fresh juice was obtained from the macerated leaves of D. metel. References from Siddha Formulary of India (listed in Schedule I of DC Act 1940) [1] and Gunapadam [10] were followed for Mathan Tailam preparation. Copper sulfate (350 g) was dissolved in D. metel leaf juice (3.500 L). Coconut oil (1.400 L) was added to this, mixed and heated (heating by using hard fire wood is preferable) on mild flame in properly seasoned clay vessel. Since the smoking point of coconut oil is approximately 171 °C, care must be taken to avoid overheating beyond this temperature. Care was taken to avoid charring and mixing of smoke of fire wood in to the formulation. Mixture in the open vessel was stirred intermittently till it attains required characters like desired smell, color, free of cracking sound, disappearance of froth and rolling of the herbal drug between the fingers. When the rolled material was ignited, it burnt without spurting and without noise. The tailam was prepared at a stretch within a day. Finally, the mixture was filtered when hot, through muslin cloth, stored in an amber colored bottle and stoppered until use.
2.2. Quality evaluation of MT
2.2.1. Physico-chemical parameters
The following physico-chemical parameters namely iodine value, saponification value, acid value, peroxide value, refractive index, specific gravity and rancidity were carried by using standard procedure [7]. Three replicates were prepared and the mean values were computed.
2.2.2. Gas chromatography mass spectrum analysis
Sample was injected into the GC–MS unit (Instrument; Agilent 5975 series, column; DB5 MS 30 m × 0.25 mm × 0.25 mm, single quadrupole detection system with carrier gas of Helium). Oven temperature and injection temperatures were maintained at 280 °C. Injection volume was 10 μl. It was separated into various constituents with different retention times which are detected by mass spectrophotometer. The chromatogram was plot for intensity against retention time and recorded by the software attached to it. From the graph, the compounds were identified comparing the data with the existing software NIST-11.
2.2.3. Extractive value
Extraction of phytochemical constituents from Mathan Tailam was done by mixing 5 ml of Mathan Tailam with 15 ml of 90% aqueous methanol and subjected to constant stirring by using magnetic stirrer on hot top at 60 °C for 1 h. It was then stored in freezer for solidification. The alcoholic portion was separated and filtered through Whatman filter paper no. 41 and filtrate was used for qualitative phytochemical testing and HPTLC profiling. The extraction was repeated for three times for effective extraction of phytochemicals from the medicated oil. Same procedure was followed for coconut oil [3].
2.2.4. Qualitative phytochemical parameters
Analyses for the following qualitative phytochemical parameters were carried out: steroids, triterpinoids, flavonoids, alkaloids, sugar, coumarine, quinine, saponine, tanic acid, furan, phenol [11].
2.2.5. High performance thin layer chromatographic (HPTLC) profile
HPTLC studies were carried out by following standard methods [12], [13], [14]. The extract prepared for extractive value was utilized for HPTLC fingerprinting of coconut oil and Mathan Tailam. D. metel leaf extract was prepared by 4 g shade dried and coarse powdered leaves. The prepared powder was extracted with 40 ml of 90% aqueous methanol. It was then filtered and concentrated to 10 ml. 15 μl of this extract was used for HPTLC fingerprinting. Aliquots of samples were applied on a pre-coated silica gel 60 F254 (E. Merck) of 2 mm thickness aluminum plates to a bandwidth of 6 mm using CAMAG HPTLC system equipped with ATS 4 applicator. The plate was developed using solvent system of Chloroform: Methanol in the proportion of 9:1 up to 80 mm, removed from the chamber and allowed to dry. The developed plate was visualized under UV 254, 366 nm using deuterium, mercury lamp source, with slit dimensions 5.00 mm × 0.45 mm and scanned using TLC Scanner 4 and analyzed with winCATS software version 1.4.4. The chromatograms were recorded. After scanning, the plate was dipped in vanillin-sulfuric acid reagent and dried at 105 °C in hot air oven till the color of the spots appears. Then the plate was scanned at 520 nm using tungsten lamp. The Rf values and fingerprint data were recorded by winCATS software.
2.2.6. Inductively coupled plasma-optical emission spectroscopic (ICP-OES) analysis
Copper and lead estimation was carried out using ICP-OES. 2 g of oil was weighed and treated with 20 ml of 0.1N HNO3 for 1 h using magnetic stirrer at 60 °C. The aqueous portion was separated by using separating funnel. This aqueous portion was filtered using Whatman filter paper number 40. The volume was made up to 50 ml using de-ionized water. This clear aqueous solution was used for analysis of copper and lead concentration by ICP-OES using the wavelength of copper – 327.393 and lead – 220.353.
