Abstract
Oral health influences general well-being and quality of life. Oral diseases can be debilitating and are a major heath concern worldwide. Medicinal plants have been used for thousands of years for treating human diseases. Considering the emergence of multi-drug resistant pathogens and financial difficulties in developing countries, there is an urgent need for developing new antimicrobial compounds which are safe, efficient and cost effective. Liquorice also known as yashtimadhu, sweetwood or mulhatti is one such herbal remedy which has shown to have immense potential in treatment of orofacial diseases. Liquorice is rich in secondary metabolites which are used in cosmetics, foods, traditional and modern medicine. It has well known properties such as antiviral, glucocorticoid, anti-inflammatory, antioxidant, anti-ulcerative, anti-carcinogenic and many more. Liquorice extracts and liquorice bioactive ingredients such as glabridin, licoricidin, licorisoflavan A, licochalcone A, and glycyrrhizin have shown beneficial effects in preventing and treating oral diseases. This paper reviews the effects of liquorice and its constituents on oral diseases such as dental caries, periodontitis, gingivitis, candidiasis, recurrent aphthous ulcer and oral cancer and its use as a root canal medicament and summarizes the results of clinical trials that investigated the potential beneficial effects of liquorice and its constituents as a prevention and treatment modality in oral diseases. Clinical trials, case reports and review of literature evaluating the effect of liquorice on oral microorganisms and oral diseases are included. Literature pertaining to the effects of liquorice on systemic diseases have been excluded from this review of literature.
Keywords: Liquorice, Dental caries, Candidiasis, Gingivitis, Periodontitis, Oral cancer, Aphthous, Dentistry
1. Introduction
In recent times, there is a rise in use of plant extracts in modern medicine. The search for effective, efficient, safe and economical alternatives have led to a rise in use of natural phytochemicals derived from plants in treating diseases of the human body. A large body of evidence exists to substantiate the use of herbs for preventing and treating human diseases. Liquorice root is one such herb which has been an integral part of Chinese medicine and Ayurveda for centuries. Liquorice is a sweet, moist, soothing herb that belongs to the glycyrrhiza species native to Mediterranean countries and Asia. The term Glycyrrhiza comes from the ancient Greek words; glycos meaning sweet and rhiza meaning root [1]. Due to its sweet taste, liquorice has been used worldwide as a sweetener and a flavouring agent in food and medicine production and is listed in the USA by the Food and Drug Administration (FDA) as Generally Recognized as Safe (GRAS) [2].
Liquorice is rich in secondary metabolites which have been associated with various health benefits. Secondary metabolites of liquorice roots have shown to have a beneficial effect in the treatment of various diseases such as cancer, tuberculosis, atherosclerosis, gastric ulcers, immunodeficiency, hepatitis and bacterial infections [3], [4], [5], [6]. Recently the benefits of liquorice in oral diseases has been of great interest. Clinical trials have been conducted worldwide to evaluate the effects of liquorice and its metabolites in preventing and treating various oral diseases such as dental caries, periodontal diseases, candidiasis, aphthous ulcers and debilitating diseases like oral cancer. Moreover, liquorice has also been studied as a root canal medicament which can prevent failed root canal therapies and lead to higher success rate of the treatment. Very little is known about these beneficial effects of liquorice in oral diseases. This manuscript aims to review, summarize and highlight these beneficial effects of liquorice and its derivatives in preventing and treating oral diseases.
2. Plant description, sources and phytochemical composition
Liquorice, also called as gancao which means “sweet herb” in Chinese and popularly known in India as Jeshthamadh (Marathi), Yashtimadhu and Madhuka (Sanskrit), Jethimadhu (Gujarati), Atimadhurum (Tamil) and Jaishbomodhu (Bengali) belongs to the genus Glycyrrhiza and has been used by humans for various purposes for at least 4000 years [7], [8]. It has been mentioned in Ayurvedic texts as Atirasa, Madhurasaa, Madhuka, Yastikavha and Madhuyashtyaahvaa [9]. The genus glycyrrhiza contains about 30 species but dried unpeeled roots of Glycyrrhiza glabra Linn. and Glycyrrhiza uralensis Fisch. (Fam. Leguminosae) are the commonest sources of liquorice. Glycyrrhiza glabra is a perennial shrub which grows to a height of 1 m and has a root system composed of taproot and stolons which are sources of commercial liquorice. Glycyrrhiza uralensis Fisch which is found in the Urals, Siberia and parts of East Asia has similar root system to G. glabra [1]. The sweetness of liquorice comes from glycyrrhizin, which constitutes 10–25% of liquorice and is 50 times sweeter than refined sugar [10].
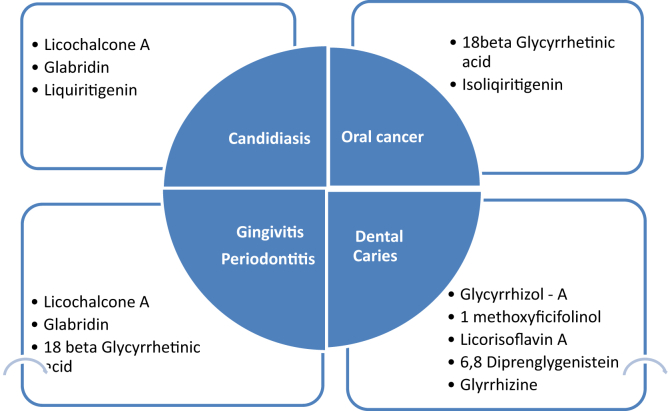
Glycyrrhiza uralensis and Glycyrrhiza inflata, which are listed in Chinese pharmacopoeia have shown to have pharmacological properties [10], [11], [12]. The biological effects are due to the presence of secondary metabolites like saponins, flavonoids, isoflavonoids, chalcones, coumarins, aurones, benzofurans, phenols, pterocarpans, and stilbenes which contribute to its pharmacological properties [10], [13], [14]. In Ayurveda, Charaka discusses the pharmacological effects of liquorice as Jivaneeya (invigorator), Varnya (complexion promoter), Kanthya (voice promoter), Kandughna (itching reliever), Snehopag (adjuvant of unctuous) Vamanopag (adjuvant for emesis), Mutravirajniya (urinary antiseptic) [9]. Susruta included liquorice under Sarivadigana, Anjanadigana, Ambashthadigana, Nyagrodhadigana, Utpaladigana [15]. Bhavmishra referred liquorice as Klitaka and mentioned its Balya (strength promoting activity), Chakshushya (beneficial to eyes), Vrishya (enhance fertility), Keshya (good for hair) properties [16]. Charadutta suggests the beneficial effects of liquorice in pain relief due to ulcers [17]. Ayurvedic texts like Charaka and Sushrutahave mentioned the role of liquorice in Dantaroga (diseases of teeth) and Dantmamsaroga (diseases of gums). Liquorice has also shown great potential in treatment of oral diseases. Based on clinical investigations, Table 1 summarizes the different phytochemical classes of liquorice extracts along with their potential benefits in oral diseases.
