Abstract
Background and Aims
GI angiodysplasia is the most common cause of small-bowel bleeding. Argon plasma coagulation (APC) is preferred for ablation because of its availability, ease of use, and perceived safety, but it has limitations. An instrument capable of repeated use through the enteroscope, which covers more area of intestinal mucosa per treatment with low risk of damage to healthy mucosa, and which improves ablation, is desirable. A series of patients treated with a through-the-scope radiofrequency ablation (RFA) catheter is reported.
Methods
Patients with a previous diagnosis of small-bowel angiodysplasia (SBA) and ongoing bleeding with melena, hematochezia, or iron-deficiency anemia were eligible for treatment. A small-bowel radiofrequency ablation (SBRFA) catheter was passed through the enteroscope instrument channel. The treatment paddle was pushed against the SBA, achieving coaptive coagulation, and the SBA was treated up to 2 times at standard settings of 10 J/cm2. The patients’ demographics, pretreatment and posttreatment hemoglobin levels, time to recurrence of bleeding, and need for more therapy were recorded. This study was approved by the institutional review boards of the respective institutions.
Results
Twenty consecutive patients were treated from March until October 2018 and followed up until March 2019. There were 6 women (average age 68 years, standard deviation ± 11.1), and 14 men (average age 73 years, standard deviation ± 10.4). All had undergone at least 1 previous EGD and colonoscopy; 14 patients (70%) had SBA on video capsule endoscopy, and 14 patients had undergone previous endoscopic treatment of SBA with APC. A median of 23 treatments were applied (range, 2-99). The median follow-up time was 195 days (range, 30-240 days). Four patients, including 3 with a left ventricular assist device (LVAD), had recurrent bleeding between 45 and 210 days after treatment, and 2 patients received repeated blood transfusions. Three of those patients underwent repeated endoscopies, including a push enteroscopy and an upper endoscopy with no treatment, and a repeated enteroscopy with SBA treated with APC, respectively. One patient with LVAD underwent arterial embolization.
Conclusions
In this case series, bleeding recurred in 20% of patients in a follow-up time of ≤240 days. Notably, 3 of the 4 patients who had recurrent bleeding had an LVAD. These rates compare favorably with reported bleeding recurrence after APC of SBA. More studies on the benefits of SBRFA, which may include reduced risk of recurrent bleeding or prolonging the time to recurrent bleeding, resource utilization, and factors associated with bleeding recurrence are needed.
Abbreviations: APC, argon plasma coagulation; GIAD, GI angiodysplasia; LVAD, left ventricular assist device; RFA, radiofrequency ablation; SBA, small-bowel angiodysplasia; SBRFA, small-bowel radiofrequency ablation
GI angiodysplasia (GIAD), also called angioectasia, arteriovenous malformation, and vascular ectasia, consists of mucosal or submucosal dilated blood vessels lined by epithelium with no overlying mucosal lesion, which result from a combination of submucosal vein obstruction, hypoxemia, and neovascularization.1, 2, 3, 4 GIAD is the most common cause of mid small-bowel bleeding and a common cause of obscure GI bleeding.1,5, 6, 7, 8 It is also a cause of persistent iron deficiency anemia in patients with valvular heart disease (especially aortic stenosis), end-stage renal disease, and von Willebrand disease.1,9 GIADs are multifocal, and although previous data indicate a predominance of disease burden in the right side of the colon, improvements in evaluation of the small bowel suggest that this location is also a major cause of chronic anemia.3,5,6,9, 10, 11 Management includes endoscopic therapy, surgery, therapeutic angiography, and pharmacologic treatment. Endoscopic therapy including thermal methods (multipolar electrocoagulation, argon plasma coagulation [APC], laser), injections (sclerosants, saline solution, epinephrine), and mechanical methods (hemostatic clips, band ligators) are widely used to treat all causes of GI bleeding. Of those, APC has emerged as the preferred mode of endoscopic therapy for GIAD because of its availability, its relative ease of use, and a perceived superficial depth of burn; however, studies suggest that deeper intestinal layers can also be secondarily ablated.1,8,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25
About half of the patients with GIAD will experience spontaneous stoppage of bleeding, but 25% to 50% will have recurrent GI bleeding manifested by overt bleeding (melena or hematochezia), persistent fecal occult blood, persistent iron deficiency anemia, and transfusion dependency despite endoscopic therapy.5,9,10 This is probably a reflection of the underlying disease process with which GIADs are associated; the multifocal and sometimes evanescent nature of GIADs, which can thus be missed during endoscopy; and limitations of endoscopic therapy.12,13,26,27
Although APC is the preferred endoscopic mode of treatment for small-bowel GIAD, there are limitations. Catheter flexibility can make it difficult to pass through the enteroscope, especially when it is angulated in the small bowel, and the sheath may bend during reinsertions. For adequate ablation, the APC beam must be directed toward the lesion, which can be a challenge when the GIADs are at a distance from the enteroscope working channel, resulting in incomplete ablation. Sometimes the catheter may need to be removed to clean accumulated debris on its tip. There is a potential for significant intestinal insufflation, given that argon gas flow is set at a rate of 0.5 to 2 L/min, which may be a concern because enteroscopies can last over 1 hour.12,26,27
Radiofrequency ablation (RFA) of Barrett's esophagus is the endoscopic criterion standard for this disease.28,29 The same catheter has been used successfully to treat transfusion-dependent gastric antral vascular ectasia, a condition with similar pathophysiologic characteristics as those of GIAD.30,31 A through-the-scope RFA catheter, which can be used in routine colonoscopies and enteroscopes with U.S. Food and Drug Administration clearance for treatment of GI bleeding has recently become available. In this series, patients with small-bowel angiodysplasia (SBA) treated by use of this small-bowel RFA (SBRFA) catheter are reported.
