Abstract
Background
Several studies have reported the efficacy of cabazitaxel in cancer therapy; however, investigations of its safety are few. The aim of this study was to retrospectively analyze the efficacy and safety of cabazitaxel based on treatment outcome data.
Methods
A questionnaire form on the use of cabazitaxel was mailed to hospitals associated with the Shinshu University. Responses were received from 11 institutions regarding 55 cases.
Results
Patients received a median of 4 courses of cabazitaxel treatment. Decreases in prostrate-specific antigen (PSA) were observed in 61.5% of cases with declines of 30%, 50%, and 90% in 36.5%, 23.0%, and 7.6% of cases, respectively. PSA progression-free survival was 5.0 months, and overall survival after the start of cabazitaxel was 13.0 months. Forty-five patients received postcabazitaxel treatment; 17 showed decreased PSA. Safety assessment indicated that white blood cell and neutrophil counts were significantly higher in the second than in the first course of treatment and Grade 3 to 4 leukopenia and neutropenia significantly decreased. Twenty-four subjects were aged ≥75 years; 79% of them had their doses reduced at the first administration. The mean dose was 20 mg/m2. However, there was no significant difference in the PSA progression-free survival between the ≥75-year-old and <75-year-old groups. Patients in the ≥75-year-old group, particularly those whose doses were not reduced, experienced several Grade 3 to 4 adverse effects. Ten patients discontinued treatment owing to adverse effects and systemic worsening.
Conclusions
To use cabazitaxel effectively, starting administration as early as possible before disease progression is important, and even if Grade 3 to 4 leukopenia and neutropenia are observed during the first course, it is important to carefully maintain the dose. Even when treating elderly patients, reducing the dose does not reduce therapeutic efficacy. However, because this cohort experienced several ≥ Grade 3 adverse effects, a great deal of caution is required.
Keywords: Cabazitaxel, Castration-resistant prostate cancer, Leukopenia, Neutropenia, Safety
Abbreviations: AE, adverse event; CRPC, castrate-resistant prostate cancer; FN, febrile neutropenia; GS, Gleason Score; HR, hazard ratio; mCRPC, metastatic castrate-resistant prostate cancer; NLR, neutrophil/lymphocyte ratio; OS, overall survival; PS, performance status; PSA, prostate-specific antigen; PSA-PFS, PSA progression-free survival; WBC, white blood cell
1. Introduction
Cabazitaxel is the first drug effective in extending overall survival in cases of metastatic castrate-resistant prostate cancer (mCRPC) after docetaxel therapy 1, 2, 3, 4. Based on these findings, cabazitaxel is currently widely used in patients with mCRPC. In Japan, cabazitaxel was included among the drugs covered by the National Health Insurance system in 2014. In the four years since then, several studies have reported its efficacy, but only a few studies have investigated its safety5, 6, 7, 8, 9. Thus, we conducted a retrospective analysis focusing on the efficacy and safety of cabazitaxel in clinical settings using treatment outcome data.
2. Methods
Questionnaires on the status of cabazitaxel treatment were mailed to hospitals associated with the Shinshu University. Responses were received from 11 institutions regarding 55 cases. mCRPC disease progression was defined as an increase of ≥25% of prostate-specific antigen (PSA) from PSA nadir and a PSA value of at least 2 ng/ml10. Bell Curve for Excel 2016, version 2.03 (Social Survey Research Information Co., Ltd., Japan) was the statistical software used for all statistical analyses. PSA progression-free survival (PFS) and the overall survival distribution were estimated via the Kaplan–Meier curve, and the analysis of difference was measured using the log-rank test. Factors that contributed to PSA-PFS [Gleason Score (GS) at the time of diagnosis, the time period from the start of treatment to castrate-resistant prostate cancer (CRPC), history of treatment with new hormone therapy, number of pharmaceuticals used until cabazitaxel use, age at the start of cabazitaxel treatment, performance status, PSA value, visceral metastasis, neutrophil/lymphocyte ratio, cabazitaxel dose, and presence of ≥ Grade 3 neutropenia] were investigated via the log-rank test using the Cox proportional-hazards model, and intergroup differences were evaluated with the Kaplan–Meier curve. Safety analysis was performed by conducting intergroup comparisons using the Mann–Whitney U test.
