Abstract
Background
Silver nanoparticles are toxic to bacteria and have widespread application in different research areas.
Objective
The aim of this study was to synthesize silver nanoparticles using an aqueous leaf extract of Cestrum nocturnum and to test its antioxidant and antibacterial activities.
Materials and methods
The silver nanoparticles were synthesized by addition of 20 ml extract (8% w/v) with 180 ml silver nitrate solution (1 mM). The synthesis of silver nanoparticles was confirmed by UV–Vis spectrophotometer. The silver nanoparticles were characterized by X-ray diffractometer, Transmission Electron Microscope, Scanning Electron Microscope and Fourier Transform Infra-Red spectroscopy. The antioxidant property of silver nanoparticles was analyzed by the 2, 2-diphenyl-1-picrylhydrazyl, hydrogen peroxide, hydroxyl radical and superoxide radical scavenging methods. The bacteriostatic and bactericidal activity of silver nanoparticles against Escherichiacoli, Enterococcusfaecalis, and Salmonellatyphi was determined using bacterial growth inhibition method. The antibacterial sensitivity and Minimum Inhibitory Concentration (MIC) of silver nanoparticles was determined against the bacteria.
Results
The results confirmed that the silver nanoparticles synthesized by C.nocturnum extract were crystalline in nature, average particle size was 20 nm and were mostly spherical in shape. The antioxidant methods confirmed that the silver nanoparticles have more antioxidant activity as compared to vitamin C. The silver nanoparticles have strong antibacterial (maximum Vibrio cholerae and minimum E. faecalis) activity. The MIC value of silver nanoparticles was 16 μg/ml (Citrobacter), 4 μg/ml (E. faecalis), 16 μg/ml (S. typhi), 8 μg/ml (E. coli), 8 μg/ml (Proteusvulgaris), and 16 μg/ml (V. cholerae).
Conclusion
Green synthesized silver nanoparticles have strong antioxidant and antibacterial activity due to the presence of bioactive molecules on the surface of silver nanoparticles.
Keywords: Cestrum nocturnum, silver nanoparticles, Green synthesis, Antioxidant, Antibacterial activity
Highlights
-
•
This study focuses on synthesis of silver nanoparticles from medicinal plant in nature.
-
•
The medicinal plant is rich in antioxidant property.
-
•
This study will help the researcher to uncover the antibacterial activity of silver nanoparticles.
1. Introduction
Nanoparticles can be created by physical, chemical or biological method. Biological method holds better chances as it is environment friendly and economical. Biological method consists of using micro-organisms or medicinal plants for the production of nanoparticles. Using medicinal plants is advantageous as their medicinal properties are added to the nanoparticles during synthesis. Phytocompounds of plants provide antioxidant property and the plants which have antibacterial activity provide additional antioxidant and antibacterial property to the nanoparticles [1].
From ancient times, silver has been used as an anti-microbial agent and silver-based compounds are much cheaper than gold based [2]. Further, silver nanoparticles (AgNPs) are non-toxic to eukaryotic cells including humans, but it has high toxicity against prokaryotic cells such as micro-organisms like bacteria, viruses, and fungi [3]. The AgNPs have unique chemical, optical, electrical, magnetic and mechanical properties. These unique properties of AgNPs are of interest to researchers in investigating its applications in nanomedicine such as anti-plasmodial [4], anti-microbial [5], targeted drug delivery [6], sensing and imaging [7], anti-fungal [8], anti-platelet [9], anti-cancerous [10] and wound healing [11]. The advancement in the synthesis of silver nanoparticles has expanded a strong impact in many scientific areas. Due to low yield and the use of toxic compounds physical and chemical methods are not suitable for the synthesis of silver nanoparticles respectively. Micro-organism based AgNPs synthesis is also not preferred because most of the microbes are pathogenic [12]. Several works already reported that plants extracts used for the synthesis of AgNPs such as Memecylonedule [13], Callicarpa maingayi [14], Terminalia chebula [15], Trachyspermum ammi and Papaver somniferum [16], Bauhinia variegate L. [17], Hevea brasiliensis [18], Aloe vera [19] and tea leaf [20]. Cestrum nocturnum belong to the family Solanaceae. This plant contains bioactive molecules such as alkaloids, flavonols glycosides, steroidal saponins, phenols, fatty acids and essential oil [21]. The extract of C. nocturnum has been used in burn and swelling, analgesic and bactericidal activity, local anesthetic effect, inhibitory effect on the central nervous system, cardiac arrhythmic, tumor inhibition and antioxidant activity [22]. Due to the above said reasons present work designed to use C. nocturnum leaves extract for the synthesis of AgNPs from silver nitrates and test its antioxidant property and antibacterial activity against human pathogenic bacteria such as Citrobacter, Salmonella typhi, Enterococcus faecalis, Escherichia coli, Proteus vulgaris and Vibrio cholerae was evaluated. Further, Bacterial Growth Inhibition (BGI) of AgNPs against E. faecalis, E. coli and P. vulgaris were determined.