Methods
In this open-label study, sequential patients with a previous diagnosis of small-bowel bleeding caused by SBA were treated by use of a SBRFA catheter. After informed consent data were captured, clinical and laboratory data were extracted. Patients underwent enteroscopy by use of a single-balloon overtube system (SIF-Q180, ST-SB1, respectively; Olympus America, Center Valley, Pa, USA) or a double-balloon overtube system (EN-450BI5, and TS-13140, respectively; Fujifilm Medical Systems, Wayne, NJ, USA). The RFA through-the-scope catheter (SBRFA; Medtronic, Minneapolis, Minn, USA) was used to treat SBA.
Endoscopic procedure
The endoscopic technique, including pretreatment and posttreatment findings, is described in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12, Figure 13, as well as in the case presentations in Video 1 (available online at www.VideoGIE.org). All patients underwent antegrade (n = 19) or retrograde (n = 1) enteroscopies.7 The RFA catheter was passed through the working channel. The manufacturer’s preselected radiofrequency energy settings of 10 J/cm2 were used to ablate the SBA with the use of up to 2 sequential pulse applications on each site.30,31 The paddle of the catheter was positioned on top of the intestinal mucosa, and pressure was applied to achieve coaptive coagulation. Examples of endoscopic therapy are demonstrated in Video 1. After recovery from anesthesia, patients were instructed to advance their diet from soft on discharge.
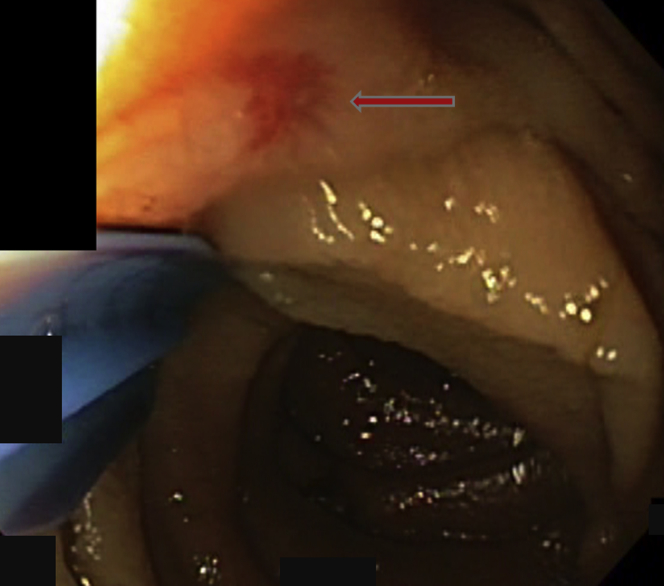
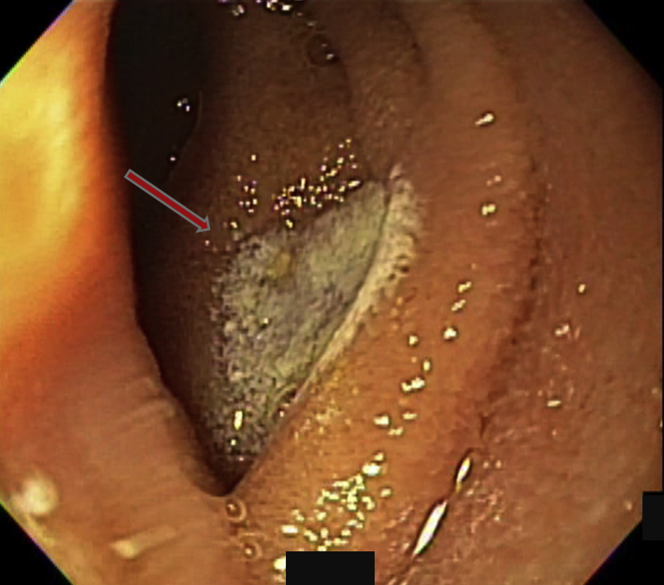
Figure 1.