Table 1.
Phytochemical classes in liquorice extracts and their potential benefits in oral diseases.
Various forms of liquorice are widely distributed worldwide in countries like Malaysia, Japan and United States of America as candies, liquid extracts, lollipops, tablets and capsules (Picture 1). A 100 gm of liquorice available as candies prices upto USD 3.
Picture 1.
Liquorice root and candy available in grocery and general stores.
3. Liquorice, a safe herb
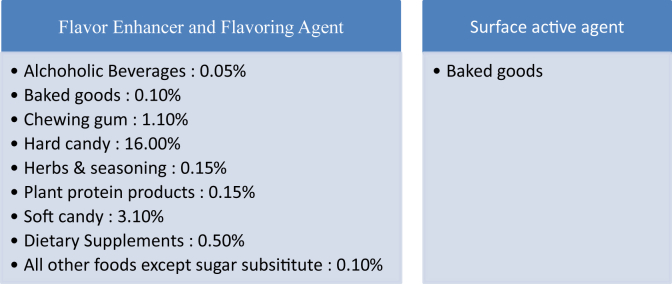
Liquorice has been labelled Generally Recognized as Safe (GRAS) by United States Food and Drug Administration (FDA) and has been considered safe for human consumption provided it is consumed in small amounts and by individuals who are not sensitive to glycyrrhizin.[1], [3]. It is proven that excessive liquorice intake can lead to hypokalemia, hypertension, rhabdomyolysis, muscle paralysis, respiratory impairment, hypertensive emergencies, hyperparathyroidism, encephalopathy and acute renal failure [18]. Touyz et al. recommended that 250–500 mg of liquorice can be safely consumed upto three times a day for medicinal purposes [19]. Sigurjonsdottir et al. reported that consumption of doses as low as 50 gm/d for a period of 2 weeks can cause a rise in blood pressure [20]. According to the World Health Organisation 100 mg/d of liquorice can be safely consumed without any ill effects, however the Dutch Nutrition Information Bureau limits the use of liquorice to 200 mg/d. In a study conducted by Sontai et al., 50% of the 14 study subjects consuming 100–200 mg/d of liquorice had to be prematurely withdrawn from the study due to hypokalemia or edema [21]. Studies have reported that liquorice metabolites can interfere with the pharmacological actions of conventional drugs. Due to the anticoagulant and antiplatelet effects of liquorice there is a potential risk of increased bleeding for patients taking conventional anti-clotting medications for cardiovascular or cerebrovascular diseases along with Chinese herbal medicines containing liquorice [22]. Table 2 summarizes the use and use limits of liquorice in food given by US Food and Drug Administration [23].
Table 2.
US Food and Drug Administration limitations of glycyrrhizin use in foods (21 CFR 184.1408c).
4. Dental caries and liquorice
Dental caries is an infectious microbial disease that results in localized dissolution and destruction of calcified tissues of teeth [24]. Mutans group of streptococci, Streptococcus sanguis, Lactobacillus spp. and Actinomyces spp. are implicated in the pathogenesis of tooth decay [25], [26], [27]. Streptococcus mutans help in the pathogenesis of dental caries by forming the extracellular polymeric substances including exopolysaccharides (EPS), eDNA and lipoteichoic acid (LTA). EPS produced by S. mutans forms a matrix which helps in the accumulation of microbes on the teeth, protects them and creates an acidic microenvironment which helps in the formation and progression of dental caries [28]. These bacteria ferment sugars in diet and produce lactic acid as a by-product which leads to the dissolution and destruction of tooth structure [25], [26], [27].
Although the anti-cariogenic properties of liquorice have been discussed for many years, few studies have been published evaluating its role as an anticariogenic agent. Recently, liquorice has been studied extensively for its anticaries properties. He et al. extracted pterocarpenes namely glycyrrhizol A and glycyrrhizol B along with four known isoflavonoids, 5-O-methylglycryol, isoglycyrol, 6,8-diisoprenyl-5,7,4′-trihydroxyisoflavone and gancaonin G from the roots of Glycyrrhiza uralensis and concluded that all these metabolites show activity against S. mutans whereas glycyrrhizol A and 6,8-diisopreny l-5,7,4′-trihydroxyisoflavone had highest antimicrobial activity against these bacteria [29]. Based on the above observations, Hu et al. developed a sugar-free orange flavoured liquorice lollipop containing glycyrrhizol A for caries prevention. They found that liquorice lollipops are safe and effective against S. mutans when consumed for 10 days (twice daily) and lead to a marked reduction in salivary S. mutans [30]. The results of an in vitro study conducted by Liu et al. reported that glycyrrhizic acid inhibits the multiplication and acid producing of S. mutans and can inhibit the growth of these bacteria in vitro [31]. A clinical study evaluated the potential of liquorice lollipops for caries control in pre-school children. In this study, the study subjects consumed liquorice lollipops twice a day for a period of 3 weeks and interestingly it was noted that there was a steep decline in the number of S. mutans. The numbers were reduced for a period 22 days after the last lollipop and then began to rebound [2]. Study conducted by Mentes on liquorice lollipops et al. observed that there was a reduction of S. mutans with consumption of two liquorice lollipops for a period of 21 days period [32]. Even though liquorice lollipops show promise further double blinded, randomized longitudinal studies using more human subjects are required before the liquorice lollipops can be used as anti caries products for caries prevention in high risk children and adults.