2. Materials and methods
All the chemicals used were of analytic grades. Silver nitrate (Sigma Aldrich, 99%), DPPH (SRL, 99%), Methanol (Molychem, 99%), Vitamin C (Himedia, 99–100.5%), Hydrogen peroxide (Fischer Scientific, 30%), NADH (SRL, 98%), PMS (SRL, 99%), NBT (SRL, 99%) deoxyribose (SRL, 98%), FeSO4–EDTA (Sigma Aldrich, 99%), trichloroacetic acid (Fischer Scientific, 98%), TBA (Himedia, 98%) and NaOH (Fischer Scientific, 97%) were purchased. This study was performed in collaboration of the Department of Biochemistry and Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
2.1. Preparation of C.nocturnum extract
C.nocturnum leaves were procured from the botanical garden of Banaras Hindu University, Varanasi, UP, India. The leaves were dried and powdered using a grinder. The aqueous extract (8% w/v) of C. nocturnum was prepared using 500 ml Erlenmeyer flask containing 8 g powder and 100 ml deionized water and heated at 70 °C using a hot plate for 2 h. The extract was obtained by centrifuge the mixture at 3000 rpm for 5 min followed the filtration using Buchner funnel and Whatman no. 1 filter paper. These filtrates were stored in the refrigerator for further use [23].
2.2. Synthesis of silver nanoparticles
20 ml extract was added to the 180 ml silver nitrate solution (1 mM) in 250 ml volumetric flask and stirred two times for 5 min using a magnetic stirrer at room temperature. The change in color of the solution after 1 week indicated the reduction of silver nitrate into AgNPs. Then 2 ml aliquots were taken every 5 h till 24 h and absorbance (λ, 200–600 nm) was analyzed using UV–Vis spectrophotometer (Systronics, AU-2701). After the completion of the reaction, the solution was centrifuged at 5000 rpm for 15 min and the pellet was collected. Then pellets were washed three times using the 5 ml deionized water and centrifuged at 5000 rpm for 15 min. Further, the pellets were dried in hot air oven at 80 °C for 5 h [23].
2.3. Characterization of silver nanoparticles
The AgNPs were powdered and characterized by X-Ray Diffraction (XRD), Transmission Electron Microscopy (TEM), Energy Dispersive Spectrophotometer (EDS), Scanning Electron Microscopy (SEM) and Fourier Transform Infra-Red spectroscopy (FTIR). XRD (Bruker Advanced D8, Eco) was performed for the phase identification of AgNPs. TEM (FEI, TECNAI G2 20 TWIN) was carried out to find the structure and size distribution of silver nanoparticles and EDS was performed to check whether the nanoparticles were made of elemental silver or not and SEM (JEOL-MODEL 6390) was used to analyze the shape and size of AgNPs. FTIR (Varian Excalibur 3000, Palo Alto, CA) was used to check the capping agents on the surface of AgNPs.
2.4. Antioxidant activity
2.4.1. 2, 2-diphenyl-1-picrylhydrazyl (DPPH) method
The method was performed according to the Bhakya et al. (2016) with slight modification [24]. The free radical scavenging activity of AgNPs and standard vitamin C was determined using the stable radical DPPH. 1 ml of different concentrations (10, 20, 30, 40, 50, 75 and 100 μg/ml) of AgNPs was mixed with 1 ml freshly prepared DPPH (1 mM in methanol) solution and vortexed thoroughly. Then the solution was incubated at room temperature in the dark for 30 min. The absorbance was recorded at 517 nm using UV—Vis spectrophotometer (Systronics, AU-2701). DPPH (all the reagent except the sample) was used as a control and methanol was used as a blank solution. The free radical scavenging activity was expressed as the percentage of inhibition which was determined using the following formula [24],
| (1) |
where Pc is the absorbance of control and Ps is the absorption of AgNPs/vitamin C.
2.4.2. Hydrogen peroxide scavenging activity
This method was performed according to the Keshari et al., 2016 [25]. 0.1 ml AgNPs (25–250 μg/ml) in phosphate buffer (50 mM, pH = 7.4) was mixed with 0.3 ml phosphate buffer (50 mM, pH = 7.4) and 0.6 ml hydrogen peroxide solution (2 mM H2O2 in phosphate buffer, 50 mM, pH = 7.4). The mixture was vortexed and after 10 min the observance was recorded at 230 nm using UV–Vis spectrophotometer (Systronics, AU-2701). Vitamin C was used as standard while phosphate buffer (50 mM, pH 7.4) was used as blank. The percentage of hydrogen peroxide scavenging activity was calculated using Formula 2: [25]
| (2) |
where Oc represents the absorbance of control (all the reagent except the test sample) and Os absorbance of AgNPs/Vitamin C.
2.4.3. Hydroxyl radical (OH−) scavenging activity
The OH− radicals scavenging activity was determined according to the Keshari et al., 2016 [25]. 0.075 ml AgNPs (25–250 μg/ml in methanol), 0.45 ml sodium phosphate buffer (200 mM, pH = 7.0), 0.15 ml deoxyribose (10 mM), 0.150 ml FeSO4–EDTA (10 mM), 0.15 ml H2O2 (10 mM) and 0.525 ml deionized water were mixed. The mixture was kept in the digital incubator for 4 h. The reaction was stopped by the addition of 0.75 ml trichloroacetic acid (2.8%) and 0.75 ml TBA (1% in 50 mM NaOH). Then the solution was kept in boiling water bath for 10 min and cooled using tap water. The absorbance of the solution was determined at 520 nm. Methanol was used as blank while vitamin C was used as a standard. The percentage of hydroxyl radical scavenging activity was calculated using Formula 3: [25]
| (3) |
whereas Hc is the absorbance of control (all the reagent except the test sample) and Hs, absorbance of AgNPs/vitamin C.