Mucosal vascular arborizing lesion consistent with an angiodysplasia was seen in the small bowel (arrow). The radiofrequency ablation catheter was used to push the intestinal fold away and expose the lesion.
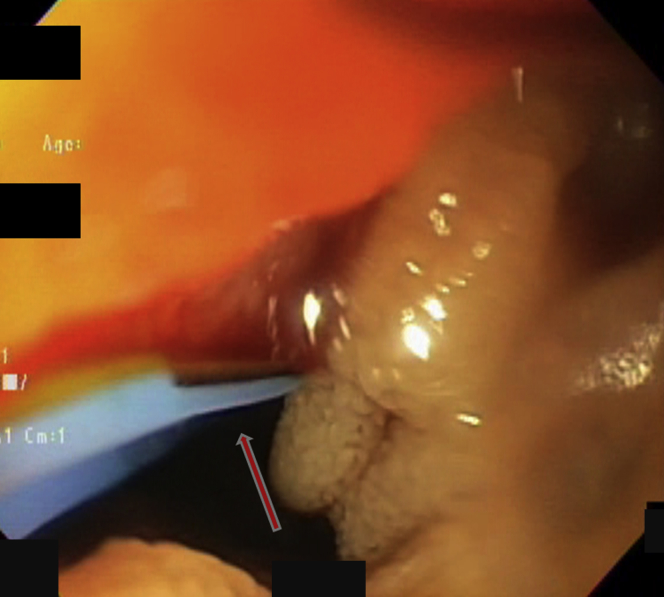
Figure 2.
During treatment the angiodysplasia bled. The rigidity of the instrument allowed us to push the paddle on the lesion in the direction indicated by the arrow and apply radiofrequency therapy.
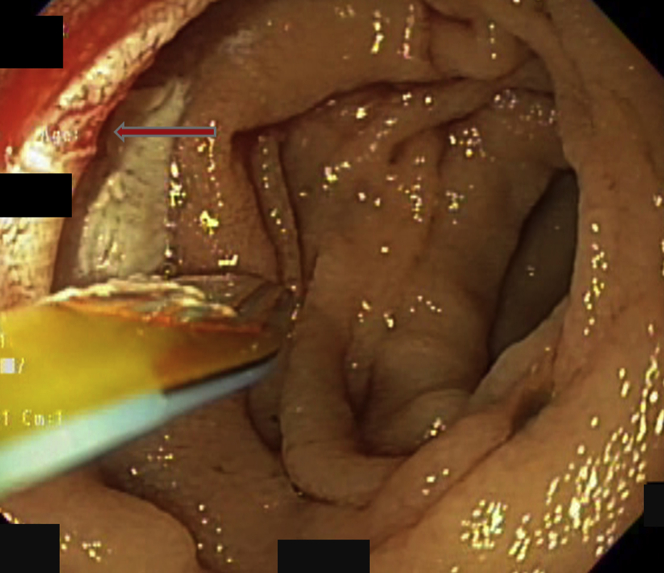
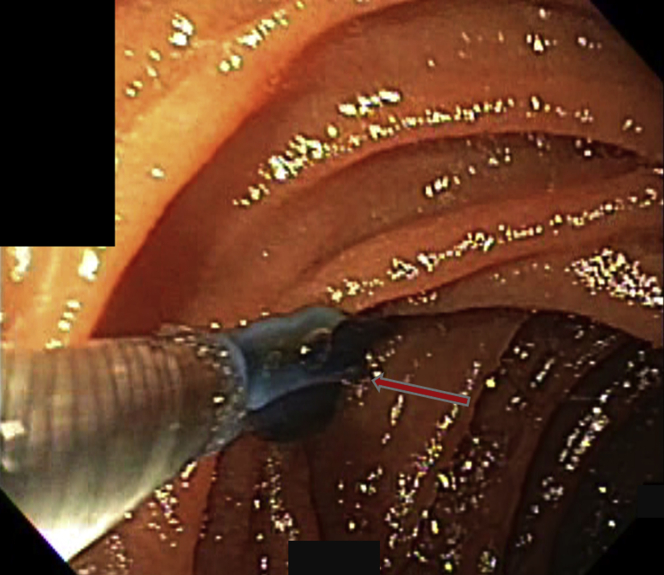
Figure 3.
