Abstract
Background
To evaluate the relationship between body composition and the oncological outcome of androgen deprivation therapy (ADT), we investigated whether body composition features including the psoas muscle may be predictive factors of ADT.
Methods
This study enrolled patients with hormone-naïve metastatic prostate cancer who were treated with primary ADT from April 1996 to November 2013 at Kyushu University Hospital and who underwent a computed tomography scan before primary ADT for calculating body fat percentage, psoas muscle ratio (psoas muscle, cm3/height, cm), and body mass index.
Results
Of the 178 patients enrolled, 60 patients died during follow-up. Median follow-up was 32 months, and progression-free survival and overall survival (OS) were 28 and 80 months, respectively. Multivariate analysis revealed that the psoas muscle ratio was correlated with OS (hazard ratio: 0.448; 95% confidence interval = 0.206–0.922; p = 0.028).
Conclusions
This study demonstrated that higher psoas muscle ratio predicts longer OS among patients with nonlocalized prostate cancer treated with primary ADT.
Keywords: Androgen deprivation therapy, Body composition, Prostate cancer, Psoas muscle, Testosterone
1. Introduction
Prostate cancer (PCa) is one of the most common cancers in men in the United States, Western Europe, and Japan.1,2 Since the 1940s, androgen deprivation therapy (ADT) has been the gold standard for primary therapy of metastatic PCa because ADT suppresses the production of testosterone.3 Most patients with PCa respond well to ADT because their tumors are dependent on androgens for their growth, but eventually become resistant to ADT, and are then defined as having castration-resistant PCa.4 Clinical stage and Gleason score are important factors for predicting outcome of ADT.5 Previously, insufficient decreases in serum testosterone levels during Luteinizing Hormone-Releasing Hormone (LHRH) agonist treatment were reported in obese men, which may detrimentally affect the outcome.6,7 Meanwhile, there are controversial reports on the effects of obesity on the prognosis of ADT.8, 9, 10 In addition, there are no reports on the influence of detailed body composition on the efficacy of ADT. Therefore, we hypothesized that body composition including the psoas muscle and distribution of adipose tissue may affect the efficacy of ADT, and as a result, contribute to the oncological outcome of patients with PCa.
In this study, we attempted to identify the parameters of body composition that influence the oncological outcome of ADT in PCa.
2. Materials and Methods
2.1. Study design
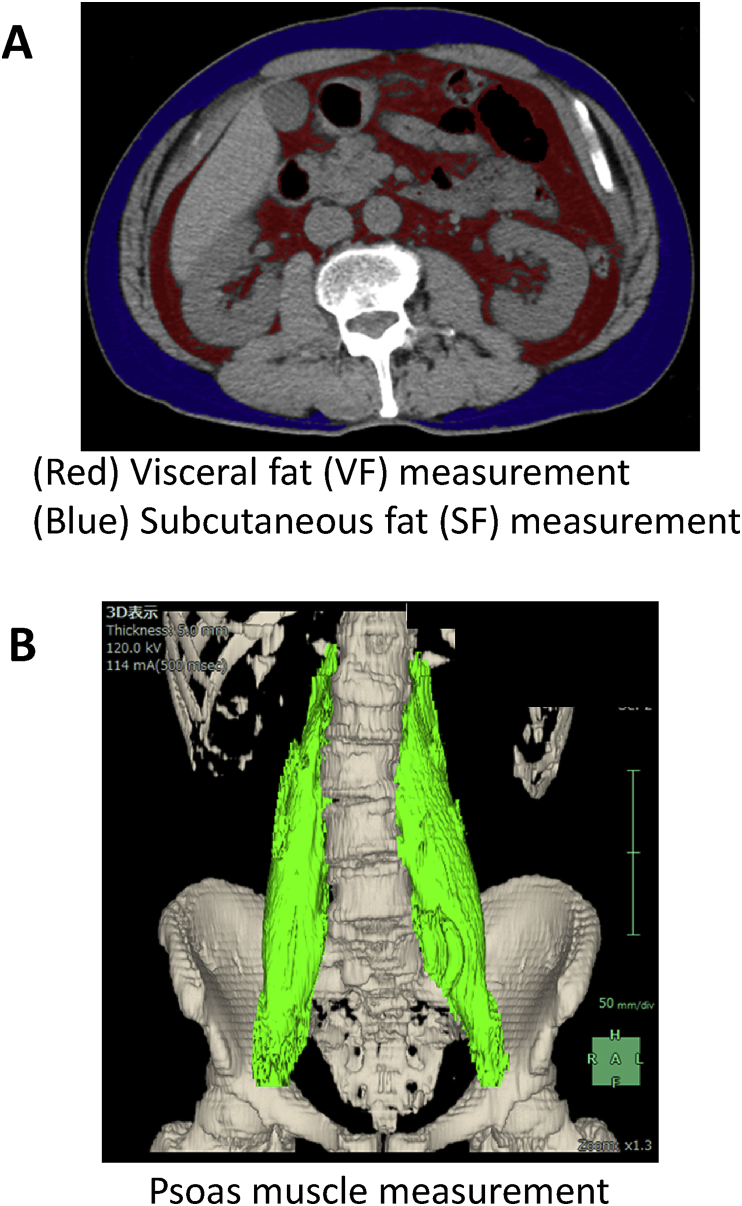
This study was retrospective and included patients with metastatic PCa treated with primary ADT at Kyushu University Hospital (Fukuoka, Japan) from April 1996 to November 2013. This study was approved by the institutional review board. All patients were histopathologically verified with adenocarcinoma via prostate biopsy. Body mass index (BMI) (kg/m2) was calculated for each patient based on weight and height values recorded before therapy. All the patients were examined by computed tomography before ADT. Adipose tissue was identified as the pixels ranging from −250 to −50 Hounsfield units. All imaging data were transferred to a computer workstation for