Highlights
-
•
As in farm animals, deficit in passive immune transfer is associated in puppies with a higher risk of neonatal mortality.
-
•
Intestinal barrier is closed after 12–16 hours of life in puppies.
-
•
Time of colostrum ingestion is the major determinant of passive immune transfer, rather than colostral IgG concentration.
-
•
Banking of colostrum collected between 24 and 48 hours of life should be encouraged.
-
•
Lactogenic immune transfer is not only systemic but also local, providing protection of the digestive tract.
Keywords: Neonatology, Colostrum, Immunoglobulins G, Growth, Digestive tract, Dog
Abstract
The puppy, born without immunoglobulins G (IgG), acquires a passive systemic immunity thanks to colostrum intake during the two first days of life. The quality of passive immune transfer (i.e. blood IgG concentration at two days of age), highly variable between litters and between puppies within litters, depends mainly on the time elapsed between birth and ingestion of colostrum, with limited influence of colostrum IgG concentration. Deficit in passive immune transfer, impacting puppy’s health and neonatal mortality rate, can be indirectly diagnosed through blood gammaglutamyltransferases assay and evaluation of growth rate over the two first days of life. In the absence of maternal colostrum, few homo- and heterospecific immune sources are available and canine colostrum banking remains the optimal solution. Whereas passive immune transfer is crucial for survival during the neonatal period, it later interferes with response to vaccination. In addition to systemic passive immune transfer, maternal antibodies (mainly IgA) would provide local (digestive) immunity, ensuring mid-term protection of the puppies’ gut together with probably long term training of the digestive immune system.
1. Introduction
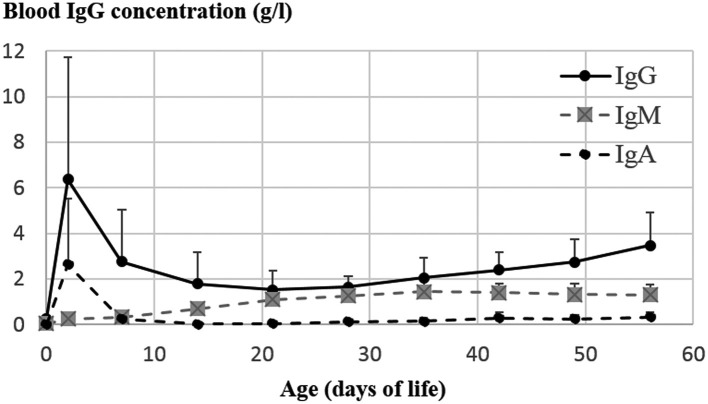
Neonatal period in the canine species, defined as the first three weeks of life, is a critical period with high risk of mortality: about 10% of all live-born puppies die between birth and 21 days of age (Mugnier et al., 2018). Survival rate during this period depends on the ability of the newborn to adapt to the extra-uterine life. Once cardiorespiratory system has been able to switch from a placental to an aerial oxygen provision, the newborn dog goes through new challenges, both immune and nutritional. From the nutritional point of view, the circulation of nutrients via placenta is interrupted at birth; nutrients supply relies on implementation of new strategies by the newborn, including mammary gland approach, suckling and milk digestion. The immune situation of the newborn dog is critical, as the endotheliochorial structure of the placenta drastically limits transplacental transfer of macromolecules, including immunoglobulin G (IgG), to the newborn’s bloodstream. Whereas exposed to high concentrations of infectious agents immediately after birth when delivered out of the uterus, puppies are born with very low systemic immunity, with mean serum IgG concentration at about 0.3 g/L versus 8–25 g/L in the adult dog (Poffenbarger et al., 1991; Bouchard et al., 1992; Chastant-Maillard et al., 2012; Mila et al., 2014a). Transfer of passive immunity from dam to the offspring is thus essentially lactogenic in the canine species, colostrum ensuring both nutrients and immunity provision: at two days of age, mean serum IgG concentration in the puppy rises up to 6–16 g/L, with 85–95% of the immunoglobulins originating from the colostral transfer (Pollock and Carmichael, 1982; Poffenbarger et al., 1991; Schäfer-Somi et al., 2005a; Greene and Schultz, 2006; Day, 2007; Chastant-Maillard et al., 2012; Fig. 1 ). Even when evaluated to its maximum (two days of age), immunoglobulins concentrations or specific antibody titers acquired by the puppy after colostrum intake remain lower than in the adult dog, reaching between 50 and 77% of the maternal level (Mila et al., 2014a; Gillespie et al., 1958).
