Abstract
Introduction
A common treatment for localized prostate cancer (PCa) is radiotherapy; however, effectiveness is hampered because of toxicities and tumor resistance. Cyclooxygenase-2 (COX-2) inhibitors have been identified as potential agents that could improve treatment outcomes and have demonstrated ability to increase the radiosensitivity of many human carcinomas. This retrospective human study aims to investigate the ability of COX-2 inhibitors, celecoxib, and meloxicam, to improve treatment outcomes after radiotherapy.
Methods
Prostate Specific Antigen (PSA) data of eligible patients were obtained from Genesis Cancer Care, Southport, Australia. The primary outcome was the percentage of patients in each group that had reached biochemical relapse at two and five years after treatment. Secondary outcomes included time to biochemical relapse and PSA velocity.
Results
At two and five years after treatment, both the celecoxib (6.7%, 18.3%) and meloxicam (0.0%, 18.9%) showed lower relapse rates than the control (8.6%, 31.0%). Although not statistically significant, these results are clinically significant. In addition, the two treatment groups were found to increase the time to relapse, 46.20 months for celecoxib and 54.15 months for meloxicam, compared with the control group, 35.53 months. A similar trend was shown for PSA velocity with both treatment groups demonstrating lower PSA velocities compared with control.
Conclusions
This study provides further evidence to the potential for COX-2 inhibitors to address gaps in localizedz PCa treatment by demonstrating high clinical significance for the use of celecoxib and meloxicam. Further research should be conducted including larger retrospective studies and prospective studies to fully evaluate the benefits of COX-2 inhibitors in combination with radiotherapy for PCa.
Keywords: Biochemical relapse, Celecoxib, COX-2, Meloxicam, Prostate cancer
1. Introduction
Prostate Cancer (PCa) is the second most commonly diagnosed solid-state tumor in men worldwide.1 In Australia, PCa is the most commonly diagnosed cancer, and by 2020, it is expected that 25,000 new cases will be diagnosed each year.2 In addition to its high prevalence, it is also the second highest killer among Australian men.3 However, early diagnosis is common because of current screening methods with up to 90% of newly diagnosed cases of PCa being localizedz.4 There is also a good prognosis for those diagnosed with PCa with a five-year survival of 95%.5 Although this survival rate is positive, if the disease progresses to the more aggressive androgen-independent disease mean survival drops to 12-24 months.6
Radiotherapy is commonly used in the treatment and management of PCa, with approximately 60% of diagnosed individuals undergoing either external beam radiotherapy (EBRT) or brachytherapy (BT).7 In addition to the potential for relapse, these treatments are also accompanied by many unwanted toxicities, with up to 80% of patients suffering acute adverse effects and up to 40% chronic. Radiotherapy is commonly associated with increased bowel frequency, rectal bleeding and pain, skin reactions, urinary frequency, and erectile dysfunction8,9 as well as the potential for unfavourable toxicities; approximately one-third to one half of men who undergo radical treatment will experience disease relapse.10 Owing to the risk of disease recurrence and presence of toxicities, there is a need for new treatments, either as monotherapy or as an adjunct to current therapies.
Several well-designed studies have demonstrated that Cyclooxygenase-2 (COX-2) inhibitors have displayed cytotoxic and antitumor properties.11, 12, 13 COX-2 is an inducible enzyme that has been implicated in the growth and survival of PCa and has been shown to be over expressed in malignant tumors.14 This enzyme has been linked to proliferation, invasion, apoptosis, host immune response, and angiogenesis of malignant tumors.15 In addition to these processes, COX-2 has also been implicated in the development of tumor radioresistance, resulting in reduced efficacy of radiation therapy modalities.16 Further support for the evidence of the role of COX-2 in the development of tumor radioresistance comes from studies that have demonstrated the ability of COX-2 inhibitors to increase radiosensitivity in lung, breast, colon, and prostate carcinomas.17, 18, 19 Past studies on patients have investigated the use of celecoxib after radiotherapy or radical prostatectomy, but at a dose of four times that of the standard therapeutic dose.10,20 To our knowledge, there have been no human studies investigating the effects of COX-2 inhibitors at standard therapeutic doses.
Therefore, this retrospective human study aims to investigate the ability of COX-2 selective inhibitors, celecoxib and meloxicam, to improve treatment outcomes of EBRT and BT when used at standard therapeutic doses. The primary objective will be to demonstrate a reduced incidence of cancer relapse at two and five years after treatment. Secondary outcomes, PSA velocity, and biochemical relapse rate, will also be used to evaluate the effectiveness of celecoxib and meloxicam.
