Abstract
Background
Withaferin-A (WA), an active principle obtained from a traditional Indian herb known as Ashwagandha or the Indian ginseng, has been shown to prevent and cure urethane-induced lung tumors in mice, and also inhibit the growth of transplanted sarcoma in mice.
Objectives
In this study, we evaluated the safety and pharmacokinetics of WA in patients with advanced stage high-grade osteosarcoma.
Methods
A phase I dose escalation study was planned using the classical 3 + 3 design (C33D). Dose escalation cohorts comprised of 72, 108, 144 and 216 mg of WA administered in two to four divided doses per day. Three patients were enrolled in each cohort and the last patient was observed for at least 30 days for any dose-limiting toxicity before progressing to a higher cohort. Pharmacokinetic studies were performed using high performance liquid chromatography (HPLC) technique with sensitivity up to 50 ng/ml. Safety evaluation including clinical examination, detailed history of adverse events, Liver Function Tests , Renal Function Tests and complete blood counts were performed at each visit. WA was administered daily till progression. Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 was used for grading adverse events.
Results
The formulation used was generally well tolerated. Eleven adverse events of grade 1 or grade 2 severity were observed. No grade 3 or grade 4 adverse events were observed. Elevation of liver enzymes (5/11) and skin rash (2/11) was the most common adverse events. Other adverse effects include fatigue, fever, edema, and diarrhea (one each). None of the patients had detectable levels of WA in circulation.
Conclusion
The formulation was well tolerated. However, WA appears to have low oral bioavailability. Further studies with improved formulations are warranted.
Keywords: Withaferin-A, Ashwagandha, Osteosarcoma, Safety, Pharmacokinetics
1. Introduction
There is an increasing need for new therapies to treat cancer, especially in the advanced stages. There is much interest in naturally derived compounds as targets for potential cancer treatment. Many plant derived principles have shown excellent anti-cancer activity in the past. The anticancer drugs such as Etoposide, Vincristine, and Taxanes to name a few were derived from plants. Withania somnifera (Ashwagandha) or the Indian Ginseng has been used in Ayurveda for centuries for the treatment of several disorders. It possesses numerous pharmacological properties including neuroprotection, anti-inflammation, aphrodisiac, rheumatism, insomnia, and goiter among others [1]. An extract from the roots contains a group of biologically active constituents known as withanolides. The chemical structures of withanolides have been studied and they are widely distributed in the family Solanaceae.
Withaferin- A (WA) is a therapeutically active withanolide reported to be present in roots and leaves of the plant. In several preclinical studies, it has shown significant anticancer activity. The structure of WA is shown in Fig. 1. WA has been shown to prevent and cure urethane-induced lung tumors in mice, and also inhibit the growth of transplanted sarcoma in mice. Ashwagandha roots possess health-promoting activities, some of the activities of the root extract and its components include inhibition of NF-kappaB and AP-1 transcription factors [2], induction of apoptosis via mitochondria mediated cytochrome c release and caspase activation [3], elicitation of humoral and cell-mediated immune responses by upregulation of Th1-dominant polarization [4] and anti-tumor effects by downregulation of p34cdc2 expression [5]. The leaf extract of Ashwagandha demonstrated an antitumor activity which was mainly associated with its component withanone that selectively activated the p53 pathway in tumor cells [6], [7].
Fig. 1.