Recently, Ahn et al. isolated three additional antimicrobial flavonoids namely 1 methoxyficifolinol, licorisoflavan A and 6,8-diprenylgenistein isolated from Glycyrrhiza uralensis which have shown to completely inhibit the formation of biofilm and recommended the use of these flavonoids in oral hygiene products for gargling solutions and in dentrifices [33]. However, pilot study conducted by Soderling et al. to find out whether liquorice containing starch gel could affect plaque accumulation and its microbial composition and concluded that tooth brushing with a liquorice gel containing 2.5% liquorice extract made no difference in the microbial counts or in the plaque index [34]. Ayurveda recommends chewing on liquorice herbal sticks, twelve angulas (9 inches) long and thickness of one's little finger to reduce dental caries and plaque. There is considerable amount of data which states that liquorice is effective as an anticaries agent, however there is a need for more randomised clinical trials before liquorice can be safely incorporated used in oral hygiene products.
5. Oral candidiasis and liquorice
Oral candidiasis (candidosis or thrush) is caused by a yeast like fungus called Candida albicans and is an opportunistic infection of the oral cavity [27], [35], [36]. Candida albicans is an inhabitant of normal flora of the mouth and the gastrointestinal tract and causes no infections in healthy individuals. Predisposing factors for oral candidial infections include medications like antibiotics and corticosteroids, systemic diseases like diabetes mellitus and hypothyroidism, nutritional deficiencies like iron and B12 deficiencies, xerostomia, immunosuppressive diseases and therapy [37], [38], [39]. Pseudomembranous candidiasis also called as thrush, is one of the most common forms of oral candidiasis. It is a white pseudomembrane consisting of desquamated epithelial cells, necrotic debris, fibrin and fungal hyphae. It occurs most frequently on the surface of the buccal mucosa and tongue but is also seen on hard and soft palate, tongue, periodontal tissue and oropharynx. Erythematous candidiasis is associated with burning mouth secondary to antibiotic therapy, corticosteroids or diseases which suppress the immune system. Asymptomatic form of erythematous candidiasis, also called as Denture stomatitis is characterized by diffuse erythema and edema of the palatal mucosa that is in contact with the denture. Denture stomatitis can be localized or generalized [27], [35], [36]. Virulence factors of C.albicans such as adhesion, proteinases secretion, yeast–hyphal transition and phenotypic switching are responsible for host damage [40], [41]. The treatment for oral candidiasis is topical or systemic use of antifungal drugs, management of predisposing factors, oral hygiene and disinfection of prosthetic appliances [42], [35].
Antimicrobial drug resistance has led to the need for development of alternative drugs. Several studies have investigated the effects of liquorice on C. albicans. Utsunomiya et al. noticed that compared to normal mice, MAIDS mice (mice infected with LP-BM5 murine leukemia virus) are 100 times more susceptible to infection with C. albicans and administration of glycyrrhizin improved the resistance of MAIDS mice to C. albicans infection [42]. Another in vitro study conducted by Motsei et al. screened liquorice extracts against C. albicans and reported the antifungal effect of fresh water extract of G. glabra on C. Albicans [43]. Interestingly, Glabridin from the roots of G. glabra showed resistance modifying activity against drug resistant mutants of C. albicans at a minimum inhibitory concentration of 31.25–250 μg/mL [44]. Animal studies conducted by Lee et al. concluded that Liquiritigenin (LG) found in liquorice can protect mice against disseminated candidiasis by the CD4+ Th1 immune response [45]. A more recent study evaluated the effects of Glabridin and Licochalcone A on growth, biofilm formation and yeast–hyphal transition of C. albicans. Results showed that Licochalcone A has a significant effect on biofilm formation, while both licochalcone A and glabridin prevented yeast–hyphal transition in C. albicans. They also stated that both these compounds can act synergistically with nystatin against C. Albicans [46]. Furthermore, research shows that 18β-glycyrrhetinic acid can be beneficial in the treatment of Th1-disordered diseases due to C. Albicans [47]. Although more in vitro and in vivo studies are required to explore the beneficial effects of liquorice on oral candidiasis, the findings of these studies suggest that liquorice can be a useful therapeutic alternative for the treatment of oral candidiasis.
6. Gingivitis and liquorice
Gingivitis is characterized by presence of clinical signs of inflammation that are confined to gingiva. Porphyromonas gingivalis does not initially colonize clean tooth surfaces and adheres to bacteria already present in plaque mass and plays a potential role in the etiology of childhood gingivitis. The presence of P. gingivalis is most strongly associated with the progression of gingivitis and onset of periodontitis in healthy children [48].
Few studies have investigated the effect of liquorice on P. gingivalis. Aqueous extracts of raw polysaccharides from G. glabra have shown to have strong anti adhesive effects against P. gingivalis [49]. Interestingly, a supercritical extract of Chinese liquorice (Glycyrrhiza uralensis), and its major isoflavans, Licoricidin and Licorisoflavan A showed to have an inhibitory effect on growth, volatile sulfur compounds (VSCs) production and protease activity of P. gingivalis therefore controlling halitosis [50]. These studies implicate that liquorice can be used in oral hygiene products to maintain gingival and oral health.
7. Periodontitis and liquorice
Periodontitis is an inflammatory disease of the supporting tissues of the teeth caused by specific microorganisms resulting in progressive destruction of periodontal ligament and alveolar bone. Periodontitis is characterised by attachment loss, increased probing depth or recession, bleeding on probing, changes in bone height and density, mobility and loss of teeth in advanced cases [48], [51]. The etiological factors of this destructive process involves both tissue damage from bacteria and bacterial products in plaque and indirect damage through bacterial induction of the host immune and inflammatory responses by the over production of inflammatory mediators and matrix metalloproteinases (MMPs) leading to the progression and severity of periodontitis [48], [52], [53], [54]. The pathogens detected in high levels in chronic form of periodontitis include P. gingivalis, Tannerella forsythia, Prevotella intermedia, Treponema denticola, Spirochetes and Aggregatibacter actinomycetemcomitans [1], [48]. The treatment of periodontitis involves removal of plaque and calculus through scaling and root planning and maintenance of good oral hygiene. This non-surgical pocket therapy helps in reduction of pocket depth, tissue inflammation, increases clinical attachment level and improves the condition of the periodontium [55], [56], [57]. Administration of low dose doxycycline has been reported to provide additional benefits [58].
