Abstract
Background
Histopathological features after radical prostatectomy (RP) provide important information for the prognosis of prostate cancer (PCa). The possible correlations between Prostate-Imaging Reporting and Data Scoring System (PIRADS) scores in multiparametric magnetic resonance imaging (mpMRI) may also be predictive for prognosis. In this study, we aimed to evaluate the correlation of PIRADS scores with histopathological data.
Methods
A total of 177 patients who underwent preoperative mpMRI and RP for PCa from eight institutions were included in the study. Correlation of PIRADS score in preoperative mpMRI with adverse histopathological factors in RP specimen was investigated using univariate and multivariate analyses.
Results
The relationship between PIRADS score and postoperative extracapsular extension, lymphovascular invasion, and seminal vesicle involvement was significant (P < 0.001, P = 0.032, and P = 0.007, respectively). Although the PIRADS score was significantly correlated with the number of dissected lymph nodes (p = 0.026), it had no significant correlation with the number of positive nodes (P = 0.611). Total Gleason score, extracapsular extension, seminal vesicle invasion, and number of lymph nodes were found to be independent factors, which correlated with high PIRADS scores in ordinal logistic regression analysis.
Conclusion
PIRADS scoring system in mpMRI showed a statistically significant correlation with adverse histopathological factors in RP specimen. A higher PIRADS score may help to predict a higher Gleason score, indicating clinically important PCa as well as poor prognotic factors such as extracapsular extension, lymphovascular invasion, and seminal vesicle invasion that may indicate a higher risk of recurrence and the need for additional treatment.
Keywords: Histopathology, Multiparametric magnetic resonance imaging prostate, PIRADS, Prostate neoplasms
1. Introduction
Prostate cancer (PCa) is the most common cancer diagnosed in men.1 The diagnosis of PCa is traditionally made by transrectal ultrasound–guided biopsy based on information obtained from the combination of transrectal ultrasound, prostate-specific antigen (PSA), and digital rectal examination.2 However, this method may lead to overdiagnosis and overtreatment and may also miss the diagnosis of clinically significant cancers in a significant number of patients.3 The clinical behavior of PCa may range from low-grade silent tumors that do not progress to lethal disease to invasive, aggressive tumors that rapidly progress and become metastatic.4 New and effective methods and biomarkers are needed to differentiate between indolent and aggressive forms of this disease.5
Accordingly, there is a need for an imaging and diagnostic method that reduces the rates of overdiagnosis and overtreatment and that can diagnose more clinically significant cancers. Prostate multiparametric magnetic resonance imaging (mpMRI) may in part confer a solution for this unmet need.6 Suspicious lesions in mpMRI are graded using the Prostate-Imaging Reporting and Data Scoring System (PIRADS) version 2, co-developed by the European Association of Urogenital Radiology and the American College of Radiology, providing important information for diagnosis and treatment planning to clinicians.7
In recent years, the use of mpMRI in the diagnosis and staging of PCa has attained wider acceptance, and it has become useful especially for the differentiation of clinically important cancers.8 Predicting the prognosis of PCa is important in terms of individualizing the treatment of the disease and the applicability of more effective treatment methods. Histopathological features of the disease at final pathology after radical prostatectomy (RP) provide important information for predicting the prognosis of the disease. Possible correlations of PIRADS scoring system with final histopathology may also be predictive for prognosis. Thus, the data obtained with mpMRI may also be identified as a prognostic factor and may in fact modulate the treatment of the patients with appropriate risk stratification.
There are a limited number of studies in the literature in which the correlation of PIRADS scores with Gleason score was investigated, and the results are conflicting.9,10 Studies, investigating the prognostic role of mpMRI by evaluating the correlation of PIRADS with histopathological factors, are needed. Thus, it is important to diagnose lesions of clinical importance in disease management to evaluate the extension of the disease at the time of diagnosis and to determine the risk of progression. In this study, we aimed to investigate the correlation of PIRADS scoring system of prostatic lesions detected by mpMRI and histopathological data obtained after RP by using the “Prostate Cancer Database (PCD)" of Urooncology Association, Turkey (UOAT).