2. Method
2.1. Participants
Human research ethics was approved by UnitingCare Health Queensland for data collection at their facility (2014.20.126) and approval was gained from the Ethics Committee of Griffith University (PHM/10/14/HREC). Participants were identified through CAS8 database at Genesis Cancer Care in Southport QLD, Australia. An initial screening of the database was conducted, and patients diagnosed with PCa, who received radiotherapy, and who were taking celecoxib or meloxicam during the time of treatment were identified. Patients who had PCa and received radiotherapy but were not taking any COX-2 inhibitors were also identified and were included in the control group. These patients had histologically confirmed adenocarcinoma of the prostate and received radiotherapy between 2004 and 2011. Patients who received radiotherapy after 2011 were not included as this did not allow for the collection of PSA results at five years after treatment. The exclusion criteria included unclear use of celecoxib or meloxicam, use of other COX inhibitors (apart from low-dose aspirin <300 mg daily), evidence of or suspected metastatic disease, previous radical treatment of PCa, evidence other malignant diseases, and insufficient PSA data (absence of PSA data at two or more years after treatment). Participants were allocated to one of three groups (celecoxib, meloxicam, or control) based on the use of COX-2 inhibitors.
All PSA values that could be obtained from the CAS8 database at Genesis Cancer Care were recorded for future analysis. For patients where only limited PSA values were available, pathology records were investigated using the online database of pathology organizations.
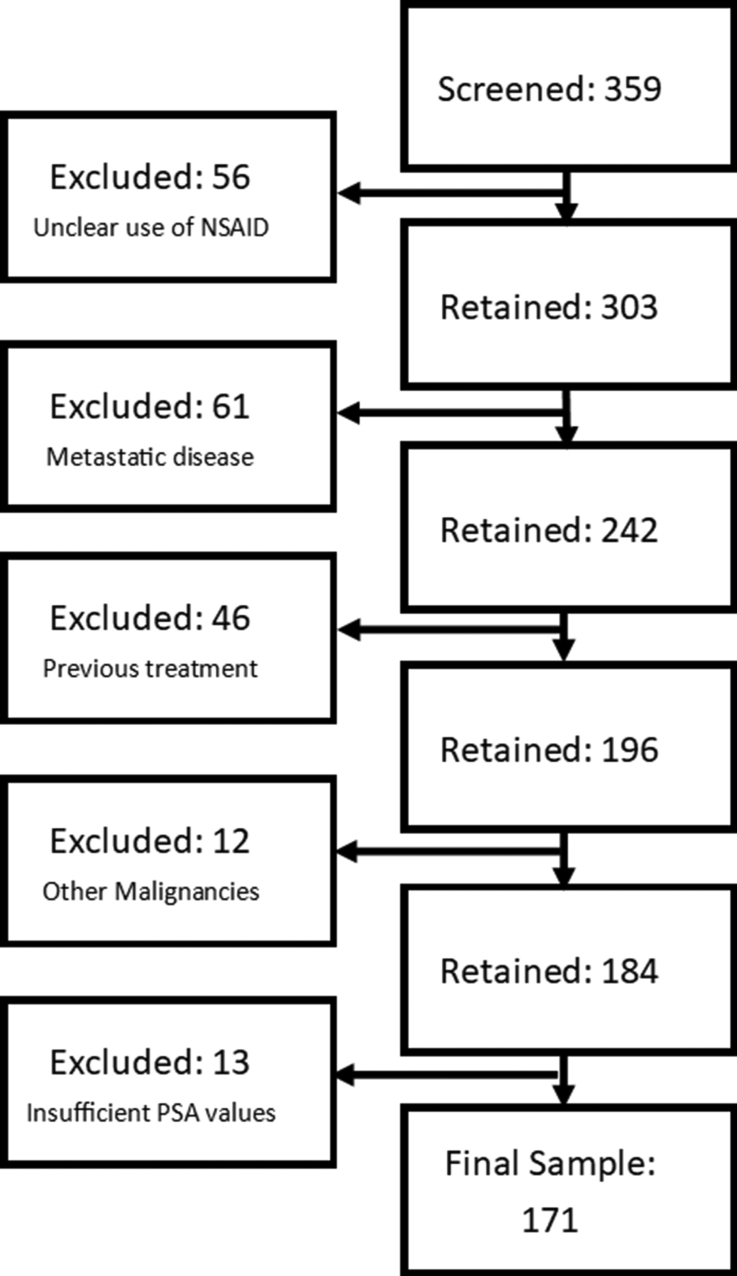
A total of 359 patients were screened for inclusion in the study. From this, a final sample population of 171 patients was obtained. The final study population comprised 60 patients in the celecoxib group, 53 in the meloxicam group, and 58 in the control group. There were 56 patients excluded because of no or unclear use of an Non-steroidal antiinflammatory drug (NSAID), 61 excluded because of having metastatic disease at the time of treatment, 46 excluded because of receiving previous radical PCa treatment, 12 excluded because of the presence of other malignancies, and 13 excluded because of insufficient PSA values. The screening process can be visualizedz in Fig. 1.