3. Results
Results of physico-chemical analyses are presented in Table 1. The results showed that the in-house prepared drug represented the values similar to that of coconut oil [15].
Table 1.
Physico-chemical analysis of in-house prepared Mathan Tailam and existing quality standards.
| S. no | Physico-chemical parameters | Values | Values as per CCRAS quality check manual [9] |
|---|---|---|---|
| 1 | Specific gravity | 0.92 | – |
| 2 | Refractive index | 1.3325 | 1.454 |
| 3 | Saponification value | 252.08 | 257.2 |
| 4 | Iodine value | 8.02 | 12.12 |
| 5 | Acid value | 2.99 | 1.62 |
| 6 | Peroxide value | 0.92 | – |
| 7 | Rancidity | Nil | – |
The result of GC-MS analysis of in-house prepared drug is given in Fig. 1 & Table 3. The results of the GC–MS analysis represented the coconut oil composition but did not reveal the composition of herbal and mineral portion of the drug.
Fig. 1.
GC–MS chromatogram of in-house prepared Mathan Tailam.
Table 3.
GC–MS profile of in-house prepared Mathan Tailam.
| RT | % Area | Molecular formula/molecular weight/structure | Compound name | Super impossible factor % |
|---|---|---|---|---|
| 10.099 | 1.65 | M. F: C8H14O2 M. W: 142.1956 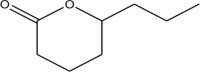
|
2H-Pyran-2-one, tetrahydro-6-propyl | 91 |
| 11.288 | 5.18 | M. F: C10H20O2 M. W: 172.2646 
|
n-Decanoic acid (Capric acid) | 97 |
| 12.966 | 4.70 | M. F: C10H18O2 M. W: 170.2487 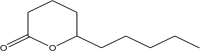
|
2H-Pyran-2-one, tetrahydro-6-pentyl | 87 |
| 13.798 | 41.90 | M. F: C12H22O2 M. W: 198.3019 
|
Dodecanoic acid (Lauric acid) | 99 |
| 15.500 | 3.48 | M. F: C12H22O2 M. W: 198.3019 
|
2H-Pyran-2-one, 6-heptyltetrahydro | 93 |
| 16.042 | 13.82 | M. F: C14H28O2 M. W: 228.3709 
|
Tetradecanoic acid (Myristic acid) | 99 |
| 16.369 | 4.16 | M. F: C22H44O2 M. W: 340.5836 
|
Eicosanoic acid, ethyl ester | 76 |
| 18.115 | 3.17 | M. F: C16H32O2 M. W: 256.4241 
|
n-Hexadecanoic acid (Palmitic acid) | 99 |
| 19.816 | 3.99 | M. F:C18H34O2 M. W:282.4614 
|
9-Octadecenoic acid, (E)-(trans-Oleic acid) | 95 |
| 20.113 | 2.02 | M. F: C13H28O M. W: 200 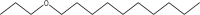
|
N-Propyl decyl ether | 86 |
Extractive value of in-house prepared sample was found to be 11.5 mg/ml. This value directly represented the phytochemicals of medicinal herb. Results of the qualitative phytochemical parameters are given in Table 2.
Table 2.
Qualitative phytochemical analyses of 90% aqueous methanolic extract of coconut oil, in-house prepared Mathan Tailam, and D. metel leaf.
| S. No | Test | Observation | Inference |
||
|---|---|---|---|---|---|
| Coconut oil | in-house prepared sample | D. metel leaf | |||
| 1 | Alkaloids (Dragendorff's reagent) | Brick red precipitate | −Absent | +Present | +Present |
| 2 | Flavonoids (Magnesium + Concentrated HCl) | Magenta color | −Absent | −Absent | −Absent |
| 3 | Sugar (Anthrone + Conc. H2SO4) | Green color | −Absent | −Absent | +Present |
| 4 | Saponins (Frothing test) | Froth formation | −Absent | +Present | +Present |
| 5 | Coumarin (10% NaOH) | Formation of yellow color, color disappears with the addition of Conc. H2SO4 | −Absent | +Present | +Present |
| 6 | Tannins (a) Ferric chloride test | Green precipitate | −Absent | −Absent | +Present |
| 7 | Steroids (Conc. H2SO4 test) | Reddish brown precipitate at interface | +Present | +Present | +Present |
| 8 | Triterpenoids (Tin + Thionyl chloride) | Red precipitate | −Absent | +Present | +Present |
| 9 | Acid compounds test | Color change in moist blue litmus | −Absent | −Absent | −Absent |
| 10 | Quinone (reaction with conc. H2SO4) | Red color | +Present | +Present | +Present |
| 11 | Phenol (Ferric chloride) | Green color | −Absent | −Absent | +Present |
| 12 | Furan Extract dissolved in alcohol P-dimethyl amino benzaldehyde and Conc. HCl was added | Pink color | +Present | +Present | +Present |
The results of HPTLC analysis are given in Fig. 2 and Supplementary file. At 254 nm, number of spots observed in coconut oil, Mathan Tailam and D. metel plant extract were 5, 15 & 12 respectively. Among these spots, the spots with Rf 0.32 and 0.87 were common for both Mathan Tailam and D. metel plant extract. At 366 nm, number of spots observed in coconut oil, Mathan Tailam and D. metel plant extract were 0, 12 and 11 respectively. Among the spots, the spot with Rf value 0.28 was common for Mathan Tailam and D. metel plant extract. At 520 nm, number of spots observed in coconut oil, Mathan Tailam and D. metel plant extract were 9, 10 and 11 respectively. Among the spots, the spot with Rf value 0.49 was common for coconut oil and Mathan Tailam. Another spot with Rf value 0.59 was common for coconut oil, Mathan Tailam and D. metel plant extract. Two other spots with Rf values 0.78 and 0.92 were common for Mathan Tailam and D. metel plant extract.