2. Patients and methods
2.1. Patient selection, inclusion, and exclusion criteria
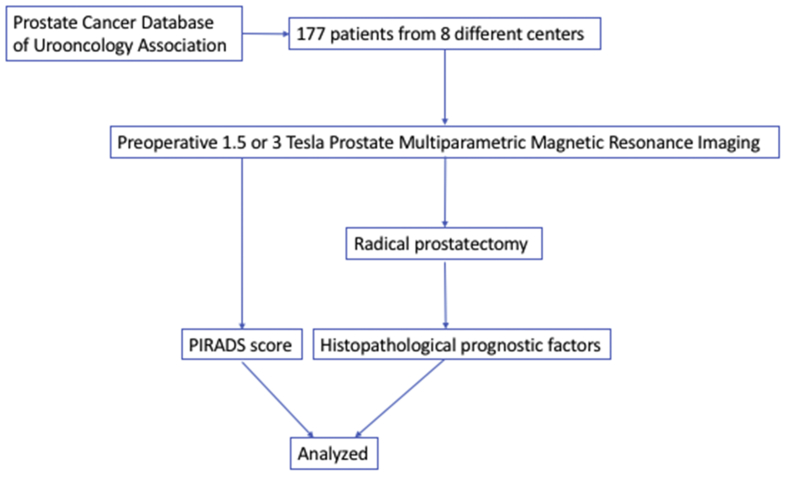
In this study, data of 177 patients from eight different centers in PCD–UOAT were collected and analyzed. All data were anonymized at each participating center before recorded in the PCD in compliance with the local regulations. Patients who underwent preoperative 1.5 or 3 Tesla mpMRI and subsequent RP (open, laparoscopic, or robotic) for PCa were included in the study. Patients whose data were missing and who did not undergo mpMRI before the operation were excluded from the study. The flowchart of the study is shown in the Fig. 1. The study protocol was approved by the institutional review board of the project planner central institution (ID: 18-10.1/7, Date: 26/10/2018.)
Fig. 1.
The flow-chart of the study. PIRADS, Prostate-Imaging Reporting and Data Scoring System.
PCD-UOAT is an online-based professionally supported software. The tab headers required for data entry according to the needs of the planned study are added to the system by the system administrators. All researchers have their own usernames and passwords. They enter the data to the system by anonymization the confidential information of their patients. After the data entry is completed, statistical analysis is performed by the expert statisticians. Then, the result of the analysis is transmitted to the central institute author and shared with other co-authors.
2.2. Analysis criteria and outcome measures
The patients were classified according to the lesion detection status in mpMRI, and the patients with lesions were graded according to PIRADS system version 1 or 2. Gleason score, pathological tumor volume, extracapsular extension, presence of positive surgical margins, pT stage, number of dissected lymph nodes, number of metastatic nodes, and perineural invasion status were recorded. Correlation of these variables with PIRADS scoring system in the preoperative mpMRI was the primary outcome measure of the study. The investigation of the ability of PIRADS score in mpMRI to predict important histopathological prognostic factors was the secondary outcome measure of the study.
2.3. Statistical analysis
Nonparametric Kruskal–Wallis test was used to determine whether there was a statistically significant difference between two or more groups of independent variables. Chi-square test was used to analyze the relationship between categorical variables. Ordinal logistic regression analysis was performed to analyze the correlation between the rising PIRADS scores and prognostic factors. P values less than 0.05 were considered statistically significant. SPSS, version 23.0, was used for all statistical analysis.
3. Results
A total of 177 patients who underwent RP with complete data were included in the study. The mean age of the patients was 65.7 ± 6.31 (50–86). The preoperative mean total PSA value was 10.91 ng/mL (range:1.21–394 ng/mL). Total PSA value was in the range of 4–10 ng/mL in 115 patients (59%). When the mpMRI data were analyzed, the mean prostate volume was 39.98 ml. The size of the tumor focus at mpMRI was in the range of 1.1–2 mm in 65 patients (53.3%). Tumor positivity at mpMRI was detected in two foci in 22 patients (16.8%). Fifty-three patients (41.7%) had PIRADS 4 lesions, and 49 patients (38.6%) had PIRADS 5 lesions. Preoperative clinical data and mpMRI characteristics of the patients are summarized in Table 1.
Table 1.
Preoperative clinical features and multiparametric magnetic resonance imaging characteristics of patients.
| Variable | n = 177 |
|---|---|
| Age, mean ± SD | 65.7 ± 6.31 |
| Total PSA (ng/mL) | 10.91 |
| Free PSA (ng/mL) | 1.4 |
| Total PSA (ng/mL) group, | n (%) |
| 0–4 | 20 (11.7%) |
| 4–10 | 101 (59%) |
| 10–20 | 42 (24.6%) |
| 20 and above | 8 (4.7%) |
| Prostate volume (ml) mean ± SD | 39.98 ± 10.21 |
| Lesion size (mm), n (%) | |
| 0-1 | 29 (23.7%) |
| 1.1-2 | 65 (53.3%) |
| 2.1-3 | 24 (19.7%) |
| 3.1 and above | 4 (3.3%) |
| Lesion side on MRI, | n (%) |
| Right | 49 (36.6%) |
| Left | 63 (47%) |
| Bilateral | 22 (16.4%) |
| Lesion location on MRI, | n (%) |
| Anterior | 31 (43.7%) |
| Posterior | 35 (49.3%) |
| Bilateral | 5 (7%) |
| Lesion number and region on MRI, | n (%) |
| Double foci | 22 (16.8%) |
| Single focus | 109 (83.2%) |
| Apex | 54 (41.2%) |
| Mid | 84 (64.1%) |
| Base | 15 (11.5%) |
| PIRADS score, | n (%) |
| 2 | 5 (3.9%) |
| 3 | 20 (15.8%) |
| 4 | 53 (41.7%) |
| 5 | 49 (38.6%) |
SD, standard deviation; PSA, prostate-specific antigen; MRI, magnetic resonance imaging; PIRADS, Prostate-Imaging Reporting and Data Scoring System.