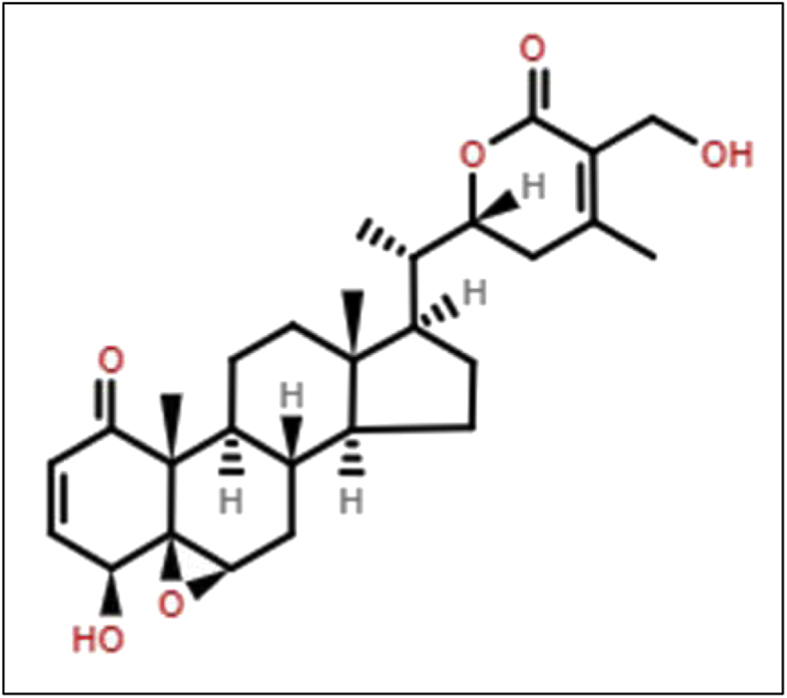
Structure of Withaferin A.
WA was tried in mouse sarcoma 180 (S-180) solid and ascites tumor cells that were treated in vivo and in vitro. On observing via an electron microscope the compound was found to affect these spindle microtubules of cells in metaphase [8]. WA demonstrated a sensitizer enhancement ratio of 1.5 for in vitro cell killing of V79 Chinese hamster cells at a non-toxic concentration of approximately 2 μM [9]. WA can inhibit T-cell and B-cell upregulation and proliferation without causing cell death. Thus demonstrating potential as an anti-inflammatory agent [10]. WA shows cytotoxicity in a variety of tumor cell lines. In a study, they have shown that it primarily induces oxidative stress in human leukemia HL-60 cells and in several other cancer cell lines [11]. Genetic studies suggest that it may present an attractive treatment option for triple negative breast cancer (TNBC) which has a poor prognosis [12]. In preclinical data, WA has shown antiproliferative potential and led to apoptosis in MG-63 and U2OS human osteosarcoma cell lines by inducing a ROS-mediated reduction in mitochondrial membrane potential and inhibiting G2/M checkpoint proteins thus fostering cell cycle arrest [13], [14]. Advanced osteosarcoma has limited treatment options. Metastatic disease presents a treatment challenge and patients often have a poor prognosis. Thereby indicating the need for new therapies and options for the advanced stage of the disease. Some of the challenges that have to be surpassed at the clinical level prior to using WA as an anticancer agent are its potency, efficacy and side effects i.e. establishing a safe and effective dosing protocol [15].
Despite being a strong candidate there are no clinical trials in cancer on this product. To determine the established benefits in a clinical setting, we carried out a phase I study in osteosarcoma patients. We selected a standardized root extract of W. somnifera containing 4.5% of WA w/w for the Phase I trial. W. somnifera is taken with clarified butter in Ayurveda, the traditional Indian medicine. We used a formula in which the extract was processed as per Ayurveda text in clarified butter. Our objectives for this study were to determine the presence of any dose limiting toxicity (DLT) at day 30 and to evaluate the safety and pharmacokinetics of WA containing formulation in patients with advanced stage high-grade osteosarcoma.
2. Materials and methods
The study was approved by our Institutional Ethics Committee which is presently registered with the Drugs Controller General of India (IEC registration number: ECR/170/Inst/MH/2013). The trial was registered in clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT00689195).
2.1. Patients and setting
It was a single-center phase I dose escalation study conducted at a tertiary care cancer hospital in western India. We recruited 13 patients who were willing to participate and who met the eligibility criteria, the enrolled patients provided their written informed consent to participate in the study.