Fig. 2.
TLC photo documentation of 90% aqueous methanolic extract of coconut oil (Track 1), in-house prepared Mathan Tailam (Track 2) and D. metel leaf extract (Track 3) at 254, 366 nm and 520 nm after derivatization with vanillin sulfuric acid. Note: 1 denotes track 1; 2 denotes track 2 and 3 denotes track 3.
Result of ICP-OES for all the samples indicated that the level of lead was below detectable limit. Copper content of in-house prepared drug and coconut oil was 33.2 and 0.55 ppm respectively.
4. Discussion
Quality of herbal medicine depends on the originality of raw drugs, method of preparation, proper storage, proper indication, correct dose and dosage regimen. In this study, the drug Mathan Tailam, was prepared from D. metel leaf juice, coconut oil and copper sulfate. D. metel Linn. (D. fastulata L; D. alba Nees) is a poisonous herb used in Siddha system for various ailments. Leaf powder taken internally and rolled leaf for smoking were indicated for respiratory ailments. The leaves wilted in coconut oil used for fomentation for the conditions like joint swelling, abscess pain and inflammatory pain [16]. Major chemical constituents of Datura are scopolamine, daturadiol, hyoscyamine, fastudine, allantoin, niacin, vitamin C, tropine, noratropine, meteolodine hyosine, fastusic acid etc. Tropane alkaloids present in all parts of Datura are toxic. The plant is common in tropical part of India to temperate parts of Himalayas. Parts used are seed, flower, root, bark and leaves. Datura, on external application improves skin quality, helps in quick wound healing, helps to relieve pain and inflammation. It also detoxifies skin and relieves itching when applied as a paste. In this preparation, leaves were used for preparing the drug. There was no mention about the purification process for leaves used in this particular formulation. Purple variety of the plant is called Krishna mentioned in Rajanigandu. Folklore claim states that the purple variety is medicinally more valued than the white variety [17]. Copper sulfate can be purified by dissolving in water and recrystallizing. For internal use, it is purified by being rubbed with honey or ghee and exposed to heat in a crucible. It is then soaked for three days in whey or water and dried in sun [18], [19]. For external application purification of copper sulfate is not much indicated. However, the drug available in local market may have iron, lead and other contaminants. Hence, in order to remove such contaminants, recrystallization is always preferred. Reference states that addition of copper sulfate in medicated oil for external application increases its shelf life [20]. In the present study, finished drug did not show the presence of copper in qualitative assays. But the ICP-OES analysis revealed the presence of copper in ppm level and the absence of lead content also ensures the safety of the product from heavy metal toxicity. Further, copper sulfate reacts with coconut oil forming a copper soap at low concentration [21] which possesses anti-fungal property. A patented combination of soluble copper fertilizer and a naturally occurring fatty acid produces copper soap which has been reported to act as a fixed copper fungicide at a concentration range of 90 ppm and is also said to be biodegradable [22]. Coconut oil is preferred for drug used in wound healing among the medicated oils used in traditional system. In allergic conditions, it is used for external application. It can be justified by the presence of wound healing constituents like capric acid and lauric acid [23]. The extractive value holds its importance in deciding the dose of effective fractions so that the drug is devoid of toxicities. Still, there is a need to test its safety upon topical application over skin and mucous membrane. In spite of the lag behind advanced treatment and awareness, a 1984 clinical study [2] revealed much about the wound healing property of Mathan Tailam in diabetic cases where the chronic ulcerative wound upon its application in diabetic ulcers had shown its quick healing potential ranging from one to three months in accordance to the depth and affected area of ulcer. Another report indicates that in the presence of metabolic activation in Salmonella/microsome mutagenicity assay, the dichloromethane