analysis of the visceral/subcutaneous fat (SF) and psoas muscle volume. Visceral fat (VF) volume, SF volume (Fig. 1A), psoas muscle volume (Fig. 1B) were calculated using SYNAPSE VINCENT software (Fuji Film, Tokyo, Japan). SF was calculated from the diaphragm to the pubic bone level. To calculate the visceral fat/SF ratio (V/S ratio), the VF volume was divided by the SF volume. To calculate the psoas muscle ratio, the psoas muscle volume was divided by the height. All patients were primarily treated with ADT by surgical castration or medical castration using an Luteinizing Hormone-Releasing Hormone (LHRH) agonist (goserelin acetate or leuprorelin acetate) with or without a traditional antiandrogen (bicalutamide, flutamide, or chlormadinone acetate). Progressive disease was defined as an increase in serum prostate-specific antigen (PSA) levels of >2 ng/ml and a 25% increase over the nadir, the appearance of a new lesion, or the progression of known lesions classified in accordane with the Response Evaluation Criteria in Solid Tumors.11
Fig. 1.
Measurement of (A) visceral and subcutaneous fat and (B) psoas muscle volume using SYNAPSE VINCENT software.
2.2. Statistical analysis
All statistical analyses were performed using JMP Pro 13 software (SAS Institute, Cary, NC, USA). Univariate and multivariate analyses were performed using the Cox proportional hazards regression model. In multivariate analysis, we analyzed VF percentage, V/S ratio, psoas muscle ratio, and BMI separately adjusted for age, Gleason score, PSA at diagnosis, cT stage, and cM stage to investigate each parameter of body composition that may influence clinical outcome.
3. Results
Table 1 summarizes the clinical and pathological characteristics of 178 patients. Clinical staging was undertaken before ADT, and all the patients were diagnosed with metastatic hormone-naive PCa (N1 and/or M1). Pretreatment testosterone was tested in 58 patients (32.6%). The median follow-up was 32 months (range, 0–190) and all-cause death occurred in 60 (33.7%) patients. All patients were diagnosed as having disease progression with PSA progression. Median progression-free survival (PFS) and overall survival (OS) were 28 and 80 months, respectively.
Table 1.
Patient characteristics (n = 178).
| variable | |
|---|---|
| Median age, years (range) | 72 (46-91) |
| PSA at diagnosis, median (range) | 164 (3.2-8740) |
| Biopsy Gleason score, n (%) | |
| ≤6 | 5 (2.8) |
| 7 | 29 (16.2) |
| ≥8 | 129 (72.4) |
| not available | 15 (8.4) |
| cT stage, n (%) | |
| T1c | 1 (0.5) |
| T2a | 4 (2.2) |
| T2b | 5 (2.8) |
| T2c | 1 (0.5) |
| T3a | 64 (35.9) |
| T3b | 43 (24.1) |
| T4 | 52 (29.2) |
| not available | 8 (4.4) |
| cN stage | |
| N0 | 59 (33.1) |
| N1 | 116 (65.1) |
| not available | 3 (1.6) |
| cM stage | |
| M0 | 19 (10.6) |
| M1 | 157 (88.2) |
| not available | 2 (1.1) |
| Median testosterone, ng/dl (range) | 400 (37-1042) |
| Median BMI, kg/m2 (range) | 22.5 (14.5-31.1) |
| Median visceral fat, % (range) | 29.4 (2.1-57.6) |
| Median V/S ratio, ratio (range) | 1.16 (0.34-19.6) |
| Psoas muscle ratio, ratio (range) | 1.95 (0.71-3.64) |
| hormonal therapy | |
| Castration | 11 (6.2) |
| Combined androgen blockage | 167 (93.8) |
Abbreviations: BMI, body mass index; PSA, prostate-specific antigen; V/S ratio, Visceral fat/subcutaneous fat ratio.