2.4.4. Superoxide (O2−) radical scavenging activity
This activity of AgNPs was determined according to Keshari et al., 2017 [25]. Superoxide radicals are generated by the oxidation of NADH in Nicotinamide adenine dinucleotide (NADH) – phenazinemethosulphate (PMS) system and analyzed by the reduction of Nitro blue tetrazolium (NBT). 0.2 ml AgNPs (100–500 μg/ml in methanol), 1 ml Tris–HCl buffer (16 mM, pH = 8), 1 ml NBT (50 μM), 1 ml NADH (78 μM) and 1 ml PMS (10 μM) were mixed and kept for 5 min at 25 °C. The absorbance was recorded at 560 nm using UV–Vis spectrophotometer (Systronics, AU-2701). Vitamin C was used as standard and prepared same as AgNPs. The inhibition percentage of superoxide generation was calculated using Formula 4: [25]
| (4) |
whereas Sc is the absorbance of control (all the reagent except the test sample) while Ss is the absorption of AgNPs/vitamin C.
2.5. Antibacterial activity
2.5.1. Analysis of anti-microbial sensitivity
The antibacterial activity of AgNPs, extracts and silver nitrates was determined against the Citrobacter, S. typhi, E. faecalis, E. coli, P. vulgaris and V. cholerae. Pure cultures of bacteria were subcultured on agar-solidified Luria broth (LB) medium. The bacteria were swabbed onto the agar plates using a swab. Then extracts, AgNPs and AgNO3 were dropped on sterile discs while deionized water was used as a control. Then the plates were incubated overnight in the incubator at 37 °C. The inhibition zone around dropping was measured using an ordinary scale [26].
2.5.2. Analysis of minimum inhibitory concentration (MIC)
The MIC of different compounds (AgNPs and AgNO3) was determined using broth microdilution method. 0.5 McFarland's bacterial suspension was poured into each microtiter plates. Then different concentration (0, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and 256 μg/ml) of AgNPs and AgNO3 was used against the selected bacteria. The bacterial strains were incubated with AgNPs by two folds serial dilution and kept at 37 °C for 24 h [27].
2.5.3. Analysis of bacterial growth inhibition
The role of AgNPs on bacterial growth was determined in LB media. Few bacterial cells (E. coli, S. typhi and E. faecalis) were inoculated in the LB media and kept at 37 °C, 250 rpm for overnight. Then culture was mixed with fresh LB media to maintain the OD 0.1 at 600 nm (OD, 0.1 represents 108 cells/ml). Then equal amounts (50 μl) of each concentration of AgNPs (0–100 μg/ml) and fresh bacterial culture was mixed in a microtiter plate. The absorbance was recorded at 600 nm using an Elisa reader (Thermo scientific, Multiskan Ex) every hour for 6 h [27].
3. Results
3.1. Formation of silver nanoparticles
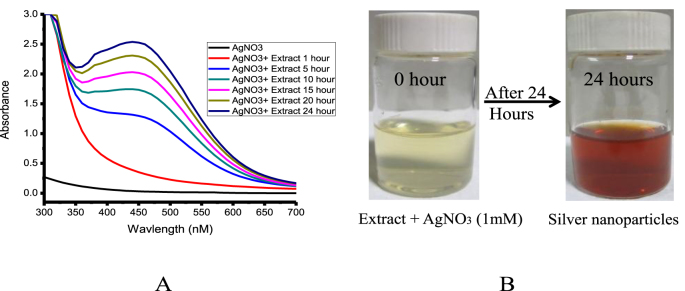
The formation of nanoparticles started after the mixing the extract with the silver nitrate solution. The visible color changes (light yellow to dark brown) of the solution and spectral analysis confirmed the formation of silver nanoparticles. The absorption band of AgNPs was observed at 442 nm due to the Localized Surface Plasmon resonance (LSPR) (Fig. 1A and B).
Fig. 1.
Spectra represent the formation of silver nanoparticles with the help of Cestrum nocturnum extract (A) and the visual observation of color changes at different time intervals (0 h–24 h) (B).
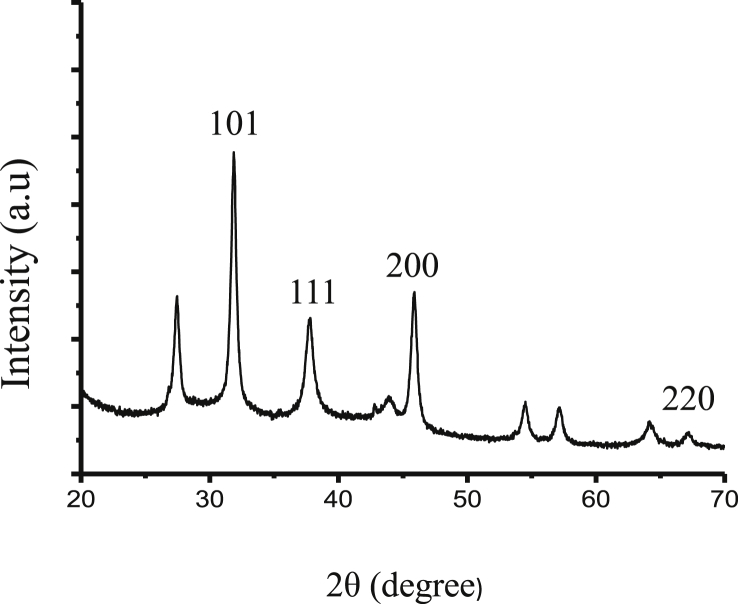
3.2. X-ray diffractometry
The peaks at the 2θ0 values 38.06, 44.23 and 67.43 represented the planes at 111, 200 and 220 respectively. These planes confirmed the AgNPs synthesized by extract was crystalline in nature (Fig. 2).