Fig. 1.
Patient eligibility screening process.
2.2. Data Collection
Demographic data, including age, Gleason score, cancer stage, the use of androgen deprivation therapy (ADT) and the type of radiotherapy received, were extracted from the CAS8 database and recorded for analysis. In addition to demographic characteristics, PSA levels were extracted and recorded for analysis of the outcomes, which are listed in the following section. PSA values were recorded from diagnosis until 120 months after treatment. If there were missing PSA values in the CAS8 database, the online reporting databases of pathology companies were used to retrieve these missing data points.
2.3. Outcomes
The primary outcome measured was the percentage of participants that displayed biochemical failure/relapse at 5 years after treatment. Disease recurrence was defined as the PSA nadir after treatment plus 2 ng/mL. This definition was based upon the Phoenix Consensus Conference definition of disease relapse after radiotherapy.21 Biochemical failure was also evaluated at 2 years after treatment.
In addition, PSA kinetics were analyzed and compared between the groups. PSA kinetics can be used as a prognostic factor in the determination of risk of cancer relapse after treatment.22 PSA velocity was used over PSA doubling time, as it is known to be a more accurate predictor for risk of relapse.23 PSA velocity was determined using the linear regression method, with the inclusion of all PSA values from 3 months after treatment up to the diagnosis of relapse, if relapse did not occur, all available PSA values were included for analysis.
2.4. Treatments
Patients were identified based their use of celecoxib or meloxicam. Patients were using these drugs for reasons other than PCa treatment, predominately for osteoarthritis or rheumatoid arthritis, and were not actively treated with these drugs for research purposes. To be included in the study, patients had to be taking therapeutic doses of either celecoxib (100-200 mg daily) or meloxicam (7.5-15 mg daily) during radiotherapy treatment.
All participants received either EBRT or BT. Participants received EBRT by means of intensity-modulated radiation therapy or 3D-conformational radiation therapy. The radiation dose ranged from 70 Gy to 80 Gy, given for more than 35 to 38 fractions. Both low-dose and high-dose patients with BT were included. For BT, a dose of 145 Gy was given.
During the study period, this ADT consisted of treatment with depot injections of a luteinizing hormone-releasing hormone agonist or oral cyproterone. The duration of ADT for individual patients was not available in the patient records; however, standard practice was for participants with intermediate risk to be treated for a six-month duration before radiotherapy, those with high risk PCa continued ADT for another 12 months after radiotherapy, giving a total of 18 months treatment and low-risk participants were not treated with ADT.
2.5. Statistical analysis
All statistical analyses were conducted using the IBM SPSS program, apart from the development of a Kaplan–Meier survival curve which was constructed using GraphPad Prism 7.04. For continuous data that was normally distributed a one-way analysis of variance, with relevant post hoc tests, was used to determine statistical significance and for continuous data that was not normally distributed, a Kruskal–Wallis analysis was used. For discrete data, the Fisher exact test was used.
3. Results
3.1. Patient demographics
Demographic characteristics of the patients were collected, which is shown in Table 1. Demographic characteristics included age, Gleason score at time of diagnosis, cancer stage at time of diagnosis (using the TNM method of staging), the use of ADT and the type of radiotherapy that was given.
Table 1.
Demographic characteristics
| Risk Group | Control (n = 58) | Celecoxib (n = 60) | Meloxicam (n = 53) | P value |
|---|---|---|---|---|
| Age [mean (SD)] | 68.49 (7.21) | 68.90 (7.60) | 69.23 (6.18) | 0.868 |
| Gleason Score sum [n (%)] | ||||
| 6 | 10 (17.2) | 4 (6.7) | 5 (9.4) | 0.908 |
| 7 | 22 (39.7) | 35 (58.3) | 23 (43.4) | |
| 8 | 7 (12.1) | 10 (16.7) | 8 (15.1) | |
| 9 | 14 (24.1) | 10 (16.7) | 10 (18.9) | |
| 10 | 1 (1.7) | 0 (0.0) | 0 (0.0) | |
| Stage (n (%)) | ||||
| T1c | 14 (24.1) | 15 (25.0) | 11 (20.8) | 0.884 |
| T2a | 6 (10.3) | 7 (11.7) | 10 (18.9) | |
| T2b | 12 (20.7) | 11 (22.0) | 10 (18.9) | |
| T2c | 7 (12.1) | 8 (16.0) | 6 (11.3) | |
| T3a | 5 (8.6) | 8 (16.0) | 6 (11.3) | |
| T3b | 3 (5.2) | 1 (2.0) | 0 (0.0) | |
| ADT use (n (%)) | ||||
| Yes | 42 (72.4) | 40 (66.7) | 34 (64.2) | 0.631 |
| No | 16 (27.6) | 20 (33.3) | 19 (35.8) | |
| RT type (n (%)) | ||||
| EBR alone | 42 (72.4) | 50 (83.3) | 27 (50.9) | <0.05 |
| BT alone | 4 (6.9) | 4 (6.7) | 9 (17.0) | A > C*, B > C* |
| BT with EBR | 10 (17.2) | 6 (10) | 17 (32.1) | C > B*, C > A* |
* = P < 0.05. ADT, ndrogen deprivation therapy; RT, adiotherapy; EBR, xternal beam radiation; BT, brachytherapy; SD, standard deviation.
