Abstract
Background
Gentamicin is widely used as an antibiotic for the treatment of gram negative infections. Evidences indicates that oxidative stress is involved in gentamicin-induced nephrotoxicity. In Ayurvedic medicine, Punica granatum Linn. is considered as 'a pharmacy unto itself”. It has been claimed in traditional literature, to treat various kidney ailments due to its antioxidant potential.
Objective
To explore the possible mechanism of action of methanolic extract of P.granatum leaves (MPGL) in exerting a protective effect on gentamicin-induced nephropathy.
Material and methods
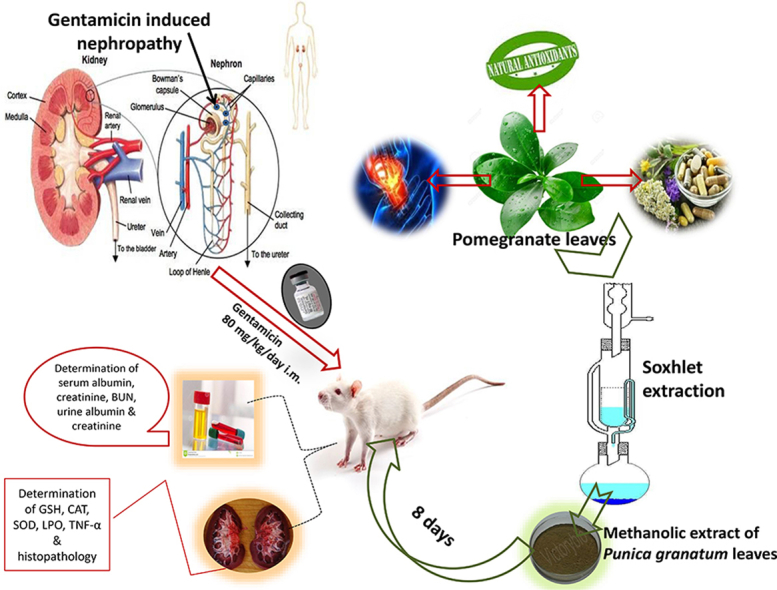
Animals were administered with gentamicin (80 mg/kg/day i.m.) and simultaneously with MPGL (100, 200 and 400 mg/kg p.o.) or metformin (100 mg/kg p.o.) for 8 days. A satellite group was employed in order to check for reversibility of nephrotoxic effects post discontinuation of gentamicin administration. At the end of the study, all the rats were sacrificed and serum–urine parameters were investigated. Antioxidant enzymes and tumor necrosis factor alpha (TNF-α) levels were determined in the kidney tissues along with histopathological examination of kidneys.
Results
Increase in serum creatinine, urea, TNF-α, lipid peroxidation along with fall in the antioxidant enzymes activity and degeneration of tubules, arterioles as revealed by histopathological examination confirmed the manifestation of nephrotoxicity caused due to gentamicin. Simultaneous administration of MPGL and gentamicin protected kidneys against nephrotoxic effects of gentamicin as evidenced from normalization of renal function parameters and amelioration of histopathological changes.
Conclusion
Data suggests that MPGL attenuated oxidative stress associated renal injury by preserving antioxidant enzymes, reducing lipid peroxidation and inhibiting inflammatory mediators such as TNF-α.
Keywords: Gentamicin, Nephropathy, Oxidative stress, Punica granatum
Graphical abstract
Highlights
-
•
MPGL preserved antioxidant enzymes and inhibited TNF-α.
-
•
MPGL attenuated oxidative stress associated renal injury.
-
•
MPGL demonstrated antioxidant and nephroprotective potential.
1. Introduction
Gentamicin is an important aminoglycoside which is used to treat gram negative bacterial infections [1]. However, it causes dose dependent nephrotoxicity which manifests at lowest therapeutic doses and accounts for 10–15% of all cases of acute renal failure [2], [3], [4]. Gentamicin's specificity for nephrotoxicity is due to its preferential accumulation in the renal proximal convoluted tubules [5], [6]. There has been a lack of clear understanding of the underlying mechanism of toxicity [7]. However, recent studies propose the role of oxidative stress along with depletion of antioxidant enzymes in the manifestation of nephrotoxicity [8], [9]. Functional alterations such as elevated serum creatinine along with morphological alterations like proximal tubular edema and tubular necrosis are observed in gentamicin-induced nephropathy [5], [10]. In addition to this, inflammatory mediators such as tumor necrosis factor alpha (TNF-α) are known to be involved in the pathological environment [11].
Punica granatum Linn. (Pomegranate), belonging to the family Punicaceae is native to the Himalayan region in North India to Iran [12]. In Ayurveda, it is considered as 'a pharmacy unto itself” [13]. It is the oldest tree known for its countless health benefits [14], [15]. Pomegranate has been claimed in traditional literature to treat various kidney ailments [16] due to its antioxidant potential which is attributed to the high polyphenolic content [17] and presence of ellagic acid [18].
Moreover, a recent study in our research lab showcased ameliorative effects of methanolic extract of P. granatum leaves (MPGL) in diabetes-induced nephropathy in rats by augmenting the antioxidant parameters [19]. However, the protective effect of MPGL against gentamicin-induced nephropathy has not been investigated so far.
In order to further explore the mechanism of action of MPGL, the present study aimed at examining the nephroprotective potential of MPGL on gentamicin-induced nephropathy in rats and its relationship with the oxidative stress.
2. Material and methods
2.1. Collection of plant material
Leaves of P. granatum were collected during the month of January, 2014 from Nashik, Maharashtra, India. The plant material was authenticated by Dr. Ganesh Iyer, Botany Department, Ruia College, Mumbai, Maharashtra. A voucher specimen (No. 2014/01) was deposited at the Department of Pharmacology, Institute of Chemical Technology, Mumbai, Maharashtra (India).