Robotic transperitoneal RP was performed in 55.6% (94 patients) and radical retropubic prostatectomy in 34.9% (59 patients) of the patients. Pathological evaluation of the specimens revealed a Gleason score of 7 in 106 patients (62.4%). Surgical margin positivity was detected in 60 patients (34.5%), extracapsular extension in 55 patients (32.4%), perineural invasion in 129 patients (74.6%), lymphovascular invasion in 10 patients (5.8%), and seminal vesicle involvement in 22 patients (12.8%). Of 79 patients who underwent lymph node dissection, 15 (18.9%) had tumor involvement. The perioperative details and histopathological features of RP specimens are as shown in Table 2.
Table 2.
Radical prostatectomy, type of surgery, and pathological features of radical prostatectomy specimens.
| Variable | n (%) |
|---|---|
| Radical prostatectomy methods | |
| Radical Retropubic Prostatectomy—n (%) | 59 (34.9%) |
| Robotic Retroperitoneal Radical Prostatectomy—n (%) | 1 (0.6%) |
| Laparoscopic Transperitoneal Radical Prostatectomy—n (%) | 15 (8.9%) |
| Robotic Transperitoneal Radical Prostatectomy—n (%) | 94 (55.6%) |
| Tumor volume (ml) mean +SD | 2.75 |
| Histological type, n (%) | |
| Adenocarcinoma | 173 (99.4%) |
| Ductal adenocarcinoma | 1 (0.6%) |
| Primary Gleason grade, n (%) | |
| 3 | 112 (65.5%) |
| 4 | 52 (30.4%) |
| 5 | 7 (4.1%) |
| Secondary Gleason grade, n (%) | |
| 3 | 69 (40.6%) |
| 4 | 87 (51.2%) |
| 5 | 14 (8.2%) |
| Tertiary Gleason grade, n (%) | |
| 1 | 7 (12.3%) |
| 2 | 26 (45.6%) |
| 3 | 10 (17.5%) |
| 4 | 2 (3.5%) |
| 5 | 12 (21.1%) |
| Total Gleason score, n (%) | |
| 6 | 37 (21.7%) |
| 7 | 106 (62.4%) |
| 8 | 7 (4.1%) |
| 9 | 19 (11.2%) |
| 10 | 1 (0.6%) |
| Positive surgical margin, n (%) | 60 (34.5%) |
| Apex | 13 (7.5%) |
| Anterior | 11 (6.3%) |
| Posterolateral | 22 (12.6%) |
| Bladder neck | 13 (7.5%) |
| Surgical margin Gleason grade, n (%) | |
| 1 | 4 (9.8%) |
| 2 | 6 (14.6%) |
| 3 | 16 (39%) |
| 4 | 8 (19.5%) |
| 5 | 7 (17.1%) |
| Tumor size at positive surgical margin, n (%) | |
| Microscopic (<1 mm) | 21 (38.9%) |
| Macroscopic (>1 mm) | 33 (61.1%) |
| Extracapsular extension, n (%) | 55 (32.4%) |
| Extracapsular extension side, n (%) | |
| Right | 15 (34.9%) |
| Left | 16 (37.2%) |
| Bilateral | 12 (27.9%) |
| Perineural invasion, n (%) | 129 (74.6%) |
| Lymphovascular invasion, n (%) | 10 (5.8%) |
| Seminal vesicle invasion, n (%) | 22 (12.8%) |
| Seminal vesicle invasion side, n (%) | |
| Right | 5 (25%) |
| Left | 6 (30%) |
| Bilateral | 9 (45%) |
| Lymph node dissection, n(%) | 79 (45.4%) |
| Number of Lymph Nodes | |
| Tumor positive lymph node side, n (%) | |
| Right | 3 (20%) |
| Left | 4 (26.7%) |
| Bilateral | 8 (53.3%) |
SD, standard deviation.