Fig. 2.
XRD graph represents the crystalline nature of silver nanoparticles.
3.3. Transmission electron microscopy
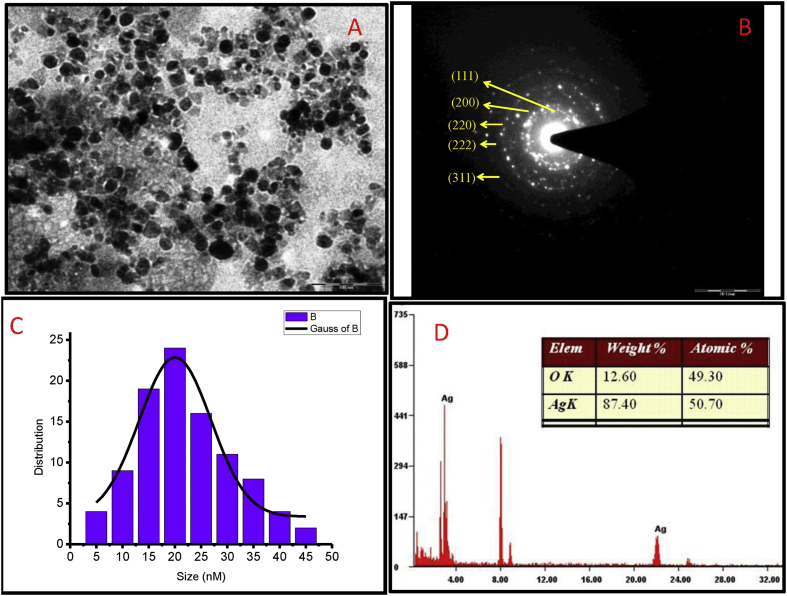
TEM results confirmed the synthesized AgNPs were spherical in shape (Fig. 5A) and the size of AgNPs ranged from 5 to 45 nm. The average mean size of AgNPs was 20 nm (Fig. 5C). The diffraction pattern confirmed the formation of metallic AgNPs (Fig. 4B). EDS spectrometer analysis confirmed the presence of silver signals. The silver signal confirmed the presence of elemental silver (peak, 3 KeV) due to localized surface plasmon resonance (Fig. 4D).
Fig. 5.
TEM results confirmed the synthesized AgNPs was spherical in shape (Figure 5, A) and the size of AgNPs ranged from 5-45 nm but the average mean size of AgNPs was 20 nm (Figure 5, C). The diffraction pattern confirmed the formation of metallic AgNPs (Fig 5, B). EDS spectrometer analysis confirmed the presence of silver and copper signals. The silver signal confirmed the presence of elemental silver (peak, 3KeV) due to localized surface plasmon resonance and copper signal due to the background of a copper grid (Figure 5, D).
Fig. 4.
SEM image indicates the formation of variable size of silver nanoparticles.
3.4. Scanning electron microscopy
The SEM results confirmed that the shape of AgNPs was spherical and variable in size. The size of AgNPs was ranged from 15 to 28 nm (Fig. 4).
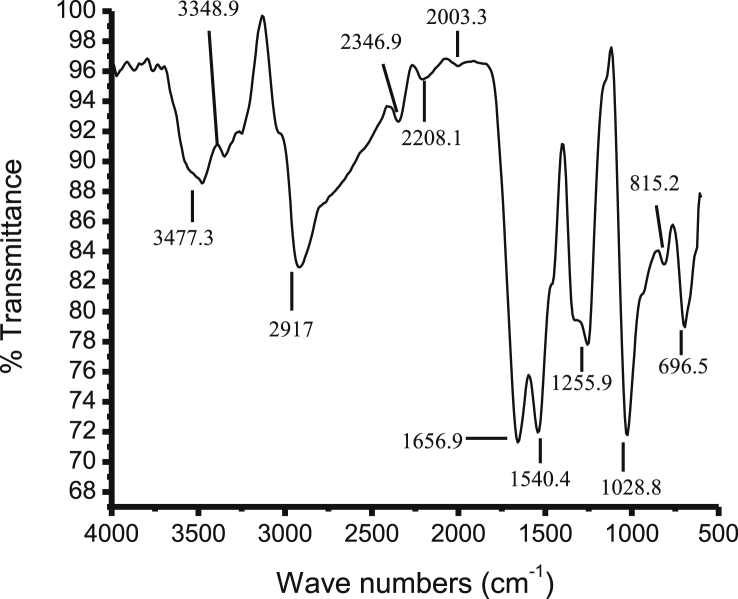
3.5. Fourier Transform Infra-Red spectroscopy
FTIR data of AgNPs provided different absorption peaks which assigned the different functional group of phyto compounds. The absorption peaks at 3477, 3348, 2917, 2346, 2208, 2003 and 1656 cm−1 were assigned the O—H stretch of phenolic compounds, N—H stretch of primary and secondary amines and amides, C—H stretch of methyl groups, H—C O: stretch of aldehydes, C N stretch of nitriles, C C stretch of alkynes, C O stretch of carbonyl groups of flavonoids and tannins respectively (Fig. 3).
Fig. 3.
FTIR graph represents the functional groups present on the surface of silver nanoparticles.