2.2. Extraction of plant material and high resolution-liquid chromatography mass spectroscopy (HR-LCMS) analysis of MPGL
Freshly collected leaves were cleaned with distilled water to remove adhering dust and shade dried. The dried samples were powdered in mixer grinder to a coarse powder. The powder was successively extracted with petroleum ether and methanol using Soxhlet apparatus. The methanolic extract was concentrated under reduced pressure using rotary evaporator (yield: 50.707%) and stored in an airtight container for subsequent analysis.
LC-MS technique was used to identify the phyto-components present in the extract. HR-LCMS analysis was carried out at Sophisticated Analytical Instrument Facility, IIT Bombay, India. The analysis was performed using Agilent Technologies 1290 Infinity UHPLC system and liquid chromatograph interfaced to a Mass Spectrometer (LC-MS) equipped with electro-spray ionization source and zorbax eclipse column (C18, 2.1 × 150 mm, 5-micron). An injection volume of 5.00 μl was employed. The sample was introduced by direct infusion at a flow rate of 0.200 ml/min and was run in both positive and negative ionization mode from 50 m/z to 1000 m/z. For LC-MS detection, a TOF/Q-TOF mass spectrometer with dual AJS ESI ion source was used. The analysis was performed using the following tuning parameters: gas temp (250 °C), gas flow (13 l/min) and nozzle voltage (1000 V). Total LC running time was 30 min. The structure, molecular weights and retention times of the compounds were deduced and compared to the METLIN database for the purpose of identification.
2.3. Animals
Male Wistar rats (weight 150–200 g) maintained on standard pellet diet and water ad libitum were obtained from Bombay Veterinary College and kept in registered central animal house with temperature of 22 ± 1 °C and relative humidity of 50–60% at TNMC and BYL Nair Ch. Hospital, Mumbai. Experimental protocol complied with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines and was approved by the Institutional Animal Ethics Committee (IAEC), TNMC and BYL Nair Ch. Hospital (IAEC project No. 2016/04 dated 29th February 2016).
2.4. Experimental protocol
The animals were randomly allocated into seven groups and treated for 8 days as follows (N = 6 for all groups): Group I (normal control rats) received water as a vehicle p.o.; Group II (negative control rats) received gentamicin, 80 mg/kg/day i.m.; Group III (positive control rats) received metformin, 100 mg/kg p.o. and gentamicin 80 mg/kg/day i.m. simultaneously; Group IV to VI (MPGL treated rats) received MPGL (100 mg/kg, 200 mg/kg and 400 mg/kg, respectively) p.o. and gentamicin 80 mg/kg/day i.m. simultaneously and Group VII (satellite control rats) received gentamicin, 80 mg/kg/day i.m. for 8 days and thereafter were further housed for 7 days in order to check for reversibility of nephrotoxic effects post discontinuation of gentamicin administration.
Body weight of each animal was determined at the initiation and end of the study. Post 8 days treatment, rats in all groups except satellite control group were placed into individual metabolic cages for 24 h urine collection. Blood was withdrawn via retro-orbital plexus. Blood samples were centrifuged at 12,000g at 4 °C for 15 min for separation of serum and stored at – 20 °C until assayed for biochemical parameters. After housing for 7 more days, rats in satellite control group were placed in individual metabolic cages for 24 h urine collection followed by blood withdrawal, centrifugation and storage of serum samples.
2.5. Preparation of kidney homogenate
At the end of the experimental period, animals were sacrificed under anesthesia. Immediately after sacrifice, both the kidneys were quickly removed, weighed and divided equally into two longitudinal sections. One half was placed in formaldehyde solution for histopathological analysis. The other half was used for preparation of 10% (w/v) homogenate in phosphate buffer (0.1 M, pH 7.4) using a homogenizer. The kidney homogenate was centrifuged at 10,000g for 15 min and the supernatant was estimated for kidney antioxidant parameters.
2.6. Renal function parameters
Serum was used for the estimation of albumin, creatinine and blood urea nitrogen (BUN). Pooled 24 h urine was evaluated for albumin and creatinine values. The estimation of the above mentioned parameters was carried out using biochemical kits (ACCUREX, Biomedical Pvt. Ltd.).
2.7. Kidney antioxidant parameters
5-5-dithiobis (2-nitrobenzoic acid) (DTNB) reagent was used to estimate reduced glutathione (GSH) level in tissue homogenates and the absorbance was read at 412 nm. The amount of GSH in the sample was calculated in microgram per ml from a standard curve obtained and represented in GSH per total tissue protein. Evaluation of kidney homogenate for lipid peroxidation levels, superoxide dismutase (SOD) and catalase (CAT) activities was carried out following the method published by Nishi et al., and Halliwell and Chirico [20], [21].
2.8. Measurement of TNF-α level
TNF- α concentrations were quantitatively determined in kidney tissue homogenates using BD OptEIA Rat TNF ELISA (enzyme-linked immunosorbent assay) kit (BD flow cytometers, BD Biosciences, Becton, Dickinson and Company). Kidney tissue homogenates were added to the 96-well plate pre-coated with a monoclonal antibody specific for rat TNF. Incubation, washing of wells, substrate additions and quenching of reactions were carried out as per the kit manufacturer's instruction manual. TNF- α levels were expressed in pg/mg wet tissue.
2.9. Histopathological examination
Kidney tissues were immediately preserved in 10% neutral buffered formalin, dehydrated through graded alcohol series, embedded in paraffin, cut into 5 μm sections and stained with hematoxylin and eosin (H and E) and periodic acid Schiff base (PAS) method. The slides were examined by light microscopy under 400× magnification for microscopic alterations of pathological significance.