The eligibility criteria for the study included: 1) Children and adults aged between 8 and 65 years 2) Histologically proven high grade osteosarcoma of the extremity and 3) Relapsed disease after primary line of treatment who are unsuitable or refuse secondary chemotherapy. Exclusion criteria included: 1) Pregnant/lactating women, 2) Those requiring concomitant treatment with CYP inducers or inhibitors and 3) Those without good venous access.
2.2. Study intervention
A standardized root extract of W. somnifera containing 4.5% of WA w/w was used for this study (AshwaMAX 400, Pharmanza Herbal Pvt Ltd., Gujarat, India). Each 400 mg capsule of AshwaMAX contained 18 mg of WA. The extract was processed in clarified butter as prescribed in the traditional texts of Ayurveda.
2.3. Study design
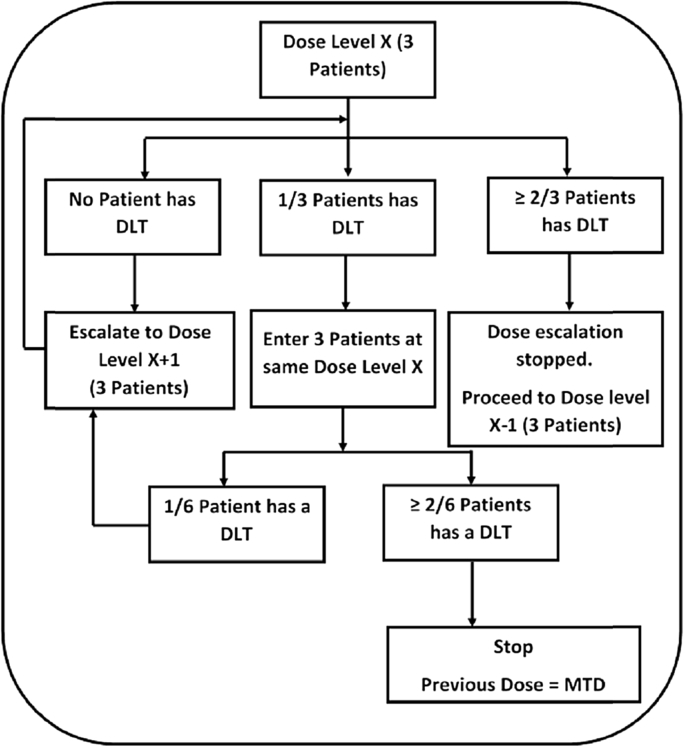
Our study design was a classic 3 + 3 dose escalation approach as shown in Fig. 2. The purpose of phase 1 trials is to determine the maximum tolerated dose (MTD). 3 + 3 designs are easy to operate and identify MTD with acceptable level of precision. A major limitation of 3 + 3 design is that it enrolls a significant proportion of patients at sub-therapeutic doses [16]. However, its conservative dose escalation strategy minimizes the risk of exposure to toxic doses of the investigational product. We had earlier conducted a 28-day repeated dose toxicity study in rats, where we used a standardized W. somnifera extract containing 4.5% of WA. The No observed adverse effect level (NOAEL) found in the study was 2000 mg/kg. The corresponding Human Equivalent Dose (HED) is approximately 325 mg/kg/day. A safety factor of five was applied to obtain the Maximum Recommended Starting Dose (MRSD) of 65 mg/kg/day of the formula in humans [17]. Considering the average human weight to be 60 kg we arrived at an MRSD of 3900 mg/day. In the present study the starting dose of W. somnifera extract used is 1600 mg, which was gradually escalated to 4800 mg/day. The highest dose of WA at which ≤1 out of 6 patients had dose limiting toxicities (DLTs) was defined as the Maximum Tolerated Dose (MTD). Dose limiting toxicity was defined as any grade 3 non-hematological or grade 4 hematological event related to Withaferin-A, based on the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. The dose escalation cohorts comprised of 2 capsules BID, 3 capsules BID, 4 capsules BID and 4 capsules TID corresponding to daily doses of 72, 108, 144 and 216 mg of WA. Three patients were enrolled in each cohort and the last patient was observed for at least 30 days for any dose limiting toxicity before progressing to a higher cohort.