leaf extract shows anti-mutagenic property [24]. This further supports the evidence of protective and preventive action of Mathan Tailam against chronic wounds that are persisting and progressing towards malignancy. In addition, another report states that the leaf extract effectively inhibits mycelia growth of ringworm fungi Epidermophyton floccosum, Trichophyton mentagrophytes and Microsporum gypseum [25]. Literature search revealed that the plant possesses anti-bacterial activity. A new anti-bacterial agent 51, 71 dimethyl 61– hydroxy 31, phenyl 3 α – amine β – yne sitosterol 1 has been isolated from D. metel leaves [26]. A study states that the methanolic and chloroform extracts showed promising results against resistant strains of pathogenic bacteria [27]. Literature survey and traditional claim states that Mathan Tailam possesses anti-spasmodic, anodyne and antiseptic properties [28]. Literature available on standardization parameters of Mathan Tailam states only about its physico-chemical properties and not much about the phytochemical constituents derived from the plant. Hence, we carried out qualitative phytochemical screening for Mathan Tailam, coconut oil and plant extract. Marker compound gives an idea about the identity of plant in many cases. Most of the time, identified marker compound may not be a biologically active marker. In drugs using herbs in crude form, certain chemical will act in targeting the specific condition; other chemicals either potentiate the dynamics and facilitate the kinetic of this compounds or nullify the toxicity of the toxic principles. Therefore, in our study, the leaves extract was used as marker instead of specific single marker. Phytochemical constituents of Mathan Tailam revealed that the ingredients were derived from both coconut oil and plant. HPTLC fingerprinting was carried out for coconut oil, Mathan Tailam and plant extract using a solvent system that detects other than alkaloids. For detecting the presence of alkaloids, qualitative phytochemical analysis with dragendorff reagent was carried out and found to be positive. GC–MS method is more suitable for quantitative analysis of natural volatile components and lipophilic compounds with low melting points and good thermal stability in medicinal herbs. Advantage of GC–MS analysis is spectrum identification along with quantification for a sample by one injection. But highly polar heat liable non-volatile macromolecular organic compounds could not be detected. In the present study the drug in its native form was used for GC-MS analysis. Majority of the constituents detected were the fatty acid constituents of the base oil. The presence of copper from copper sulfate, lauric and capric acids from coconut oil and other phyto chemicals from leaves of D. metel ensures wound healing and counteract the bacterial and fungal infection thereby decides the quality of the drug.
5. Conclusion
All the parameters and result of this study provides quality standards for Mathan Tailam representing its source from both herbal and mineral origin. This can be utilized for the overall quality check over its preparation and formulation.
Sources of funding
None.
Conflict of interest
None.
Acknowledgement
The authors are highly thankful to Director General CCRAS, Assistant Director (S-III) In-charge, CSMRADDI, Bureau Veritas India Pvt Ltd and SAIF IIT Madras for providing facility to carry out the work.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2017.08.011
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.The Siddha formulary of India, Part I. 1st ed. Government of India, Ministry of Health and Family welfare, Department of AYUSH; 1992. pp. 99–109. [Google Scholar]
- 2.Kalavathy N., Rajalakshmi S., Sundharam M., Veluchami G. A siddha remedy for chronic ulcer. Indian J Hosp Pharm. 1984;21(1):30–35. [Google Scholar]
- 3.Lahorkar P., Ramitha K., Bansal V., Anantha Narayana D.B. A comparative evaluation of medicated oils prepared using Ayurvedic and modified processes. Indian J Pharm Sci. 2009;71(6):656–662. doi: 10.4103/0250-474X.59548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formulary of Siddha medicine. 4th ed. The Indian Medical Practitioner's Co-Operative Pharmacy and Stores Ltd; Madras 41: 1993. p. 279. [Google Scholar]