3. Results
3.1. HR-LCMS analysis
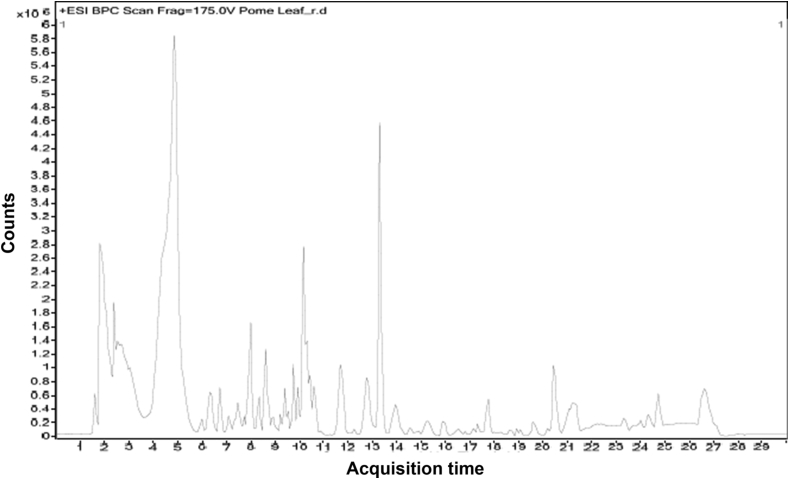
The results from HR-LCMS analysis showed the presence of following compounds like dulcitol, loganin, bergenin, quercitrin, cosmosiin, folic acid and khayanthone in MPGL (Fig. 1).
Fig. 1.
LC-ESI-MS-MS Chromatogram of MPGL by HR-LCMS. MPGL: methanolic extract of P. granatum leaves. HR-LCMS: high resolution liquid chromatography mass spectroscopy.
3.2. Body weight
Reduction in the body weight of negative control group was not significant as compared to the normal control group. Similarly, no significant change was observed in the body weight of positive control group, treatment groups and satellite control group as compared to the negative control group (Table 1).
Table 1.
Effect of MPGL on body weight, kidney weight and kidney hypertrophy in gentamicin-induced nephropathy (Mean ± SEM).
| Groups | Body weight (g) |
Kidney weight (g) | Kidney weight/Body weight (%) | |
|---|---|---|---|---|
| Initial (0 day) | 8th day | |||
| Normal control | 150.833 ± 8.7 | 190.5 ± 12.899 | 1.383 ± 0.075 | 0.365 ± 0.006 |
| Negative control | 148.333 ± 9.28 | 174.667 ± 9.646 | 1.867 ± 0.067### | 0.541 ± 0.032### |
| Positive control | 158.333 ± 4.014 | 171.833 ± 5.338 | 1.683 ± 0.079 | 0.492 ± 0.029 |
| 100 mg/kg MPGL | 158.333 ± 10.541 | 175.167 ± 10.842 | 1.8 ± 0.073 | 0.59 ± 0.041 |
| 200 mg/kg MPGL | 151.667 ± 3.073 | 166 ± 3.183 | 1.683 ± 0.111 | 0.494 ± 0.035 |
| 400 mg/kg MPGL | 151.667 ± 9.189 | 155 ± 9.132 | 1.65 ± 0.145 | 0.48 ± 0.009 |
| Satellite control | 160.833 ± 5.54 | 157.833 ± 11.72 | 2.067 ± 0.128 | 0.663 ± 0.037 |
###: Negative control group was compared with normal control, p < 0.001, using One-way ANOVA with Dunnett's test.
MPGL: Methanolic extract of P. granatum leaves.
SEM: Standard error of mean.
ANOVA: Analysis of variance.
3.3. Kidney weight and kidney hypertrophy index
Kidney weight and kidney hypertrophy index were found to be significantly increased in negative control group as compared to the normal control rats. Similarly, a marked increase in the kidney weight and kidney hypertrophy index was observed in the satellite control group. MPGL at 100 mg/kg did not show any reduction in the kidney weight and kidney hypertrophy index. However, these alterations were attenuated by MPGL at the dose of 200 and 400 mg/kg (Table 1).
3.4. Renal function parameters
A marked increase in serum creatinine, BUN and urine albumin was noted in the negative control and satellite control rats. Excretion of creatinine in urine was significantly reduced in negative control and satellite control rats. Serum albumin in negative control group and satellite group fell significantly. Positive control group and treatment groups normalized the altered serum and urine parameters. MPGL at all doses significantly prevented the fall in serum albumin levels. Serum creatinine and BUN levels in rats treated with MPGL 100 mg/kg were slightly decreased whereas serum albumin and urine creatinine values were slightly increased but not significant when compared to normal control group. A significant decrease in the excretion of albumin in urine was noted in positive control and treatment groups when compared to negative control rats. MPGL at the dose of 400 mg/kg significantly increased the concentration of albumin in serum and decreased the concentration of albumin and BUN in urine and serum respectively (Table 2).
Table 2.
Effect of MPGL on serum and urine parameters in gentamicin-induced nephropathy (Mean ± SEM).
| Groups | Serum albumin (gm%) | Urine albumin (gm/ml/24 h) | BUN (mg/dl) | Serum creatinine (mg/dl) | Urine creatinine (mg/ml/24 h) |
|---|---|---|---|---|---|
| Normal control | 5.574 ± 1.156 | 0.036 ± 0.002 | 13.173 ± 0.959 | 0.915 ± 0.156 | 20.596 ± 2.686 |
| Negative control | 1.548 ± 0.246### | 1.274 ± 0.037### | 53.173 ± 5.488### | 2.507 ± 0.182### | 5.373 ± 0.597### |
| Positive control | 2.137 ± 0.685 | 0.131 ± 0.037*** | 40.961 ± 5.748 | 1.910 ± 0.308 | 7.347 ± 0.004 |
| 100 mg/kg MPGL | 1.794 ± 0.209 | 0.254 ± 0.165*** | 55.865 ± 4.331 | 1.990 ± 0.201 | 5.910 ± 0.537 |
| 200 mg/kg MPGL | 1.802 ± 0.103 | 0.130 ± 0.001*** | 41.154 ± 6.626 | 1.950 ± 0.179 | 6.716 ± 0.268 |
| 400 mg/kg MPGL | 4.714 ± 0.772** | 0.083 ± 0.002*** | 24.038 ± 4.241** | 1.751 ± 0.219 | 8.283 ± 0.552 |
| Satellite control | 1.490 ± 0.391 | 1.267 ± 0.375 | 47.981 ± 6.664 | 2.587 ± 0.226 | 7.435 ± 0.152 |
**: Treatment group was compared with negative control group, p < 0.01.
***: Treatment group was compared with negative control group, p < 0.001.
###: Negative control group was compared with normal control, p < 0.001, using One-way ANOVA with Dunnett's test.