The lesion was treated twice, producing the discolored rectangular treatment footprint. After treatment with the radiofrequency ablation catheter, the lesion appeared cherry-red, with a hint of the small cluster of vessels that formed the lesion (arrow). Bleeding stopped.
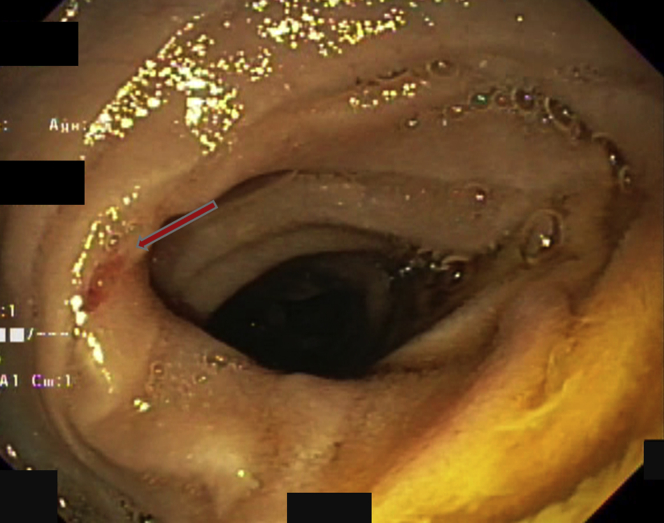
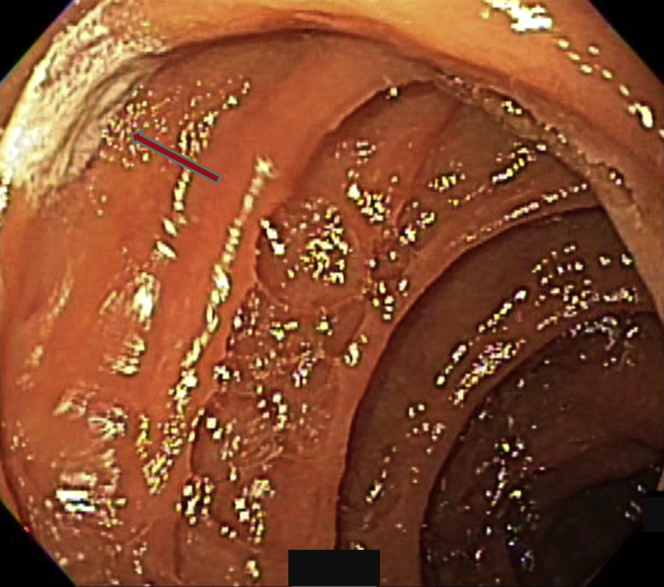
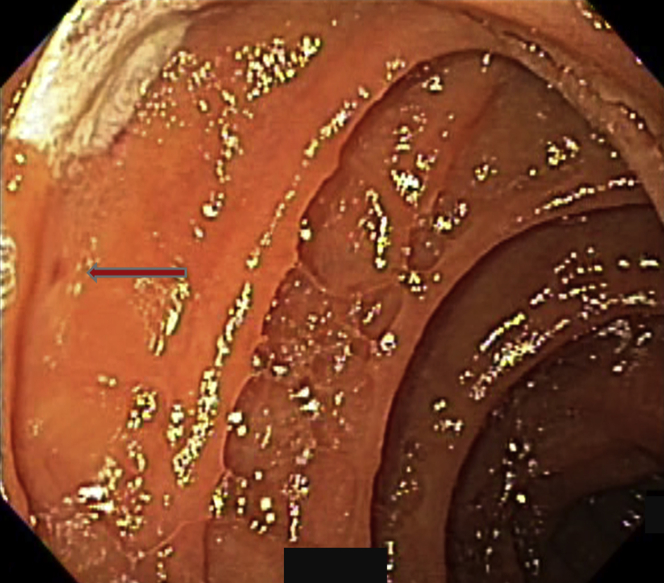
Figure 4.
A lesion was indicated by the arrow.
Figure 5.
The positions of the lesions were noted to sometimes change because of peristalsis or change in enteroscope dynamics when the instrument was passed. The paddle was pushed against the lesion, flattening the intestinal mucosa to enhance exposure of the lesion to the treatment paddle and maximize therapy in the direction of the arrow.
Figure 6.
The treatment footprint was visible, and the angiodysplasia appeared completely obliterated as indicated by the arrow.