- 5.Qualitity standard for certain Siddha formulation . Ministry of health and Family welfare, Govt of India; New Delhi: 1991. Central council for research in Ayurveda and Siddha; p. 131. [Google Scholar]
- 6.Nair N.C., Henry A.N. Botanical Survey of India; 1987. Flora of Tamilnadu; p. 113. [Google Scholar]
- 7.The ayurvedic pharmacopoeia of India, Part II. 1st ed. vol. 1. Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homeopathy; National Institute of Science Communication and Information Resources (NISCAIR) CSIR; Dr. K. S. Krishnan Marg, New Delhi 110012: 2008. pp. 190–203. The Controller of Publication Civil Line Delhi 110054 New Delhi. [Google Scholar]
- 8.Nadkarni K.M. 3rd ed. vol. 2. Popular Prakasham Pvt Ltd; Mumbai India: 1976. p. 52. (Copper purification. Indian materia medica). [Google Scholar]
- 9.Quality control standards for certain Siddha formulation . Ministry of Health and Family Welfare, Govt. of India; New Delhi: 1991. Central Council for Research in Ayurveda and Siddha; p. 59. [Google Scholar]
- 10.Thiyagarajan R. Department of Indian medicine and homeopathy; Chennai 600 106: 2013. Gunapadam part 2 and 3; p. 555. [Google Scholar]
- 11.Harborne J.B. 2nd ed. Chapman & Hall; London: 1973. Phytochemical methods; pp. 49–188. London. [Google Scholar]
- 12.Sethi P.D. 1st ed. vol. 10. CBS Publishers and Distributors; New Delhi: 1996. High performance thin layer chromatography; pp. 4–28. [Google Scholar]
- 13.Stahl I. Student edition. Springer-Verlag; Berlin: 1969. Thin layer chromatography. A laboratory hand book; pp. 52–105. [Google Scholar]
- 14.Wagner H., Baldt S., Zgainski E.M. vol. 54. Springer; Berlin: 1996. pp. 35–118. (Plant drug analysis). [Google Scholar]
- 15.Saraswathy A., Girijarani M., Joy S., Alam M.M. Analytical studies on Mattan Tailam. Anc Sci Life. 1999;18(3&4):199–204. [PMC free article] [PubMed] [Google Scholar]
- 16.Murugesa mudhaliyar . 9th ed. Department of Indian medicine and homeopathy; Chennai-106: 2013. Siddha material Medica (Gunappadam) part 1; p. 143. [Google Scholar]
- 17.Dhatura Shastri J.L.N. vol. 83. Choukhamba publication; Varanasi, India: 2005. p. 384. (Text book of dravyaguna vinjanan). [Google Scholar]
- 18.Nadkarni K.M. 3rd ed. popular prakasham pvt ltd; Mumbai India 2: 1976. Copper purification. Indian materia medica; p. 52. [Google Scholar]
- 19.Thiyagarajan R. 4th ed. Department of Indian medicine and homeopathy; Chennai-106: 1992. Gunapadam, Thadhu - Jeva vaguppu Part II and III. English version by Dr, Anaivaari, Anandhan R. Dr. M. Thulasimani, Siddha material medica, Mineral and Animal Kingdom. 1st ed. Translation and Publication Wing. Department of Indian medicine and homeopathy, Chennai-106 (2008) 418. [Google Scholar]
- 20.Thottam P.J. vol. 89. Sura Books; Chennai: 2005. (Modernizing Ayurveda). [Google Scholar]
- 21.James E.T. 1946. US patent entitled “Process for making copper soaps” Patent No. US 2397767 A. [Google Scholar]
- 22.http://www.faithful-to-nature.co.za/Biogrow-Copper-Soap-Fungicide-p-3825.html.
- 23.Srivastava P., Durgaprasad S. Burn wound healing property of Cocos nucifera: an appraisal. Indian J Pharmacol. 2008;40:144–146. doi: 10.4103/0253-7613.43159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reid K.A., Maes A., Van Staden J., De Kimpe N., Mulholl D.A., Vereschaeve L. Evaluation of the mutagenic and anti mutagenic effects of South African plants. J ethanopharmacol. 2006;106:44–50. doi: 10.1016/j.jep.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 25.Mishra D.N., Dixit V., Mishra A.K. Mycotoxic evaluation of some higher plants against ring worm causing fungi. Indian drugs. 1991;2:300–303. [Google Scholar]
- 26.Donatus E.O., Ephraim C.I. Isolation, characterization and antibacterial activity of alkaloid from Datura metel Linn leaves. Afr J Pharm Pharmacol. 2009;3(5):277–281. [Google Scholar]
- 27.Varahalarao V., Kaladhar D.S.V.G.K. Antimicrobial study of plant extracts of Datura metel L. against some important disease causing pathogens. Asian Pac J Trop Dis. 2012:S94–S97. [Google Scholar]
- 28.Saroja P.R., Veluchamy G. Simple medicine in Siddha system. In: Subramanian S.V., Madhaven V.R., editors. Heritage of Tamil Siddha medicine. vol. 193. International Institute of Tamil Studies; Madras, India: 1983. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





