To the Editor:
The importance of the immune system in pulmonary arterial hypertension (PAH) pathogenesis is increasingly recognized by clinicians and scientists (1). Immune cells are recruited to the lungs of patients with PAH (2), and preclinical studies have demonstrated their requirement for disease progression (3). The importance of the human immune system in PAH pathogenesis is underscored by our recent finding that mice, which do not develop robust experimental pulmonary hypertension, are rendered susceptible to severe disease when reconstituted with human immune tissue (4). Consequently, several clinical trials are investigating immunomodulation as PAH treatment (1).
The ubiquitously expressed damage-associated molecular pattern HMGB1 (high-mobility group box-1) is gaining recognition as a mediator of PAH. HMGB1 can be secreted from immune cells in response to stress, and it mediates many paracrine and autocrine effects in inflammatory conditions (5). HMGB1, by binding TLR4 (Toll-like receptor 4), activates macrophages and lymphocytes; induces TNF (tumor necrosis factor), IL-6, and IL-1β; and triggers autoimmunity (5). All of these factors are hallmarks of PAH (1). Expression of both HMGB1 and TLR4 is elevated in lungs of patients with PAH (6), and HMGB1 is secreted in response to hypoxia (7). Conversely, mice treated with neutralizing antibodies against HMGB1 (6) or deficient in TLR4 are protected from hypoxia-induced pulmonary hypertension (8). Although targeting HMGB1/TLR4 signaling may be a promising new treatment for PAH, this strategy can be expected to come at a cost, given the multiple roles of these molecules in infection and immunity.
In this study, we first consolidated the critical role of HMGB1 in PAH in human samples and animal models. Next, we tested the therapeutic efficacy of a novel peptide, P5779, which specifically targets extracellular HMGB1 in its disulfide form, disrupting its interaction with the TLR4 adaptor MD-2 (9). Hence, P5779 does not affect the epigenetic functions of intracellular HMGB1 or non–HMGB1-mediated TLR4 signaling, thus minimizing potential off-target effects of anti-HMGB1 therapies for PAH. Some of these results were previously reported in abstract form (10).
Methods