The mean age in the control group was 68.49 (7.21) years, compared with 68.90 (7.60) and 69.23 (6.18) in the celecoxib and meloxicam groups, respectively. No significant difference was found between the groups with respect to age (P = 0.868).
The proportion of tumor biopsy results that had a Gleason score of 8-10, was higher in the control group, 37.9% (n = 22), compared with the celecoxib and meloxicam groups, 33.4% (n = 20) and 34.0% (n = 18), respectively. A Gleason score within this range indicates high-grade, poorly differentiated carcinoma.24 In addition, the celecoxib group had higher proportion of locally advanced tumors (Table 2), 18.0% (n = 9) compared with the control group, 13.8% (n = 8), and the meloxicam group, 11.3% (n = 6). The definition of locally advanced PCa was adopted from the European Association of Urology, defining it as a cancer staging diagnosis of T3 or T4.25 However, there were no statistically significant differences between the groups with respect to Gleason score (P = 0.908).
Table 2.
Risk stratification
| Risk Group | Control (n = 58) | Celecoxib (n = 60) | Meloxicam (n = 53) | P value |
|---|---|---|---|---|
| Risk group | ||||
| Low | 13.8% (n = 8) | 3.3% (n = 2) | 6.2% (n = 3) | 0.189 |
| Intermediate | 31.0% (n = 18) | 40.0% (n = 24) | 39.6% (n = 19) | |
| High | 55.2% (n = 32) | 56.7% (n = 34) | 54.2% (n = 26) | |
Low risk = Diagnostic PSA <10 ng/mL, Gleason score ≤6 and stage T1c or T2a, Intermediate = Diagnostic PSA of 10-20 ng/mL or Gleason score of 7 or cancer stage T2b, High = Diagnostic PSA > 20 ng/mL or Gleason score of 8-10 or cancer stage of T2c/T3.
Finally, the treatment modalities varied between the three groups. ADT use was found to be higher in the control group, 72.4% (n = 42), compared with the celecoxib and meloxicam groups, 66.7% (n = 40) and 64.2% (n = 34), however these differences were not statistically significant (0.631). Furthermore, the percentage of patients that received EBR alone was similar between the control and celecoxib groups with 72.4% (n = 42) and 83.3% (n = 50), respectively; however, it was much lower in the meloxicam group (P < 0.05) with only 50.9% (n = 27) of patients receiving this treatment modality. The meloxicam group showed the highest use of BT (P < 0.05) with 49.1% (n = 26) of patients receiving either BT alone or BT combined with EBR.
3.2. Risk stratification
Demographic data were used to determine risk groups within the treatment samples. Risk stratification followed the risk groups established by the National Comprehensive Cancer Network.26 Low risk was classified as diagnostic PSA <10 ng/mL, Gleason score of ≤6, and cancer stage of T1c or T2a. To be defined as intermediate risk, patients had either a diagnostic PSA of 10-20 ng/mL or Gleason score of 7 or cancer stage of T2b. High-risk patients were defined as diagnostic PSA>20 ng/mL or Gleason score of 8-10 or cancer stage of T2c/T3. The risk group can be used to predict a 5-year survival with low-, intermediate-, and high-risk groups having a 5-year survival rate of 85-94%, 68-84%, and 43-67%, respectively.26
The spread of patients across the three risk groups was relatively similar. The control group had the highest percentage of low-risk patients, 13.8% (n = 8) and the lowest percentage of intermediate-risk patients, 31.0% (n = 18). The percentage of high-risk patients was similar across the three groups with rates of 55.2%, 56.7%, and 54.2% across the control, celecoxib, and meloxicam groups, respectively. No significant difference in risk stratification was found between the groups (P = 0.189).
