Abstract
Purpose
The aim of the present study was to evaluate the pathological and oncological outcomes of laparoscopic radical prostatectomy (LRP) and robot-assisted radical prostatectomy (RARP) performed by one surgeon at a single center.
Subjects
We evaluated 700 patients with localized prostate cancer (i.e., 250 received LRP and 450 received RARP) in the study. The clinicopathological outcomes, positive surgical margin (PSM) frequency, and biochemical recurrence (BCR)–free survival were compared between LRP and RARP.
Results
At diagnosis, the median patient age and level of prostate-specific antigsen in the serum for LRP were 68 years and 8.1 ng/ml, respectively, while those for RARP were 66 years and 7.7 ng/ml, respectively. In the LRP group, the overall PSM rate was 31.2% (11.1% for pT2a, 19.0% for pT2b, 25.0% for pT2c, 60.0% for pT3a, 64.3% for pT3b, and 50% for pT4). In the RARP group, the overall PSM rate was 20.7% (4.8% for pT2a, 15.9% for pT2b, 12.9% for pT2c, 36.9% for pT3a, 46.2% for pT3b, and 100% for pT4). The PSM rate was significantly lower for RARP in men with pT2c, pT3a, or pT3b disease (p = 0.006, p = 0.009, and p = 0.027, respectively). Based on the multivariate analysis, RARP reduced the risk of BCR (hazard ratio = 0.8, p = 0.014).
Conclusions
We compared the pathological findings and rates of BCR-free survival between patients who received LRP and those who received RARP at a single center. The rate of BCR-free survival was significantly higher in men classified as D'Amico high-risk patients who received RARP versus that reported in D'Amico high-risk patients who received LRP.
Keywords: Laparoscopic radical prostatectomy, Oncological outcome, Robot-assisted radical prostatectomy
1. Introduction
Robot-assisted radical prostatectomy (RARP)—an advancement of minimally invasive surgery—is aimed at reducing the difficulty associated with complex laparoscopic surgery. Given the technological advances in the surgical field (e.g., three-dimensional views, instruments with seven degrees of freedom, and optical power), RARP has become a widely used to manage surgical approach for the treatment of localized prostate cancer (PC) in Japan. However, thus far, there are no large randomized controlled trials showing the superiority of RARP over laparoscopic radical prostatectomy (LRP) [1,2]. A systematic review comparing the oncological outcomes associated with these two surgical approaches was inconclusive regarding the superiority of RARP versus LRP [3, 4, 5].
The aim of the present study was to compare the pathological and oncological outcomes—including localization of a positive surgical margin (PSM)—in men receiving LRP or RARP performed by one surgeon at a single center.
2. Subjects
2.1. Patient characteristics
The study included patients who received radical prostatectomy for localized PC between April 1, 2007 and March 31, 2018 at the Kyorin University Hospital, Tokyo, Japan. The study was approved by the ethics committee (approval number: H30-053) of the hospital. Written informed consent was provided by all patients prior to surgery. Treatment-naïve patients (n = 700; LRP, n = 250; and RARP, n = 450) with localized prostate cancer enrolled in the study (Table 1). All patients were not received therapy (radiotherapy and/or androgen therapy) before surgery. In Japan, RARP that covered by insurance since April 2012 was performed in all patients with localized PC at our hospital. A significant difference was observed only in median follow-up (35 months for LRP vs 61 months for RARP, p = 0.02).
Table 1.
Patient's characteristics in patients with localized prostate cancer received LRP or RARP.
| LRP (n = 250) | RARP (n = 450) | P value | |
|---|---|---|---|
| Median age (ranges) | 68 (51–76) | 66 (48–82) | 0.13 |
| BMI(kg/m2) | 24.0 (20.8-27.6) | 24.2 (20.2-26.5) | 0.24 |
| Median prostate volume (g) (ranges) | 46.9 (27-65.1) | 49.5 (31-82.5) | 0.15 |
| Median preoperative membranous urethral length (MUL)mm | 10.3 (8.7-12.6) | 10.8 (7.9-13.3) | 0.21 |
| Median serum PSA (ng/mL) (ranges) | 8.1 (4.2–46) | 7.7 (3.8–49) | 0.26 |
| Gleason score (%) | |||
| 6 | 108 (43) | 144 (32) | |
| 7 | 113 (45) | 234 (52) | 0.27 |
| 8–10 | 29 (12) | 72 (16) | |
| D'Amico classification (%) | |||
| Low | 85 (34) | 129 (29) | |
| Intermediate | 134 (54) | 252 (56) | 0.32 |
| High | 31 (12) | 69 (15) | |
| Median follow-up (months) | 61 | 35 | 0.02 |
LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; PSA, prostate-specific antigen; BMI, body mass index.