Fig. 2.
3 + 3 Dose escalation study design for phase I clinical trials. DLT = Dose Limiting Toxicity; MTD = Maximum Tolerated Dose. All DLTs were evaluated at day 30.
2.4. Safety and pharmacokinetics sampling and analysis
Pharmacokinetic samples were collected on day 1 at the following time points – 0.0 (predose), 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 6.0, 7.0, 8.0, 10, 12.0 and 24.0 h after administration and plasma levels were measured using the validated High Performance Liquid Chromatography (HPLC) technique as described by Patial et al. having a limit of quantitation of 50 ng/mL [18]. The chromatographic system used a reverse-phase C18 column with UV–visible detection at 225 nm. The mobile phase consisted of water and acetonitrile applied in a gradient flow. Withaferin-A was extracted by simple protein-precipitation technique. The calibration curve was linear in the concentration range of 0.05–1.6 μg/mL. The method has the desired sensitivity to detect the plasma concentration range of Withaferin-A that is likely to show biological activity based on in vitro data. Patients were followed-up at 3 monthly intervals. Safety evaluation, including clinical examination, detailed history of adverse events, liver function tests, renal function tests and complete blood counts were performed at each visit. WA was administered daily till progression. CTCAE version 3.0 was used for grading adverse events.
3. Results
Thirteen patients meeting the eligibility criteria were enrolled in the dose-escalation trial. Baseline characteristics of the study participants are summarized (Table 1).
Table 1.
Baseline characteristics of the study population. ALP – Alkaline Phosphatase.
| Patient Characteristics | |
|---|---|
| Number Of Patients | 13 |
| Male/Female | 10/3 |
| Median Age (yrs) | 16 (13–43) |
| Mean Hemoglobin (±SD)a (g/dL) | 10.62 (±2.53) |
| Mean Sr. Creatinine (±SD)a (mg/dL) | 0.9 (±0.14) |
| Mean Sr. ALPa (IU/L) | 597.5 (±849.5) |
Hemoglobin values in g/dL, Serum creatinine values in mg/dL and ALP values in IU/L are those measured at baseline.
A flow chart depicting patient enrollment and allocation procedure that was followed in this study as well as the number of patients starting therapy at each dose is shown in Fig. 3.
Fig. 3.
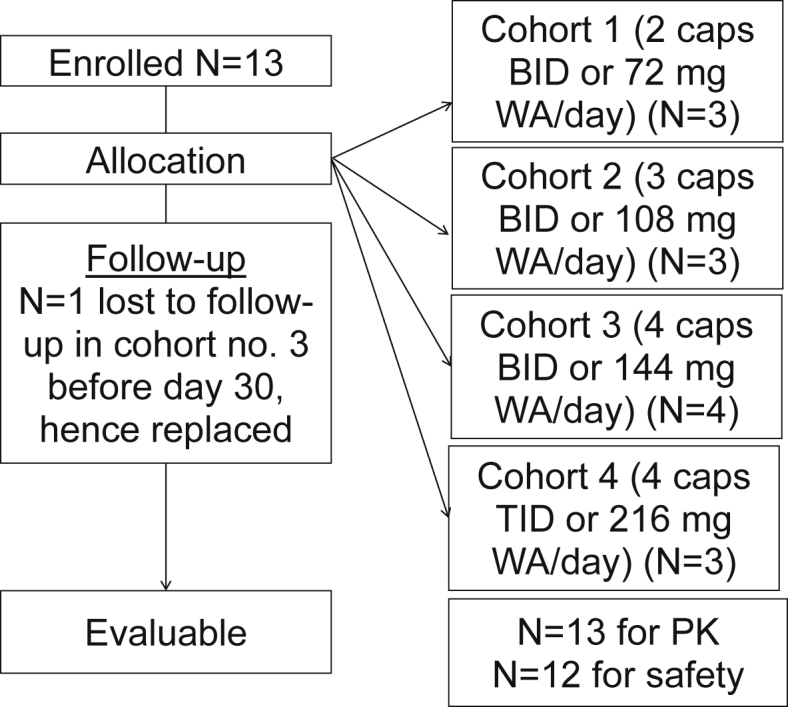
Flow chart displaying patient enrollment, allocation, follow-up and evaluation. WA = Withaferin-A, PK = Pharmacokinetics.