3.6. Antioxidant activity
3.6.1. 2, 2-diphenyl-1-picrylhydrazyl (DPPH) method
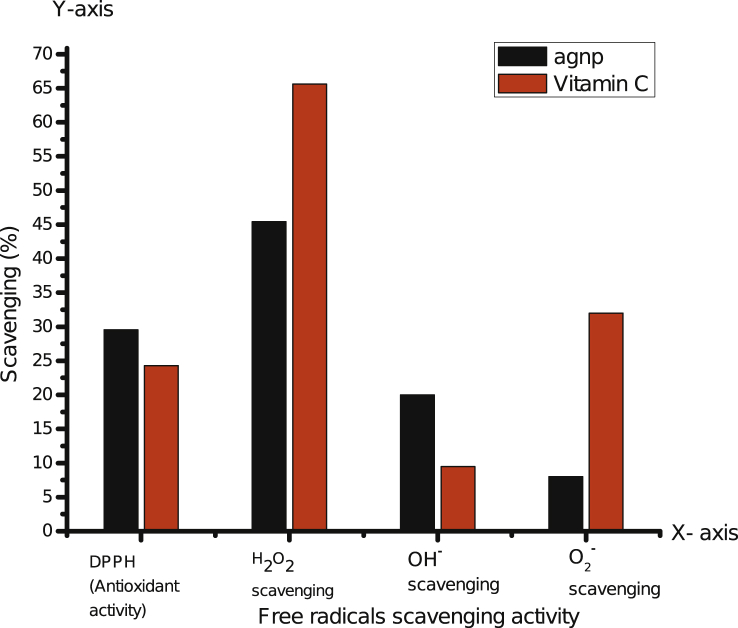
The results confirmed the AgNPs and vitamin C has the antioxidant activity. The AgNPs has 29.55% antioxidant activity while vitamin C has 24.28% antioxidant activity. The results confirmed the AgNPs has greater antioxidant activity as compared with vitamin C (Fig. 6).
Fig. 6.
Graph represents the percentage of Antioxidant (DPPH), and Free radicals (hydrogen peroxide, hydroxyl and superoxide radical) scavenging activity of silver nanoparticles and vitamin C.
3.6.2. Hydrogen peroxide scavenging activity
The results confirmed the AgNPs has 45.41% hydrogen peroxide scavenging activity while the standard vitamin C has 65.63% hydrogen peroxide scavenging activity. This result proved the vitamin C has strong hydrogen peroxide scavenging activity as compared with AgNPs (Fig. 6).
3.6.3. Hydroxyl radicals scavenging activity
The results proved the AgNPs has 20% hydroxyl radical scavenging activity and vitamin C has 9.47% hydrogen peroxide scavenging activity. The results showed the AgNPs has strong hydrogen peroxide scavenging activity as compared with standard vitamin C (Fig. 6).
3.6.4. Superoxide scavenging activity
The results confirmed the AgNPs has 8% superoxide scavenging activity while standard vitamin C has 32% superoxide scavenging activity. The results indicated the vitamin C has greater superoxide scavenging activity when compared with AgNPs (Fig. 6).
3.7. Anti-microbial activity of silver nanoparticles
3.7.1. Screening of anti-microbial activity
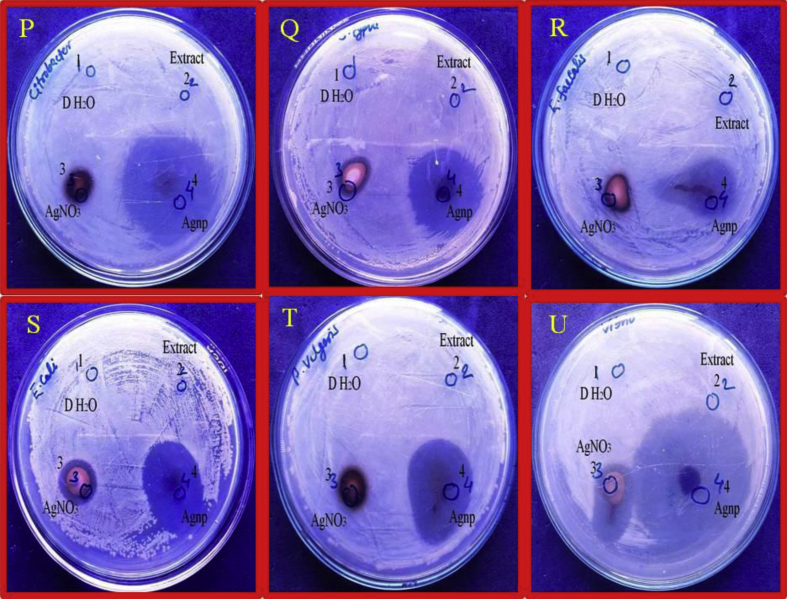
The results confirmed that the antibacterial activity was present in AgNPs, AgNO3 and no antibacterial activity was observed in the extract. The zone of inhibition confirmed the AgNPs has greater antibacterial activity as compared with AgNO3 (Fig. 7).
Fig. 7.
The images represent that silver nanoparticles have strong antibacterial activity as compared with AgNO3 while Cestrum nocturnum extract have no antibacterial activity and deionized H2O used as control (P–U).