MPGL: Methanolic extract of Punica granatum leaves.
BUN: Blood Urea Nitrogen.
SEM: Standard error of mean.
ANOVA: Analysis of variance.
3.5. Kidney antioxidant parameters
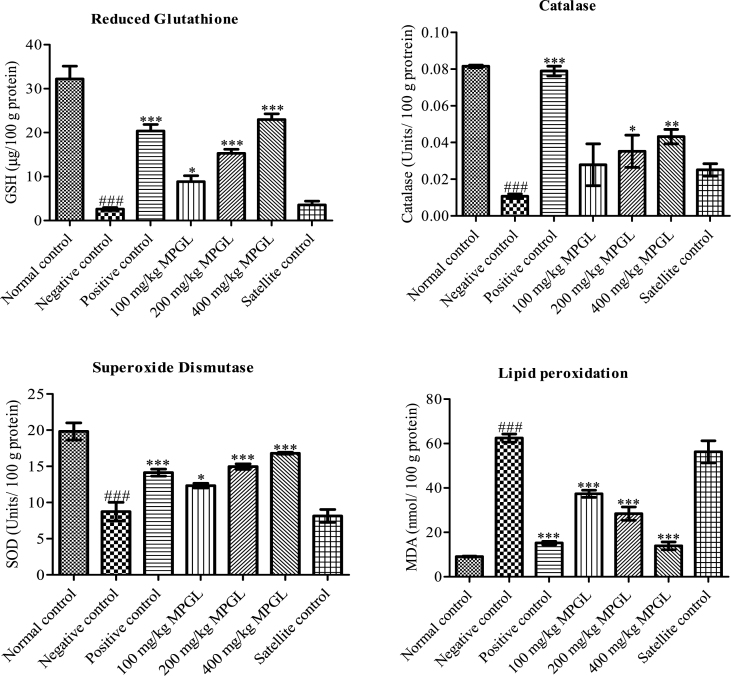
Administration of gentamicin caused a significant decrement in the GSH levels, catalase and SOD activities of negative and satellite control rats. This reduction was significantly attenuated by MPGL in a dose dependent manner. Lipid peroxidation levels were augmented in negative control and satellite control group; however, MPGL in a dose dependent manner significantly decreased lipid peroxidation levels in rats (Fig. 2).
Fig. 2.
Effect of MPGL on antioxidant parameters in gentamicin-induced nephropathy. (A) GSH in kidney homogenate of normal control, negative control, positive control, satellite control and MPGL treated rats. (B) Catalase in kidney homogenate of normal control, negative control, positive control, satellite control and MPGL treated rats. (C) SOD in kidney homogenate of normal control, negative control, positive control, satellite control and MPGL treated rats. (D) Lipid peroxidation in kidney homogenate of normal control, negative control, positive control, satellite control and MPGL treated rats. ∗: Treatment group was compared with negative control group, p < 0.05; ∗∗: Treatment group was compared with negative control group, p < 0.01; ∗∗∗: Treatment group was compared with negative control group, p < 0.001; ###: Negative control group was compared with normal control, p < 0.001, using one-way ANOVA with Dunnett's test. MPGL: methanolic extract of P. granatum leaves. GSH: Reduced glutathione. SOD: Superoxide dismutase. ANOVA: Analysis of variance.
3.6. TNF-α level in kidney tissue homogenate
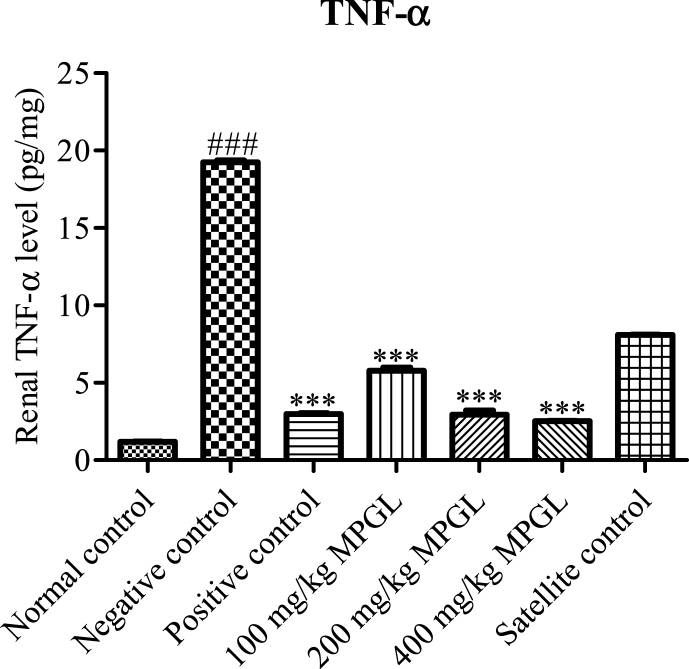
The negative control rats showcased a significant increase in the renal TNF-α level as compared to the normal control rats. MPGL in a dose dependent manner decreased the renal TNF-α level in gentamicin induced nephrotoxic rats. A significant decrease in the renal TNF-α level was observed in the positive control group whereas increased levels of TNF- α were found in the satellite group as compared to the normal control rats (Fig. 3).
Fig. 3.
Effect of MPGL on renal TNF-α level in gentamicin induced nephropathy. ∗∗∗: Treatment group was compared with negative control group, p < 0.001; ###: Negative control group was compared with normal control, p < 0.001, using One-way ANOVA with Dunnett's test. TNF-α: Tumor necrosis factor – alpha. ANOVA: Analysis of variance.