3.1. Safety laboratory results
One subject in cohort 1 developed worsening of anemia with hemoglobin levels decreasing to 6.9 g/dL at the 3 month follow-up period compared to 7.7 g/dL at baseline. Five subjects (38.46%) had elevations in liver enzymes – aspartate aminotransferase (AST) and alanine aminotransferase (ALT). All liver enzyme level elevations were grade 1 in severity. AST alone was elevated in one patient, ALT alone in one patient, while 3 patients had combined elevation of ALT and AST (Table 2).
Table 2.
Frequency of adverse events across cohorts is shown.
| Adverse Event | 72 mg (n = 3) | 108 mg (n = 3) | 144 mg (n = 4) | 216 mg (n = 3) | Overall (n = 13) |
|---|---|---|---|---|---|
| Number of adverse events | |||||
| Abnormal LFTs | 1 | 2 | – | 2 | 5 |
| Fatigue | 1 | – | – | – | 1 |
| Fever | – | 1 | – | – | 1 |
| Rash | – | 1 | 1 | – | 2 |
| Diarrhea | – | – | 1 | – | 1 |
| Edema | – | – | – | 1 | 1 |
| DLT | – | – | – | – | – |
AE- Adverse Event, DLT- Dose Limiting Toxicity, LFT- Liver Function Tests.
3.2. Adverse events
Eleven adverse events occurred in 8 subjects during the course of the study. Two events occurred in cohort 1 and cohort 3 each, while four events were observed in cohort 2 and three in cohort 4. The adverse events fall into 6 major categories: Fatigue (1), Fever (1), Rash (2), Diarrhea (1), Edema (1) and abnormal LFTs (5). The events were either grade 1 or grade 2 in nature. The adverse events that occurred during the study across the 4 cohorts are shown above in (Table 2).
3.3. Pharmacokinetic data
All patients (N = 13) enrolled in the study contributed samples for pharmacokinetic analysis of WA. There were no detectable levels of Withaferin-A in any of the samples collected.
4. Discussion
W. somnifera (WS) has been studied for treatment of several conditions. In one study, subjects with cognitive decline were randomized to either WS 300 mg orally twice daily or placebo for 8 weeks. The study found that WS was effective at improving the immediate and general memory in patients having mild cognitive impairment while also enhancing the executive function, attention, and information processing speed [19]. Another study showed that treatment with the Ashwagandha root extract in adults with chronic stress helped in body weight management and resulted in a significant decrease in body weight, serum cortisol levels and body mass index (BMI) [20]. WA a component of Ashwagandha has been studied for its anticancer activity in many in vitro as well as in vivo preclinical models. However, to date, there have been no clinical studies of WA in cancer have been performed.
Advanced osteosarcoma has limited treatment options and phytochemicals have demonstrated useful anticancer activity in the past. The phytochemical WA is a promising anticancer agent. At the molecular level, it has shown to target multiple cell survival kinase pathways, including NF-kB, PI3 kinase, Protein kinase, Mitogen activated protein kinase among others. WA has been reported to induce apoptosis via intrinsic and extrinsic pathways in human prostate, breast and leukemic cancer cells among others. It has also demonstrated its potential to contribute to its anti-tumor effect both in vitro as well as in vivo [21].
Preclinical studies using orthotropic murine models have demonstrated that oral administration of Withaferin-A is effective against Glioblastoma Multiform (GBM), an aggressive malignancy [22]. It has also shown the ability to induce apoptosis in osteosarcoma U2OS cell lines by generating ROS, also causing cell cycle arrest in osteosarcoma cell lines by inhibition of G2/M checkpoint proteins [13], [14].