The follow-up including trimonthly visits for 5 years and annual visits thereafter. Biochemical recurrence (BCR) was defined as a consecutive increase in the level of prostate-specific antigen (PSA) in the serum > 0.2 ng/ml. Several patients who developed BCR subsequently received radiotherapy and/or androgen therapy.
2.2. Surgical techniques
We performed LRP using the posterior approach to the seminal vesicle based on the method described in the Montsouris technique [6]. The same approach was also adopted for the RARP procedure [7]. For those who received surgery, preservation of cavernous nerves was performed on the cancer-negative lobe. Bilateral preservation was limited in patients in whom the cancer was located at the transitional zone. Limited dissection of the lymph nodes was performed for all patients. Preservation of the neurovascular bundle was performed in 23 (9.2%) and 47 (10.4%) patients who received LRP and RARP, respectively. In our center, most of the patients think that complete cancer resection is more important than function preservation.
2.3. Statistical analysis
The results are expressed as means and range values. Continuous variables were compared between the two groups using the Student t test and Mann–Whitney U test. The Kaplan–Meier method was used to plot BCR-free survival curves, which were verified using the Wilcoxon test. In addition, the χ2 test was used to analyze nominal data. All statistical analyses were performed using the JMP 12.0 software (SAS Institute, Cary, NC, USA).
3. Results
The characteristics of patients (e.g., age, levels of PSA in the serum, Gleason score, and D'Amico risk classification) are shown in Table 1 [8]. In accordance with the D'Amico classification, in the LRP group, there were 85, 134, and 31 patients at low, intermediate, and high risk, respectively. In the RARP group, these numbers were 129, 252, and 69 patients, respectively. In accordance with the Gleason score and D'Amico risk classification, the RARP group did not exhibit significantly advanced PC versus the LRP group.
The data (i.e., pathological results, statistical analyses, and salvage therapy) of the two groups are summarized in Table 2. Based on the Gleason score and pathological stage, the RARP group did not demonstrate significantly advanced PC versus the LRP group. In the LRP group, the overall PSM rate was 31.2% (11.1% for pT2a, 19.0% for pT2b, 25.0% for pT2c, 60.0% for pT3a, 64.3% for pT3b, and 50% for pT4) (Table 3). In the RARP group, the overall PSM rate was 20.7% (4.8% for pT2a, 15.9% for pT2b, 12.9% for pT2c, 36.9% for pT3a, 46.2% for pT3b, and 100% for pT4). For RARP, the PSM rate was significantly lower in men with pT2c, pT3a, or pT3b disease (p = 0.006, p = 0.009, and p = 0.027, respectively) (Table 3). The PSM site was classified as follows: the base, lateral lobe, apex, anterior, posterior, periprostatic fat tissues, or seminal vesicle. In the RARP group, PSM localization was significantly less frequent versus the LRP group at the lateral lobe (13% vs. 5.1%, respectively, p < 0.0001) and apex (28% vs. 10%, respectively, p < 0.0001).
Table 2.
Pathological and oncological outcomes in men received LRP or RARP.