Tumor volumes at final pathology showed a statistically significant difference between PIRADS 4 and 5 lesions (P < 0.001) and between PIRADS 3 and 5 lesions (P = 0.034). Postoperative Gleason scores were also statistically significantly differed between PIRADS 3 and 5 lesions (P = 0.001) and PIRADS 4 and 5 lesions (P < 0.001). There was a significant correlation between the PIRADS scores and extracapsular extension, lymphovascular invasion, and seminal vesicle involvement (P < 0.001, P = 0.032, and P = 0.007, respectively). Although the PIRADS score was significantly correlated with the number of dissected lymph nodes (P = 0.026), it was not significantly correlated with the number of positive lymph nodes (P = 0.611). The relationship between PIRADS scores and postoperative histopathological data is summarized in Table 3.
Table 3.
The relationship between postoperative histopathological data and Prostate-Imaging Reporting and Data Scoring System scores.
| Variable | PIRADS score |
P | |||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | ||
| Age | 65 (64-69) | 65.5 (57-74) | 66 (50-76) | 66 (53-86) | 0.999 |
| Tumor volume (ml) | 0.58 (0.50-6.25) | 1.00 (0.10-8.42) | 1.32 (0.10-23.50) | 4.3 (0.20-19.97) | <0.001 |
| Primary Gleason grade | 3 (3-4) | 3 (3-4) | 3 (3-5) | 4 (3-5) | <0.001 |
| Secondary Gleason grade | 3 (3-4) | 4 (3-5) | 4 (3-5) | 4 (3-5) | 0.083 |
| Tertiary Gleason grade | Ø | 2.5 (2-5) | 3 (1-5) | 3 (1-5) | 0.801 |
| Total Gleason score | |||||
| 6 | 4 (80%) | 14 (70%) | 22 (42%) | 14 (28%) | <0.001 |
| 7 | 1 (20%) | 6 (30%) | 21 (39%) | 22 (45%) | |
| 8 | - | - | 7 (14%) | 6 (12%) | |
| 9 | - | - | 3 (5%) | 5 (10%) | |
| 10 | - | - | - | 2 (5%) | |
| Surgical margin | 2.4% | 14.6% | 31.7% | 51.2% | 0.234 |
| Extracapsular extension | 0% | 4.9% | 26.8% | 68.3% | <0.001 |
| Perineural invasion | 60% | 65% | 64.7% | 79.2% | 0.379 |
| Lymphovascular invasion | Ø | Ø | Ø | 100% | 0.032 |
| Seminal vesicle invasion | Ø | Ø | 15.4% | 84.6% | 0.007 |
| Number of dissected lymph nodes | Ø | 11.5% | 36.5% | 51.9% | 0.026 |
| Number of tumor positive lymph nodes | Ø | 100% | 89.5% | 81.5% | 0.611 |
PIRADS, Prostate-Imaging Reporting and Data Scoring System. Significant p values are given in bold.
Total Gleason score, extracapsular extension, seminal vesicle invasion, and number of dissected lymph nodes were found to have significant correlations with elevated PIRADS scores in ordinal logistic regression analysis. The correlation between prognostic factors and elevated PIRADS scores is given in Table 4.
Table 4.
Correlation of prognostic factors and elevated Prostate-Imaging Reporting and Data Scoring System.
| Variables | Estimate | Std. Error | Wald | df | P | 95% Confidence interval |
||
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Prognostic factors | Total Gleason score | 1.047 | 0.243 | 18.504 | 1 | <0.001 | 0.821 | 1.179 |
| Surgical margin positivity | 0.648 | 0.363 | 3.188 | 1 | 0.074 | 0.359 | 1.244 | |
| Extracapsular extension | 1.933 | 0.410 | 22.220 | 1 | <0.001 | 1.737 | 2.016 | |
| Seminal vesicle invasion | 2.436 | 0.801 | 9.254 | 1 | 0.002 | 2.121 | 2.735 | |
| Number of dissected lymph nodes | 0.999 | 0.352 | 8.046 | 1 | 0.005 | 0.690 | 1.123 | |
Significant p values are given in bold.