Psoas muscle ratio: Psoas muscle/height (cm3/cm).
In these patients treated with ADT, we attempted to identify parameters associated with PFS and OS by univariate and multivariate analyses using the Cox proportional hazard regression model. Among several parameters, Gleason score, cT stage, cM stage, and VF percentage were identified as significant factors for PFS in univariate analysis (Table 2). Gleason score, cT stage, VF percentage, psoas muscle ratio, and BMI were revealed as significant or marginally significant factors for OS in univariate analysis (Table 2).
Table 2.
Univariate analysis of metastatic PCa.
| Variate | No. | Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age (per 1 year) | |||||||
| 1.002 | 0.976-1.029 | 0.850 | 1.014 | 0.979-1.051 | 0.439 | ||
| Gleason score | |||||||
| ≤6 | 5 | 1 | 1 | ||||
| 7 | 29 | 9.49 E+08 | 0.980-infinity | 0.052 | 3.97 E+08 | 0.569-infinity | 0.130 |
| ≥8 | 129 | 1.70 E+09 | 1.840-infinity | 0.008 | 5.80 E+08 | 0.899-infinity | 0.062 |
| PSA at diagnosis (per 1 ng/ml) | |||||||
| 1 | 0.999-1.000 | 0.128 | 1 | 0.999-1.000 | 0.237 | ||
| cT stage | |||||||
| T1c T2a T2b T2c | 11 | 1 | 1 | ||||
| T3a T3b | 107 | 1.802 | 0.664-7.405 | 0.277 | 4.73 E+08 | 1.543-infinity | 0.015 |
| T4 | 52 | 3.392 | 1.221-14.082 | 0.015 | 7.56 E+08 | 2.432-infinity | 0.003 |
| cN stage | |||||||
| N0 | 59 | 1 | 1 | ||||
| N1 | 116 | 1.443 | 0.939-2.277 | 0.095 | 1.104 | 0.646-1.941 | 0.720 |
| cM stage | |||||||
| M0 | 19 | 1 | 1 | ||||
| M1 | 157 | 2.365 | 1.125-6.079 | 0.020 | 2.111 | 0.856-7.032 | 0.112 |
| Testosterone (per 1 ng/dl) | 1.001 | 0.998-1.003 | 0.388 | 1.001 | 0.998-1.004 | 0.442 | |
| Visceral fat percentage (per 1%) | 0.979 | 0.965-0.994 | 0.006 | 0.979 | 0.961-0.997 | 0.029 | |
| V/S ratio (per 1) | 1.010 | 0.975-1.029 | 0.446 | 1.009 | 0.921-1.038 | 0.722 | |
| Psoas muscle ratio (per 1 cm3/cm) | 1.109 | 0.729-1.651 | 0.901 | 0.593 | 0.316-1.066 | 0.082 | |
| BMI (per 1 kg/m2) | 0.943 | 0.881-1.007 | 0.084 | 0.883 | 0.802-0.968 | 0.007 | |
Abbreviations: BMI, body mass index;PSA, prostate-specific antigen; V/S ratio, Visceral fat/subcutaneous fat ratio.
In this study, we focused on body composition and wanted to determine whether each parameter, such as VF percentage, V/S ratio, psoas muscle ratio, and BMI had any impact on PFS and OS considering pathological and clinical information. Therefore, we performed multivariate analysis using each of these four variables separately adjusted for age, Gleason score, PSA at diagnosis, cT stage, and cM stage. As a result, psoas muscle ratio was significantly associated with OS (hazard ratio = 0.448; 95% confidence interval (CI) = 0.206–0.922; p = 0.028) (Table 3).
Table 3.