Clinical trials aiming to find natural resources for treatment of periodontitis have looked into the phytochemicals of Liquorice to prevent and treat periodontitis. The ability of liquorice root polysaccharides to reduce bacterial binding to host cells was observed after pre-treatment of P. gingivalis by Wittschier et al. The data suggested that polysaccharides from G. glabra are a potent agent against bacterial adhesion and are able to block the initial step of an infection and thus can be potential prophylactic tools in alternative treatment regimens against bacterial infection [49]. Patients with periodontal inflammation have high concentration of proinflammatory mediators such as interleukin (IL)-1beta, IL-2, IL -6, IL-8 receptor activator of nuclear factor kappa-B ligand (RANKL) and tumour necrosis factor – alpha in macrophages of inflamed gingival tissues [48], [54]. Bodet et al. investigated the response of liquorice on periodontopathogen-induced inflammatory response and found that liquorice extract exhibited potent anti-inflammatory properties by inhibiting the periodontopathogen LPS-induced IL-1beta, IL-6, and IL-8 and TNF-alpha responses of macrophages stimulated with A. actinomycetemcomitans and P. gingivalis lipopolysaccharide (LPS) [59]. According to La et al. licoricidin and licorisoflavan A effectively inhibit inflammatory cytokines and matrix metalloproteinases (MMPs) and can be used in the treatment of cytokine and/or MMP-mediated disorders such as periodontitis [60]. Licochalcone A inhibits P. gingivalis biofilm formation and the host immune response, the two principal etiological factors of periodontitis [61].18 alpha-glycyrrhetinic acid appears to significantly reduces P. gingivalis LPS-induced vascular permeability by repressing NF-κB-dependent endothelial IL-8 production, suggesting its therapeutic potential in P. gingivalis-related vascular diseases [62]. Recently an in vivo study demonstrated that liquorice extract can prevent the production of MMPs by host cell and can be as effective as doxycycline in patients with chronic periodontitis [63].
One of the prominent features of periodontitis is resorption of the alveolar bone. Receptor activator of nuclear factor kappa-B ligand RANKL is an important factor in bone resorption as it is involved in osteoclast differentiation, activation and survival [48]. Therapeutic potential of liquorice on RANKL has been evaluated. 18β-Glycyrrhetinic acid administered in interleukin-10-deficient mice which are highly disease susceptible, resulted in a dramatic reduction of in interleukin-10-deficient mice [64]. Zhu et al. noted that administration of isoliquiritigen prevented inflammatory bone loss in mice by attenuating osteoclast activity [65]. It has been suggested that glabridin can be used in preventing osteoclastogenesis by inhibiting RANKL-induced activation of signalling molecules and subsequent transcription factors in osteoclast precursors [66].
8. Recurrent aphthous ulcer and liquorice
Recurrent aphthous ulcers or canker sores are among the most common oral mucosal disease seen in children and adults [1]. Recurrent aphthous ulcers have varied etiology which includes bacterial infection by S. sanguis, genetic history, autoimmune response, iron and folic acid deficiency. Recurrent aphthous ulcers have a wide variety of precipitating factors which includes trauma, psychological problems, endocrine conditions and systemic diseases [27]. Recurrent aphthous ulcer has been classified by many investigators into four varieties namely recurrent aphthous minor, major, herpetiform and associated with Behcets syndrome. Recurrent aphthous minor are small and painless and referred to as canker sores. Recurrent aphthous major which is a severe form of aphthous minor are larger, slower to heal and painful. Recurrent herpetiform ulcerations which are multiple small, shallow ulcers often upto 100 in number [67], [68], [26]. Treatment of recurrent aphthous ulcers aims to relieve pain, promote healing and prevent secondary infection [67].
Recently, investigators have focussed their investigation towards the effect of liquorice on pain control and reducing the healing time of aphthous ulcers. Burgess et al. reported that over the counter Canker Melts GX patches which contain liquorice extract alter the course of the aphthous ulcers by reducing lesion duration, size and pain therefore speed healing [69]. In a randomized, double-blind clinical trial with 23 subjects, Martin et al. observed an improvement in ulcer size and pain compared to the use of a placebo patch using a dissolving oral patch containing a liquorice extract for up to 8 days [70]. A more recent in vivo study evaluated the efficacy of licorice bioadhesive hydrogel patches in controlling pain and reducing the healing time of recurrent aphthous ulcer. According to the results of the study liquorice bioadhesive can be effective in the reduction of pain and the inflammatory halo and necrotic center of aphthous ulcers [71]. The data obtained from these studies highlights the healing effect of liquorice on aphthous ulcers. Liquorice should be further investigated as a treatment modality in aphthous ulcers.
9. Oral cancer and liquorice
A neoplasm can be defined as an abnormal mass of tissue, the growth of which exceeds and is uncoordinated with the normal tissues and persists in the same excessive manner after cessation of the stimuli which evoked the change. Oral squamous cell carcinoma is the most common histopathological type of malignant neoplasm of the oral cavity which is characterized by indurated and ulcerated margins [27]. Liquorice has been investigated as a chemotherapeutic agent for its beneficial role in management of oral squamous cell carcinoma. Isoliquiritigenin (ISL), a flavonoid isolated from liquorice is a novel inhibitor of tumor angiogenesis and possesses great therapeutic potential for Adenoid cystic carcinoma and can be a potential cancer chemotherapeutic agent [72], [73]. Licochalcone A has shown promise in treatment of oral cancers. Investigations show that Licochalcone A induces apoptotic cell death of oral squamous cell carcinoma cells via down regulation of Sp1 expression and can be used for the treatment of human oral squamous cell carcinoma [74]. Kim et al. suggested that Licochalcone A decreases the number of viable KB oral cancer cells [75]. Recently Shen et al. subjected SCC-25 oral cancer cells to a treatment with licochalcone A at indicated concentrations (25, 50, and 100 μg/mL) for 36 h and analyzed for the effect of licochalcone A on the cell migration and invasion. Results of the study showed that licochalcone A significantly inhibited the cell migration/invasion capacities of SCC-25 cells [76]. Moreover, the same group of investigators reported that water-soluble polysaccharide (GIP1) from the roots of Glycyrrhiza inflata at a concentration of 50, 100, and 200 μg/mL specifically decreased cell viability of human oral cancer SCC-25 cells in a concentration-dependent manner via the induction of apoptosis [77]. Hsia et al. also reported that Isoliquiritigenin has anticancer effect on human oral squamous cell carcinoma [78]. In a recent study Das et al. concluded that Yashtimadhu Ghrita (processed ghee) can be effectively used to reduce chemotherapy induced mucositis in patients with cancers [79]. Taken together, all the above studies show that liquorice has the potential to be a safe, effective chemotherapeutic agent and additional research is needed to conclude its effective role in oral cancer.