| LRP (n = 250) | RARP (n = 450) | Pvalue | ||
|---|---|---|---|---|
| Gleason score (%) | 6 | 71 (28.4) | 132 (29.3) | 0.173 |
| 7 | 147 (58.8) | 238 (52.9) | ||
| 8–10 | 32 (12.8) | 80 (17.8) | ||
| Pathological T stage (%) | 0 | 0 (0) | 1 (0.2) | N.A. |
| 2a | 27 (10.8) | 41 (9.1) | 0.276 | |
| 2b | 42 (16.8) | 69 (18.3) | 0.342 | |
| 2c | 120 (48.0) | 201 (44.7) | 0.396 | |
| 3a | 45 (18.0) | 111 (24.7) | 0.025 | |
| 3b | 14 (5.6) | 26 (5.8) | 0.535 | |
| 4 | 2 (0.8) | 1 (0.1) | 0.293 | |
| Lymphovascular invasion (%) | 0 | 182 (72.8) | 272 (60.4) | 0.001 |
| 1 | 68 (27.2) | 178 (49.6) | ||
| Perineural invasion | 0 | 102 (40.8) | 130 (28.9) | 0.001 |
| 1 | 148 (59.2) | 320 (71.1) | ||
| N stage (%) | 0 | 246 (98.4) | 419 (93.1) | 0.002 |
| Positive surgical margin (%) | Total | 78 (31.2) | 93 (20.7) | 0.002 |
| BCR (%) | 53 (21.2) | 46 (10.2) | <0.0001 | |
| Salvage therapy | RT | 3 | 16 | |
| ADT | 16 | 14 | ||
| RT + ADT | 9 | 12 | ||
| Surveillance | 0 | 0 | ||
LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; BCR, biochemical recurrence; RT, radiation therapy; ADT, androgen deprivation therapy.
Table 3.
Comparison of the location of PSM sites between LRP and RARP.
| LRP (n = 250) | RARP (n = 450) | Pvalue | ||
|---|---|---|---|---|
| PSM (%) | Total | 78 (31.2) | 93 (20.7) | 0.002 |
| pT2a | 3/27 (11.1) | 2/41 (4.8) | 0.335 | |
| pT2b | 8/42 (19.0) | 11/69 (15.9) | 0.674 | |
| pT2c | 30/120 (25.0) | 26/201 (12.9) | 0.006 | |
| pT3a | 27/45 (60.0) | 41/111 (36.9) | 0.009 | |
| pT3b | 9/14 (64.3) | 12/26 (46,2) | 0.027 | |
| pT4 | 1/2 (50.0) | 1/1 (100) | 0.667 | |
| Sites of PSM (%) | Base | 33 (6.7) | 29 (6.4) | 0.003 |
| Lateral lobe | 62 (13) | 23 (5.1) | <0.0001 | |
| Apex | 136 (28) | 45 (10.0) | <0.0001 | |
| Anterior | 5 (1) | 4 (0.8) | 0.183 | |
| Posterior | 3 (0.6) | 1 (0.2) | 0.133 | |
| Fat tissues | 2 (0.4) | 5 (1.1) | 0.516 | |
| Seminal vesicle | 1 (0.2) | 6 (1.3) | 0.22 | |
LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; PSA, prostate-specific antigen.
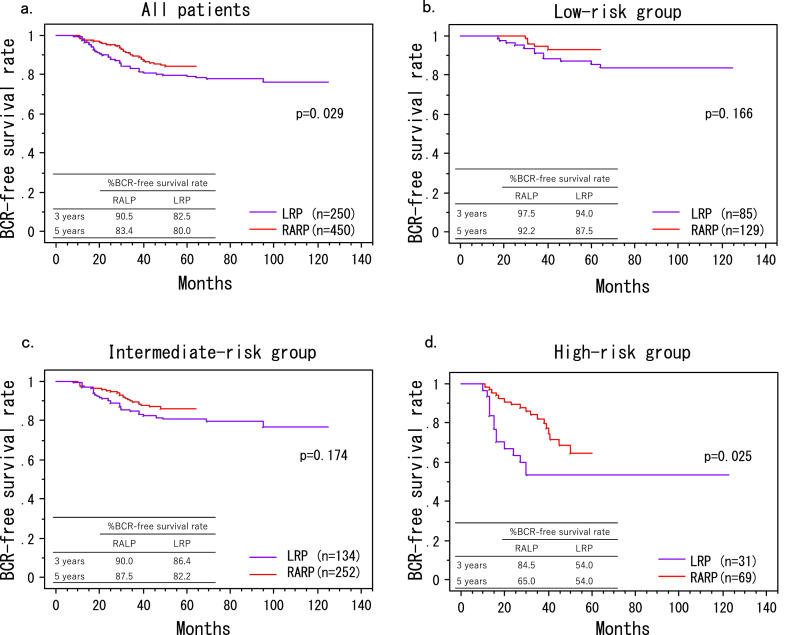
At the end of the follow-up period, 53 (21.2%) and 46 (10.2%) patients developed BCR in the LRP and RARP groups, respectively. The BCR-free survival rate was significantly higher among men who received RARP compared with that observed in patients who received LRP (p = 0.029, Fig. 1a). Moreover, there were statistically significant differences in the number of patients classified as D'Amico high-risk between the LRP and RARP groups (p = 0.024) (Fig. 1d). However, there were no statistically significant differences in the number of patients classified as D'Amico low- or intermediate-risk (p = 0.166, p = 0.174; Fig. 1b and c, respectively).