3.7. Histopathological examination
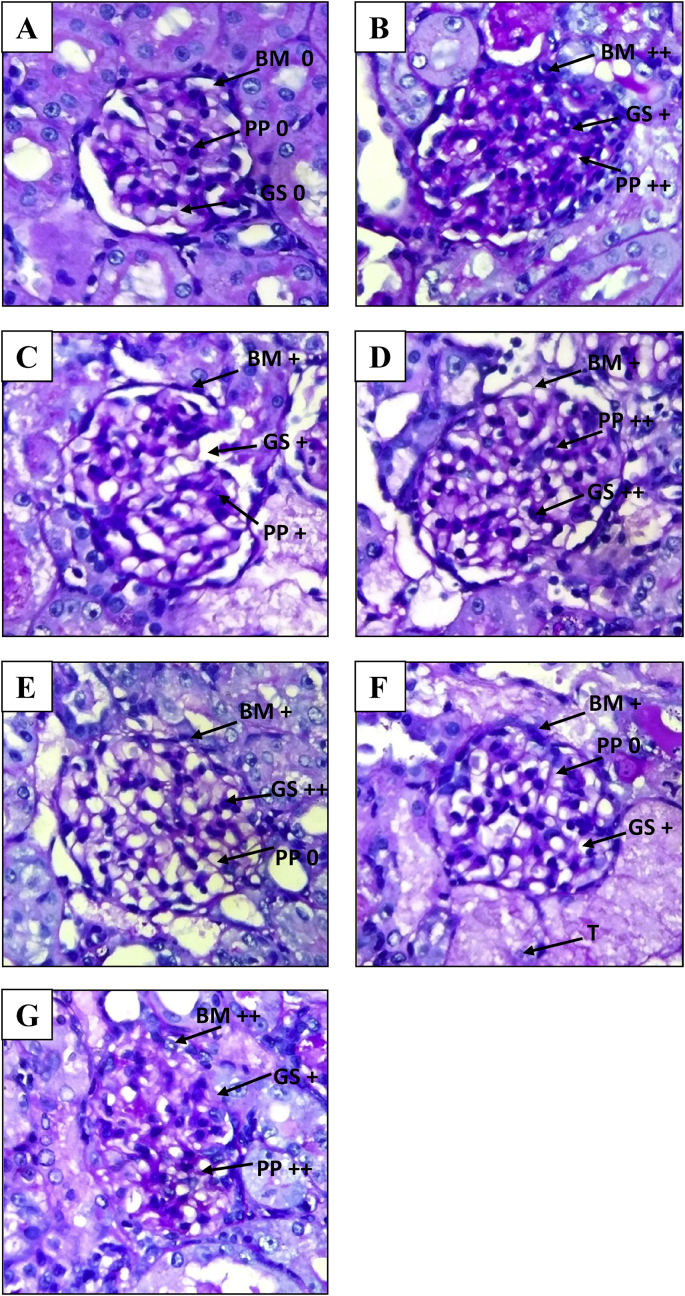
Stained renal tissue samples are shown in Fig. 4 (H and E staining) and Fig. 5 (PAS staining) respectively.
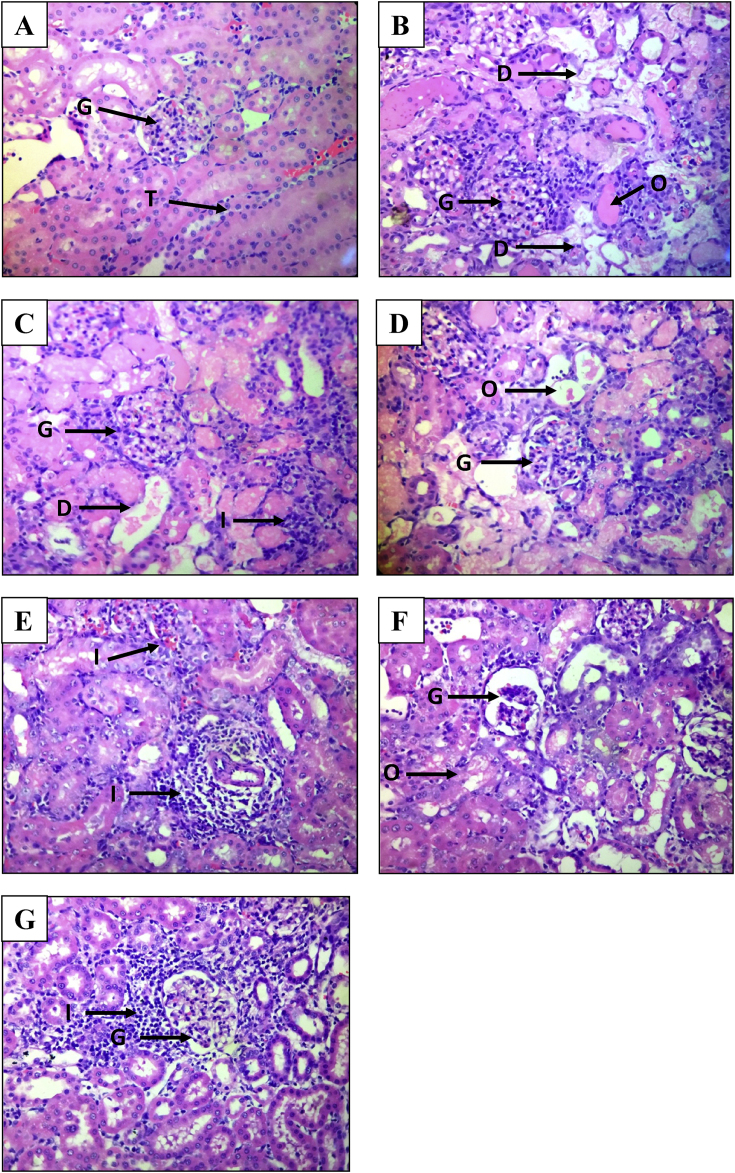
Fig. 4.
Photomicrographs of kidney (hematoxylin and eosin staining under a light microscope at 400× magnification) A: Normal control group; B: Negative control group; C: Positive control group; D: 100 mg/kg MPGL treated group; E: 200 mg/kg MPGL treated group; F: 400 mg/kg MPGL treated group; G: Satellite control group. G: Glomeruli, I: Infiltration of inflammatory MNCs, D: Tubular degeneration, O: Edema and proteinaceous casts, T: Tubules. MPGL: Methanolic extract of P. granatum leaves. MNCs: Mononuclear cells.