In this trial, our aim was to evaluate the safety and pharmacokinetics of WA in an advanced stage, high grade osteosarcoma. The results demonstrated that the formulation was well tolerated by patients up to a dose of 4800 mg, equivalent to 216 mg of WA per day, without any dose limiting toxicity. This was the highest dose that could be administered with reasonable tolerability and patient compliance since patients in the highest dose cohort (216 mg) were receiving a regimen of 4 capsules of WA TID. No DLTs were observed even at the highest dose indicating that higher doses may be tried in patients, but the total dose is limited by the number of capsules a patient can reasonably consume in a day. A standardized extract with higher WA content may circumvent this problem leading to higher doses administered per day at acceptable number of capsules and frequency of administration.
Common side effects observed include elevation of liver enzymes (grade 1) in 5 out of 11 patients and skin rash in 2 out of 11 patients. Other side effects seen include fever, fatigue, edema, and diarrhea (one event each). No grade 3 or grade 4 adverse events were observed.
WA was not detectable in any of the study samples. The method used was an HPLC assay with a sensitivity of 50 ng/mL. At the time of the study only an HPLC system capable of detecting 50 ng/ml was available. Bioavailability of withanolides depends on the extent of their absorption and transportation through the intestinal epithelium. A study conducted to assess the bioavailability of withanolides using an in vitro absorption model i.e the Mandin Darby Canine Kidney system discovered that WA was impermeable or metabolized on passing through the cell layer [23]. However, another study that attempted to analyze the PK of WA and withanolide A (WEA) in mouse plasma after oral administration found that oral absorption of the withanolides was rapid. It also demonstrated that the relative bioavailability of WA was one and a half times more than WEA. The study used the HPLC-ESI-MS/MS assay, which was found to have a sensitivity of 0.484 ng/ml [24], thus suggesting more sensitive analytical techniques are required to characterize the PK of WA in humans. We could not establish bioavailability of WA due to the above reasons.
The IC50 of WA lies in the submicromolar level, which translates to a concentration of approximately 470 ng/mL. However, WA is unlikely to be used as a stand-alone agent. The best way to use WA would be in combination with standard chemotherapy. The synergistic effects of Withaferin A were demonstrated in a study where WA, when combined with cisplatin, acted synergistically and was found to enhance antitumor effects on ovarian cell lines by inhibiting cell proliferation, generation of ROS and causing DNA damage leading to apoptosis [25]. Another study shows the potential of WA as a radiosensitizer, the study was performed on mouse melanoma tumor which was, pretreated with WA and followed by local gamma radiation. WA produced a dose dependent increase in growth delay and volume doubling time [26]. The transcription factor NFκB, which regulates inflammation, cell survival and P-glycoprotein expression, plays a major role in inducing resistance to anticancer drugs [27]. It is observed that WA inhibiits NFκB in doxorubicin-resistant K562/Adr cells, and can relieve attenuated caspase activation and induce apoptosis [27]. This makes WA is an attractive compound for chemosensitization and to elicit cell death in chemoresistant cell types. Thus WA can synergize with other anticancer agents and its P-glycoprotein modulating activity can be exploited for improving the oral bioavailability and intratumoral concentrations of co-administered anticancer drugs [27]. Further clinical studies of WA as an adjunctive to chemotherapy in osteosarcoma are warranted. There is also preliminary evidence related to the activity of WA in gastric, thyroid and ovarian cancer. Here again, the utility of WA as an adjunctive treatment to standard modalities could be evaluated.
Other benefits of WA including immunomodulatory effects suggest that it is likely to mitigate/protect for toxicity and side effects of chemotherapy. Being economical as well as a natural phytochemical, development of an improved formulation of Withaferin-A could turn out to be an attractive anticancer treatment option in both the developed and developing countries.
4.1. Limitation
One of the limitations of this study is that the maximum tolerated dose (MTD) was not achieved. The maximum dose used in this study was 216 mg which was likely an underestimation of the possible MTD. Higher doses can be given WA has an improved formulation of higher strength that allows a simpler dosing regimen and frequency of administration is reduced.