3. Results
At the start of cabazitaxel treatment, patients had a median age of 73.0 years (56–84 years); the median PSA was 34.14 ng/ml (0.06–1159.70 ng/ml), and the median total GS at diagnosis was 9 (6–10). Thirty-four of the 36 cases that underwent imaging evaluation immediately before the start of treatment were found to have metastasis.
Of the 34 metastasis cases, 29 had bone metastases, 19 had lymph node metastases, and 9 had internal organ metastases. The median time to CRPC was 13 months (2–116 months). The median number of drugs used in treatment before cabazitaxel therapy was 4 (1–10 drugs). Fifteen cases had no history of new hormone treatment, whereas 20 cases were previously treated with one new hormone, and 20 cases were previously treated with two new hormones. Eight patients did not have a history of docetaxel treatment, whereas 47 did. The median initial cabazitaxel dose was 20 mg/m2 (15–25 mg/m2), and the administration cycle was a median of 4 weeks (3–5 weeks). About 95% of cases were treated with pegfilgrastim (Table 1).
Table 1.
Characteristics of patients treated with cabazitaxel (n = 55).
| Cases | 55 |
| Median age (years, range) | 73, (56-84) |
| Median baseline PSA (ng/ml, range) | 34.14, (0.06-1159) |
| ECOG performance status (cases) | 0:27 1:22 2:5 3:1 |
| Median total Gleason score (Gleason score: cases) | 9 (6:2/7:5/8:8/9:20/10:9/unknown:11) |
| Cases with metastasis at baseline (%, cases) | 94, (34/36) |
| Metastatic part (%) | Bone (81) Lymph node (53) Visceral (25) |
| Median duration of castrate-resistant prostate cancer (months, range) | 13, (2-116) |
| Median number of pretreatment drugs (number, range) | 4, (1-10) |
| Number of new hormonal agents used (number: cases) | 0:15/1:20/2:20 |
| Docetaxel therapy history (%, cases) | 85, (47/55) |
| Cabazitaxel dose (mg/m2: cases) | 15:1/17.5:3/20:29/21:1/25:21 |
| Dosing interval (weeks: cases) | 3:22/4:28/5:1/unknown:4 |
| Using pegfilgrastim (%, cases) | 95, (52/55) |
PSA, prostate-specific antigen; ECOG, eastern cooperative oncology group.
The drug was still being administered for four cases, whereas 51 cases had already completed their course of treatment. The reasons for discontinuing cabazitaxel included disease progression (37 cases), discovery of progressive esophageal cancer (1 case), and adverse effects or worsening of general condition (13 cases).
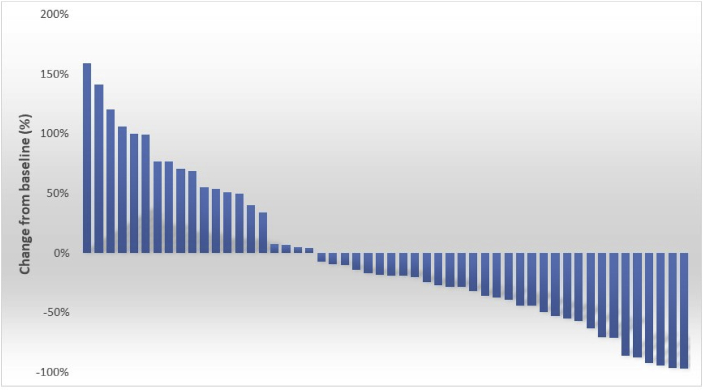
The patients received a median 4 courses of cabazitaxel treatments (1–28 courses). Thirty-two (61.5%) of 52 patients (three patients who did not measure PSA were excluded) experienced decreases in PSA, and 19 (36.5%), 12 (23.0%), and 4 (7.6%) patients experienced PSA declines of ≥30%, ≥50%, and ≥90%, respectively, (Fig. 1).
Fig. 1.