3.3. Biochemical relapse
Relapse rates were analyzed at two and five years after treatment (Table 3). It was initially aimed to analyze relapse rates at 10 years after treatment; however, only a limited number of PSA values could be obtained at this time point. Relapse was defined as >2 ng/mL PSA rise from the PSA nadir, this was in line with the Phoenix Definition of biochemical relapse.21
Table 3.
Percentage relapse at 2 and 5 years after treatment.
| % Relapse | Control (n = 58) | Celecoxib (n- = 60) | Meloxicam (n = 53) | P value |
|---|---|---|---|---|
| Relapse at 2 years | 8.6% (n = 5) | 6.7% (n = 4) | 0.0% (n = 0) | 0.730 |
| Relapse at 5 years | 31.0% (n = 19) | 18.3% (n = 11) | 18.9% (n = 10) | 0.145 |
Within two years after treatment, the control group and celecoxib groups had 8.6% (n = 5) and 6.7% (n = 4) of patients reach biochemical relapse, respectively. In contrast, the meloxicam group had no patient reach relapse in this time. At five years after treatment, the control group had the highest relapse rate of 31.0% (n = 19). The two intervention groups showed similar relapse rates at 18.3% (n = 11) for celecoxib and 18.9% (n = 10) for meloxicam.
Fisher' exact test was used to assess statistical significance between the groups at two and five years after treatment. No significant difference was found between the three groups at two years after treatment (P = 0.730). The difference in percentage relapse rates at five years after treatment between the groups were also found to not be statistically significant (P = 0.145).
When stratified based on the type of radiotherapy given, there were no statistically significant differences in relapse rates between the groups at both two and five years after treatment. However, no patients (0%) who received BT displayed relapse at both the two- and five-year time points.
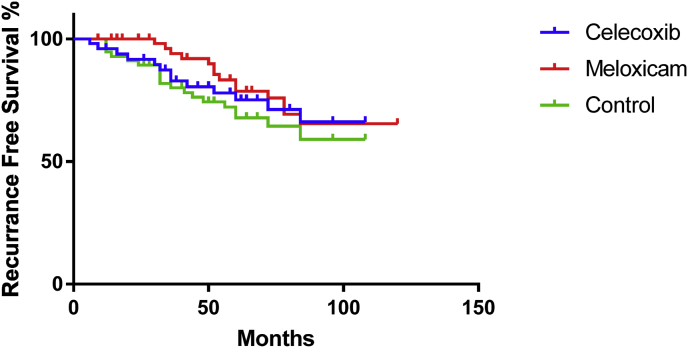
KaplanMeier survival curves (Fig. 2) were used to compare the percentage relapse between the study groups. The results displayed a recurrence-free survival, i.e., no relapse, over time. The curves demonstrated similar recurrence-free survival between the celecoxib and meloxicam groups, and both of these groups showed a higher recurrence-free survival than the control group. To analyze if these differences were statistically significant, a log-rank (Mantel-Cox) test was used. This test found that there were no statistically significant differences in recurrence-free survival between the three groups (P = 0.451).
Fig. 2.
KaplanMeier survival curves of the control, celecoxib, and meloxicam groups.
3.4. Time to relapse
In addition to the percentage of patients who had relapsed the time, in months, to relapse was also analyzed (Table 4). This analysis found that the control group had the shortest time to relapse with a mean relapse of 35.53 ± 20.21 months. The celecoxib group was found to have a mean time to relapse of 46.20 ± 31.70 months, and the meloxicam group had a mean time to relapse of 54.15 ± 16.08 months. These results were promising, as they indicated that both celecoxib and meloxicam increased the time to biochemical relapse, with a difference in mean relapse time of 10.67 and 18.62 months, respectively.
Table 4.
Time to relapse (months)
| Time to relapse (months) | Control (n = 19) | Celecoxib (n -= 15) | Meloxicam (n = 13) | P value |
|---|---|---|---|---|
| Mean (SD) | 35.53 (20.21) | 46.20 (31.70) | 54.15 (16.08) | 0.097 |
| Median (minimum, maximum) | 32 (12, 84) | 36 (6, 120) | 54 (30, 84) | 0.061 |
SD, standard deviation.
A one-way analysis of variance was conducted to determine the statistical significance of the difference. No statistically significant difference in the time to relapse was found between any of the groups (P = 0.097).
When stratified based on the type of radiotherapy given, there were no statistically significant differences in time to biochemical relapse. As no patients who receive BT demonstrated biochemical relapse, a mean time to biochemical relapse could be determined for this group.
3.5. PSA velocity
PSA velocity of all patients was analyzed as a secondary outcome (Table 5). Of the three groups, the control was found to have the highest median PSA velocity. Both the celecoxib and meloxicam groups were found to have lower PSA velocities. A Kruskal–Wallis analysis was conducted to determine the statistical significance of the differences between the three groups. A statistically significant difference was found between the celecoxib group and the control group (P < 0.05) as well as between the meloxicam and control groups (P < 0.05). There was no statistically significant difference found between the celecoxib and meloxicam groups (P = 0.708).