Multivariate analysis of metastatic PCa.
| Variate | No. | Progression-free survival |
Overall survival |
||||
|---|---|---|---|---|---|---|---|
| HRa | 95% CIa | p value | HRa | 95% CIa | p value | ||
| Visceral fat percentage (per 1%) | 0.990 | 0.973-1.006 | 0.238 | 0.992 | 0.221-2.258 | 0.544 | |
| V/S ratio (per 1) | 1.004 | 0.980-0.029 | 0.714 | 1.003 | 0.958-1.049 | 0.899 | |
| Psoas muscle ratio (per 1 cm3/cm) | 1.035 | 0.642-1.630 | 0.882 | 0.448 | 0.206-0.922 | 0.028 | |
| BMI (per 1 kg/m2) | 0.972 | 0.904-1.043 | 0.441 | 0.915 | 0.823-1.013 | 0.091 | |
Abbreviations: BMI, body mass index; HR, hazard ratio; CI, confidence interval; PSA, prostate-specific antigen.
Adjusted for age, GS, PSA at diagnosis, cT stage and cM stage.
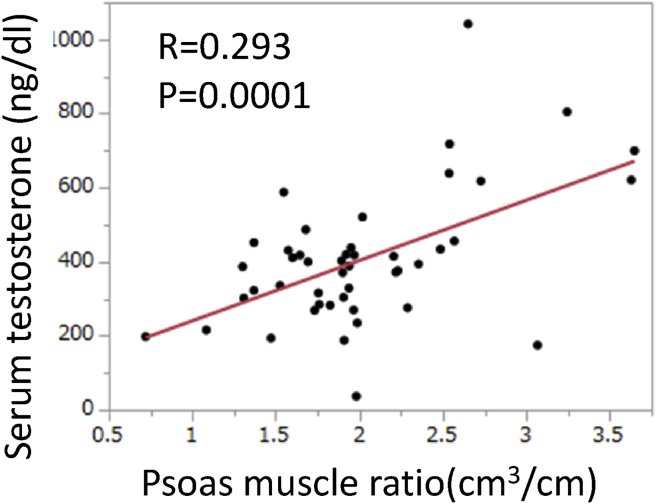
Finally, we investigated the relationship between serum testosterone levels and psoas muscle ratio. Serum testosterone data were available for 58 patients. The correlation coefficient was low, but the psoas muscle ratio was positively correlated with serum testosterone before ADT treatment (R = 0.293; p = 0.0001; Fig. 2).
Fig. 2.
Relationship between serum testosterone (ng/dl) and psoas muscle ratio (cm3/cm).
4. Discussion
Numerous epidemiological studies have examined the relationship between BMI and PCa incidence, but the findings remain inconclusive.12,13 Obesity increases the risk of high-grade PCa.13 However, higher BMI is a good prognostic factor in patients with androgen-dependent metastatic PCa14 and reduces the risk of metastasis after radical prostatectomy.15 Obese patients tend to be medicated for diabetes (e.g., metformin), which might have some antitumor effects.16 In a multivariate analysis in our study, BMI was not significant but showed a good tendency in OS (HR = 0.915; 95% CI: 0.823–1.013; p = 0.091). In our study cohort, median BMI was 22.5 and almost the same as the older Japanese population.17 Among Japanese adults aged 65 and older, a lower BMI was a risk factor of all-cause mortality, and our BMI results may support the speculation that having a thin body increases the risk of mortality.
Intriguingly, higher muscle strength is reported to be associated with improved survival in older patients with advanced cancer including PCa.18 In line with this notion, to our knowledge, this is the first study to show that increased muscle volume was associated with prognosis in ADT. We also demonstrated that the psoas muscle ratio was correlated with serum testosterone level. Testosterone has a critical role in PCa development and progression, and many studies have investigated the relationship between serum testosterone levels and PCa. Low testosterone is associated with high Gleason score19, 20, 21 and higher pathological stage.22,23 Before ADT, higher testosterone levels in advanced PCa are correlated with prolonged OS.24 For Japanese patients, testosterone reduction (≥480 ng/dl) during ADT therapy is a significant prognostic factor for OS.25 We focused on the psoas muscle because it is a core muscle and is considered to reflect the general health and mortality of different diseases.26, 27, 28 In healthy men, serum testosterone levels have a positive correlation with lean body mass29 and muscle strength.30 Furthermore, control of serum testosterone by Gonadotropin releasing hormone (GnRH) agonist and testosterone administration positively affects muscle size and strength.31 Consistent with this, our study suggested that the psoas muscle is a predictive marker of serum testosterone levels. Because high testosterone levels in serum is a well-known factor of OS9, the psoas muscle may be associated with OS via serum testosterone levels. However, in univariate analysis, serum testosterone was not a significant predictor of OS. This study included 178 patients, but serum testosterone data were only available for 58 patients, and hence we could not show a significant association with oncological outcome. Furthermore, other factors may be involved in better prognosis among men with larger psoas muscle volumes, and this area warrants further research.