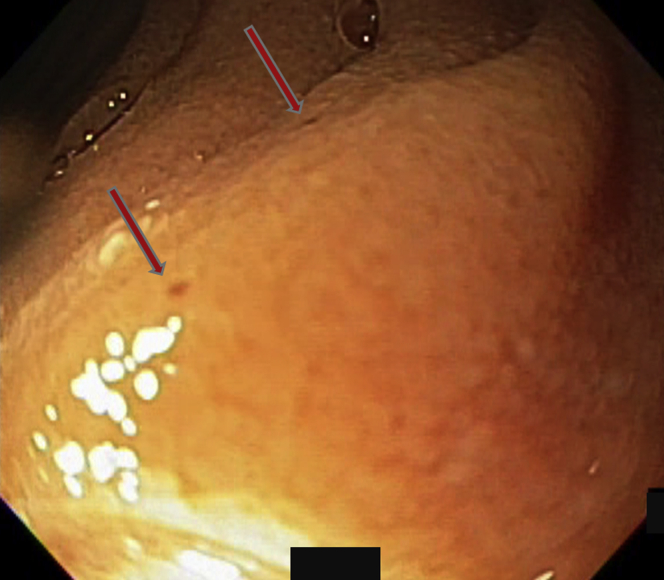
Figure 7.
Two diminutive lesions, which on video capsule endoscopy could sometimes be seen oozing blood as smokestacks, were identified as indicated by the arrows.
Figure 8.
The treatment paddle was used to flatten the mucosa and treat them in the direction indicated by the arrow.
Figure 9.
The posttreatment footprint is indicated by the arrow.
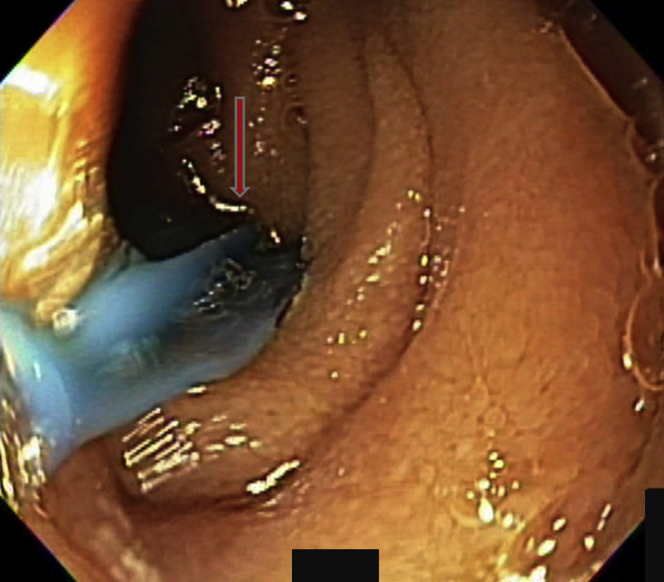
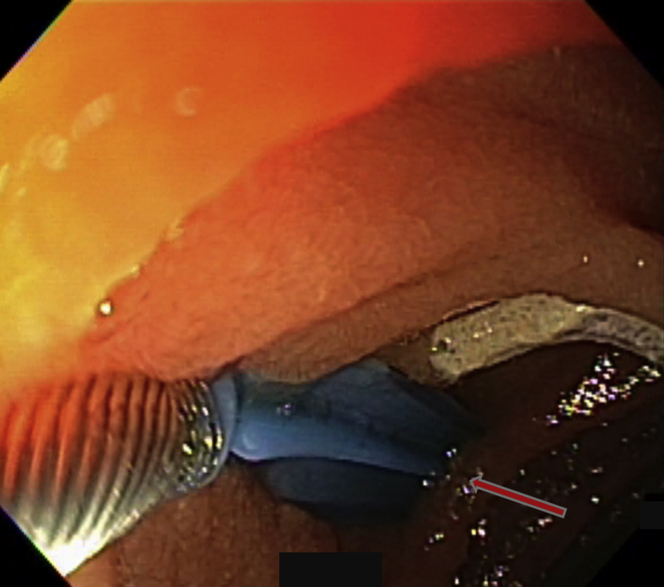
Figure 10.
One lesion had already been treated, as evidenced by the footprint. A second angiodysplasia was identified (arrow).
Figure 11.
The treatment paddle was pushed against the lesion to enhance tissue apposition and coaptive coagulation in the direction of the arrow while avoiding the previously treated site.
Figure 12.
The second treatment footprint was visible. The cherry-red island within the treatment site indicated the location of the treated angiodysplasia (arrow).