Table 5.
PSA velocity (ng/mL/year)
| PSA velocity (ng/mL/year) | Control (A) (n = 58) | Celecoxib (B) (n -= 60) | Meloxicam (C) (n = 53) | P value | Post hoc |
|---|---|---|---|---|---|
| Mean (SD) | 1.12 (3.05) | 0.828 (3.15) | 0.197 (0.939) | 0.176 | |
| Median (minimum, maximum) | 0.0846 (−0.48, 19.70) | 0.0174 (−1.81, 17.80) | 0.0036 (−2.60, 5.23) | <0.05 | B < A* C < A* |
* = P < 0.05. SD, standard deviation.
After stratification based on radiotherapy type, no statistically significant differences were observed between the treatment groups. However, the difference in PSA velocity in patients who received EBR was nearing significance (P = 0.051), Table 6. The mean and median PSA velocities for patients who received BT and BT plus EBR are seen in Table 7 and Table 8, respectively. The PSA velocity for patients who received EBR and BT plus EBRT was found to be positive whereas for patients who received BT alone displayed a negative PSA velocity.
Table 6.
PSA velocity EBR (ng/mL/year)
| PSA velocity (ng/mL/year) | Control (A) (n = 58) | Celecoxib (B) (n -= 60) | Meloxicam (C) (n = 53) | P value |
|---|---|---|---|---|
| Mean (SD) | 1.407 (3.48) | 0.935 (3.40) | 0.281 (0.587) | 0.327 |
| Median (minimum, maximum) | 0.291 (−0.476, 19.704) | 0.020 (−1.812, 17.800) | 0.0516 (−1.033, 1.816) | 0.051 |
EBR, external beam radiation; SD, standard deviation.
Table 7.
PSA velocity BT (ng/mL/year)
| PSA velocity (ng/mL/year) | Control (A) (n = 58) | Celecoxib (B) (n -= 60) | Meloxicam (C) (n = 53) | P value |
|---|---|---|---|---|
| Mean (SD) | −0.317 (0.054) | −0.315 (0.376) | −0.467 (0.819) | 0.892 |
| Median (minimum, maximum) | −0.313 (−0.392, −0.260) | −0.131 (−0.880, −0.118) | −0.221 (−2.597, −0.095) | 0.484 |
BT, brachytherapy; SD, standard deviation.
Table 8.
PSA velocity BT + EBR (ng/mL/year)
| PSA velocity (ng/mL/year) | Control (A) (n = 58) | Celecoxib (B) (n -= 60) | Meloxicam (C) (n = 53) | P value |
|---|---|---|---|---|
| Mean (SD) | 0.446 (1.19) | 0.697 (1.68) | 0.416 (1.28) | 0.903 |
| Median (minimum, maximum) | 0.033 (0.005, 3.817) | 0.047 (−0.151, 4.126) | 0.001 (−0.178, 5.233) | 0.424 |
EBR, external beam radiation; BT, brachytherapy; SD, standard deviation.
4. Discussion
To our knowledge, this is the first study investigating the use of the COX-2 inhibitors, celecoxib and meloxicam, at approved therapeutic doses as an adjunct treatment in patients undergoing radiotherapy for PCa. The primary outcome of this study was the percentage of patients that showed cancer relapse, this was recorded at both two years and five years after treatment. In addition, time to biochemical relapse and PSA velocity were analyzed as secondary outcomes.
In this study, it was demonstrated that both celecoxib and meloxicam resulted in a reduced proportion of patients displaying biochemical relapse. At the two-year time point, meloxicam showed the most favorable results with no patients in this group relapsing. The celecoxib group also demonstrated a reduced incidence of PCa relapse compared with the control group. The percentage relapse at five years showed similar promise with both the celecoxib and meloxicam groups being found to have a lower percentage relapse than the control group. These results are of high clinical relevance as the percentage relapse of the control group was almost twice that of the celecoxib and meloxicam group at the five-year mark. This suggests that the risk of relapse in patients taking either celecoxib or meloxicam may be almost half that of those that are not taking either of these drugs. Interestingly, it was discovered that no patients who received BT alone displayed biochemical relapse across all groups. This finding may suggest the BT alone is superior in preventing biochemical relapse to the other treatment modalities; however, only 17 of the total 171 patients receive BT alone.