Waterfall plot of maximal percent changes in the value of prostate-specific antigen from the baseline in 55 Japanese patients with castration-resistant prostate cancer who received cabazitaxel.
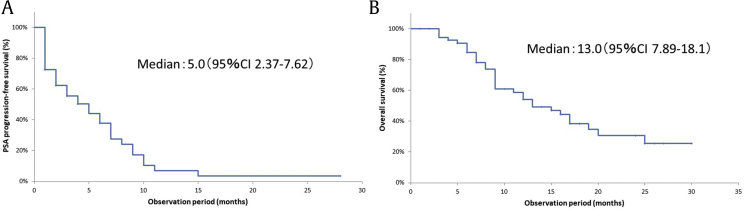
In our investigation of the 32 cases that experienced declines in PSA, we found that the median number of required courses until PSA nadir was 3.5 (1–14 courses). The median PSA-PFS was 5.0 months (95% confidence interval (CI), 2.37–7.62; Fig. 2A) and the median overall survival was 13.0 months (95% CI, 7.89–18.1; Fig. 2B).
Fig. 2.
Kaplan–Meier analysis for the time to prostate-specific antigen progression-free survival (A) and overall survival (B) in the total population (n = 55).
The adverse effects ≥ Grade 3 were as follows: Grade 3 leukopenia and neutropenia were reported in 10 and 6 cases, respectively, and Grade 4 leukopenia and neutropenia in 15 and 23 cases, respectively. Seven and 2 cases of Grade 3 febrile neutropenia and Grade 4 febrile neutropenia were noted, respectively. Three, 2, and 1 case of Grade 3 anorexia, vomiting, and fatigue were noted, respectively. Three cases of Grade 3 thrombocytopenia were noted, whereas 1 case of Grade 4 thrombocytopenia was noted. Grade 3 anemia was present in 2 cases, and pneumonia, Grade 4 sepsis, and Grade 4 gastrointestinal perforation were found in 1 case each; 1 case of Grade 5 diarrhea was reported (Supplemental Data Table S1).
After the completion of cabazitaxel treatment, a total of 45 patients underwent pharmaceutical posttreatment. A decrease in PSA was observed in 7 of the 11 patients who were administered enzalutamide (4/6 at initial administration and 3/5 at readministration), 4 of the 17 patients who were administered abiraterone (4/14 at initial administration, 0/3 at readministration), and 3 of the 3 patients who were administered estramustine. A decrease in PSA was also observed in 1 of the 5 patients who were administered alpharadin, 1 of the 3 patients who were administered ethinylestradiol, and 1 of the 2 patients who were administered immunotherapy. There was no effect on PSA in 2 patients who were readministered cabazitaxel; 1 patient was readministered docetaxel, and the other was administered chlormadinone (Supplemental Data Table S2).
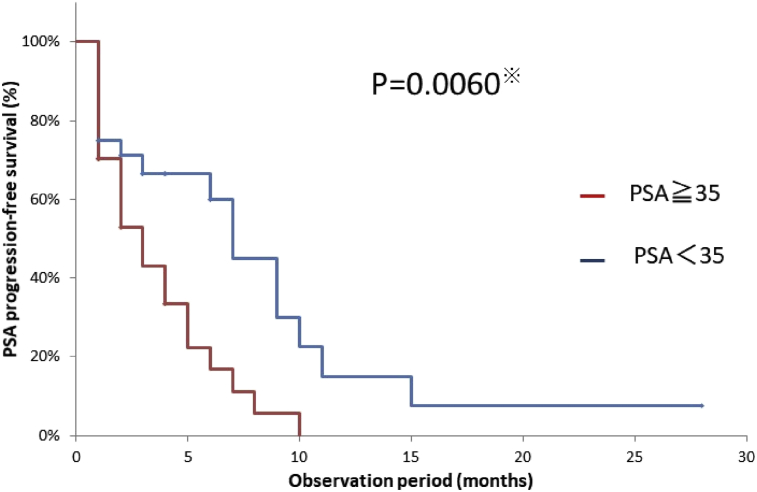
In our investigation of the efficacy of cabazitaxel, the results indicated that only the PSA values at start of treatment showed a significant difference [hazard ratio (HR), 2.32; 95% CI, 1.18–4.57; p = 0.015; p = 0.006]. No significant differences were observed for the other values (Table 2, Fig. 3).