Fig. 1.
Pattern of immunoglobulins G, M and A concentrations in puppies’ blood. n=57 Beagle puppies from one kennel, with free suckling. Mean ± standard deviation. ELISA assay (method described in Chastant-Maillard et al., 2012).
Unlike in the piglet or calf, the passive immune transfer (PIT) was investigated in the dog only recently. The aim of this review is to present different factors influencing quality of the PIT (defined as the serum IgG concentration acquired at two days of age), to describe the impact of PIT on puppies’ health, and to comment on the alternatives to colostrum. Physiology of PIT in the dog and the methods of its evaluation will be also addressed.
2. Definition of passive immune transfer in the dog
Adequate PIT is essential for the puppy as its low quality is associated with an increased risk of neonatal mortality: mortality between birth and three weeks of life in puppies with serum IgG concentration at or below 2.3 g/L was 44.4% versus 4.9% in puppies with higher IgG concentration (p = 0.001; Mila et al., 2014a) ; mean blood IgG concentration at Day2 of life was 2.32 g/L in puppies dying between Day 2 and 21 vs 6.94 g/L for puppies still alive at 21 days of age). The minimal protective level of IgG concentrations are very different depending on species: 4–8 g/L in the foal, 10 g/L in the calf and 15 g/L in the piglet (Weaver et al., 2000; Cabrera et al., 2012; Liepman et al., 2015).
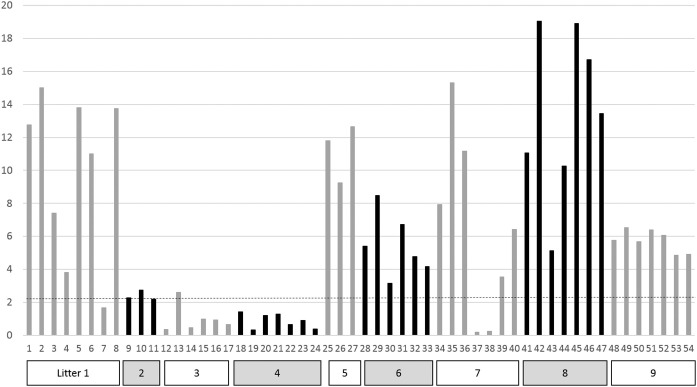
In order to determine a real prevalence of the deficit in PIT, data on large number of puppies from different kennel conditions still remain to be obtained. This deficit was diagnosed in one out of 20 puppies (5%) according to Gooding and Robinson (1982) and in 26 out of 149 puppies (17.4%) according to Mila et al. (2014a). Indeed, the prevalence of the deficit in PIT varies strongly among kennels, but may vary also among breeds, as only 4 out of 90 Labrador puppies (4.4%) presented IgG concentration below 2.3 g/L (unpublished data). In case of free suckling (uncontrolled by the breeder), quality of PIT is strongly variable among litters, but also among puppies from the same litter, both evaluated via serum IgG concentration (general immunity) and via CPV2-specific antibody titer (specific immunity; Fig. 2 ) (Mila et al., 2014b).
Fig. 2.
Heterogeneity of passive immune transfer (evaluated through blood IgG concentration at Day 2 of age) inter and intra litter. n = 54 Beagle puppies from 9 litters from one kennel, with free suckling. The horizontal line indicates the threshold defining failure of passive immune transfer (2.3 g/L). In Litters 5, 6 and 9, all puppies reached a sufficient passive immune transfer; in Litter 4, all were in failure of passive immune transfer; within Litter 1 and 7, passive immune transfer was heterogeneous, some above, others below the threshold.