ADT has numerous side effects including loss of libido, cardiovascular disease, osteoporosis, metabolic syndrome, and sarcopenia.32 Sarcopenia is a risk factor of frailty and falls33 and leads to reduced quality of life. To prevent the side effects of ADT and for better quality of life, exercise should be recommended.34,35 In addition, this study supported the hypothesis that exercise may improve the outcome of ADT via an increase in psoas muscle. However, it is inconclusive as to whether exercise can extend OS in patients who received ADT treatment, and more research is required.
Our study is not devoid of limitations. This is a retrospective study, and the patient number was relatively small. In addition, data on serum testosterone were available for only 58 patients (32.6%).
In conclusion, we showed that the psoas muscle ratio is a good prognostic factor of OS in patients with metastatic PCa and can be mediated by serum testosterone levels.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank H. Nikki March, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Wong M.C., Goggins W.B., Wang H.H., Fung F.D., Leung C., Wong S.Y. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. 2016;70(5):862–874. doi: 10.1016/j.eururo.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 2.Kimura T., Takahashi H., Okayasu M., Kido M., Inaba H., Kuruma H. Time trends in histological features of latent prostate cancer in Japan. J Urol. 2016;195(5):1415–1420. doi: 10.1016/j.juro.2015.11.068. [DOI] [PubMed] [Google Scholar]
- 3.Huggins C., Hodges C.V. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. J Urol. 2002;168(1):9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 4.Shiota M., Yokomizo A., Naito S. Pro-survival and anti-apoptotic properties of androgen receptor signaling by oxidative stress promote treatment resistance in prostate cancer. Endocr Relat Cancer. 2012;19(6):243–253. doi: 10.1530/ERC-12-0232. [DOI] [PubMed] [Google Scholar]
- 5.Ross R.W., Xie W., Regan M.M., Pomerantz M., Nakabayashi M., Daskivich T.J. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112(6):1247–1253. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 6.Oefelein M.G., Cornum R. Failure to achieve castrate levels of testosterone during luteinizing hormone releasing hormone agonist therapy: the case for monitoring serum testosterone and a treatment decision algorithm. J Urol. 2000;164(3):726–729. doi: 10.1097/00005392-200009010-00025. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.R. Obesity and sex steroids during gonadotropin-releasing hormone agonist treatment for prostate cancer. Clin Cancer Res. 2007;13(1):241–245. doi: 10.1158/1078-0432.CCR-06-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keto C.J., Aronson W.J., Terris M.K., Presti J.C., Kane C.J., Amling C.L. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110(4):492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiota M., Takeuchi A., Sugimoto M., Kashiwagi E., Dejima T., Kiyoshima K. Prognostic Impact of Serum Testosterone and Body Mass Index Before Androgen-deprivation Therapy in Metastatic Prostate Cancer. Anticancer Res. 2015;35(12):6925–6932. [PubMed] [Google Scholar]
- 10.Shiota M., Fujimoto N., Yokomizo A., Takeuchi A., Kashiwagi E., Dejima T. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 2016;19(2):191. doi: 10.1038/pcan.2016.2. [DOI] [PubMed] [Google Scholar]
- 11.Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright M.E., Chang S.C., Schatzkin A., Albanes D., Kipnis V., Mouw T. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 13.Gong Z., Neuhouser M.L., Goodman P.J., Albanes D., Chi C., Hsing A.W. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomark Prev. 2006;15(10):1977–1983. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery R.B., Goldman B., Tangen C.M., Hussain M., Petrylak D.P., Page S. Association of body mass index with response and survival in men with metastatic prostate cancer: Southwest Oncology Group trials 8894 and 9916. J Urol. 2007;178(5):1946–1951. doi: 10.1016/j.juro.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Schiffmann J., Karakiewicz P.I., Rink M., Manka L., Salomon G., Tilki D. Obesity paradox in prostate cancer: increased body mass index was associated with decreased risk of metastases after surgery in 13,667 patients. World J Urol. 2018;36:1067–1072. doi: 10.1007/s00345-018-2240-8. [DOI] [PubMed] [Google Scholar]