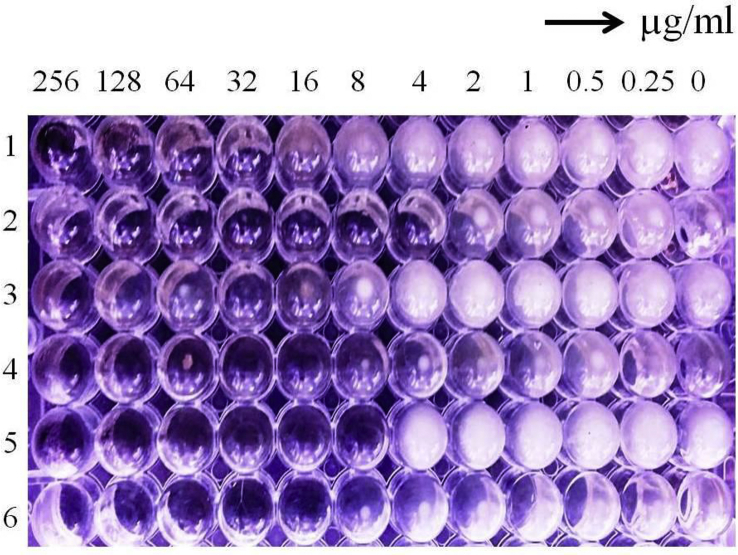
3.7.2. Minimum inhibitory concentration (MIC)
The results proved the MIC value of AgNPs against selected bacteria was varied and this variability depends upon the bacterial strains. The MIC values of AgNPs against bacteria were 16 μg/ml (Citrobacter), 4 μg/ml (E. faecalis), 16 μg/ml (S. typhi), 16 μg/ml (E. coli), 8 μg/ml (P. vulgaris) and 8 μg/ml (V. cholerae) (Fig. 8).
Fig. 8.
Image represent the Minimum Inhibitory Concentration of silver nanoparticles against Citrobacter (1), E. faecalis (2), S. typhi (3), E. coli (4), P. vulgaris (5) and V. cholerae (6) bacteria.
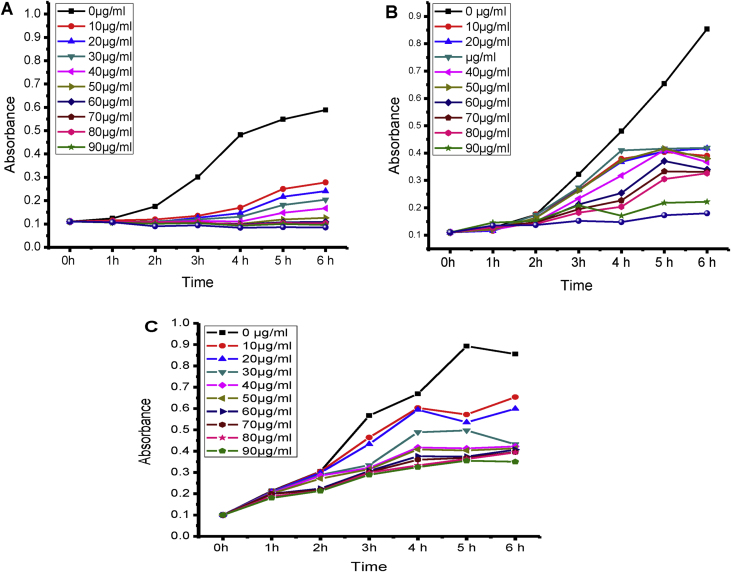
3.7.3. Bacterial growth inhibition (BGI)
The BGI results confirmed the AgNPs has bacterial growth inhibition property. When the variable concentration of AgNPs (0–100 μg/ml) was used against the E. coli, E. faecalis and S. typhi bacteria, the slope of bacterial growth was decreased (Fig. 9).
Fig. 9.
The graphs represent the effect of silver nanoparticles on the growth pattern of E. faecalis (A), E. coli (B) and P. vulgaris (C) bacteria.
4. Discussion
The present work explains the synthesis of AgNPs using C. nocturnum leaves extract. When the extract was added to the silver nitrate solution the color of the solution was changed from light yellow to dark brown color. The dark brown color of solution confirmed the reduction of silver nitrates into the silver nanoparticles. Further, the formation of silver nanoparticles was confirmed by the UV–Vis spectrophotometer (λ, 300–700 nm). The absorbance band at 442 nm was recorded due to localized surface plasmon resonance (LSPR) and confirmed the formation of silver nanoparticles (Fig. 1). However, lack of LSPR suggests the formation of ultrasmall silver nanoparticles or the silver cluster, which contains a small number of atoms [28]. The synthesis of AgNPs by green route becomes popular because of no use of toxic chemicals, cheap, eco-friendly and suitable for pharmaceutical and biomedical applications [15]. Recently, several kinds of literature proved that bacteria have been resistant to antibiotics and alternative antibiotics required [29]. The XRD results confirmed the formation of crystalline silver nanoparticles (Fig. 2). The SEM results proved that the variable size silver nanoparticles were synthesized (15–28 nm) (Fig. 4). The TEM results confirmed silver nanoparticles was spherical and few are oval in shape, and silver nanoparticles are variable in size and maximum 20 nm size silver nanoparticles were synthesized. The EDS results confirmed the presence of elemental silver and EDS signal between the 3 KeV confirmed the elemental silver [14] (Fig. 5). The bioactive compounds of the extract have been responsible for the reduction and formation of silver nanoparticles [30]. FTIR results confirmed the various functional group presents on the surface of bioactive compounds. This functional group is responsible for the capping of silver nanoparticles and stable in nanosize (Fig. 3). The DPPH, Hydrogen peroxide, hydroxyl radicals, superoxide scavenging methods confirmed the silver nanoparticles has antioxidant, hydrogen peroxide, hydroxyl radicals and superoxide scavenging activities (Fig. 6). These properties of silver nanoparticles occur due to the presence of functional groups on the surface of silver nanoparticles. The results of antibacterial sensitivity confirmed the silver nanoparticles and silver nitrates have antibacterial activity. The AgNPs have the maximum zone of inhibition against the Vibrio choleraee (41 mm) and minimum against E. faecalis (15 mm) bacteria (Fig. 7, Table 1). The MIC results proved the MIC value of AgNPs against the Citrobacter, E. faecalis, S. typhi, E. coli, P. vulgaris and V. cholerae bacteria were 16, 4, 16, 16, 8, 8 and 16 μg/ml respectively (Fig. 8). Furthermore, the BGI result confirmed the bacteriostatic and bactericidal activity of AgNPs against the E. faecalis, E. coli and P. vulgaris bacteria and AgNPs behaves dual behavior as bacteriostatic in lower concentration and bactericidal at higher concentration.