The bioanalytical method used in this study had a sensitivity of 50 ng/mL. Sensitivity and specificity of the analytical technique were a limitation of our study. Bioanalytical techniques with higher sensitivity are recommended in subsequent studies.
5. Conclusion
Our phase I study found that oral Withaferin-A in patients with advanced stage, high grade osteosarcoma has a good safety profile. Further phase II studies of WA can be conducted at a dose of 216 mg/day.
Sources of funding
Intramural Grant of Tata Memorial Centre, India (Grant ID: 381/07.).
Conflict of interest
None.
Acknowledgements
The authors acknowledge Tata Memorial Centre for conducting this study.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Dar N.J., Hamid A., Ahmad M. Pharmacologic overview of Withania somnifera, the Indian ginseng. Cell Mol Life Sci. 2015;72:4445–4460. doi: 10.1007/s00018-015-2012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh D., Aggarwal A., Maurya R., Naik S. Withania somnifera inhibits NF-kB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phyther Res. 2007;21:905–913. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 3.Senthil V., Ramadevi S., Venkatakrishnan V., Giridharan P., Lakshmi B.S., Vishwakarma R.A. Withanolide induces apoptosis in HL-60 leukemia cells via mitochondria mediated cytochrome c release and caspase activation. Chem Biol Interact. 2007;167:19–30. doi: 10.1016/j.cbi.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Malik F., Singh J., Khajuria A., Suri K.A., Satti N.K., Singh S. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007;80:1525–1538. doi: 10.1016/j.lfs.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Singh D. Downregulation of p34cdc2 expression with aqueous fraction from for a possible molecular mechanism of anti-tumor and other pharmacological effects. Phytomedicine. 2001;8:492–494. doi: 10.1078/S0944-7113(04)70072-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaur K., Rani G., Widodo N., Nagpal A., Taira K., Kaul S.C. Evaluation of the anti-proliferative and anti-oxidative activities of leaf extract from in vivo and in vitro raised Ashwagandha. Food Chem Toxicol. 2004;42:2015–2020. doi: 10.1016/j.fct.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Widodo N., Kaur K., Shrestha B.G., Takagi Y., Ishii T., Wadhwa R. Selective killing of cancer cells by leaf extract of Ashwagandha: identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin Cancer Res. 2007;13:2298–2306. doi: 10.1158/1078-0432.CCR-06-0948. [DOI] [PubMed] [Google Scholar]
- 8.Shohat B., Shaltiel A., Ben-Bassat M., Joshua H. The effect of withaferin A, a natural steroidal lactone, on the fine structure of S-180 tumor cells. Cancer Lett. 1976;2:71–77. doi: 10.1016/s0304-3835(76)80014-6. [DOI] [PubMed] [Google Scholar]
- 9.Devi P.U. Withania somnifera Dunal (Ashwagandha): potential plant source of a promising drug for cancer chemotherapy and radiosensitization. Indian J Exp Biol. 1996;34:927–932. [PubMed] [Google Scholar]
- 10.Gambhir L., Checker R., Sharma D., Thoh M., Patil A., Degani M. Thiol dependent NF-κB suppression and inhibition of T-cell mediated adaptive immune responses by a naturally occurring steroidal lactone Withaferin A. Toxicol Appl Pharmacol. 2015;289:297–312. doi: 10.1016/j.taap.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Malik F., Kumar A., Bhushan S., Khan S., Bhatia A., Suri K.A. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 12.Szarc Vel Szic K., Declerck K., Crans R.A.J., Diddens J., Scherf D.B., Gerhäuser C. Epigenetic silencing of triple negative breast cancer hallmarks by Withaferin A. Oncotarget. 2017;8:40434–40453. doi: 10.18632/oncotarget.17107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv T.-Z., Wang G.-S. Antiproliferation potential of withaferin A on human osteosarcoma cells via the inhibition of G2/M checkpoint proteins. Exp Ther Med. 2015;10:323–329. doi: 10.3892/etm.2015.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li A.-X., Sun M., Li X. Withaferin-A induces apoptosis in osteosarcoma U2OS cell line via generation of ROS and disruption of mitochondrial membrane potential. Eur Rev Med Pharmacol Sci. 2017;21:1368–1374. http://www.ncbi.nlm.nih.gov/pubmed/28387888 accessed July 11, 2017. [PubMed] [Google Scholar]