The secondary outcome, time to biochemical relapse, also showed promising results, with both celecoxib and meloxicam groups extending time to biochemical relapse by approximately 11 and 19 months, respectively, compared with the control group. This translates to an average of 11 and 19 extra months of diagnosed cancer-free life for patients taking celecoxib or meloxicam compared with those not taking these drugs.
The final end point of the study was the difference in PSA velocity between each group, and this end point displayed the most striking results. In the past, PSA monitoring and PSA doubling time have been used as a prognostic tool for the diagnosis of PCa and PCa relapse after treatment, PSA velocity is favored because of its increased specificity in PCa detection.27 Both celecoxib and meloxicam were found to have a significantly lower median PSA velocity compared with the control group. Adding strength to this finding was that the both the difference between celecoxib and control and meloxicam and control were found to be statistically significant. When the results were stratified based on radiotherapy type, it was found that patients who received BT alone displayed a negative PSA velocity, whereas the other two treatments resulted in positive PSA velocities. This negative PSA velocity translates to PSA levels that were still falling at 5 years after treatment, suggesting that BT alone leads to long-lasting disease control. However, as mentioned previously, the number of patients who received BT alone was small, leading to potential bias in the results.
Translating these statistically significant results into a clinically relevant finding, a PSA velocity between 0.35 and 2 ng/mL/year results in a 5.3- to 10-fold increased PCa risk.27 This correlation between PSA velocity and increased risk was also found to translate in the risk of PCa recurrence after radiotherapy.28 Of the groups in the study, both the celecoxib and control groups were found to fit within this range. Importantly, the meloxicam group showed an average PSA velocity of 0.197, meaning it fell below the range. This translates into a much lower risk of PCa relapse when a patient is taking meloxicam at the same time as receiving radiotherapy.
5. Conclusion
Being the first of its kind, this study demonstrated the potential ability of COX-2 inhibitors to influence response to radiotherapy in localizedz patients with PCa. All the end points in the study favored the use of celecoxib or meloxicam, showing high levels of clinical significance. The change in PSA velocity was shown to not only be clinically but also statistically significant between the control and treatment groups.
Conclusions from this work provide an interesting preliminary insight into the role of COX-2 inhibitors as potential adjuncts in radiation treatment of PCa. However, large-scale prospective research with greater patient numbers is required to confirm the findings of this research.
5.1. Compliance with ethical standards
All methods performed in this study in accordance with the seventh revision (2013) of the Declaration of Helsinki and approval was granted by the UnitingCare Health HREC (2014.20.126).
Conflict of interest
All authors have no conflict of interest to declare.
Acknowledgments
No funding was received for this project. The authors would like to acknowledge Genesis Cancer Care for the use of their facility and access to patient data during the study.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Autstralian Institute of Health and Welfare . 2013. Prostate Cancer in Australia. Cancer Series 79.http://aihw.gov.au/publication-detail/?id=60129545134&tab=2 Available from: [Google Scholar]
- 3.Australian Institute of Health and Welfare . 2016. Australian Cancer Incidence and Mortality (ACIM) books: Prostate Cancer 2016.https://www.aihw.gov.au/acim-books Available from: [Google Scholar]
- 4.Penney K.L., Stampfer M.J., Jahn J.L., Sinnot J.A., Flavin R., Rider J.R. Gleason grade progression is uncommon. Cancer Res. 2013;73(16):5163–5168. doi: 10.1158/0008-5472.CAN-13-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autstralian Institute of Health and Welfare . 2017. Cancer in Australia. in Cancer Series no.101. 2017: Canberra. [Google Scholar]
- 6.Kirby M., Hirst C., Crawford E.D. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65(11):1180–1192. doi: 10.1111/j.1742-1241.2011.02799.x. [DOI] [PubMed] [Google Scholar]
- 7.Hall S., Radrawar S., Zunk M., Bernaitis N., Arora D., McDermott C. Protection against Radiotherapy-Induced Toxicity. Antioxidants. 2016;5(3) doi: 10.3390/antiox5030022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchesne G. Localised prostate cancer - current treatment options. Aust Fam Physician. 2011;40(10):768–771. [PubMed] [Google Scholar]