Fig. 1.
Rates of biochemical recurrence (BCR)-free survival rates forin men who underwentreceived RARP or LRP. (a) all patients, (b) the D’Amico low--risk groups, (c) intermediate-risk groups, (d) high-risk groups. The rate of BCR-free survival was significantly higher among men who received RARP compared with that reported in patients who received LRP (A: p = 0.029), especially for those classified as D'Amico high-risk patients (D: p = 0.024). There were no significant differences in the number of patients classified as D'Amico low- or intermediate-risk patients (B: p = 0.166, C: p = 0.174). LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy.
Univariate and multivariate proportional analyses were performed to investigate the association between the rate of BCR-free survival following these surgical procedures and clinicopathological characteristics of patients (Table 4). In the univariate analysis, the levels of PSA in the serum (p = 0.033), Gleason score (p = 0.042), extra prostatic extension (p < 0.001), lymphovascular invasion (p = 0.034), perineural invasion (p < 0.001), resection margin (p < 0.001), lymph node metastasis (p < 0.001), and RARP (p = 0.029) were identified as significant prognostic predictors. In the multivariate analysis, RARP, perineural invasion, and the resection margin were identified as independent predictors of BCR (p = 0.014, p = 0.001, and p = 0.002, respectively) (see Table 5).
Table 4.
Univariate and multivariate proportional hazard analyses of PSA relapse (n = 800).
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% index | p value | Hazard ratio | 95% index | p value | |
| Serum PSA level (ng/mL) (≥7.9 vs. <7.9) | 1.6 | 1.1–2.4 | <0.001 | |||
| Gleason score (≥8 vs. ≤7) | 2.1 | 1.4–3.1 | 0.009 | - | - | - |
| Extra prostatic extension (1 vs. 0) | 2.3 | 1.6–3.6 | <0.001 | - | - | - |
| Lymphovascular invasion (1 vs. 0) | 1.4 | 1.2–2.5 | 0.006 | 1.5 | 0.8–1.7 | 0.053 |
| Perineural invasion (1 vs. 0) | 1.8 | 1.2–2.9 | <0.001 | 1.6 | 1.4–2.2 | 0.001 |
| Surgical margin (1 vs. 0) | 2.6 | 2.1–4.3 | <0.001 | 1.7 | 1.4-2.5 | 0.002 |
| Lymph node metastasis (1 vs.0) | 3.1 | 1.1–7.1 | <0.001 | - | - | - |
| RARP vs LRP | 0.8 | 0.3–1.2 | 0.004 | 0.8 | 0.3-0.9 | 0.014 |
LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; PSA, prostate-specific antigen.
Table 5.
Characteristics, positive surgical rates, and biochemical recurrence in comparative studies evaluating LRP and RARP.
| Author, years country (ref) |
Asimakopoulos, 201116) |
Asimakopoulos, 201318) |
Hakimi, 200919) |
Papachristos, 201520) |
Park, 201321) |
Ploussard, 201422) |
Porpiglia, 201317) |
Stolzenburg, 201323) |
Wolanski, 201324) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study design |