4. Discussion
The use of mpMRI in the evaluation of prostate cancer is attaining a wider acceptance. However, the contribution of mpMRI to clinical practice is still not entirely clear and deserves a detailed assessment. In a retrospective analysis of MRI-guided biopsy performed on 343 men, it was shown that the PIRADS score correlated with the clinically significant disease defined as Gleason score ≥3 + 4 disease.9 On the other hand, in the study of Wang et al, it was shown that the lesion diameter, area, and proximity of the capsule were also correlated with MRI visibility in low-grade PCa, which is defined as GS 3 + 3 and 3 + 4.11 In another retrospective analysis, PIRADS score and Gleason score of RP specimens after MRI fusion targeted biopsy of 74 patients were compared, and the relationship between PIRADS score and Gleason score was not statistically significant. Moreover, concordance of Gleason score in both MRI-targeted and classical biopsies with RP specimens’ Gleason scores of the target areas was weak.10 In contrast, analysis of our data indicates an important correlation of the PIRADS scoring system with the histopathology of the RP specimens. In our study, a clinically significant prostate cancer (csPCa), defined as the presence of Gleason grade group ≥ 2 disease, was found in 30% of patients with PIRADS 3 score. This observation clearly emphasizes the importance of PIRADS 3 lesions and is higher than previously reported both by Osses et al (10%) and Felker et al (10%) and Venderink et al (17%).12, 13, 14. A possible explanation may be evaluation of MRI reports and scoring by different radiologists. As a result of this finding, it is possible to speculate that PIRADS 3 lesions may be subdivided and evaluated in two categories as PIRADS 3a and 3b. PIRADS 3a may indicate clinically less important cancers, whereas 3b may indicate clinically more important cancers, similar to the definition reflected by Gleason 3 + 4 and 4 + 3. Schlenker et al also tried to answer this important question and evaluated the rates of significant PCa marking of lesions appearing as PIRADS 3 in mpMRI with a real world-based and different radiologist reports. In their series, PIRADS 3 lesions correlated with overall and csPCa at 26.8% and 14.6%, respectively. This finding questions the surveilliability of PIRADS 3 lesions and needs to be confirmed in large series.15 On the other hand, the csPCa rates for PIRADS 4 and 5 was 43% and 67% in our study. Those were similar to previously reported by three different studies, 34%–45% in PIRADS 4 and 67–84% in PIRADS 5, respectively.12, 13, 14.
In the present study, tumor volume, total Gleason score, extracapsular extension, seminal vesicle invasion, and the number of dissected lymph nodes were statistically correlated with elevated PIRADS scores. The clinical significance of adverse pathological factors was evaluated by Jung et al, and perineural invasion and lymphovascular invasion were associated with a higher pathological stage, a higher Gleason score, a higher tumor volume in the robotic-assisted prostatectomy specimen, a higher frequency of positive surgical margin, and a higher frequency of seminal vesicle invasion.16 Another study by Koca et al showed that PSA level at the time of diagnosis, Gleason score, prostatic capsule invasion, extracapsular extension, seminal vesical invasion, and surgical margin positivity were important factors predicting recurrence in their 238 case series, with PSA level and Gleason score at the time of diagnosis as independent factors predicting biochemical recurrence after RP.17 Consequently, the significant correlation between PIRADS scores and adverse histopathological factors observed in our study indicates the possibility of MRI use as a surrogate for outcomes after definitive treatment. However, there is no clear consensus in the literature. In a single-center study of 100 consecutive patients undergoing RP after mpMRI, it was shown that 21% of the pathological lesions could be missed or underestimated in size by MRI. Therefore, the authors cautioned about the need for new approaches to reduce this false-negative rate.12, 13, 14. Tumor size and grade appears important factors for proper detection of cancers by mpMRI when the results were verified by prostatectomy. Apparently, mpMRI is often capable of detecting the worst cancer focus in multifocal cancers.18 In our study, multifocality data were not available, but there was a significant correlation between increasing PIRADS scores, tumor volume, and Gleason score.
An interesting finding that stands out in our study was that although the PIRADS score was significantly correlated with the number of dissected lymph nodes, it was not correlated with the number of positive nodes. Certainly, multiple pathologist interpretations from different centers may affect this result. However, it is also possible that some of the negative lymph nodes are false-negative. Recently, Brembilla et al concluded that mpMRI could identify suitable candidates for extended pelvic node dissection by predicting nodal metastasis in PCa patients in a retrospective analysis evaluating the role of preoperative mpMRI in the estimation of nodal metastasis in patients undergoing extended dissection.19 However, as in the aforementioned studies, it should be kept in mind that mpMRI can produce false-negative results in prostate tumor foci as well as similar results in lymph nodes, and this is the subject of further research.