- 9.Martin N.E., D'Amico A.V. Progress and controversies: Radiation therapy for prostate cancer. CA A Cancer J Clin. 2014;64(6):389–407. doi: 10.3322/caac.21250. [DOI] [PubMed] [Google Scholar]
- 10.Smith M.R., Manola J., Kaufman D.S., Oh W.K., Bubley G.J., Kantoff P.W. Celecoxib versus placebo for men with prostate cancer and a rising serum prostate-specific antigen after radical prostatectomy and/or radiation therapy. J Clin Oncol. 2006;24(18):2723–2728. doi: 10.1200/JCO.2005.03.7804. [DOI] [PubMed] [Google Scholar]
- 11.Chen W.S., Wei S.J., Liu J.M., Hsiao M., Kou-Lin J., Yang W.K. Tumor invasiveness and liver metastasis of colon cancer cells correlated with cyclooxygenase-2 (COX-2) expression and inhibited by a COX-2-selective inhibitor, etodolac. Int J Cancer. 2001;91(6):894–899. doi: 10.1002/1097-0215(200102)9999:9999<894::aid-ijc1146>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Grosch S., Tegeder I., Niederberger E., Brautigam L., Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15(14):2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi Y., Kamijo R., Takizawa K., Hatori M., Nagumo M. Inhibitors of cyclooxygenase-2 (COX-2) suppressed the proliferation and differentiation of human leukaemia cell lines. Eur J Cancer. 2001;37(12):1570–1578. doi: 10.1016/s0959-8049(01)00160-5. [DOI] [PubMed] [Google Scholar]
- 14.Milas L., Mason K.A., Crane C.H., Liao Z., Masferrer J. Improvement of radiotherapy or chemoradiotherapy by targeting COX-2 enzyme. Oncology. 2003;17(5 Suppl 5):15–24. [PubMed] [Google Scholar]
- 15.Toomey D.P., Murphy J.F., Conlon K.C. COX-2, VEGF and tumour angiogenesis. The Surgeon. 2009;7(3):174–180. doi: 10.1016/s1479-666x(09)80042-5. [DOI] [PubMed] [Google Scholar]
- 16.Milas L. Cyclooxygenase-2 (COX-2) enzyme inhibitors as potential enhancers of tumor radioresponse. Semin Radiat Oncol. 2001;11(4):290–299. doi: 10.1053/srao.2001.26018. [DOI] [PubMed] [Google Scholar]
- 17.Kishi K., Petersen S., Petersen C., Hunter N., Mason K., Masferrer J.L. Preferential enhancement of tumor radioresponse by a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60(5):1326–1331. [PubMed] [Google Scholar]
- 18.Pyo H., Choy H., Amorino G.P., Kim J.S., Cao Q., Hercules S.K. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin Cancer Res. 2001;7(10):2998–3005. [PubMed] [Google Scholar]
- 19.Shin Y.K., Park J.S., Kim H.S., Jun H.J., Kim G.E., Suh C.O. Radiosensitivity enhancement by celecoxib, a cyclooxygenase (COX)-2 selective inhibitor, via COX-2-dependent cell cycle regulation on human cancer cells expressing differential COX-2 levels. Cancer Res. 2005;65(20):9501–9509. doi: 10.1158/0008-5472.CAN-05-0220. [DOI] [PubMed] [Google Scholar]
- 20.Pruthi R.S., Derksen J.E., Moore D., Carson C.C., Grigson G., Watkins C. Phase II trial of celecoxib in prostate-specific antigen recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. Clin Cancer Res. 2006;12(7 Pt 1):2172–2177. doi: 10.1158/1078-0432.CCR-05-2067. [DOI] [PubMed] [Google Scholar]
- 21.Roach M., 3rd, Hanks G., Thames H., Jr., Schellhammer P., Shipley W.U., Sokol G.H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(4):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Vickers A.J., Brewster S.F. PSA Velocity and Doubling Time in Diagnosis and Prognosis of Prostate Cancer. Br J Med Surg Urol. 2012;5(4):162–168. doi: 10.1016/j.bjmsu.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng M.K., Van As N., Thomas K., Woode-Amissah R., Horwich A., Huddart R. Prostate-specific antigen (PSA) kinetics in untreated, localized prostate cancer: PSA velocity vs PSA doubling time. BJU Int. 2009;103(7):872–876. doi: 10.1111/j.1464-410X.2008.08116.x. [DOI] [PubMed] [Google Scholar]
- 24.van Poppel H. Locally advanced and high risk prostate cancer: The best indication for initial radical prostatectomy? Asian J Urol. 2014;1(1):40–45. doi: 10.1016/j.ajur.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heidenreich A., Bastian P.J., Bellmunt J., Bolla M., Joniau S., van der Kwast T. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–137. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 26.Mohler JL A.A., Bahnson R.R., D'Amico A.V., Davis B.J., Eastham J.A., Enke C.A. NCCN Practice Guidelines in Oncology; 2016. Prostate Cancer Version 3. [Google Scholar]
- 27.Bjurlin M.A., Loeb S. PSA Velocity in Risk Stratification of Prostate Cancer. Rev Urol. 2013;15(4):204–206. [PMC free article] [PubMed] [Google Scholar]
- 28.D’Amico A.V., Renshaw A.A., Sussman B., Chen M.H. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. J Am Med Assoc. 2005;294(4):440–447. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]