10. Root canal irrigant, medicament and liquorice
Thorough debridement and complete elimination of micro-organisms are objectives of an effective endodontic treatment. For many years, intracanal irrigants have been used as an adjunct to enhance antimicrobial effect of cleaning and shaping in endodontics to reduce bacterial load in the root canal. Enterococcus faecalis is the predominant micro-organism recovered from root canals of the teeth where previous endodontic therapy has failed and is the major cause of failure of root canal therapy. Virulence factor of Enterococcus faecalis in failed endodontically treated teeth is attributed to the ability of E. faecalis cells to invade dentinal tubules and adhere to collagen in the presence of human serum [80]. Sodium hypochlorite and chlorhexidine which are the most common irrigants used in endodontics show limited ability to eradicate E. faecalis from infected root canals [81]. Despite the desirable properties of sodium hypochlorite, it has drawbacks like unpleasant taste, tendency to bleach clothes and is corrosive. Whereas chlorhexidine can cause skin irritation and impair the regeneration potential of periapical tissues [82].
Recent laboratory studies have focused on finding herbal alternatives which are safe to use and effective against E. faecalis. Few in vitro studies have evaluated the effectiveness of liquorice as root canal irrigant and medicament. Badr et al. evaluated the antibacterial and cytotoxic effects of liquorice as a root canal medicament and compared its action to the commonly used root canal medicament calcium hydroxide Ca(OH)2 The results of the study showed that liquorice extract either by itself or in combination with Ca(OH)2 had a significant inhibitory effect against E. faecalis compared to Ca(OH)2 alone. The use of Liquorice extract followed by Liquorice/Ca(OH)2 mixture retained significantly more viable periodontal ligament cells than Ca(OH)2, which had a strong lethal effect on the cells [83]. Presently there is limited evidence supporting the use of liquorice in root canal therapy, further research should be directed to explore the beneficial effect of liquorice on E. faecalis and as a root canal irrigant and medicament.
11. Conclusion
The anti-inflammatory, anti-adhesive, anti-microbial properties of Liquorice have shown beneficial effects in oral diseases like dental caries, ginigivitis, periodontitis, aphthous ulcers and oral cancer. The useful phytochemicals of liquorice should be explored so as to integrate herbs in dental products which can be beneficial to oral care. Further in vivo studies should be directed to explore and evaluate the therapeutic benefits of liquorice in dentistry.
Sources of funding
None declared.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Messier C., Epifano F., Genovese S., Grenier D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012;18(1):32–39. doi: 10.1111/j.1601-0825.2011.01842.x. [DOI] [PubMed] [Google Scholar]
- 2.Peters M.C., Tallman J.A., Braun T.M., Jacobson J.J. Clinical reduction of S. mutans in pre-school children using a novel liquorice root extract lollipop: a pilot study. Eur Arch Paediatr Dent. 2010;11(6):274–278. doi: 10.1007/BF03262762. [DOI] [PubMed] [Google Scholar]
- 3.Isbrucker R.A., Burdock G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul Toxicol Pharmacol. 2006;26:167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Shen X.P., Xiao P.G., Liu C.X. Research and application of Radix Glycyrrhizae. Asian J Drug Metab Pharmacokinet. 2007;7:181–200. [Google Scholar]
- 5.Nassiri-Asl M., Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalani K., Chaturvedi V., Alam S., Khan F., Srivastava S.K. Anti-tubercular agents from Glycyrrhiza glabra. Curr Top Med Chem. 2015;15(11):1043–1049. doi: 10.2174/1568026615666150317223323. [DOI] [PubMed] [Google Scholar]
- 7.Nomura T., Fukai T., Akiyama T. Chemistry of phenolic compounds of licorice (Glycyrrhiza species) and their estrogenic and cytotoxic activities. Pure Appl Chem. 2002;74 1199–2002. [Google Scholar]
- 8.Chopra R.N., Nayar S.L., Chopra I.C. NISCAIR, CSIR; New Delhi: 2002. Glossary of Indian medicinal plants. [Google Scholar]
- 9.Anilkumar D., Hemang J., Nishteswar K. Review of glycyrrhiza glabra (Yahtimadhu) – a broad spectrum herbal drug. Pharma Sci Monit. 2012;12(3):3171. [Google Scholar]
- 10.Kondo K., Shiba M., Nakamura R., Morota T., Shoyama Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol Pharm Bull. 2007;30:p.1271–1277. doi: 10.1248/bpb.30.1271. [DOI] [PubMed] [Google Scholar]
- 11.Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D. Antiviral effects of Glycyrrhiza species. Phytother Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka A., Horiuchi M., Umano K., Shibamoto T. Antioxidant and anti-inflammatory activities of water distillate and its dichloromethane extract from licorice root (Glycyrrhiza uralensis) and chemical composition of dichloromethane extract. J Sci Food Agric. 2008;88:1158–1165. [Google Scholar]
- 13.Simons R., Vinc1ken J.P., Bakx E.J., Verbruggen M.A., Gruppen H. A rapid screening method for prenylated flavonoids with ultra-highperformance liquid chromatography/electrospray ionisation mass spectrometry in licorice root extracts. Rapid Commun Mass Spectrom. 2009;23:3083–3093. doi: 10.1002/rcm.4215. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J Chromatogr A. 2009;13(3) doi: 10.1016/j.chroma.2008.07.072. 1216(11): p.1954-1969. [DOI] [PubMed] [Google Scholar]
- 15.Susruta: Susruta Samhita, Nibh and hasangrah commentary by Dalhan acharya, edited by Yadavji Trikamji Acharya, 7th ed., Sutrasthana 38 Varanasi: Chaukhambha Orientelia 2002.