Human studies were approved by the University Health Network Research Ethics Board, and the patient characteristics and microarray methods have been published elsewhere (11). Animal experiments were approved by the animal care committee at St. Michael’s Hospital. Sugen/hypoxia- or monocrotaline-treated rats were given anti-HMGB1 antibody (2G7; 2 mg/kg i.p. every 48 h), P5779 (20 mg/kg i.p. daily), or control (isotype-matched antibody or scrambled peptide). Right ventricular systolic pressure (RVSP) was recorded by microtip catheter, and right ventricular hypertrophy was measured by Fulton’s condition index. Pulmonary vascular remodeling was determined in hematoxylin and eosin–stained sections as a ratio [(external area − internal area)/external area]. Data are shown as mean ± SEM, and statistical significance was tested by ANOVA.
Results
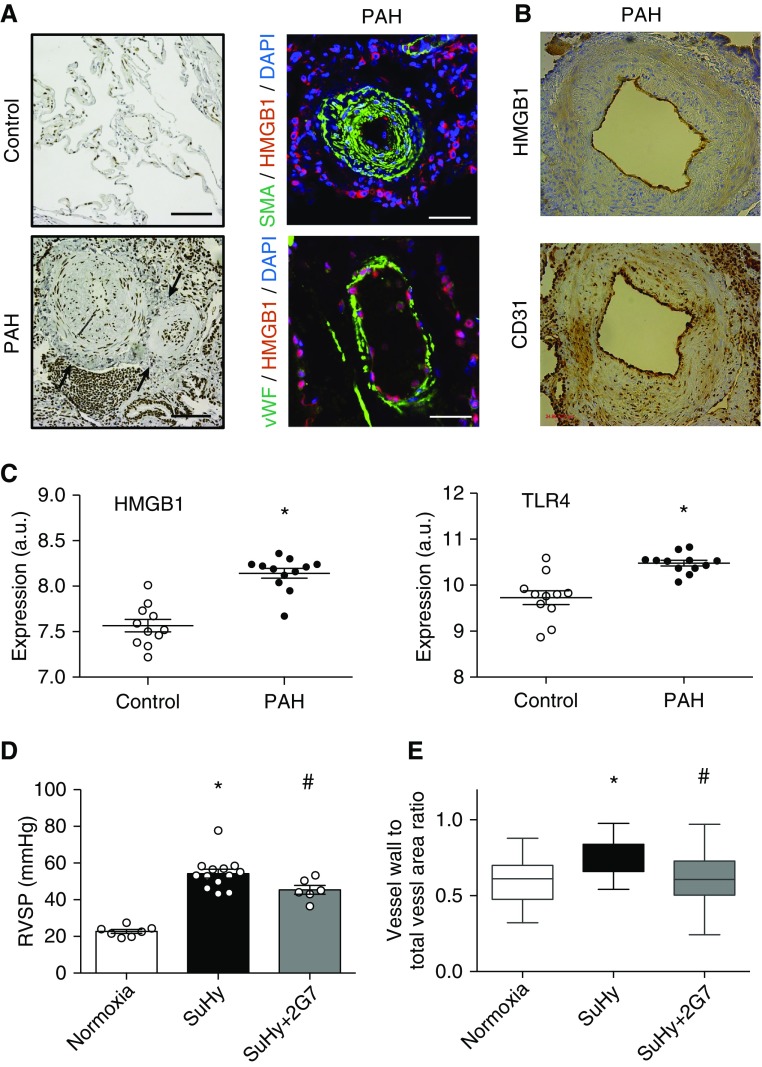
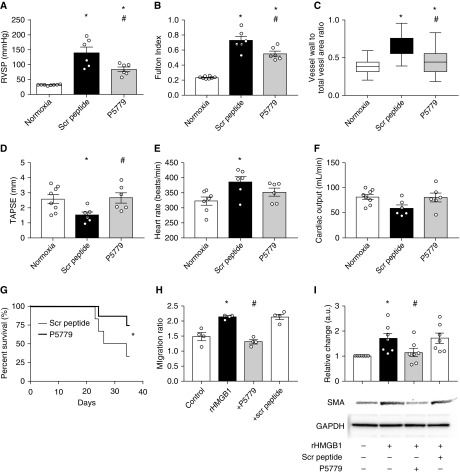
In lungs of patients with PAH, HMGB1 mRNA was upregulated, and staining was increased predominantly in the perivascular adventitia and intima (Figures 1A and 1B). Similarly, TLR4 was upregulated in lungs of patients with PAH (Figure 1C). To assess the importance of HMGB1 in Sugen/hypoxia-induced pulmonary hypertension in rats, we used a neutralizing anti-HMGB1 antibody. Anti-HMGB1 antibody–treated animals had lower RVSP and less lung vascular remodeling than isotype control–treated animals (Figures 1D and 1E). Because HMGB1 is ubiquitously expressed and acts through multiple receptors, affecting a multitude of cellular functions (5), we specifically targeted the interaction of disulfide HMGB1 and TLR4 using the peptide P5779 (9). When given to monocrotaline-treated rats from Day 1, P5779 treatment resulted in lower RVSP (35.6 vs. 50.5 mm Hg; P = 0.0002) and less vascular remodeling (0.5 vs. 0.7; P < 0.0001; not shown) than scrambled peptide. P5779, when given in a delayed fashion (from Day 21) to Sugen/hypoxia rats, similarly reduced RVSP, Fulton’s condition index, and lung vascular remodeling (Figures 2A–2C). was unchanged between groups, and heart rate showed minor changes, so that differences in pulmonary vascular resistance and right ventricular hypertrophy could not be attributed to changes in cardiac output or left ventricular function (because left ventricular ejection fraction was unchanged; not shown) (Figures 2D–2F). Furthermore, delayed administration of P5779 for 2 weeks to rats with monocrotaline-induced severe pulmonary hypertension conferred a striking survival advantage (Figure 2G). In vitro, P5779 blocked HMGB1-induced migration of pulmonary artery smooth muscle (Figure 2H) and endothelial cells (not shown), as well as smooth muscle hypertrophy (Figure 2I).
Figure 1.