RCT |
Prospective |
Retrospective |
Retrospective |
Retrospective |
Prospective |
RCT |
Prospective |
Retrospective |
|||||||||
| Procedure | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP | LRP | RARP |
| No. of cases | 60 | 52 | 91 | 136 | 75 | 75 | 100 | 100 | 144 | 183 | 1377 | 1009 | 60 | 60 | 100 | 100 | 87 | 73 |
| Age, years medians/means | 61.1 ± 5.1 | 59.6 ± 5.4 | 63 ± 4.9 | 60 ± 5.5 | 59.6 (43-72) | 59.8 (42-71) | 62.5 (45-72) | 60.5 (45-75) | 67 (38-77) | 67 (44-71) | 62.7 | 62.7 | 64.7 ± 5.9 | 63.9 ± 6.7 | 61.33 ± 7.4 | 61.21 ± 7.7 | 61.3 ± 6.5 | 61.4 ± 7.2 |
| PSA, ng/ml, median/means | 7.37 (1.5-9.15) | 8.9 (5.8-92) | 6 | 6.4 | 7.5 | 8.4 | 7.1 (1.8-37) | 5.5 (0.72-35) | 5.84 (0.008-41.26) | 4.98 (0.05-51.46) | 9.8 | 9.2 | 8.3 ± 6.5 | 6.9 ± 4.2 | 10.7 ± 11.49 | 8.75 ± 7.1 | 6.4 | 6 |
| Pathology stage, n (%) | ||||||||||||||||||
| ≦pT2 | 52 (86.7%) | 43 (82.7%) | 77 (84.6%) | 118 (86.8%) | 71 (84.6%) | 64 (85.3%) | 90 (62.5%) | 127 (69.4%) | 815 (59.6%) | 585 (58.0%) | 38 (63.3%) | 38 (63.3%) | 77 (77.0%) | 67 (67.0%) | 63 (72.4%) | 49 (67.1%) | ||
| ≥pT3 | 8 (13.3%) | 9 (17.3%) | 14 (15.4%) | 18 (13.2%) | 4 (5.3%) | 11 (14.7%) | 54 (37.5%) | 56 (30.6%) | 562 (40.8%) | 424 (42.0%) | 22 (36.7%) | 22 (36.7%) | 23 (23.0%) | 33 (33.0%) | 24 (27.6%) | 24 (33.9%) | ||
| Overall PSM, n (%) | 6 (10.0%) | 8 (15.4%) | 6 (6.6%) | 21 (15.4%) | 10 (13.3%) | 9 (12.0%) | 22 (15.3%) | 25 (13.7%) | 366 (26.6%) | 316 (31.3%) | 12 (20.0%) | 16 (26.6%) | 14 (14.0%) | 19 (19.0%) | 12 (13.8%) | 9 (12.3%) | ||
| pT2, PSM, n/M(%) | 4/52 (7.7%) | 3/43 (7.0%) | 9/71 (12.7%) | 7/64 (10.9%) | 6/90 (6.7%) | 14/127 (11.0%) | 6/37 (16.2%) | 5/37 (13.5%) | 5/77 (6.5%) | 6/67 (9.0%) | 0/63 (0%) | 0/49 (0%) | ||||||
| >pT3 PSM, n/M(%) | 2/8 (25.0%) | 5/9 (55.6%) | 1/4 (25.0%) | 2/11 (18.2%) | 16/54 (29.6%) | 11/56 (19.6%) | 6/22 (27.3%) | 11/22 (50.0%) | 9/23 (39.1%) | 13/33 (39.4%) | 12/24 (50.0%) | 9/24 (37.5%) | ||||||
| Follow-up, mo | 12 | 12 | 21 | 18 | 48 | 17 | 12 | 12 | 19 | 13 | 39 | 15.4 | 12 | 12 | 3 | 3 | 3 | 3 |
| BCR definition, ng/ml | PSA ≥0.2 | PSA ≥0.2 | PSA ≥0.2 | PSA >0.1 | PSA ≥0.2 | PSA ≥0.2 | PSA ≥0.2 | PSA ≥0.2 | PSA ≥0.4 | |||||||||
| Overall BCR, n/N (%) | 2/60 (3.3%) | 4/52 (7.7%) | 0/91 (0%) | 6/136 (4.4%) | 4.75 (6.7) | 5/75 (5.3) | 10/89 (11.2) | 5/97 (5.3) | 24/144 (16.7) | 24/183 (13.1) | 248/1377 (18.0) | 104/1009 (10.3) | 4/53 (7.5) | 1/50 (2.0) | 6/100 (6.0) | 8/100 (8.0) | 2/87 (2.3) | 1/73 (1.4) |
LRP, laparoscopic radical prostatectomy; RARP, robot-assisted radical prostatectomy; PSM, positive surgical margin; BCR, biochemical recurrence; PSA, prostate-specific antigen; RCT, randomized controlled trial.