Table 2.
Univariate analysis of associations between various parameters and prostate-specific antigen progression-free survival in patients with castrate-resistant prostate cancer who received cabazitaxel.
| Variables (n, %) | Univariate analysis |
||
|---|---|---|---|
| HR 95% CI | p value | ||
| Age (years) | 0.83 | 0.5818 | |
| ≧75 | 24 (43.6) | (0.42-1.62) | |
| ˂75 | 31 (56.4) | ||
| PS | 1.24 | 0.6908 | |
| 2-3 | 6 (12.7) | (0.43-3.55) | |
| 0-1 | 49 (87.3) | ||
| PSA (ng/ml) | 2.32 | 0.0150* | |
| ≧35 | 27 (49.1) | (1.18-4.57) | |
| ˂35 | 28 (50.9) | ||
| Time to CRPC (months) | 1.05 | 0.8886 | |
| ≧12 | 28 (50.9) | (0.54-2.04) | |
| ˂12 | 27 (49.1) | ||
| Number of pretreatment drugs | 1.04 | 0.8484 | |
| ˃4 | 29 (52.3) | (0.51-1.84) | |
| ≦4 | 26 (47.7) | ||
| Number of new hormonal agents used | 0.97 | 0.9324 | |
| 2 | 20 (36.4) | (0.70-1.55) | |
| 1 | 20 (36.4) | ||
| 0 | 15 (27.2) | ||
| Cabazitaxel dose(mg/m2) | 0.85 | 0.6201 | |
| ˃20 | 33 (60.0) | (0.44-1.63) | |
| ≦20 | 22 (40.0) | ||
| Grade 3/4 neutropenia | 1.09 | 0.7995 | |
| Yes | 29 (52.3) | (0.57-2.07) | |
| No | 26 (47.7) | ||
| NLR (n = 48) | 1.55 | 0.2400 | |
| ˃3.6 | 24 (50.0) | (0.75-3.22) | |
| ≦3.6 | 24 (50.0) | ||
| Total Gleason score (n = 44) | 1.03 | 0.9437 | |
| ˃8 | 29 (65.9) | (0.50-2.09) | |
| ≦8 | 15 (34.1) | ||
| Visceral metastasis (n = 34) | 2.23 | 0.1171 | |
| Yes | 9(26.5) | (2.82-6.11) | |
| No | 17(73.5) | ||
*p < 0.05.
PS, performance status; CRPC, castrate-resistant prostate cancer; NLR, neutrophil/lymphocyte ratio; HR, hazard ratio; PSA, prostate-specific antigen.
Fig. 3.
Kaplan–Meier analysis for the time to prostate-specific antigen (PSA)-progression-free survival of the 55 patients who received cabazitaxel in the PSA ≥ 35 group vs the PSA < 35 group.
At the beginning period of cabazitaxel treatment, the patient was hospitalized for treatment and was discharged after confirming of rising white blood cell (WBC) counts. After the doctors get used to administration of cabazitaxel, they tended to follow-up at outpatient.