Fig. 5.
Photomicrographs of kidney (Periodic Acid Schiff staining under light microscope at 400× magnification). A: Normal control group; B: Negative control group; C: Positive control group; D: 100 mg/kg MPGL treated group; E: 200 mg/kg MPGL treated group; F: 400 mg/kg MPGL treated group; G: Satellite control group. PP: PAS positivity, BM: Basement membrane thickness, G: Glomeruli, +: mild change, ++: moderate change. MPGL: Methanolic extract of P. granatum leaves. PAS: Periodic Acid Schiff.
The H and E and PAS stained kidney specimens from normal control rats showed no structural alterations in renal tissues. In the H and E stained kidney specimens, negative control group renal tissue samples showed severe vacuolar degeneration, hyalinization and destruction of arterioles, edema, tubular collapse, scarring and matrix rich expansion of interstitium and distorsion of tubules as compared to normal control group tissue samples. Severe inflammatory infiltrate in the form of mononuclear cells (MNCs) were observed in the renal sections of this group. Both positive control group and MPGL treatment groups decreased the severity of vacuolar degeneration, edema and MNC infiltration and improved renal morphology in gentamicin administered rats. Kidney specimens from rats treated with gentamicin and MPGL at 400 mg/kg revealed significant improvement in renal tubules and reversed most of the histopathological alterations induced by gentamicin as evidenced by preservation of tubular histology as compared to the negative control group.
Kidneys stained with PAS stain demonstrated severe PAS positivity staining intensity in glomeruli, increased basement membrane thickening and increased glomerular size in the negative control group. MPGL at 100 mg/kg dose reduced the PAS positivity but could not control the increase in the glomerular size and basement membrane thickening. Positive control rats and MPGL treated rats at the dose of 200 mg/kg and 400 mg/kg significantly reduced the increase in basement membrane thickening and glomerular size and prevented PAS positivity staining intensity in glomeruli.
Satellite control group continued to demonstrate vacuolar degeneration, MNC infiltration and edema in H and E stained kidney specimens as well as increased PAS positivity, basement membrane thickening and glomerular size in PAS stained kidney specimens, thus showcasing no significant reversal of gentamicin- induced nephrotoxicity in rats discontinued with gentamicin treatment post 8 days.
4. Discussion
Gentamicin nephrotoxicity is one of the most common causes of acute renal failure. Several studies have documented the role of oxidative stress in renal damage [22]. Previous studies strongly suggest the mediation of reactive oxygen species (ROS) in the renal effects of gentamicin. Moreover, ROS are also involved in proximal tubular necrosis and acute renal failure caused by gentamicin [23].
ROS, mainly superoxide anions and hydroxyl radicals, cause cellular damage and death through diverse mechanisms including inhibition of electron transport chain, suppression of cellular respiration and ATP production; DNA damage; lipid peroxidation and destabilization of the cellular membrane. Accumulation of gentamicin in the renal cortex induces renal morphological changes wherein the overall syndrome is very similar in humans and experimental animals. Hence, gentamicin-induced nephropathy model was chosen to study the pathophysiological and molecular mechanisms underlying gentamicin's nephrotoxicity [23].
For past many years, research has been carried out on plant-derived natural compounds as potential therapeutic agents for various human ailments [24]. P. granatum leaves have been recognized since ancient times for their anti-diabetic, anti-hyperlipidemic [25] and antioxidant activities [18]. The aim of the present study was to elucidate whether MPGL promotes a nephroprotective effect in gentamicin-induced nephropathy model via attenuation of oxidative stress and inflammation.
Several studies have demonstrated pleiotropic effects of metformin wherein it has been found to improve mitochondrial homeostasis; diminish apoptosis induced by oxidative stress and prevent cell death; boost the antioxidant system and significantly decrease the depletion of respiratory components [26]. Due to its nephroprotective potential as showcased by earlier studies, metformin was used as a positive control in this study [2], [27].
Our study revealed that gentamicin had no effect on the body weight of the rats. These findings are consistent with the results of Zeeni et al. [28]. Kidneys are extremely susceptible to deterioration due to ROS and hence, increased kidney weight was evident in the negative control rats which might be due to the inflammation, edema and oxidative stress after gentamicin treatment [11]. Decrease in kidney weight indices was observed in treatment groups as compared to negative control rats.
Gentamicin induced renal function deterioration is reflected as escalation in serum levels of creatinine and urea beyond normal limits as well as loss of albumin in urine and decline in glomerular filtration rate [26]. In our study, the negative control rats as well as the satellite control group showed abnormal BUN, serum and urine creatinine and albumin values. MPGL treatment groups showed variable levels of nephroprotection. Rats receiving MPGL at the dose of 100 mg/kg along with gentamicin did not show complete nephroprotection as indicated by the serum and urine levels of urea, creatinine and albumin respectively. However, rats receiving metformin and MPGL at 200 and 400 mg/kg doses demonstrated attenuation of the nephrotoxic insult as evidenced by a comparative decline in the serum and urine values. Moreover, the protective effect of MPGL on creatinine clearance could be attributed to its antioxidant potential as ROS has been found to be involved in the impairment of glomerular filtration rate [29].
GSH is a scavenger of hydroxyl radicals and singlet oxygen. Gentamicin-induced nephropathy is associated with low activity of antioxidant enzymes such as catalase, SOD in the renal cortex [30]. Furthermore, this decreased renal antioxidant enzymatic defense can aggravate oxidative damage in rats [29]. Also, increased malondialdehyde (MDA) levels suggest enhanced oxidative stress in gentamicin induced nephropathy [1]. MDA levels increased significantly whereas the levels of antioxidant enzymes, GSH, CAT and SOD were found to decrease in the kidney of rats exposed to gentamicin compared to normal control group. The protective effect of MPGL in this experimental model was associated to an increased activity of the antioxidant enzymes GSH, CAT and SOD and a decrease in oxidative stress. MPGL was found to attenuate MDA which is a lipid peroxidation marker. Furthermore, MPGL diminished the expression of TNF-α, an inflammatory cytokine which is closely involved not only in inflammation but also in tissue remodeling which eventually leads to renal fibrosis [24].