4. Discussion
RARP is widely used for the management of localized PC. The perioperative advantages offered by laparoscopic surgery—apart from minimal invasiveness—include a lower surgical margin rate for patients with intermediate- and high-risk disease [9, 10, 11, 12, 13, 14, 15]. Our study demonstrated that the overall PSM rate for LRP (31.2%) was higher than that observed for RARP (20.7%). In addition, the PSM rate was significantly lower for RARP in men with pT2c, pT3a, or pT3b disease (p = 0.006, p = 0.009, and p = 0.027, respectively). In addition, the PSM rates after LRP were higher versus those reported in other studies (Table 4) [16, 17, 18, 19, 20, 21, 22, 23, 24]. In a systematic review investigating PSM after RARP, Yossepowitch et al [25] reported that the average PSM rate for RARP was 15% (range: 6.5 – 32%). Of note, this rate was higher in men with disease of a more advanced pathological stage. Tozawa et al [26] reported that the distributions of PSM following LRP and RARP are significantly different. The investigators found that apical PSM was more commonly observed in LRP versus RARP because of the unclear visualization of the apex in the LRP. The results of the present study are consistent with this finding. In this study, the significantly reduced PSM rate may be attributed to our careful incision at the apex and lateral sites during RARP. There are several reasons for the improved surgical outcomes with RARP versus LRP, especially at the apex and lateral sites. First, apical dissection was easily performed during RARP. During both procedures, the bunching technique was used for the preparation of the dorsal vein complex (DVC). In LRP, DVC resection was performed using LigaSure™ (Medtronic, Minneapolis, Minn). However, this technique may alter the shape of the apex or tear the prostatic capsule, resulting in exposure of the tumor. In contrast, in RARP, the DVC was cut using scissors. Therefore, a large or complex-shaped prostate in the apex may have been more accurately dissected. Second, during RARP, the lateral side of the prostate—including the periprostatic tissues—was dissected using a fourth arm for counter traction. Notably, the most relevant predictors of PSM rates in LRP and RARP include the skill of the surgeon, tumor stage, preoperative level of PSA, Gleason score, and volume of the prostate [27, 28, 29].
Regarding BCR, Huang performed a meta-analysis showing that the overall BCR rates for LRP and RARP were similar. [30] In the present study, the rate of BCR-free survival was significantly higher in men who received RARP versus that observed in patients who received LRP. The rate of BCR-free survival was significantly higher in men classified as D'Amico high-risk patients who received RARP versus that reported in D'Amico high-risk patients who received LRP. However, there were no significant differences between those classified as D'Amico low-risk or intermediate-risk patients. Moreover, PSM following surgery was identified as a significant independent predictor of BCR [31, 32]. The PSM was associated with an increased risk of BCR after surgery, and the PSM rate was higher in men with disease of a more advanced pathological stage. In theory, the BCR rate for advanced tumors is expected to be higher than that observed for early stage tumors. In the present study, the outcomes recorded after RARP were better versus those observed after LRP in terms of PSM in patients with pT2c, pT3a, or pT3b disease. The rate of BCR-free survival in those classified as D'Amico high-risk patients may be significantly higher after RARP versus that reported after LRP. Further studies are required to determine the oncological benefit of RARP in high-risk patients.
The limitations of this study were its retrospective design, single-center investigation, one surgeon, and the relatively short follow-up period for the RARP group. Therefore, future studies with longer follow-up periods, comparing BCR at different stages of tumors, are warranted.
5. Conclusions
In this study, we observed improved oncological outcomes for patients who received RARP versus those reported in patients who received LRP. Additional follow-up is necessary to determine the importance of these results for the rate of mortality related to PC.
Funding
There was no funding support for this study.
Availability of data and materials
The datasets of this study are available at the Department of Urology, Kyorin University School of Medicine, Tokyo, Japan.
Author contributions
T.O. collected the data and assisted in designing the study and writing the manuscript. H.F., S.O., M.S., and N.N. collected the data and assisted in designing the study. T.O. assisted in performing the statistical analysis. S.T., Y.N., T.Y., and M.T. assisted in designing the study and provided important suggestions in writing the manuscript. All the authors read and approved the final manuscript.
Ethics approval
The study was approved by the Ethics Committee of Kyorin University School of Medicine, Tokyo, Japan (approval number: H30-053), and written informed consent was provided by all individual patients.