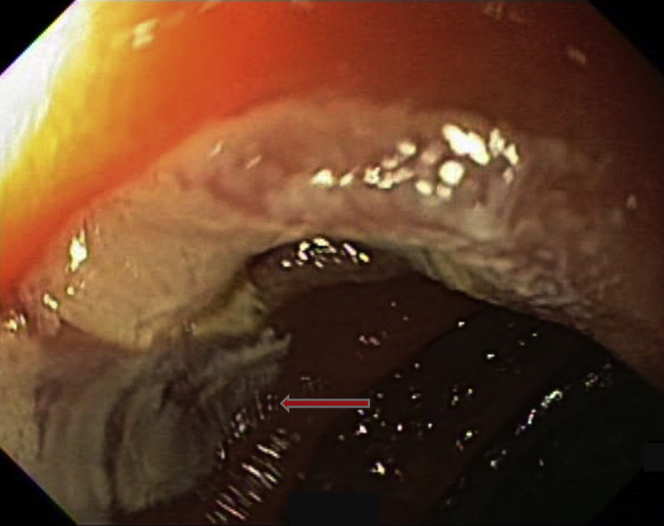
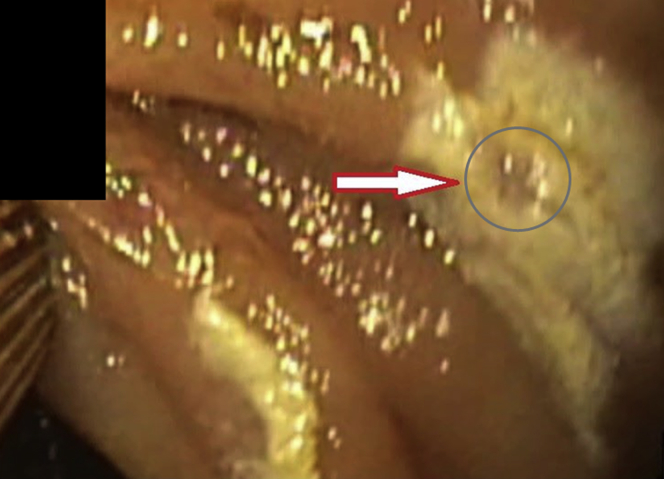
Figure 13.
This image highlights the discolored treatment footprint (arrow). The cherry-red color of the treated angiodysplasia within the site after radiofrequency ablation is highlighted in the circle.
Results
Twenty patients were treated from March until October 2018 and were followed up until March 2019. There were 6 women (average age, 68 years; SD ± 11.1) and 14 men (average age 73 years; SD ± 10.4). All had undergone at least 1 previous EGD and colonoscopy; 14 patients (70%) had SBA on video capsule endoscopy, and 14 patients had previous endoscopic treatment of SBA with APC. A median of 23 SBA were treated (range, 2-99). The median follow-up time was 195 days (range, 30-240 days). Four patients, including 3 with a left ventricular assist device (LVAD), had recurrent bleeding between 45 and 210 days after RFA treatment. Two patients required repeated blood transfusions: 2 and 1 units, respectively. Mean pretreatment hemoglobin was 8.3 g/dL (range, 5.1-14.4 g/dL), and mean posttreatment hemoglobin available in 16 patients was 9.4 g/dL (range, 5-13 g/dL). Three of the 4 patients underwent repeated endoscopies including a push enteroscopy and an upper endoscopy with no treatment, and a repeated enteroscopy with SBA treated with APC, respectively. One patient with LVAD had recurrent overt GI bleeding and underwent arterial embolization. There were no endoscopic adverse events. Two patients died 60 and 180 days after the procedures of causes unrelated to GI hemorrhage.
Discussion
GIADs are the most common cause of small-bowel bleeding and will probably remain a major cause of anemia as the population ages and anticoagulation use increases.13 Despite the general availability of endoscopy, enteroscopy, and APC, recurrent bleeding rates remain high. This is probably a result of numerous factors, including the pathologic features that provoked the GIAD and associated hemorrhage, which may not be reversible, such as aortic stenosis or chronic kidney disease, but the problem also highlights the limitations of available therapies, including APC therapy.5,12,13
APC therapy may not be uniform because the adequacy and depth of ablation depend on the distance and the presence of debris, mucus, or blood between the probe and tissue. The beam must also be directed toward the lesion, which can be challenging because of the inability to target the tissue in the presence of breathing, intestinal peristalsis, and endoscope position. The APC catheter itself has shortcomings. It is very flexible, which sometimes makes it difficult to pass through the enteroscope, and it may bend during repeated insertions, which is sometimes needed to remove tissue debris buildup on the tip, or to suction the intestinal lumen contents.