Safety analysis revealed that there was a median of 8 days (5–10 days) until leukocyte nadir was achieved, and 2 days (1–6 days) until the WBC rose from the nadir. In the 37 cases in which pegfilgrastim was used and in which we had enough data for WBC and neutrophil counts during both the first and second courses of cabazitaxel, the median WBC and neutrophil counts at nadir in the first course were 2140 and 763, respectively; Grade 3 and Grade 4 leukocytopenia was observed in 8(21.6%) and 10(27.0%) cases, respectively, and neutropenia was observed in 5(13.5%) and 15(40.5%) cases, respectively. WBC and neutrophil counts at nadir during the second course were 4900 and 3630, respectively, indicating a significant increase compared with values in the first course (p = 0.0013 and p < 0.001, respectively). There were 4(10.8%) and 1(2.7%) case of Grade 3 and 4 of leukocytopenia, respectively, and 2(5.4%) and 3(8.1%) cases of Grade 3 and 4 of neutropenia, respectively, indicating a significant decrease compared with the values in the first course of treatment (p < 0.001, p = 0.0021). However, because the dose was reduced in 5 of the 37 cases at the second course, we repeated our investigation on the 32 cases wherein doses were not reduced. The median WBC and neutrophil counts of this cohort at nadir during course one of treatment were 2535 and 1028, respectively; Grade 3 and 4 leukocytopenia was observed in 6(18.8%) and 8(25.0%) cases, respectively, and Grade 3 and 4 neutropenia was observed in 5(15.6%) and 11(34.4%) cases, respectively. The WBC and neutrophil counts at nadir during course two of treatment, compared with the first course, were 5100 and 3662, respectively, indicating a significant increase (p = 0.0156, p = 0.0034). Grade 3 and 4 leukocytopenia was observed in 4(12.5%) and 1(3.1%) case, respectively, and Grade 3 and 4 neutropenia in 2(6.2%) and 3(9.3%) cases, respectively, indicating significant decreases (p = 0.0051, p < 0.001). Thus, the difference in doses did not change the results (Table 3).
Table 3.
Safety of cabazitaxel, cases with data on white blood cell count and neutrophil count for the first and second courses of treatment (n = 37), excluding cases in which the cabazitaxel dose was reduced in the second course (n = 32).
| Median time to nadir of white blood cells (days, range) (n = 55) | 8, (5–10) | ||
| Median time to leukocyte ascent (days, range) (n = 55) |
2, (1–6) |
||
| Number of courses (n = 37) |
1st |
2nd |
p value |
| Nadir of white blood cells (/μl) (median, range) |
2140, (590–12820) | 4900, (780–19030) | 0.0013** |
| Grade 3/4 Leukopenia (cases, %) | G3: 8, (21.6) G4: 10, (27.0) |
G3: 4, (10.8) G4: 1, (2.7) |
˂ 0.001** |
| Nadir of neutrophils (median, range) | 763, (20-9700) | 3630, (120-14700) | ˂ 0.001** |
| Grade 3/4 neutropenia (cases, %) |
G3: 5, (13.5) G4: 15, (40.5) |
G3: 2, (5.4) G4: 3, (8.1) |
0.0021** |
| Number of courses (n = 32) |
1st |
2nd |
p value |
| Nadir of white blood cells (/μl) (median, range) |
2535, (730-12820) | 5100, (780-19030) | 0.0156* |
| Grade 3/4 leukopenia (Cases, %) | G3: 6, (18.8) G4: 8, (25.0) |
G3: 4, (12.5) G4: 1, (3.1) |
0.0051* |
| Nadir of neutrophils (median, range) | 1028, (20-9700) | 3662, (120-14700) | 0.0034** |
| Grade 3/4 neutropenia (cases, %) | G3: 5, (15.6) G4: 11, (34.4) |
G3: 2, (6.2) G4: 3, (9.3) |
˂ 0.001** |
*p < 0.05; **p < 0.005.
In the third course, there are many cases that have passed with a value similar to that of the second course. In cases where the disease did not progress, the tendency of decreasing WBC and neutrophil counts were not seen even when additional courses continued.
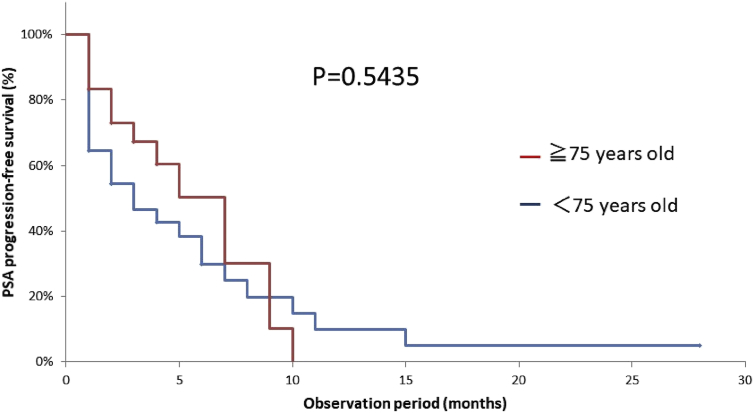
In this study, many of the patients (24 of 55) were aged ≥75 years, and their median age was 77.5 years. Nineteen of the 24 patients in the ≥75-year-old group (79%) had their doses reduced at the initial administration, and their median dose was 20 mg/m2. This dose was significantly smaller than that in the <75-year-old group (p = 0.0098), but no significant difference was observed in PSA-PFS (Fig. 4).