Conflicts of interest
The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2019.09.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. European Association of Urology EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative inteßnt-update 2013. Eur Urol. 2014;65:124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 2.Bianco F.J., Jr., Scardino P.T., Eastham J.A. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function (“trifecta”) Urology. 2005;66:83–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 3.Robertson C., Close A., Fraser C., Gurung T., Jia X., Sharma P. Relative effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localised prostate cancer: a systematic review and mixed treatment comparison meta-analysis. BJU Int. 2013;112:798–812. doi: 10.1111/bju.12247. [DOI] [PubMed] [Google Scholar]
- 4.Ficarra V., Novara G., Artibani W., Cestari A., Galfano A., Graefen M. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55:1037–1063. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 5.Moran P.S., O'Neill M., Teljeur C., Flattery M., Murphy L.A., Smyth G. Robot-assisted radical prostatectomy compared with open and laparoscopic approaches: a systematic review and meta-analysis. Int J Urol. 2013;20:312–321. doi: 10.1111/iju.12070. [DOI] [PubMed] [Google Scholar]
- 6.Guillonneau B., Vallancien G. Laparoscopic radical prostatectomy: the Montsouris technique. J Urol. 2000;163:1643–1649. doi: 10.1016/s0022-5347(05)67512-x. [DOI] [PubMed] [Google Scholar]
- 7.Zorn K.C. Robotic radical prostatectomy: advantages of an initial posterior dissection. J Robot Surg. 2008;2:135–137. doi: 10.1007/s11701-008-0091-9. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico A.V., Whittington R., Malkowicz S.B., Schultz D., Blank K., Broderick G.A. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. J Am Med Assoc. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 9.Yaxley J.W., Coughlin G.D., Chambers S.K., Occhipinti S., Samaratunga H., Zajdlewicz L. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: early outcomes from a randomised controlled phase 3 study. Lancet. 2016;388:1057–1066. doi: 10.1016/S0140-6736(16)30592-X. [DOI] [PubMed] [Google Scholar]
- 10.Alemozaffar M., Sanda M., Yecies D., Mucci L.A., Stampfer M.J., Kenfield S.A. Benchmarks for operative outcomes of robotic and open radical prostatectomy: results from the Health Professionals Follow-up Study. Eur Urol. 2015;67:432–438. doi: 10.1016/j.eururo.2014.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandaglia G., Sammon J.D., Chang S.L., Choueiri T.K., Hu J.C., Karakiewicz P.I. Comparative effectiveness of robot-assisted and open radical prostatectomy in the postdissemination era. J Clin Oncol. 2014;32:1419–1426. doi: 10.1200/JCO.2013.53.5096. [DOI] [PubMed] [Google Scholar]
- 12.Novara G., Ficarra V., Rosen R.C., Artibani W., Costello A., Eastham J.A. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol. 2012;62:431–452. doi: 10.1016/j.eururo.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Tewari A., Srivasatava A., Menon M., Members of the VIP Team A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2013;92:205–210. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 14.Trinh Q.D., Sammon J., Sun M., Ravi P., Ghani K.R., Bianchi M. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol. 2012;61:679–685. doi: 10.1016/j.eururo.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Leow J.J., Chang S.L., Meyer C.P., Wang Y., Hanske J., Sammon J.D. Robot-assisted versus open radical prostatectomy: a contemporary analysis of an all-payer discharge database. Eur Urol. 2016;70:837–845. doi: 10.1016/j.eururo.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 16.Asimakopoulos A.D., Pereira Fraga C.T., Annino F., Pasqualetti P., Calado A.A., Mugnier C. Randomized comparison between laparoscopic and robot-assisted nerve-sparing radical prostatectomy. J Sex Med. 2011;8:1503–1512. doi: 10.1111/j.1743-6109.2011.02215.x. [DOI] [PubMed] [Google Scholar]
- 17.Porpiglia F., Morra I., Lucci Chiarissi M., Manfredi M., Mele F., Grande S. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013;63:606–614. doi: 10.1016/j.eururo.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Asimakopoulos A.D., Miano R., Di Lorenzo N., Spera E., Vespasiani G., Mugnier C. Laparoscopic versus robot-assisted bilateral nerve-sparing radical prostatectomy: comparison of pentafecta rates for a single surgeon. Surg Endosc. 2013;27:4297–4304. doi: 10.1007/s00464-013-3046-9. [DOI] [PubMed] [Google Scholar]