The relationship between preoperative PIRADS scores and adverse pathological features of 206 patients undergoing RP was evaluated, and differential transcriptomic analysis was performed with PIRADS scores. Similar to our study, the authors concluded that the PIRADS scores were associated with adverse pathological features, increased metastatic risk, and differential genomic pathway activation.20 Recently, radiomic properties measured by mpMRI have been shown to predict PCa aggressiveness.21 The records of 64 patients who underwent mpMRI before prostatectomy were reviewed, and a total of 14 radiomic features correlated with the Gleason score. The authors concluded that MRI radiomic features were promising markers of PCa aggressiveness at histopathological and genomic levels. By adding mpMRI information to established nomograms such as Partin and Memorial Sloan Kettering Cancer Center pre-RP nomograms, useful information could be obtained in the prediction of poor pathological factors in PCa. With the addition of mpMRI findings to systematic biopsy-based Memorial Sloan Kettering Cancer Center and Partin nomograms, AUC for extraprostatic invasion and seminal vesicle invasion was significantly increased.22 This information can help the urologists to make preoperative planning and inform their patients about the risk of future treatment although there are contradictory reports in the literature.23 In a recent study, the authors could predict side-specific extracapsular extension in 353 patients by adding mpMRI to the nomogram. They claimed a better evaluation of extracapsular extension could be done by this combined nomogram and could aid urologists to decide whether preserve or resect the neurovascular bundles.24 The majority of our patients consisted of patients with a PSA value of 4–10 ng/mL, which is described as the gray zone. It is emphasized that mpMRI can provide more favorable information and more csPCa diagnosis in this population. Ross et al highlighted the importance of the use of mpMRI to enhance the diagnosis of clinically important PCa with fewer unnecessary investigations during imaging and biopsy. The authors reported that a cut-off value of 1.5 ng/mL should be used in conjunction with digital rectal examination, risk calculation, and PSA adjuncts.25 However, in the study of Petrillo et al, it was emphasized that MRI has a high negative predictive value in patients with PSA ≤10 ng/mL and can predict significant PCa.26
In a review analyzing three different studies evaluating the role of mpMRI in the diagnosis and staging of PCa, it was found that mpMRI could provide valuable information about the histopathological aggressiveness and tumor stage of a PCa lesion and to determine a possible extracapsular extension in the pretreatment stage for optimal patient-tailored treatment planning.27 On the other hand, Almeida et al have underlined that a lesion seen in the mpMRI of patients eligible for active surveillance according to Prostate Cancer Research International: Active Surveillance criterion may indicate significant clinical significant cancer and mpMRI should be used as the clinical selection criterion for active surveillance.28
In a retrospective analysis comparing the diagnostic accuracy of mpMRI and 68Ga-PSMA-11 PET/MRI for extracapsular extension and seminal vesicle invasion, data from 40 patients undergoing both imaging modalities were evaluated, and local staging capacity of both modalities in intermediate-risk to high-risk PCa patients was found similar.29 This result seems to be important in favor of mpMRI, especially considering the high cost of PET/MRI.
Two different mpMRI T techniques were used in our study (1.5 T and 3 T). However, the majority of patients underwent 1.5 mpMRI (159/177). Therefore, no statistical comparison was made between these two techniques. On the other hand, there are reports in the literature that 1.5 T MRI can save time and contrast material, indicating cancer location, extraprostatic extension, and seminal vesicle invasion similar rates to 3 T MRI.30,31
Limitations of our study include its retrospective analysis and absence of a centralized radiological review. However, it is valuable in terms of establishing its role in real life clinical practice. Effect of evaluation of MRI reports by different radiologists may be considerable as there may be a certain degree of interobserver variability. However, in most of the current studies, MRI reports have been evaluated by multiple radiologists, and no data are available to compare single and multiple radiologist evaluations. Another point is that the reports were evaluated with a mixture of PIRADS version 1 and 2. This bias has become inevitable with data presented by different radiologists from many centers. However, the available data in the literature suggest that the effect of this will be minimal to the outcomes.32
5. Conclusion
The results of our study showed that PIRADS scoring system in mpMRI has statistically significant correlation with RP histopathological adverse prognostic features. PIRADS 3-5 lesions are also likely to indicate clinically significant cancer. The high PIRADS scores may help to predict poor prognotic factors indicative of csPCa as well as disease recurrence and need for additional treatment.
Ethical approval
The study was carried out in accordance with the principles of Declaration of Helsinki and all patients included in the study were granted their consent to use their data for scientific studies, provided that their identities are confidential.
Funding
None declared.
Conflicts of interest
All authors have no conflict of interest to declare.