Fig. 4.
Kaplan–Meier analysis for the time to prostate-specific antigen progression-free survival of the 55 patients who received cabazitaxel in the ≥75-year-old group vs < 75-year-old group.
Adverse effects ≥ Grade 3/4 in the ≥75 year-old group were as follows: Grade 3 febrile neutropenia in 7 cases, Grade 4 febrile neutropenia in 2 cases, Grade 4 gastrointestinal perforation in 1 case, Grade 4 sepsis in 1 case, Grade 3 thrombocytopenia in 1 case, Grade 3 anorexia in 3 cases, Grade 3 vomiting in 2 cases, and Grade 3 fatigue and pneumonia in 1 case each. Thus, there was a tendency for more adverse effects in the ≥75-year-old group compared with that in the <75-year-old group. There were a particularly high number of adverse effects in the ≥75-year-old patient group whose dose was not reduced. Ten patients discontinued treatment owing to adverse effects and systemic worsening, a higher number of patients than in the <75-year-old group (Table 4).
Table 4.
Comparison by age, ≥75 years old vs < 75 years old.
| Age | <75 | ≧75 |
| n | 31 | 24 |
| Median age (years) | 67.0 | 77.5 |
| Reduction of drug (cases, %) | 15, (48.4) | 19, (79.2) |
| Median cabazitaxel dose (mg/m2) | 25 | 20 |
| Number of treatments discontinued owing to adverse events and deterioration of general condition (number, %) | 3, (9.7) | 10, (41.3) |
| Adverse events ≧ G3 (number) | Thrombocytopenia G3 (2), G4 (1), Anemia G3 (2), Diarrhea G5 (1) |
All adverse events Thrombocytopenia G3 (1), Anorexia G3 (3), Vomiting G3 (2), Fatigue G3 (1), FNG3 (7), FNG4 (2), Pneumonia G3 (1), Gastrointestinal perforation G4 (1), Sepsis G4 (1). 25 mg/m2(n = 5) Thrombocytopenia G3 (1), Anorexia G3 (3), Vomiting G3 (2), Gastrointestinal perforation G4 (1), FNG3 (3) |
FN, febrile neutropenia. To make it easier to see that the cabazitaxel dose is changing is given in bold.
4. Discussion
Some limitations exist in this study because the study is retrospective study in associated other hospitals, there is no definitive protocol in both treatment and follow-up schedules, and pathological diagnosis was diagnosed by pathologists at each hospital.
Our investigation of the efficacy of cabazitaxel indicated there were no significant differences in PSA-PFS regarding GS at the time of diagnosis, the period from the start of treatment to CRPC, or the number of medications administered before the use of cabazitaxel. This suggests that cabazitaxel is consistently effective regardless of whether it is administered to cases with short-term hormone treatment effect or cases with a high degree of malignancy. We did not observe significant differences in PSA-PFS based on past history of new hormone therapy. This suggests that, as reported previously11, 12, cabazitaxel either has no cross-resistance with new hormonal agents or has cross-resistance to a small extent. Our investigation of a low-dose cabazitaxel group (≤20 mg/m2) and a high-dose cabazitaxel group (≥21 mg/m2), likewise, indicated no significant difference in PSA-PFS, which is consistent with previous studies13, 14. However, despite previous reports4,6,9,15 that cabazitaxel is effective in cases with Grade 3/4 neutropenia and low neutrophil/lymphocyte ratio values, in the present study, no significant differences were observed. The only significant difference observed was the PSA value. Cabazitaxel was more effective in cases with low PSA values, indicating that treatment should be administered as soon as possible before prostate cancer progresses. This is a method for extending PSA-PFS of cabazitaxel; however, it is unclear from this study whether this action contributes to prolonging overall survival.