- 16.Chunekar K.C, Hota N.P. Plants of Bhavprakash, New Delhi; National Academy of Ayurveda: 382.
- 17.Datta Chakrapani. 2006. Chakradatta of Shri Chakrapani Datta with the Padarthabodhini Hindi commentary btVd; p. 160. Ravidatta Shastri Reprint Varanasi; Chaukhambha Surbharti Prakashan. [Google Scholar]
- 18.Yasue H., Itoh T., Mizuno Y. Severe hypokalemia, rhabdomyolysis, muscle paralysis, and respiratory impairment in a hypertensive patient taking herbal medicines containing licorice. Intern Med. 2007;46(9):575–578. doi: 10.2169/internalmedicine.46.6316. [DOI] [PubMed] [Google Scholar]
- 19.Touyz L.Z. Liquorice health check, oro-dental implications, and a case report. Case Rep Med. 2009;2009 doi: 10.1155/2009/170735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigurjonsdottir H.A., Franzson L., Manhem K., Ragnarsson J., Sigurdsson G., Wallerstedt S. Liquorice-induced rise in blood pressure: a linear dose-response relationship. J Hum Hypertens. 2001;15:549–552. doi: 10.1038/sj.jhh.1001215. [DOI] [PubMed] [Google Scholar]
- 21.Sontia B., Mooney J., Gaudet L., Touyz R.M. Pseudohyperaldosteronism, liquorice, and hypertension. J Clin Hypertens (Greenwich) 2008;10(2):153–157. doi: 10.1111/j.1751-7176.2008.07470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai H.H., Lin H.W., Lu Y.H., Chen Y.L., Mahady G.B. A review of potential harmful interactions between anticoagulant/antiplatelet agents and Chinese herbal medicines. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burdock George. vol 1. CRC Press. Inc; 1997. p. 1236. (Encyclopedia of food and colour additives). [Google Scholar]
- 24.Herald O.H., Edward J., Andre V.R. 6th ed. Elsevier Inc; 2013. Sturdevants art and science of operative dentistry; p. 274. [Google Scholar]
- 25.Takahashi N., Nyvad B. Caries ecology revisited: microbial dynamics and the caries process. Caries Res. 2008;42:409–418. doi: 10.1159/000159604. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N., Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 27.Shafer Hine. 7th ed. Elsevier Inc; 2012. Levy. Shafer's textbook of oral pathology. [Google Scholar]
- 28.Klein M.I., Hwang G., Santos P., Campanella O.H., Koo H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front Cell Infect Microbiol. 2015;13(2):5–10. doi: 10.3389/fcimb.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J., Chen L., Heber D., Shi W., Lu Q.Y. Antibacterial compounds from Glycyrrhiza uralensis. J Nat Prod. 2006;69:121–124. doi: 10.1021/np058069d. [DOI] [PubMed] [Google Scholar]
- 30.Hu C.H., He J., Eckert R. Development and evaluation of a safe and effective sugar-free herbal lollipop that kills cavity-causing bacteria. Int J Oral Sci. 2011;3:13–20. doi: 10.4248/IJOS11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G., He Y.H., Zhang F.F., Kong X.L., Wen Y.L., Ma Q.R. Effects of glycyrrhizic acid on the growth and acid-producing of Streptococcus mutansinvitro. Sichuan Da Xue Xue Bao Yi Xue Ban. 2010;41(4):634–637. [PubMed] [Google Scholar]
- 32.Mentes J.C., Kang S., Spackman S., Bauer J. Can a licorice lollipop decrease cariogenic bacteria in nursing home residents? Res Gerontol Nurs. 2012;5(4):233–237. doi: 10.3928/19404921-20120906-07. [DOI] [PubMed] [Google Scholar]
- 33.Ahn S.J., Park S.N., Lee Y.J., Cho E.J., Lim Y.K., Li X.M. Invitro antimicrobial activities of 1-methoxyficifolinol, licorisoflavan A, and 6, 8-diprenyl genistein against Streptococcus mutans. Caries Res. 2015;49(1):78–89. doi: 10.1159/000362676. [DOI] [PubMed] [Google Scholar]
- 34.Soderling E., Karjalainen S., Lille M., Maukonen J., Saarela M., Autio K. The effect of liquorice extract-containing starch gel on the amount and microbial composition of plaque. Clin Oral Invest. 2006;10:108–113. doi: 10.1007/s00784-006-0040-9. [DOI] [PubMed] [Google Scholar]
- 35.McCullough M.J., Savage N. Oral candidosis and the therapeutic use of antifungal agents in dentistry. Aust Dent J. 2005;50:S36–S39. doi: 10.1111/j.1834-7819.2005.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 36.Samaranayake L.P., Keung Leung W.K., Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 37.Zunt S.L. Oral candidiasis: diagnosis and treatment. J Pract Hyg. 2000;9:31–36. [Google Scholar]
- 38.Pontes H.A., Neto N.C., Ferreira K.B., Fonseca F.P., Vallinoto G.M., Pontes F.S. Oral manifestations of vitamin B12 deficiency: a case report. J Can Dent Assoc. 2009;75:p.533–537. [PubMed] [Google Scholar]
- 39.Belazi M., Velegraki A., Fleva A., Gidarakou I., Papanaum L., Baka D. Candidal overgrowth in diabetic patients: potential predisposing factors. Mycoses. 2005;48:192–196. doi: 10.1111/j.1439-0507.2005.01124.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y.L. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36:223–228. [PubMed] [Google Scholar]
- 41.Ford C.B., Funt J.M., Abbey D., Issi L. The evolution of drug resistance in clinical isolates of Candida albicans. Elife. Feb 3 2015;4 doi: 10.7554/eLife.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Utsunomiya T., Kobayashi M., Herndon D.N., Pollard R.B., Suzuki F. Glycyrrhizin improves the resistance of MAIDS mice to opportunistic infection of Candida albicans through the modulation of MAIDS-associated type 2 T cell responses. Clin Immunol. 2000;95(2):145–155. doi: 10.1006/clim.2000.4854. [DOI] [PubMed] [Google Scholar]