An improvement in our ability to treat GIAD is desirable. The instrument needs to be easy to use repeatedly through the enteroscope and to cover more area of intestinal mucosa per treatment; it must be easier to manipulate into position on the GIAD to achieve ablation, and it should carry a low risk for damage to the surrounding healthy intestinal mucosa.
This initial series of RFA of small-bowel angiodysplasia with the use of a newly available catheter may have a few potential advantages. The catheter rigidity was not difficult to pass through the endoscope. The rotatability of the paddle made it easier to direct therapy to the intestinal wall without a need for major changes to the position of the tip of the endoscope, and it was possible to semiblindly treat lesions that were on opposing intestinal walls or behind folds because it was easy to direct the probe. The platform remained stable during the ablation. The surface area of the paddle was adequate to ablate most lesions in a focal manner, respecting normal surrounding mucosa, and posttreatment inspection of the mucosa showed complete ablation of the lesions in most cases. Recurrent bleeding was mostly associated with patients with LVAD, and overall it compared favorably with published rates of bleeding recurrence due to intestinal angiodysplasia. An important variable that merits further investigation is time to bleeding recurrence, which may be an important feature of angiodysplasia bleeding that is rarely reported.
The toolbox for dedicated enteroscopy is limited. Most instruments are derivations or adaptations developed for upper endoscopies and colonoscopies. Inasmuch as the major reason to perform overtube-assisted enteroscopy is to evaluate and treat small-bowel bleeding due to angiodysplasia, enhanced therapies are desirable. Further outcomes including ease of use, dedicated procedure time, hemoglobin levels before and after treatment, blood and iron supplementation needs, and frequency of repeated endoscopy should be captured to determine efficacy and efficiency.
Conclusions
This case series shows the feasibility of using an RFA catheter to treat angiodysplasia. There may be advantages over what is currently available, but studies to determine improved efficacy of therapy, recurrent bleeding rates, and time to recurrence of bleeding are needed.
Disclosure
Dr Lara and Dr Samarasena are consultants for Medtronic. All other authors disclosed no financial relationships relevant to this publication.
Supplementary data
Video 1. Patients with small-bowel angiodysplasias treated with a small-bowel radiofrequency ablation catheter.
References
- 1.Sami S.S., Al-Araji S.A., Ragunath K. Review article: gastrointestinal angiodysplasia -pathogenesis, diagnosis and management. Aliment Pharmacol Ther. 2013;39:15–34. doi: 10.1111/apt.12527. [DOI] [PubMed] [Google Scholar]
- 2.Boley S.J., Sammartano R., Adams A. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650–660. [PubMed] [Google Scholar]
- 3.Heer M., Sulser H., Hany A. Angiodysplasia of the colon: an expression of occlusive vascular disease. Hepatogastroenterology. 1987;34:127–131. [PubMed] [Google Scholar]
- 4.Junquera F., Saperas E., de Torres I. Increased expression of angiogenic factors in human colonic angiodysplasia. Am J Gastroenterol. 1999;94:1070–1076. doi: 10.1111/j.1572-0241.1999.01017.x. [DOI] [PubMed] [Google Scholar]
- 5.Raju G.S., Gerson L., Das A. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697–1717. doi: 10.1053/j.gastro.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6.Liao Z., Gao R., Xu C. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Lara L.F., Ukleja A., Pimentel R. Change in airway management for overtube assisted enteroscopy following identification of adverse events by a permanent endoscopy quality program. Endoscopy. 2014;46:927–932. doi: 10.1055/s-0034-1390852. [DOI] [PubMed] [Google Scholar]
- 8.Barkun A., Bardou M., Marshall J.K. Nonvariceal upper GI bleeding consensus conference group. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 9.Diggs N.G., Holub J.L., Lieberman D.A. Factors that contribute to blood loss in patients with colonic angiodysplasia from a population-based study. Clin Gastroenterol Hepatol. 2011;9:415–420. doi: 10.1016/j.cgh.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foutch P.G., Rex D.K., Lieberman D.A. Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 1995;90:564–567. [PubMed] [Google Scholar]
- 11.Clouse R.E., Costigan D.J., Mills B.A. Angiodysplasia as a cause of upper gastrointestinal bleeding. Arch Intern Med. 1985;145:458–461. [PubMed] [Google Scholar]