- 19.Hakimi A.A., Blitstein J., Feder M., Shapiro E., Ghavamian R. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: single-surgeon experience. Urology. 2009;73:119–123. doi: 10.1016/j.urology.2008.08.491. [DOI] [PubMed] [Google Scholar]
- 20.Papachristos A., Basto M., Te Marvelde L., Moon D. Laparoscopic versus robotic-assisted radical prostatectomy: an Australian single-surgeon series. ANZ J Surg. 2015;85:154–158. doi: 10.1111/ans.12602. [DOI] [PubMed] [Google Scholar]
- 21.Park B., Kim W., Jeong B.C., Jeon S.S., Lee H.M., Choi H.Y. Comparison of oncological and functional outcomes of pure versus robotic-assisted laparoscopic radical prostatectomy performed by a single surgeon. Scand J Urol. 2013;47:10–18. doi: 10.3109/00365599.2012.696137. [DOI] [PubMed] [Google Scholar]
- 22.Ploussard G., de la Taille A., Moulin M., Vordos D., Hoznek A., Abbou C.C. Comparisons of the perioperative, functional, and oncologic outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014;65:610–619. doi: 10.1016/j.eururo.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 23.JU Stolzenburg, Qazi H.A., Holze S., Mende M., Nicolaus M., Franz T. Evaluating the learning curve of experienced laparoscopic surgeons in robot-assisted radical prostatectomy. J Endourol. 2013;27:80–85. doi: 10.1089/end.2012.0262. [DOI] [PubMed] [Google Scholar]
- 24.Wolanski P., Chabert C., Jones L., Mullavey T., Walsh S., Gianduzzo T. Preliminary results of robot-assisted laparoscopic radical prostatectomy (RALP) after fellowship training and experience in laparoscopic radical prostatectomy (LRP) BJU Int. 2012;110:64–70. doi: 10.1111/j.1464-410X.2012.11479.x. [DOI] [PubMed] [Google Scholar]
- 25.Yossepowitch O., Briganti A., Eastham J.A., Epstein J., Graefen M., Montironi R. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303–313. doi: 10.1016/j.eururo.2013.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Tozawa K., Yasui T., Umemoto Y., Mizuno K., Okada A., Kawai N. Pitfalls of robot-assisted radical prostatectomy: a comparison of positive surgical margins between robotic and laparoscopic surgery. Int J Urol. 2014;21:976–979. doi: 10.1111/iju.12492. [DOI] [PubMed] [Google Scholar]
- 27.Kasraeian A., Barret E., Chan J., Sanchez-Salas R., Validire P., Cathelineau X. Comparison of the rate, location and size of positive surgical margins after laparoscopic and robot-assisted laparoscopic radical prostatectomy. BJU Int. 2011;108:1174–1178. doi: 10.1111/j.1464-410X.2010.10077.x. [DOI] [PubMed] [Google Scholar]
- 28.Novara G., Ficarra V., Mocellin S., Ahlering T.E., Carroll P.R., Graefen M. Systematic review and meta-analysis of studies reporting oncologic outcomes after robot-assisted radical prostatectomy. Eur Urol. 2012;62:382–404. doi: 10.1016/j.eururo.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 29.Karavitakis M., Ahmed H.U., Abel P.D., Hazell S., Winkler M.H. Margin status after laparoscopic radical prostatectomy and the index lesion: implications for preoperative evaluation of tumor focality in prostate cancer. J Endourol. 2012;26:503–508. doi: 10.1089/end.2011.0345. [DOI] [PubMed] [Google Scholar]
- 30.Huang X., Wang L., Zheng X., Wang X. Comparison of perioperative, functional, and oncologic outcomes between standard laparoscopic and robotic-assisted radical prostatectomy: a systemic review and meta-analysis. Surg Endosc. 2017;31:1045–1060. doi: 10.1007/s00464-016-5125-1. [DOI] [PubMed] [Google Scholar]
- 31.Blute M.L., Bostwick D.G., Bergstralh E.J., Slezak J.M., Martin S.K., Amling C.L. Anatomic site-specific positive margins in organ-confined prostate cancer and its impact on outcome after radical prostatectomy. Urology. 1997;50(5):733–739. doi: 10.1016/S0090-4295(97)00450-0. [DOI] [PubMed] [Google Scholar]
- 32.Aydin H., Tsuzuki T., Hernandez D., Walsh P.C., Partin A.W., Epstein J.I. Positive proximal (bladder neck) margin at radical prostatectomy confers greater risk of biochemical progression. Urology. 2004;64(3):551–555. doi: 10.1016/j.urology.2004.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets of this study are available at the Department of Urology, Kyorin University School of Medicine, Tokyo, Japan.