- 15.Palliyaguru D.L., Singh S.V., Kensler T.W. Withania somnifera : from prevention to treatment of cancer. Mol Nutr Food Res. 2016;60:1342–1353. doi: 10.1002/mnfr.201500756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen A., Graham D., Pond G., Siu L. Phase I trial design: is 3+3 the best? Cancer Contr. 2014;21:200–208. doi: 10.1177/107327481402100304. [DOI] [PubMed] [Google Scholar]
- 17.Patel S., Rao N., Hingorani L. Safety Assessment of Withania somnifera extract standardized for Withaferin A : acute and sub-acute toxicity study. J Ayurveda Integr Med. 2016:30–37. doi: 10.1016/j.jaim.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patial P., Gota V. Rapid and sensitive method for determination of withaferin-A in human plasma by HPLC. Bioanalysis. 2011;3:285–289. doi: 10.4155/bio.10.207. [DOI] [PubMed] [Google Scholar]
- 19.Choudhary D., Bhattacharyya S., Bose S. Efficacy and safety of Ashwagandha ( Withania somnifera (L.) Dunal ) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;14:599–612. doi: 10.1080/19390211.2017.1284970. [DOI] [PubMed] [Google Scholar]
- 20.Choudhary D., Bhattacharyya S., Joshi K. Body weight management in adults under chronic stress through treatment with Ashwagandha root extract. J Evid Based Complementary Altern Med. 2017;22:96–106. doi: 10.1177/2156587216641830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chirumamilla C.S., Pérez-Novo C., Van Ostade X., Vanden Berghe W. Molecular insights into cancer therapeutic effects of the dietary medicinal phytochemical Withaferin A. Proc Nutr Soc. 2017;76:96–105. doi: 10.1017/S0029665116002937. [DOI] [PubMed] [Google Scholar]
- 22.Chang E., Pohling C., Natarajan A., Witney T.H., Kaur J., Xu L. AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models. J Neurooncol. 2016;126:253–264. doi: 10.1007/s11060-015-1972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Devkar S., Kandhare A., Sloley B., Jagtap S., Lin J., Tam Y. Evaluation of the bioavailability of major withanolides of Withania somnifera using an in vitro absorption model system. J Adv Pharm Educ Res. 2015;6:159–164. doi: 10.4103/2231-4040.165023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil D., Gautam M., Mishra S., Karupothula S., Gairola S., Jadhav S. Determination of withaferin A and withanolide A in mice plasma using high-performance liquid chromatography-tandem mass spectrometry: application to pharmacokinetics after oral administration of Withania somnifera aqueous extract. J Pharmaceut Biomed Anal. 2013;80:203–212. doi: 10.1016/j.jpba.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Kakar S.S., Jala V.R., Fong M.Y. Synergistic cytotoxic action of cisplatin and withaferin A on ovarian cancer cell lines. Biochem Biophys Res Commun. 2012;423:819–825. doi: 10.1016/j.bbrc.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devi P.U., Kamath R., Rao B.S. Radiosensitization of a mouse melanoma by withaferin A: in vivo studies. Indian J Exp Biol. 2000;38:432–437. [PubMed] [Google Scholar]
- 27.Suttana W., Mankhetkorn S., Poopimon W., Palagani A., Zhokhov S., Gerlo S. Differential chemosensitization of P-glycoprotein overexpressing K562/Adr cells by withaferin A and Siamosis polyphenols. Mol Cancer. 2010;9:1–22. doi: 10.1186/1476-4598-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]