Our investigation of postcabazitaxel treatment was conducted in 45 cases. Of these, 17 (38%) experienced decreases in PSA. This is consistent with previous research16. In particular, PSA decreases were observed in 4 of 6 cases that were initially administered enzalutamide and in 3 of 5 cases of readministered enzalutamide, and in all 3 cases that underwent initial administration of estramustine. These results suggest that administration of these drugs after cabazitaxel may be effective.
Our investigation of safety indicated that, in cases where cabazitaxel was administered continuously at the same dose, both the WBC and the neutrophil counts significantly increased (p = 0.0156 and p = 0.0034, respectively) during the second course compared with the first course of treatment, and that the adverse effects of leukocytopenia and neutropenia significantly declined (p = 0.0051 and p < 0.001, respectively). Although the mechanism of action remains unknown, most of the 37 cases were administered with pegfilgrastim on the second day of the first course. Therefore, this may have influenced the increase in the number of WBC and neutrophil counts. These results suggest that even if Grade 3 or 4 leukocytopenia and neutropenia are observed during the first course of treatment, the treatment should be carefully continued at the same dose.
In this study, there were many patients aged ≥75 years (24/55). Investigation of the effectiveness of treatment indicated no significant difference in PSA-PFS, and although there was no decrease in treatment efficacy with low doses, a high number of ≥ Grade 3 adverse effects were observed in those aged ≥75 years. There was also a tendency toward both disease progression and systemic worsening as the number of treatments increased. By properly reducing the dose, it is possible to treat patients aged ≥75 years without any loss in drug efficacy, but there is a tendency for many ≥ Grade 3 adverse effects to occur. Thus, when treating elderly patients, it is necessary to exercise extreme caution.
5. Conclusion
Cabazitaxel does not exhibit resistance to new hormonal agents, and it has been shown to be consistently effective in cases of highly malignant cancer and cases in which hormone therapy was insufficiently effective. To use cabazitaxel effectively, it is important to begin use as soon as possible and before disease progression, and it is important to carefully maintain the same dose even if Grade 3/4 leukocytopenia and neutropenia occurs during the first course. In addition, by reducing the dose, cabazitaxel can treat patients aged ≥75 years without a decrease in the therapeutic effect, but there is a tendency for many ≥ Grade 3 adverse effects to occur; as a result, extreme caution must be exercised.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
Ethics approval and consent to participate in the present study were provided by the Institutional Review Board (IRB) of the Nagano Municipal Hospital (Approval No. 29 City Hospital Ethics Committee No. 0059). Consent was obtained from all participants included in the study through the opt-out method in accordance with the national regulations and the ethical guidelines for clinical studies in Japan. The IRB waived the requirement for written informed consent owing to the retrospective nature of the present analysis.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
The authors are grateful to all doctors at the hospitals associated with the Shinshu University who provided the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2019.10.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mukherji D., Omlin A., Pezaro C., Shamseddine A., de Bono J. Metastatic castration-resistant prostate cancer (CRPC): preclinical and clinical evidence for the sequential use of novel therapeutics. Cancer Metastasis Rev. 2014;33(2-3):555–566. doi: 10.1007/s10555-013-9473-1. [DOI] [PubMed] [Google Scholar]
- 2.Fitzpatrick J.M., Bellmunt J., Fizazi K., Heidenreich A., Sternberg C.N., Tombal B. Optimal management of metastatic castration-resistant prostate cancer: highlights from a European Expert Consensus Panel. Eur J Cancer. 2014;50(9):1617–1627. doi: 10.1016/j.ejca.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 3.Chi K., Hotte S.J., Joshua A.M., North S., Wyatt A.W., Collins L.L. Treatment of mCRPC in the AR-axis-targeted therapy-resistant state. Ann Oncol. 2015;26(10):2044–2056. doi: 10.1093/annonc/mdv267. [DOI] [PubMed] [Google Scholar]