Acknowledgments
The authors declare that they have no relevant financial interests and no conflict of interest.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer Statistics, 2017. CA Canc J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Emberton M. Is a negative prostate biopsy a risk factor for a prostate cancer related death? Lancet Oncol. 2017;18(2):162–163. doi: 10.1016/S1470-2045(17)30024-4. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S. When is a Negative Prostate Biopsy Really Negative? Repeat Biopsies in Detection and Active Surveillance. J Urol. 2017;197(4):973–974. doi: 10.1016/j.juro.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Shoag J., Barbieri C.E. Clinical variability and molecular heterogeneity in prostate cancer. Asian J Androl. 2016;18(4):543–548. doi: 10.4103/1008-682X.178852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang A.Y., Chiam K., Haupt Y., Fox S., Birch S., Tilley W. An analysis of a multiple biomarker panel to better predict prostate cancer metastasis after radical prostatectomy. Int J Canc. 2019;144(5):1151–1159. doi: 10.1002/ijc.31906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M., Huang Z., Yu H., Wang Y., Zhang Y., Song B. Comparison of PET/MRI with multiparametric MRI in diagnosis of primary prostate cancer: A meta-analysis. Eur J Radiol. 2019;113:225–231. doi: 10.1016/j.ejrad.2019.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Weinreb J.C., Barentsz J.O., Choyke P.L., Cornud F., Haider M.A., Macura K.J. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69(1):16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borofsky S., George A.K., Gaur S., Bernardo M., Greer M.D., Mertan F.V. What Are We Missing? False-Negative Cancers at Multiparametric MR Imaging of the Prostate. Radiology. 2018;286(1):186–195. doi: 10.1148/radiol.2017152877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastian-Jordan M. Magnetic resonance imaging of the prostate and targeted biopsy, Comparison of PIRADS and Gleason grading. J Med Imag Radiat Oncol. 2018;62(2):183–187. doi: 10.1111/1754-9485.12678. [DOI] [PubMed] [Google Scholar]
- 10.Slaoui H., Neuzillet Y., Ghoneim T., Rouanne M., Abdou A., Lugagne-Delpon P.M. Gleason Score within Prostate Abnormal Areas Defined by Multiparametric Magnetic Resonance Imaging Did Not Vary According to the PIRADS Score. Urol Int. 2017;99(2):156–161. doi: 10.1159/000468947. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Janaki N., Buzzy C., Bukavina L., Mahran A., Mishra K. Whole mount histopathological correlation with prostate MRI in Grade I and II prostatectomy patients. Int Urol Nephrol. 2019;51(3):425–434. doi: 10.1007/s11255-019-02083-8. [DOI] [PubMed] [Google Scholar]
- 12.Osses D.F., van Asten J.J., Kieft G.J., Tijsterman J.D. Prostate cancer detection rates of magnetic resonance imaging-guided prostate biopsy related to Prostate Imaging Reporting and Data System score. World J Urol. 2017;35(2):207–212. doi: 10.1007/s00345-016-1874-7. [DOI] [PubMed] [Google Scholar]
- 13.Felker E.R., Lee-Felker S.A., Feller J., Margolis D.J., Lu D.S., Princenthal R. In-bore magnetic resonance-guided transrectal biopsy for the detection of clinically significant prostate cancer. Abdom Radiol (NY) 2016;41(5):954–962. doi: 10.1007/s00261-016-0750-7. [DOI] [PubMed] [Google Scholar]
- 14.Venderink W., van Luijtelaar A., Bomers J.G., van der Leest M., Hulsbergen-van de Kaa C., Barentsz J.O. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur Urol. 2017;73(3):353–360. doi: 10.1016/j.eururo.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Schlenker B., Apfelbeck M., Armbruster M., Chaloupka M., Stief C.G., Clevert D.A. Comparison of PIRADS 3 lesions with histopathological findings after MRI-fusion targeted biopsy of the prostate in a real world-setting. Clin Hemorheol Microcirc. 2019;71(2):165–170. doi: 10.3233/CH-189407. [DOI] [PubMed] [Google Scholar]
- 16.Jung J.H., Lee J.W., Arkoncel F.R., Cho N.H., Yusoff N.A., Kim K.J. Significance of perineural invasion, lymphovascular invasion, and high-grade prostatic intraepithelial neoplasia in robot-assisted laparoscopic radical prostatectomy. Ann Surg Oncol. 2011;18(13):3828–3832. doi: 10.1245/s10434-011-1790-4. [DOI] [PubMed] [Google Scholar]
- 17.Koca O., Un S., Turk H., Zorlu F. The factors predicting biochemical recurrence in patients with radical prostatectomy. Arch Ital Urol Androl. 2016;87(4):270–275. doi: 10.4081/aiua.2015.4.270. [DOI] [PubMed] [Google Scholar]