- 43.Motsei M.L., Lindsey K.L., Van Staden J., Jager A.K. Screening of traditionally used South African plants for antifungal activity against Candida albicans. J Ethno Pharmacol. 2003;86:235–241. doi: 10.1016/s0378-8741(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 44.Fatima A., Gupta V.K., Luqman S. Antifungal activity of Glycyrrhiziaglabra extracts and its active constituent glabridin. Phytother Res. 2009;23:1190–1193. doi: 10.1002/ptr.2726. [DOI] [PubMed] [Google Scholar]
- 45.Lee J.Y., Lee J.H., Park J.H., Kim S.Y., Choi J.Y. Liquiritigenin, a licorice flavonoid, helps mice resist disseminated candidiasis due to Candida albicans by Th1 immune response, whereas liquiritin, its glycoside form, does not. Int Immuno Pharmacol. 2009;9(5):632–638. doi: 10.1016/j.intimp.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Messier C., Grenier D. Effect of licorice compounds licochalcone A, glabridin and glycyrrhizic acid on growth and virulence properties of Candida albicans. Mycoses. 2011;54(6):e801–e806. doi: 10.1111/j.1439-0507.2011.02028.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim J., Joo I., Kim H., Han Y. 18β-glycyrrhetinic acid induces immunological adjuvant activity of Th1 against Candida albicans surface mannan extract. Phytomedicine. 2013;20(11):951–955. doi: 10.1016/j.phymed.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 48.Newman M.G., Takei H., Carranza F.A. Carranza's clinical Periodontology. 10th ed. WB Saunders Company; Philadelphia: 2007. Carranza's classification and epidemiology of periodontal diseases. [Google Scholar]
- 49.Wittschier N., Faller G., Beikler T. Polysaccharides from Glycyrrhiza glabra L. exert significant anti-adhesive effects against Helicobacter pylori and Porphyromonas gingivalis. Planta. 2006;72:238. [Google Scholar]
- 50.Tanabe S., Desjardins J., Bergeron C., Gafner S., Villinski J.R., Grenier D. Reduction of bacterial volatile sulfur compound production by licoricidin and licorisoflavan A from licorice. J Breath Res. 2012;6(1) doi: 10.1088/1752-7155/6/1/016006. [DOI] [PubMed] [Google Scholar]
- 51.Pihlstrom B. Periodontal risk assessment, diagnosis and treatment planning. J Periodontol. 2001;25:37–58. doi: 10.1034/j.1600-0757.2001.22250104.x. [DOI] [PubMed] [Google Scholar]
- 52.O'Brien-Simpson N.M., Veith P.D., Dashper S.G., Reynolds E.C. Antigens of bacteria associated with periodontitis. Periodontol 2000. 2004;35:p.101–134. doi: 10.1111/j.0906-6713.2004.003559.x. [DOI] [PubMed] [Google Scholar]
- 53.Feng Z., Weinberg A. Role of bacteria in health and disease of periodontal tissue. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 54.Garlet G.P. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89:1349–1363. doi: 10.1177/0022034510376402. [DOI] [PubMed] [Google Scholar]
- 55.Cobb C.M. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29:6–16. [PubMed] [Google Scholar]
- 56.Matthews D. Conclusive support for mechanical nonsurgical pocket therapy in the treatment of periodontal disease. How effective is mechanical nonsurgical pocket therapy? Evid Base Dent. 2005;6(3):68–69. doi: 10.1038/sj.ebd.6400338. [DOI] [PubMed] [Google Scholar]
- 57.Suvan J.E. Effectiveness of mechanical nonsurgical pocket therapy. Periodontol 2000. 2005;37:48–71. doi: 10.1111/j.1600-0757.2004.03794.x. [DOI] [PubMed] [Google Scholar]
- 58.Preshaw P.M., Hefti A.F., Novak M.J., Michalowicz B.S., Pihlstrom B.L., Schoor R. Subantimicrobial dose doxycycline enhances the efficacy of scaling and root planing in chronic periodontitis: a multicenter trial. J Periodontol. 2004;75:1068–1076. doi: 10.1902/jop.2004.75.8.1068. [DOI] [PubMed] [Google Scholar]
- 59.Bodet C., La V.D., Gafner S., Bergeron C., Grenier D. A licorice extract reduces lipopolysaccharide-induced proinflammatory cytokine secretion by macrophages and whole blood. Periodontol. 2008;79:1752–1761. doi: 10.1902/jop.2008.080052. [DOI] [PubMed] [Google Scholar]
- 60.La V.D., Tanabe S., Bergeron C., Gafner S., Grenier D. Modulation of matrix metalloproteinase and cytokine production by licorice isolates licoricidin and licorisoflavan A: potential therapeutic approach for periodontitis. J Periodontol. 2011;82:122–128. doi: 10.1902/jop.2010.100342. [DOI] [PubMed] [Google Scholar]
- 61.Feldman M., Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113(2):438–447. doi: 10.1111/j.1365-2672.2012.05329.x. [DOI] [PubMed] [Google Scholar]
- 62.Kim S.R., Jeon H.J., Park H.J., Kim M.K., Choi W.S., Jang H.O. Glycyrrhetinic acid inhibits Porphyromonasgingivalis lipopolysaccharide-induced vascular permeability via the suppression of interleukin-8. Inflamm Res. 2013;62(2):145–154. doi: 10.1007/s00011-012-0560-5. [DOI] [PubMed] [Google Scholar]