- 4.Meisel A., von Felten S., Vogt D.R., Liewen H., de Wit R., de Bono J. Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration-resistant prostate cancer (mCRPC): A post-hoc analysis of the TROPIC phase III trial. Eur J Cancer. 2016;56:93–100. doi: 10.1016/j.ejca.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Miyake H., Sugiyama T., Aki R., Matsushita Y., Tamura K., Motoyama D. No significant impact of prior treatment profile with docetaxel on the efficacy of cabazitaxel in Japanese patients with metastatic castration-resistant prostate cancer. Med Oncol. 2017;34(8):141. doi: 10.1007/s12032-017-1005-3. [DOI] [PubMed] [Google Scholar]
- 6.Uemura K., Kawahara T., Yamashita D., Jikuya R., Abe K., Tatenuma T. Neutrophil-to-Lymphocyte ratio predicts prognosis in castration-resistant prostate cancer patients who received cabazitaxel chemotherapy. BioMed Res Int. 2017;2017:7538647. doi: 10.1155/2017/7538647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hongo H., Kosaka T., Oya M. Analysis of cabazitaxel-resistant mechanism in human castration-resistant prostate cancer. Cancer Sci. 2018;109(9):2937–2945. doi: 10.1111/cas.13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosaka T., Hongo H., Watanabe K., Mizuno R., Kikuchi E., Oya M. No significant impact of patient age and prior treatment profile with docetaxel on the efficacy of cabazitaxel in patient with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2018;82(6):1061–1066. doi: 10.1007/s00280-018-3698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kosaka T., Shinojima T., Morita S., Oya M. Prognostic significance of grade 3/4 neutropenia in Japanese prostate cancer patients treated with cabazitaxel. Cancer Sci. 2018;109(5):1570–1575. doi: 10.1111/cas.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.General Rule for Clinical and Pathological Studies on Prostate Cancer. 4th ed. The Japanese Society of Pathology, Japan Radiological Society; 2010. The Japanese Urological Association. [Google Scholar]
- 11.Pezaro C.J., Omlin A.G., Altavilla A., Lorente D., Ferraldeschi R., Bianchini D. Activity of cabazitaxel in castration-resistant prostate cancer progressing after docetaxel and next-generation endocrine agents. Eur Urol. 2014;66(3):459–465. doi: 10.1016/j.eururo.2013.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Al Nakouzi N., Le Moulec S., Albiges L., Wang C., Beuzeboc P., Gross-Goupil M. Cabazitaxel remains active in patients progressing after docetaxel followed by novel androgen receptor pathway targeted therapies. Eur Urol. 2015;68(2):228–235. doi: 10.1016/j.eururo.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberger M., Hardy-Bessard A.C., Kim C.S., Geczi L., Ford D., Mourey L. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m(2)) and the currently approved dose (25 mg/m(2)) in postdocetaxel patients with metastatic castration-resistant prostate cancer-PROSELICA. J Clin Oncol. 2017;35(28):3198–3206. doi: 10.1200/JCO.2016.72.1076. [DOI] [PubMed] [Google Scholar]
- 14.Oudard S., Fizazi K., Sengelov L., Daugaard G., Saad F., Hansen S. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III trial-FIRSTANA. J Clin Oncol. 2017;35(28):3189–3197. doi: 10.1200/JCO.2016.72.1068. [DOI] [PubMed] [Google Scholar]
- 15.Lorente D., Mateo J., Templeton A.J., Zafeiriou Z., Bianchini D., Ferraldeschi R. Baseline neutrophil-lymphocyte ratio (NLR) is associated with survival and response to treatment with second-line chemotherapy for advanced prostate cancer independent of baseline steroid use. Ann Oncol. 2015;26(4):750–755. doi: 10.1093/annonc/mdu587. [DOI] [PubMed] [Google Scholar]
- 16.Badrising S.K., van der Noort V., Hamberg P., Coenen J.L., Aarts M.J., van Oort I.M. Enzalutamide as a fourth- or fifth-line treatment option for metastatic castration-resistant prostate cancer. Oncology. 2016;91(5):267–273. doi: 10.1159/000448219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.