- 16.Raval A.D., Thakker D., Vyas A., Salkini M., Madhavan S., Sambamoorthi U. Impact of metformin on clinical outcomes among men with prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2015;18(2):110–121. doi: 10.1038/pcan.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamakoshi A., Yatsuya H., Lin Y., Tamakoshi K., Kondo T., Suzuki S. BMI and all-cause mortality among Japanese older adults: findings from the Japan collaborative cohort study. Obesity. 2010;18(2):362–369. doi: 10.1038/oby.2009.190. [DOI] [PubMed] [Google Scholar]
- 18.Versteeg K.S., Blauwhoff-Buskermolen S., Buffart L.M., de van der Schueren M.A., Langius J.A., Verheul H.M. Higher Muscle Strength Is Associated with Prolonged Survival in Older Patients with Advanced Cancer. The Oncologist. 2018;23(5):580–585. doi: 10.1634/theoncologist.2017-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lane B.R., Stephenson A.J., Magi-Galluzzi C., Lakin M.M., Klein E.A. Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology. 2008;72(6):1240–1245. doi: 10.1016/j.urology.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 20.San Francisco I.F., Regan M.M., DeWolf W.C., Olumi A.F. Low age adjusted free testosterone levels correlate with poorly differentiated prostate cancer. J Urol. 2006;175(4):1341–1346. doi: 10.1016/S0022-5347(05)00680-4. [DOI] [PubMed] [Google Scholar]
- 21.Schatzl G., Madersbacher S., Thurridl T., Waldmüller J., Kramer G., Haitel A. High-grade prostate cancer is associated with low serum testosterone levels. The Prostate. 2001;47(1):52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 22.Isom-Batz G., Bianco F.J., Jr., Kattan M.W., Mulhall J.P., Lilja H., Eastham J.A. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173(6):1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imamoto T., Suzuki H., Fukasawa S., Shimbo M., Inahara M., Komiya A. Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. Eur Urol. 2005;47(3):308–312. doi: 10.1016/j.eururo.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Claps M., Petrelli F., Caffo O., Amoroso V., Roca E., Mosca A. Testosterone levels and prostate cancer prognosis: A systematic review and meta-analysis. Clin Genitourin Cancer. 2018;16(3):165–175. doi: 10.1016/j.clgc.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto S., Sakamoto S., Minhui X., Tamura T., Otsuka K., Sato K. Testosterone reduction of≥ 480 ng/dl predicts favorable prognosis of Japanese men with advanced prostate cancer treated with androgen-deprivation therapy. Clin Genitourin Cancer. 2017;15(6):e1107–e1115. doi: 10.1016/j.clgc.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 26.Pahor M., Manini T., MJJ-TJoN Cesari, Health, Aging Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13(8):724–728. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith A.B., Deal A.M., Yu H., Boyd B., Matthews J., Wallen E.M. Sarcopenia as a predictor of complications and survival following radical cystectomy. J Urol. 2014;191(6):1714–1720. doi: 10.1016/j.juro.2013.12.047. [DOI] [PubMed] [Google Scholar]
- 28.Englesbe M.J., Patel S.P., He K., Lynch R.J., Schaubel D.E., Harbaugh C. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermeulen A., Goemaere S., Kaufman J. Testosterone, body composition and aging. J Endocrinol Investig. 1999;22(5 Suppl):110–116. [PubMed] [Google Scholar]
- 30.Roy T.A., Blackman M.R., Harman S.M., Tobin J.D., Schrager M., Metter E.J. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283(2):E284–E294. doi: 10.1152/ajpendo.00334.2001. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Bhasin D., Berman N. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 32.Isbarn H., Boccon-Gibod L., Carroll P.R., Montorsi F., Schulman C., Smith M.R. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol. 2009;55(1):62–75. doi: 10.1016/j.eururo.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 34.Galvao D., Taaffe D., Spry N., Newton R. Exercise can prevent and even reverse adverse effects of androgen suppression treatment in men with prostate cancer. Prostate Cancer Prostatic Dis. 2007;10(4):340–346. doi: 10.1038/sj.pcan.4500975. [DOI] [PubMed] [Google Scholar]
- 35.Keogh J.W., MacLeod R.D. Body composition, physical fitness, functional performance, quality of life, and fatigue benefits of exercise for prostate cancer patients: a systematic review. J Pain Symptom Manag. 2012;43(1):96–110. doi: 10.1016/j.jpainsymman.2011.03.006. [DOI] [PubMed] [Google Scholar]