- 18.Le J.D., Tan N., Shkolyar E., Lu D.Y., Kwan L., Marks L.S. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol. 2015;67(3):569–576. doi: 10.1016/j.eururo.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 19.Brembilla G., Dell'Oglio P., Stabile A., Ambrosi A., Cristel G., Brunetti L. Preoperative multiparametric MRI of the prostate for the prediction of lymph node metastases in prostate cancer patients treated with extended pelvic lymph node dissection. Eur Radiol. 2018;28(5):1969–1976. doi: 10.1007/s00330-017-5229-6. [DOI] [PubMed] [Google Scholar]
- 20.Beksac A.T., Cumarasamy S., Falagario U., Xu P., Takhar M., Alshalalfa M. Multiparametric Magnetic Resonance Imaging Features Identify Aggressive Prostate Cancer at the Phenotypic and Transcriptomic Level. J Urol. 2018;200(6):1241–1249. doi: 10.1016/j.juro.2018.06.041. [DOI] [PubMed] [Google Scholar]
- 21.Hectors S.J., Cherny M., Yadav K.K., Beksac A.T., Thulasidass H., Lewis S. Radiomics Features Measured with Multiparametric Magnetic Resonance Imaging Predict Prostate Cancer Aggressiveness. J Urol. 2019;202(3):498–505. doi: 10.1097/JU.0000000000000272. [DOI] [PubMed] [Google Scholar]
- 22.Rayn K.N., Bloom J.B., Gold S.A., Hale G.R., Baiocco J.A., Mehralivand S. Added Value of Multiparametric Magnetic Resonance Imaging to Clinical Nomograms for Predicting Adverse Pathology in Prostate Cancer. J Urol. 2018;200(5):1041–1047. doi: 10.1016/j.juro.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver J.K., Kim E.H., Vetter J.M., Shetty A., Grubb R.L., 3rd, Strope S.A. Prostate Magnetic Resonance Imaging Provides Limited Incremental Value Over the Memorial Sloan Kettering Cancer Center Preradical Prostatectomy Nomogram. Urology. 2018;113:119–128. doi: 10.1016/j.urology.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y., Yu W., Fan Y., Zhou L., Yang Y., Wang H. Development and comparison of a Chinese nomogram adding multi-parametric MRI information for predicting extracapsular extension of prostate cancer. Oncotarget. 2017;8(13):22095–22103. doi: 10.18632/oncotarget.11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ross T., Ahmed K., Raison N., Challacombe B., Dasgupta P. Clarifying the PSA grey zone: The management of patients with a borderline PSA. Int J Clin Pract. 2016;70(11):950–959. doi: 10.1111/ijcp.12883. [DOI] [PubMed] [Google Scholar]
- 26.Petrillo A., Fusco R., Setola S.V., Ronza F.M., Granata V., Petrillo M. Multiparametric MRI for prostate cancer detection: performance in patients with prostate-specific antigen values between 2.5 and 10 ng/mL. J Magn Reson Imag. 2014;39(5):1206–1212. doi: 10.1002/jmri.24269. [DOI] [PubMed] [Google Scholar]
- 27.Boesen L. Multiparametric MRI in detection and staging of prostate cancer. Dan Med J. 2017;64(2) [PubMed] [Google Scholar]
- 28.Almeida G.L., Petralia G., Ferro M., Ribas C.A., Detti S., Jereczek-Fossa B.A. Role of Multi-Parametric Magnetic Resonance Image and PIRADS Score in Patients with Prostate Cancer Eligible for Active Surveillance According PRIAS Criteria. Urol Int. 2016;96(4):459–469. doi: 10.1159/000444197. [DOI] [PubMed] [Google Scholar]
- 29.Muehlematter U.J., Burger I.A., Becker A.S., Schawkat K., Hotker A.M., Reiner C.S. Diagnostic Accuracy of Multiparametric MRI versus (68)Ga-PSMA-11 PET/MRI for Extracapsular Extension and Seminal Vesicle Invasion in Patients with Prostate Cancer. Radiology. 2019:190687. doi: 10.1148/radiol.2019190687. [DOI] [PubMed] [Google Scholar]
- 30.Van Nieuwenhove S., Saussez T.P., Thiry S., Trefois P., Annet L., Michoux N. Prospective comparison of a fast 1.5-T biparametric with the 3.0-T multiparametric ESUR magnetic resonance imaging protocol as a triage test for men at risk of prostate cancer. Bju Int. 2019;123(3):411–420. doi: 10.1111/bju.14538. [DOI] [PubMed] [Google Scholar]
- 31.Shah Z.K., Elias S.N., Abaza R., Zynger D.L., DeRenne L.A., Knopp M.V. Performance comparison of 1.5-T endorectal coil MRI with 3.0-T nonendorectal coil MRI in patients with prostate cancer. Acad Radiol. 2015;22(4):467–474. doi: 10.1016/j.acra.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasel-Seibert M., Lehmann T., Aschenbach R., Guettler F.V., Abubrig M., Grimm M.O. Assessment of PI-RADS v2 for the Detection of Prostate Cancer. Eur J Radiol. 2016;85(4):726–731. doi: 10.1016/j.ejrad.2016.01.011. [DOI] [PubMed] [Google Scholar]