Gentamicin administration leads to renal morphological alterations such as tubular atrophy, necrosis, vascularization and peritubular blood vessel congestion [31]. In our study, the histological alterations produced by gentamicin were ameliorated by MPGL.
Accumulating evidences have shown the reversibility of the alterations caused by gentamicin on its withdrawal [30]. Thus, in order to study whether withdrawal of gentamicin leads to reversibility of the nephrotoxic effects, a satellite group was incorporated in the experimental design. However, in our study no significant reversal of nephrotoxicity was observed in the satellite group which was evident from the renal function parameters and histological analysis.
Due to their antioxidant properties, flavonoid compounds have been explored for their protective role against oxidative stress. HR-LCMS analysis of MPGL showcased the presence of cosmosiin which has been previously revealed to have antioxidant activity [32]. Quercitrin, a glycoside form of quercetin has been reported to exhibit a scavenger and antioxidant role and prevent lipid peroxidation in vitro [33]. Quercetin has also been examined for its nephroprotective activities [34] which might have resulted in impeding the oxidative stress mediated renal damage in the gentamicin-induced nephropathy model. Further, study carried out by Khan et al. [35] has demonstrated bergenin to be an effective antioxidant whereas research carried out by Liu et al. [36] has established the nephroprotective potential of loganin as evidenced by its ability to attenuate diabetic nephropathy. Thus, the nephroprotective effect of MPGL in gentamicin-induced nephropathy could be associated to the presence of the aforementioned phytoconstituents in the extract.
5. Conclusion
We can conclude that the protective effect of MPGL was associated with its ability to ameliorate renal function parameters; prevent the increase in lipid peroxidation and the fall in the antioxidant enzymes activity observed in the kidney of rats with gentamicin-induced nephropathy. Taking together these studies attribute the protective effect of MPGL in gentamicin induced-nephropathy to the attenuation of inflammatory response, preservation of antioxidant enzymes and prevention of oxidative stress. Hence, the present research work identifies MPGL as a promising renoprotective agent against renal injury.
Sources of funding
INSPIRE (Innovation in Science Pursuit for Inspired Research) Program, Department of Science and Technology (DST), New Delhi (No. DST/INSPIRE Fellowship/2013/1152).
Conflict of interest
None.
Acknowledgements
The authors would like to acknowledge Dr. Sangale for providing the guidance for planning the study; Dr. Ganesh Iyer for carrying out authentication of the plant; Dr. Sanjay Pawar for assisting in histopathological examination and Mr. Sanjay Devare who arranged and provided plant material for this research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jaim.2017.09.006.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Boroushaki M.T., Asadpour E., Sadeghnia H.R., Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J Food Sci Tech. 2014;51(11):3510–3514. doi: 10.1007/s13197-012-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morales A.I., Detaille D., Prieto M., Puente A., Briones E., Arevalo M. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77(10):861–869. doi: 10.1038/ki.2010.11. [DOI] [PubMed] [Google Scholar]
- 3.Vijay Kumar K., Naidu M.U.R., Shifow A.A., Ratnakar K.S. Probucol protects against gentamicin-induced nephrotoxicity in rats. Ind J Pharmacol. 2000;32:108–113. [Google Scholar]
- 4.Ramhariya R., Ganeshpurkar A., Ayachi C., Kanojia P., Bansal D., Dubey N. Ameliorative effect of rutin on gentamicin-induced nephrotoxicity in murine model. Austin J Pharmacol Ther. 2015;3(1):1066–1070. [Google Scholar]
- 5.Rodrigues F.A., Prata M.M., Oliveira I.C., Alves N.T., Freitas R.E., Monteiro H.S. Gingerol fraction from Zingiber officinale protects against gentamicin-induced nephrotoxicity. Antimicrob Agents Chemother. 2014;58(4):1872–1878. doi: 10.1128/AAC.02431-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J.W., Bae E.H., Kim I.J., Ma S.K., Choi C., Lee J. Renoprotective effects of paricalcitol on gentamicin-induced kidney injury in rats. Am J Physiol Ren Physiol. 2010;298(2):F301–F313. doi: 10.1152/ajprenal.00471.2009. [DOI] [PubMed] [Google Scholar]
- 7.Bello S.O., Chika A. Dose-dependent amelioration of gentamicin-induced nephrotoxicity in adult swiss albino rats by vitamin B-complex – a preliminary study. Trop J Pharm Res. 2009;8(2):111–116. [Google Scholar]
- 8.Ozbek E. Induction of oxidative stress in kidney. Int J Nephrol. 2012;2012:1–9. doi: 10.1155/2012/465897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balakumar P., Chakkarwar V.A., Kumar V., Jain A., Reddy J., Singh M. Experimental models for nephropathy. J Renin Angiotensin Aldosterone Syst. 2008;9(4):189–195. doi: 10.1177/1470320308098343. [DOI] [PubMed] [Google Scholar]
- 10.Moreira M.A., Nascimento M.A., Bozzo T.A., Cintra A., da Silva S.M., Dalboni M.A. Ascorbic acid reduces gentamicin-induced nephrotoxicity in rats through the control of reactive oxygen species. Clin Nutr. 2014;33(2):296–301. doi: 10.1016/j.clnu.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 11.El-Kashef D.H., El-Kenawi A.E., Rahim M.A., Suddek G.M., Salem H.A. Agmatine improves renal function in gentamicin-induced nephrotoxicity in rats. Can J Physiol Pharmacol. 2016;94(3):278–286. doi: 10.1139/cjpp-2015-0321. [DOI] [PubMed] [Google Scholar]