- 63.Farhad S.Z., Aminzadeh A., Mafi M., Barekatain M., Naghney M., Ghafari M.R. The effect of adjunctive low-dose doxycycline and licorice therapy on gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. Dent Res J (Isfahan) 2013;10(5):624–629. [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki H., Suzuki N., Alshwaimi E. 18β-glycyrrhetinic acid inhibits periodontitis via glucocorticoid-independent nuclear factor-κB inactivation in interleukin-10-deficient mice. J Periodontal Res. 2010;45:757–763. doi: 10.1111/j.1600-0765.2010.01296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L., Wei H., Wu Y., Yang S., Xiao L., Zhang J. Licorice isoliquiritigenin suppresses RANKL-induced osteoclastogenesis invitro and prevents inflammatory bone loss invivo. Int J Biochem Cell Biol. 2012;44(7):1139–1152. doi: 10.1016/j.biocel.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 66.Kim H.S., Suh K.S., Sul D., Kim B.J., Lee S.K., Jung W.W. The inhibitory effect and the molecular mechanism of glabridin on RANKL-induced osteoclastogenesis in RAW264.7 cells. Int J Mol Med. 2012;29(2):169–177. doi: 10.3892/ijmm.2011.822. [DOI] [PubMed] [Google Scholar]
- 67.Zunt S.L. Recurrent aphthous ulcers: prevention and treatment. J Pract Hyg. 2001;10:17–24. [Google Scholar]
- 68.Munoz-Corcuera M., Esparza-Gomez G., Gonzalez-Moles M.A., Bascones-Martınez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin Exp Dermatol. 2009;34:289–294. doi: 10.1111/j.1365-2230.2009.03220.x. [DOI] [PubMed] [Google Scholar]
- 69.Burgess J.A., Van der Ven P.F., Martin M., Sherman J., Haley J. Review of over-the-counter treatments for aphthous ulceration and results fromuse of a dissolving oral patch containing glycyrrhiza complex herbal extract. J Contemp Dent Pract. 2008;9(3):88–98. [PubMed] [Google Scholar]
- 70.Martin M.D., Sherman J., van der Ven J.,P., Burgess J. A controlled trial of a dissolving oral patch concerning glycyrrhiza (licorice) herbal extract for the treatment of aphthous ulcers. Gen Dent. 2008;56:206–210. [PubMed] [Google Scholar]
- 71.Moghadamnia A.A., Motallebnejad M., Khanian M. The efficacity of the bioadhesive patches containing licorice extract in the management of recurrent aphthous stomatitis. Phytother Res. 2009;23:246–250. doi: 10.1002/ptr.2601. [DOI] [PubMed] [Google Scholar]
- 72.Sun S.J., Chen G., Zhang W., Hu X., Huang C.F., Wang Y.F. Mammalian target of rapamycin pathway promotes tumor-induced angiogenesis in adenoid cystic carcinoma: its suppression by isoliquiritigenin through dual activation of c-Jun NH2-terminal kinase and inhibition of extracellular signal-regulated kinase. J Pharmacol ExpTher. 2010;334(2):500–512. doi: 10.1124/jpet.110.167692. [DOI] [PubMed] [Google Scholar]
- 73.Chen G., Hu X., Zhang W., Xu N., Wang F.Q., Jia J. Mammalian target of rapamycin regulates isoliquiritigenin-induced autophagic and apoptotic cell death in adenoid cystic carcinoma cells. Apoptosis. 2012;17(1):p.90–101. doi: 10.1007/s10495-011-0658-1. [DOI] [PubMed] [Google Scholar]
- 74.Cho J.J., Chae J.I., Yoon G., Kim K.H., Cho J.H., Cho S.S. Licochalcone A, a natural chalconoid isolated from Glycyrrhiza inflata root, induces apoptosis via Sp1 and Sp1 regulatory proteins in oral squamous cell carcinoma. Int J Oncol. 2014;45(2):667–674. doi: 10.3892/ijo.2014.2461. [DOI] [PubMed] [Google Scholar]
- 75.Kim J.S., Park M.R., Lee S.Y., Kim do K., Moon S.M. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol Rep. 2014;31(2):755–762. doi: 10.3892/or.2013.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen H., Zeng G., Tang G., Cai X., Bi L., Huang C. Antimetastatic effects of licochalconeA on oral cancer via regulating metastasis-associated proteases. Tumour Biol. 2014;35(8):7467–7474. doi: 10.1007/s13277-014-1985-y. [DOI] [PubMed] [Google Scholar]
- 77.Shen H., Zeng G., Sun B., Cai X., Bi L., Tang G. A polysaccharide from Glycyrrhiza inflate Licorice inhibits proliferation of human oral cancer cells by inducing apoptosis via mitochondrial pathway. TumourBiol. 2015;36(6):p.4825–4831. doi: 10.1007/s13277-015-3135-6. [DOI] [PubMed] [Google Scholar]
- 78.Hsia S.M., Yu C.C., Shih Y.H., Chen M.Y., Wang T.H., Huang Y.T. Isoliquiritigenin causes DNA damage and inhibits ATM expression leading to G2/M phase arrest and apoptosis in oral squamous cell carcinoma. Head Neck. 2016;38(Suppl. 1):E360–E371. doi: 10.1002/hed.24001. [DOI] [PubMed] [Google Scholar]
- 79.Love R.M. Enterococcus faecalis–a mechanism for its role in endodontic failure. Int Endod J. 2001;34(5):399–405. doi: 10.1046/j.1365-2591.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 80.Das D., Agarwal S.K., Chandola H.M. Protective effect of Yashtimadhu (Glycyrrhiza glabra) against side effects of radiation/chemotherapy in head and neck malignancies. Ayu. 2011;32(2):196–199. doi: 10.4103/0974-8520.92579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Estrela C., Silva J.A., de Alencar A.H., Leles C.R., Decurcio D.A. Efficacy of sodium hypochlorite and chlorhexidine against Enterococcus faecalis–a systematic review. J Appl Oral Sci. 2008;16(6):364–368. doi: 10.1590/S1678-77572008000600002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luddin N., Ahmed H.M.A. The antibacterial activity of sodium hypochlorite and chlorhexidine against Enterococcus faecalis: a review on agar diffusion and direct contact methods. J Conserv Dent. 2013;16(1):9–16. doi: 10.4103/0972-0707.105291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badr A.E., Omar N., Badria F.A. A laboratory evaluation of the antibacterial and cytotoxic effect of Liquorice when used as root canal medicament. Int Endod J. 2011;44(1):51–58. doi: 10.1111/j.1365-2591.2010.01794.x. [DOI] [PubMed] [Google Scholar]