Table 1.
The antibacterial activity and zone of inhibition of silver nanoparticles, silver nitrates and extract against bacteria.
| S. no | Bacteria | Extract | Zone of inhibition (mm) |
|
|---|---|---|---|---|
| Silver nitrate | Silver nanoparticles | |||
| 1 | Citrobacter | Nil | 6 | 36 |
| 2 | S. typhi | Nil | 10 | 28 |
| 3 | E. faecalis | Nil | 7 | 15 |
| 4 | E. coli | Nil | 14 | 23 |
| 5 | P. vulgaris | Nil | 8 | 26 |
| 6 | Vibrio cholerae | Nil | 14 | 41 |
5. Conclusion
Present work describes the formation of silver nanoparticles with the help of C. nocturnum extract. The leaves of C. nocturnum have bioactive compounds which responsible for the reduction and capping of silver nitrates into silver nanoparticles. The capping agent provides stability to the AgNPs. The synthesized AgNPs has antioxidant, hydrogen peroxide, hydroxyl radicals, and superoxide scavenging activity. This activity occurs due to the presence of functional groups on the surface of AgNPs. Moreover, AgNPs have strong antibacterial activity against the selected bacteria due to the small size and presence of capping agents. These silver nanoparticles might be used as antibiotics in future due to non-toxic, cheap, eco-friendly and highly effective against the bacteria.
Sources of funding
None declared.
Conflict of interest
None.
Acknowledgment
The authors of this work would like to thank Dr. Rajeev Prakash, in charge, Central Instrument Facility, Indian Institute of Technology, Banaras Hindu University, Varanasi, India for providing TEM, SEM and XRD facility. The authors also thanks to the Head, Department of Chemical Engineering, Indian Institute of Technology, Banaras Hindu University, Varanasi, India for providing FTIR facility.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Pasupuleti V.R., Prasad T.N.V.K.V., Sheikh R.A., Balam S.K., Narasimhulu G., Reddy C.S., et al. Biogenic silver nanoparticles using Rhinacanthusnasutus leaf extract: synthesis, spectral analysis, and antimicrobial studies. Int J Nanomed. 2008;8:3355–3364. doi: 10.2147/IJN.S49000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Srikar S.K., Giri D.D., Pal DB,Mishra P.K., Upadhyay S.N. Green synthesis of silver nanoparticles: a review. Green Sustain Chem. 2016;6:34–56. [Google Scholar]
- 3.Hussain I., Singh N.B., Singh A., Singh H., Singh S.C. Green synthesis of nanoparticles and its potential application. Biotechnol Lett. 2016;38:545–560. doi: 10.1007/s10529-015-2026-7. [DOI] [PubMed] [Google Scholar]
- 4.Mishra A., Kaushik N.K., Sardar M., Sahal D. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf B Biointerfaces. 2013;111:713–721. doi: 10.1016/j.colsurfb.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Singh P.K., Bhardwaj K., Dubey P., Prabhune A. UV-assisted size sampling and antibacterial screening of Lantana camara leaf extract synthesized silver nanoparticles. RSC Adv. 2015;5:24513–24519. [Google Scholar]
- 6.Praveen S., Misra R., Sahoo S.K. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed – Nanotechnol Biol Med. 2012;8:147–166. doi: 10.1016/j.nano.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Lee K.S., El- Sayed M.A. Gold and silver nanoparticles in sensing and imaging: sensitivity of plasmon response to size, shape and metal composition. J Phys Chem B. 2006;110:19220–19225. doi: 10.1021/jp062536y. [DOI] [PubMed] [Google Scholar]
- 8.Nasrollahi A., Pourshamsian K.H., Mansourkiaee P. Antifungal activity of silver nanoparticles on some of fungi. Int J Nano Dimens. 2011;1:233–239. [Google Scholar]
- 9.NavaniethaKrishnaraj R., Berchmans S. In vitro antiplatelet activity of silver nanoparticles synthesized using the microorganism Gluconobacter roseus: an AFM-based study. RSC Adv. 2013;3:8953–8959. [Google Scholar]
- 10.Castro-Aceituno V., Ahn S., Simu S.Y., Singh P., Mathiyalagan R., Lee H.A., et al. Anticancer activity of silver nanoparticles from Panax ginseng fresh leaves in human cancer cells. Biomed Pharmacother. 2016;84:158–165. doi: 10.1016/j.biopha.2016.09.016. http://www.sciencedirect.com/science/article/pii/S075333221630395X [DOI] [PubMed] [Google Scholar]
- 11.Rigo C., Ferroni L., Tocco I., Roman M., Munivrana I., Gardin C., et al. Active silver nanoparticles for wound healing. Int J Mol Sci. 2013;14:4817–4840. doi: 10.3390/ijms14034817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindhani B.K., Panigrahi A.K. Biosynthesis and characterization of silver nanoparticles (Snps) by using leaf extracts of Ocimum sanctum L. (Tulsi) and study of its antibacterial activities. J Nanomed Nanotechnol. 2015;6:1–5. [Google Scholar]