HMGB1 (high-mobility group box-1) in pulmonary arterial hypertension (PAH). (A) HMGB1 is upregulated in human PAH. Healthy lung tissue from patients with lung cancer at the time of resection (control) is devoid of high-intensity extracellular HMGB1 staining, whereas patients with PAH show elevated HMGB1 expression in pulmonary vessels and adventitial lymphoid tissue. Left panels depict HMGB1 staining, and right panels show immunofluorescence of SMA (smooth muscle actin) or vWF (von Willebrand factor) together with HMGB1. Note the cytosolic HMGB1 staining in the vascular adventitia, indicating activation and HMGB1 secretion (arrows). Scale bars, 100 μm. (B) Serial 4-μm sections showing colocalization of HMGB1 and CD31 in endothelial cells in a patient with PAH. (C) mRNA concentrations of HMGB1 and TLR4 (Toll-like receptor 4) are upregulated in lungs of patients with PAH compared with control lungs (*P < 0.001). (D) Right ventricular systolic pressure (RVSP) and (E) vessel wall thickness in pulmonary arterioles of normoxic (n = 7), Sugen/hypoxia (SuHy) isotype control antibody–treated (n = 13), and SuHy anti-HMGB1 antibody–treated (n = 6) rats. Anti-HMGB1 antibody or isotype control treatment started at the time of model induction. For histological analysis, at least 74 vessels were measured per treatment group. *P < 0.05 versus normoxia; #P < 0.05 versus isotype control antibody.
Figure 2.
Targeted inhibition of HMGB1 (high-mobility group box-1)/TLR4 (Toll-like receptor 4) signaling with P5779 treats pulmonary hypertension in rats. (A) Right ventricular systolic pressure (RVSP), (B) Fulton’s condition index [right ventricular/(left ventricular + septal) weight], and (C) relative wall thickness of pulmonary arterioles for normoxic (n = 8), Sugen/hypoxia scrambled (Scr) peptide–treated (n = 6), and Sugen/hypoxia P5779-treated (n = 6) rats. Treatment was initiated 3 weeks after model induction (Day 21), and animals were assessed 2 weeks later (Day 35). For histological analysis, at least 88 vessels per treatment group were measured. *P < 0.05 versus normoxia; #P < 0.05 versus scrambled peptide (ANOVA). (D–F) Echocardiographic analysis was performed on Day 34. Sugen/hypoxia treatment resulted in a decrease in right ventricular function as evidenced by diminished tricuspid annular plane systolic excursion (TAPSE), and this effect was reversed by delayed administration of P5779. Additional echocardiographic analyses showed a significant increase in heart rate in Sugen/hypoxia animals, with no significant effect of P5779, whereas was unchanged in all groups. *P < 0.05 versus normoxia (ANOVA). (G) P5779 treatment for 2 weeks, beginning 3 weeks after monocrotaline administration, confers a survival advantage over treatment with scrambled peptide (*P < 0.05, log-rank Mantel-Cox test). (H) Treatment of primary human pulmonary artery smooth muscle cells (PASMC) with P5779 blocks HMGB1-induced migration in a modified Boyden chamber assay. PASMC were treated with recombinant HMGB1 (rHMGB1; 10 ng/ml) in the presence or absence of scrambled peptide or P5779 (10 μg/ml), and the ratio of cells in the chamber to those migrating through was determined. *P < 0.05, rHMGB1 versus control; #P < 0.05, P5779 versus rHMGB1 (n = 4). Similar results were achieved with pulmonary artery endothelial cells (not shown). (I) P5779 blocks HMGB1-induced smooth muscle cell hypertrophy. PASMC were treated with rHMGB1, scrambled peptide, or P5779 as indicated. Cells were lysed and immunoblotted for SMA (smooth muscle actin) or loading control (GAPDH). Shown are pooled data for n = 5 experiments (mean ± SEM), as well as a representative Western blot. *P < 0.05, rHMGB1 versus control; #P < 0.05, P5779 versus rHMGB1.
Discussion
Our findings demonstrate a fundamental role for HMGB1 in pulmonary hypertension pathogenesis. Importantly, delivery of a peptide that specifically blocks the interaction between disulfide HMGB1 and the TLR4 adaptor MD-2 improved disease severity and mortality, even when given to animals with established disease. These results further buttress immune-centric concepts of PAH pathogenesis.