- 12.Kalshetti P., Alluri R., Thakurdesai P. A review on phytochemistry and pharmacological profile of Punica granatum. J Curr Pharma Res. 2015;5(4):1607–1614. [Google Scholar]
- 13.Garachh D., Patel A., Chakraborty M., Kamath J.V. Phytochemical and pharmacological profile of Punica granatum: an overview. Int Res J Pharm. 2012;3(2):65–68. [Google Scholar]
- 14.Sreekumar S., Sithul H., Muraleedharan P., Azeez J.M., Sreeharshan S. Pomegranate fruit as a rich source of biologically active compounds. Biomed Res Int. 2014;2014:1–12. doi: 10.1155/2014/686921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma J., Maity A. Pomegranate phytochemicals: nutraceutical and therapeutic values. Fruit Veg Cereal Sci Biotech. 2010;4(2):56–76. [Google Scholar]
- 16.Bhowmik D., Gopinath H., Kumar B.P., Duraivel S., Aravind G., Samnath Kumar K.P. Medicinal uses of Punica granatum and its health benefits. J Pharmacogn Phytochem. 2013;1(5):28–35. [Google Scholar]
- 17.Chaturvedula V.S.P., Indra P. Bioactive chemical constituents from Pomegranate (Punica granatum) juice, seed and peel-a review. Int J Res Chem Environ. 2011;1(1):1–18. [Google Scholar]
- 18.Amjad L., Shafighi M. Antioxidant activity of leaf different extracts in Punica granatum. Int J Biol Med Res. 2012;3(3):2065–2067. [Google Scholar]
- 19.Mestry S.N., Dhodi J.B., Kumbhar S.B., Juvekar A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med. 2017;7(3):273–280. doi: 10.1016/j.jtcme.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishi, Ahad A., Kumar P. Protective effect of chlorogenic acid against diabetic nephropathy in high fat diet/streptozotocin induced type-2 diabetic rats. Int J Pharm Pharm Sci. 2013;5:489–495. [Google Scholar]
- 21.Halliwell B., Chirico S. Lipid peroxidation: its mechanism, measurement and significance. Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 22.Singh A.P., Junemann A., Muthuraman A., Jaggi A.S., Singh N., Grover K. Animal models of acute renal failure. Pharmacol Rep. 2012;64(1):31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Salgado C., Eleno N., Morales A.I., Perez-Barriocanal F., Arevalo M., Lopez-Novoa J.M. Gentamicin treatment induces simultaneous mesangial proliferation and apoptosis in rats. Kidney Int. 2004;65(6):2161–2171. doi: 10.1111/j.1523-1755.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 24.Trujillo J., Chirino Y.I., Molina-Jijon E., Anderica-Romero A.C., Tapia E., Pedraza-Chaverri J. Renoprotective effect of the antioxidant curcumin: recent findings. Redox Biol. 2013;1(1):448–456. doi: 10.1016/j.redox.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das S., Barman S. Antidiabetic and antihyperlipidemic effects of ethanolic extract of leaves of Punica granatum in alloxan-induced non-insulin-dependent diabetes mellitus albino rats. Indian J Pharmacol. 2012;44(2):219–224. doi: 10.4103/0253-7613.93853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janjua A., Waheed A., Bakhtiar S. Protective effect of metformin against gentamicin induced nephrotoxicity in rabbits. Pak J Pharm Sci. 2014;27(6):1863–1872. [PubMed] [Google Scholar]
- 27.Chou C., Li Y., Chen S., Shih Y., Lee J. Chitosan prevents gentamicin-induced nephrotoxicity via a carbonyl stress-dependent pathway. Biomed Res Int. 2015;2015:1–8. doi: 10.1155/2015/675714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeeni N., Selmaoui B., Beauchamp D., Labrecque G., Thibault L. Dietary protein level alters gentamicin-induced nephrotoxicity in rats. Physiol Behav. 2007;90(5):760–770. doi: 10.1016/j.physbeh.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Raheem I.T., Abdel-Ghany A.A., Mohamed G.A. Protective effect of quercetin against gentamicin-induced nephrotoxicity in rats. Biol Pharm Bull. 2009;32(1):61–67. doi: 10.1248/bpb.32.61. [DOI] [PubMed] [Google Scholar]
- 30.Al-Yahya M.A., Mothana R.A., Al-Said M.S., Al-Dosari M., Al-Sohaibani M., Parvez M.K. Protective effect of Citrus medica ‘OTROJ’ extract on gentamicin induced nephrotoxicity and oxidative damage in rat kidney. Dig J Nanomater Biostructures. 2015;10:19–29. [Google Scholar]
- 31.Kumar A., Suchetha Kumari N., D'souza P., Bhargavan D. Evaluation of renal protective activity of Adhatoda zeylanica (Medic) leaves extract in wistar rats. Nitte Univ J Health Sci. 2013;3(4):45–56. [Google Scholar]
- 32.Rao Y.K., Lee M., Chen K., Lee Y., Wu W., Tzeng Y. Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) Osbeck leaves: enhanced adiponectin secretion and insulin receptor phosphorylation in 3T3-L1 cells. Evidenced-Based Complementary Altern Med. 2011;2011:1–9. doi: 10.1093/ecam/nep204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner C., Fachinetto R., Corte C.L., Brito V.B., Severo D., Dias G.O.C. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006;1107(1):192–198. doi: 10.1016/j.brainres.2006.05.084. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhary S., Ganjoo P., Raiusddin S., Parvez S. Nephroprotective activities of quercetin with potential relevance to oxidative stress induced by valproic acid. Protoplasma. 2015;252(1):209–217. doi: 10.1007/s00709-014-0670-8. [DOI] [PubMed] [Google Scholar]
- 35.Khan H., Amin H., Ullah A., Saba S., Rafique J., Khan K. Antioxidant and antiplasmodial activities of bergenin and 11-O-galloylbergenin isolated from Mallotus philippensis. Oxidative Med Cell Longev. 2016;2016:1–6. doi: 10.1155/2016/1051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K., Xu H., Lv G., Liu B., Lee M.K., Lu C. Loganin attenuates diabetic nephropathy in C57BL/6J mice with diabetes induced by streptozotocin and fed with diets containing high level of advanced glycation end products. Life Sci. 2015;123:78–85. doi: 10.1016/j.lfs.2014.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.