- 12.Gerson L.B., Batenic M.A., Newsom S.L. Long-term outcomes after double-balloon enteroscopy for obscure gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2009;7:664–669. doi: 10.1016/j.cgh.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Jackson C.S., Gerson L.B. Management of gastrointestinal angiodysplastic lesions (GIADs): a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:474–483. doi: 10.1038/ajg.2014.19. [DOI] [PubMed] [Google Scholar]
- 14.Vargo J.J. Clinical applications of the argon plasma coagulator. Gastrointest Endosc. 2004;59:81–88. doi: 10.1016/s0016-5107(03)02296-x. [DOI] [PubMed] [Google Scholar]
- 15.Goulet C.J., Disario J.A., Emerson L. In vivo evaluation of argon plasma coagulation in a porcine model. Gastrointest Endosc. 2007;65:457–462. doi: 10.1016/j.gie.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Norton I.D., Wang L., Levine S.A. In vivo characterization of colonic thermal injury caused by argon plasma coagulation. Gastrointest Endosc. 2002;55:631–636. doi: 10.1067/mge.2002.123418. [DOI] [PubMed] [Google Scholar]
- 17.Olmos J.A., Marcolongo M., Pogorelsky V. Long-term outcome of argon plasma ablation therapy for bleeding in 100 consecutive patients with colonic angiodysplasia. Dis Colon Rectum. 2006;49:1507–1516. doi: 10.1007/s10350-006-0684-1. [DOI] [PubMed] [Google Scholar]
- 18.Lewis B.S., Salomon P., Rivera-MacMurray S. Does hormonal therapy have any benefit for bleeding angiodysplasia? J Clin Gastroenterol. 1992;15:99–103. doi: 10.1097/00004836-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Junquera F., Feu F., Papo M. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073–1079. doi: 10.1053/gast.2001.28650. [DOI] [PubMed] [Google Scholar]
- 20.Brown C., Subramanian V., Wilcox C.M. Somatostatin analogues in the treatment of recurrent bleeding from gastrointestinal vascular malformations: an overview and systematic review of prospective observational studies. Dig Dis Sci. 2010;55:2129–2134. doi: 10.1007/s10620-010-1193-6. [DOI] [PubMed] [Google Scholar]
- 21.Bon C., Aparicio T., Vincent M. Long-acting somatostatin analogues decrease blood transfusion requirements in patients with refractory gastrointestinal bleeding associated with angiodysplasia. Aliment Pharmacol Ther. 2012;36:587–593. doi: 10.1111/apt.12000. [DOI] [PubMed] [Google Scholar]
- 22.Junquera F., Saperas E., Videla S. Long-term efficacy of octreotide in the prevention of recurrent bleeding from gastrointestinal angiodysplasia. Am J Gastroenterol. 2007;102:254–260. doi: 10.1111/j.1572-0241.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- 23.Scaglione G., Pietrini L., Russo F. Long-acting octreotide as rescue therapy in chronic bleeding from gastrointestinal angiodysplasia. Aliment Pharmacol Ther. 2007;26:935–942. doi: 10.1111/j.1365-2036.2007.03435.x. [DOI] [PubMed] [Google Scholar]
- 24.Nardone G., Rocco A., Balzano T. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429–1436. doi: 10.1046/j.1365-2036.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 25.Ge Z.Z., Chen H.M., Gao Y.J. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology. 2011;141:1629–1637. doi: 10.1053/j.gastro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Shinozaki S., Yamamoto H., Yano T. Long-term outcome of patients with obscure gastrointestinal bleeding investigated by double-balloon endoscopy. Clin Gastroenterol Hepatol. 2010;8:151–158. doi: 10.1016/j.cgh.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 27.Williamson J.B., Judah J.R., Gaidos J.K.J. Prospective evaluation of the long-term outcomes after deep small-bowel spiral enteroscopy in patients with obscure GI bleeding. Gastrointest Endosc. 2012;76:771–778. doi: 10.1016/j.gie.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Shaheen N.J., Sharma P., Overholt B.F. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 29.Ganz R.A., Overholt B.F., Sharma V.K. Circumferential ablation of Barrett’s esophagus that contains high-grade dysplasia: a U.S. multicenter registry. Gastrointest Endosc. 2008;68:35–40. doi: 10.1016/j.gie.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 30.McGorisk T., Krishnan K., Keefer L. Radiofrequency ablation for refractory gastric antral vascular ectasia (with video) Gastrointest Endosc. 2013;78:584–588. doi: 10.1016/j.gie.2013.04.173. [DOI] [PubMed] [Google Scholar]
- 31.Gross S.A., Al-Haddad M., Gill K.R.S. Endoscopic mucosal ablation for the treatment of gastric antral vascular ectasia with the HALO90 system: a pilot study. Gastrointest Endosc. 2008;67:324–327. doi: 10.1016/j.gie.2007.09.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Patients with small-bowel angiodysplasias treated with a small-bowel radiofrequency ablation catheter.