Although previous studies have implicated HMGB1 in PAH pathogenesis (6), we targeted HMGB1 signaling in a potentially clinically feasible fashion. Owing to the multiplicity of HMGB1 functions in normal homeostasis and host defenses (5), global inhibition of HMGB1 in humans is likely to be plagued by off-target effects. Indeed, HMGB1-deficient mice die perinatally because of profound metabolic instability (5). Once secreted, HMGB1 can bind several receptors, including RAGE (receptor for advanced glycation end products), TLR4, TLR2, and CXCR4 (C-X-C chemokine receptor type 4). Owing to this variety of HMGB1–receptor pairs, HMGB1 can affect differential changes in a context-dependent manner. Further complexity in this system arises because HMGB1 exists in three different redox states (5). Specifically, disulfide HMGB1 is capable of binding TLR4 in an MD-2–dependent manner and of inducing cytokine secretion from immune cells. In contrast, fully reduced HMGB1 cannot bind TLR4 but instead activates CXCR4 through CXCL12 (C-X-C motif chemokine ligand 12), stimulating leukocyte chemotaxis.
To exploit the role of HMGB1 in PAH, one must be able to specifically inhibit HMGB1 in an appropriate cellular context. In this letter, we describe such a technique. P5779 possesses critical characteristics for precisely targeted HMGB1 manipulation. First, it can only interact with cytokine-inducing disulfide HMGB1 (9). Second, it specifically interferes with HMGB1/TLR4 signaling and no other known HMGB1 receptors. Third, it does not globally depress signaling through TLR4, a multifunctional immune receptor. Last, the short half-life of P5779 may prove advantageous because immunosuppression can be “turned off” by ceasing drug delivery. The summation of these properties make P5779 a promising therapeutic agent with far more specificity than other anti-HMGB1 therapies tested to date, such as monoclonal antibodies or glycyrrhizin. Our results represent a promising avenue for focused research aimed at translating immunomodulation by inhibition of HMGB1/TLR4 signaling into clinical practice for patients with PAH.
Footnotes
Supported by grants-in-aid from the Heart and Stroke Foundation of Canada and the Canadian Institutes of Health Research (W.M.K.) and by the International Anesthesia Research Society Mentored Research Award (N.M.G.).
Author Contributions: N.M.G., B.E.S., and W.M.K. conceived of the study, performed experiments, analyzed data, and wrote the manuscript. Y.H., X.H., A.V., Y.D.Z., M.M.K., and C.K. performed experiments and analyzed data. M.d.P., K.J.T., and Y.A.-A. conceived of the experiments and wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201808-1597LE on April 2, 2019
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuebler WM, Bonnet S, Tabuchi A. Inflammation and autoimmunity in pulmonary hypertension: is there a role for endothelial adhesion molecules? (2017 Grover Conference Series) Pulm Circ. 2018;8:2045893218757596. doi: 10.1177/2045893218757596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu Y, Zabini D, Gu W, Goldenberg NM, Breitling S, Kabir G, et al. The role of the human immune system in chronic hypoxic pulmonary hypertension. Am J Respir Crit Care Med. 2018;198:528–531. doi: 10.1164/rccm.201711-2175LE. [DOI] [PubMed] [Google Scholar]
- 5.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 6.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, et al. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2013;18:1509–1518. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young KC, Hussein SMA, Dadiz R, deMello D, Devia C, Hehre D, et al. Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp Lung Res. 2010;36:111–119. doi: 10.3109/01902140903171610. [DOI] [PubMed] [Google Scholar]
- 9.Yang H, Wang H, Ju Z, Ragab AA, Lundbäck P, Long W, et al. MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med. 2015;212:5–14. doi: 10.1084/jem.20141318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldenberg NM, Hu Y, Volchuk A, Gu W, Zhao YD, Hu X, et al. Therapeutic targeting of high mobility group box 1 in pulmonary hypertension [abstract] Am J Respir Crit Care Med. 2018;197:A4627. doi: 10.1164/rccm.201808-1597LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Peng J, Lu C, Hsin M, Mura M, Wu L, et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One. 2014;9:e88727. doi: 10.1371/journal.pone.0088727. [DOI] [PMC free article] [PubMed] [Google Scholar]