- 13.Elavazhagan T., Arunachalam K.D. Memecylonedule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomed. 2011;6:1265–1278. doi: 10.2147/IJN.S18347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shameli K., Bin Ahmad M., Jaffar Al-Mulla E.A., Ibrahim N.A., Shabanzadeh P., Rustaiyan A., et al. Green biosynthesis of silver nanoparticles using Callicarpa maingayi stem bark extraction. Molecules. 2012;17:8506–8517. doi: 10.3390/molecules17078506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar K.M., Sinha M., Mandal B.K., Ghosh A.R., Kumar K.S., Reddy P.S. Green synthesis of silver nanoparticles using Terminalia chebula extract at room temperature and their antimicrobial studies. Spectrochim Acta A Mol Biomol Spectrosc. 2012;91:228–233. doi: 10.1016/j.saa.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Vijayaraghavan K., Nalini S.P., Prakash N.U., Madhankumar D. One step green synthesis of silver nano/microparticles using extracts of Trachyspermum ammi and Papaver somniferum. Colloids Surf B Biointerfaces. 2012;94:114–117. doi: 10.1016/j.colsurfb.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Kumar V., Yadav S.K. Synthesis of different-sized silver nanoparticles by simply varying reaction conditions with leaf extracts of Bauhinia variegata L. IET Nanobiotechnol. 2012;6:1–8. doi: 10.1049/iet-nbt.2010.0015. [DOI] [PubMed] [Google Scholar]
- 18.Guidelli E.J., Ramos A.P., Zaniquelli M.E., Baffa O. Green synthesis of colloidal silver nanoparticles using natural rubber latex extracted from Hevea brasiliensis. Spectrochim Acta A Mol Biomol Spectrosc. 2011;82:140–145. doi: 10.1016/j.saa.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Yang D., Kong Y., Wang X., Pandoli O., Guo Gao, et al. Synergetic antibacterial effects of silver nanoparticles aloe vera prepared via a green method. Nano Biomed Eng. 2010;2:252–257. [Google Scholar]
- 20.Loo Y.Y., Chieng B.W., Nishibuchi M., Radu S. Synthesis of silver nanoparticles by using tea leaf extract from Camellia sinensis. Int J Nanomed. 2012;7:4263–4267. doi: 10.2147/IJN.S33344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil C.D., Patil S.V., Salunke B.K., Salunkhe R.B. Bioefficacy of Plumbago zeylanica (plumbaginaceae) and Cestrum nocturnam (Solanaceae) plant extracts against Aedes aegypti (Diptera: Culicide) and nontarget fish Poecilia reticulata. Parasitol Res. 2011;108:1253–1263. doi: 10.1007/s00436-010-2174-6. [DOI] [PubMed] [Google Scholar]
- 22.Khan M.A., Inayat H., Khan H., Saeed M., Khan I., Rahman I.U. Antimicrobial activities of whole plant of Cestraum nocturnum against pathogenic microorganism. Afr J Microbiol Res. 2011;5:612–616. [Google Scholar]
- 23.Ashour A.A., Raafat D., El-gowelli, El-Kamel A.H. Green synthesis of silver nanoparticles using cranberry powder aqueous extract: characterization and antimicrobial properties. Int J Nanomed. 2015;10:7207–7221. doi: 10.2147/IJN.S87268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhakya S., Muthukrishnan S., Sukumaran M., Muthukumar M. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci. 2016;6:755–766. [Google Scholar]
- 25.Keshari A.K., Srivastava A., Verma A.K., Srivastava R. Free radicals scavenging and protein protective property of Ocimum sanctum (L) Br J Pharmaceut Res. 2016;14:1–10. [Google Scholar]
- 26.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Clinical and Laboratory standards Institute, CLSI; Wayne, PA: 2003. Approved standard M7–A6. [Google Scholar]
- 27.Maiti S., Krishnan D., Barman G., Ghosh S.K., Laha J.K. Antimicrobial activities of silver nanoparticles synthesized from Lycopersicon esculentum extract. J Anal Sci Technol. 2014;5:1–7. [Google Scholar]
- 28.Santos E.D.B., Madalossi N.V., Sigoli F.A., Mazali I.O. Silver nanoparticles: green synthesis, self assembled nanostructures and their application as SERS substrates. New J Chem. 2015;39:2839–2846. [Google Scholar]
- 29.Krychowiak M., Grinholc M., Banasiuk R., Krauze-Baranowska M., Głod D., Kawiak A., et al. Combination of silver nanoparticles and Drosera binata extract as a possible alternative for antibiotic treatment of burn wound infections caused by resistant Staphylococcus aureus. PLoS One. 2014;9:1–20. doi: 10.1371/journal.pone.0115727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra A., Garg S. Effect of varying concentration of herbal extract of Nyctanthes arbor-tristis leaf on synthesis of silver nanoparticles and its evaluation. Int J Pharm Pract. 2014;7:143–147. [Google Scholar]