3. Evaluation of passive immune transfer
Although IgG is not the only molecule with immune activity provided to the newborn via colostrum intake (see below), its absorption witnesses colostrum intake, making serum IgG concentration assay at 2 days of age the reference method for PIT evaluation (Poffenbarger et al., 1991; Chastant-Maillard et al., 2010; Mila et al., 2014a). IgG assay performed via an immuno-enzymatic method (ELISA - Enzyme-Linked ImmunoSorbent Assay) is not an automated process and requires about six hours of laboratory work, limiting its use to scientific purposes. In practice, PIT can be evaluated indirectly through blood gamma-glutamyltransferases (GGT) activity at two days of age: GGT activity in canine colostrum is ten times higher than that in the female blood, whereas GGT activity in puppies’ serum at birth is almost absent. Any increase of GGT activity in the newborn’s serum is indicative of an ingestion of colostrum (Center et al., 1991), with an activity below 62U/L diagnosing a deficit in PIT with a 87.5% sensitivity and a 80% specificity (Mila et al., 2017b). PIT can be also evaluated via specific antibody titer. Indeed, at two days of age, serum IgG concentration and CPV2 specific-antibody titer are positively correlated, with concordant diagnosis of adequate PIT in 88% of puppies; adequate PIT was defined as IgG concentration >2.3 g/L for general immunity and CPV2-antibody titer >1:80 for specific immunity evaluation (Mila et al., 2018).
Easy-to-use, but also non-invasive, tests of the evaluation of the quality of PIT remain still to be developed for the canine species. Namely, all above mentioned methods require blood sampling of the newborn, in Newfoundland puppy of mean birth weight of 630 g, as well as in a 120 g Chihuahua (mean birth weight in France, A. Mugnier, personal communication). Knowing that dog breeders are forbidden to draw blood samples in most countries, and taking into account the cost of veterinary consultation (ideally at the kennel, since speaking of two-day-old puppies) and the cost of the blood test itself, such blood screening cannot be implemented in practice. An easier and costless method of indirect PIT evaluation is early growth monitoring, i.e. the percentage of weight gain from birth to two days of age, since colostrum provides together energy and immunoglobulins. A growth rate over the first two days of life below −2.7% allowed to identify the deficit of PIT in 87–96% of cases, both in terms of general (IgG) and specific (CPV2-specific antibodies) immune transfer (Table 1 ; Mila et al., 2018).
Table 1.
Diagnostic value of growth rate over the first two days of life at or below −2.7% for the diagnostic of failure of passive immune transfer. n=151 puppies of various breeds within one kennel. HI: hemagglutination inhibition.
| Parameter | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| Serum IgG concentration (<2.3 g/L) | 96.3 | 83.1 | 55.3 | 99.0 |
| CPV2-specific antibody titer (HI < 1:160) | 87.2 | 88.4 | 72.3 | 95.2 |
4. Factors determining the quality of passive immune transfer
In any species, final blood IgG concentration at two days of age depends on the quantity of IgG ingested by the newborn and on the proportion of IgG absorbed from the newborn gut into its bloodstream. Three factors thus influence the quality of PIT: i) the immunological quality of the colostrum (evaluated via IgG concentration), ii) the volume of colostrum ingested by the newborn, and iii) the time elapsed between birth and colostrum ingestion.
4.1. Immunological quality of the colostrum
4.1.1. Colostrogenesis
Immunoglobulin concentrations in canine mammary secretions are elevated during the first two days post-partum compared with later in lactation, defining this period as the colostral phase in the canine species. Three immunoglobulin classes are present in the canine colostrum (IgA, IgM and IgG), IgE remaining at undetectable concentrations (Chastant-Maillard et al., 2010).
IgA represent between 16 and 40% of total colostral immunoglobulins at the onset of lactation, becoming the principal immunoglobulin class in the mature milk (Schäfer-Somi et al., 2005b; Chastant-Maillard et al., 2010). As IgM, IgA are mainly produced locally by the lymphocytes of the mammary tissue (Hurley and Theil, 2011). Once ingested into the gut, IgA participate locally in the immune defense mechanisms of the digestive epithelium. Apart from this local role, a part of IgA passes through the intestinal wall, being absorbed into the bloodstream and subsequently redistributed onto the mucous surfaces, digestive or not (Salmon et al., 2009; Chastant-Maillard et al., 2012); they thereby participate for example in the immune defense of the respiratory tract. IgA concentration in the colostrum is between five and ten times higher than in adult serum, whereas that of IgM is much lower than in the serum, only about 15–25% of the serum concentration (Day, 2007).
IgG, main actor of the systemic immunity, is the major immunoglobulin class of the colostrum (60–75% of total immunoglobulins). Only a very small proportion of colostral IgG are produced by the mammary tissue, with the vast majority originating from the maternal blood. At the end of gestation, blood IgG circulating in the maternal bloodstream are trapped into the mammary tissue and stored until lactation onset. The involvement of the FcγRn receptors (Fragment, crystallizable receptor, neonatal) in the IgG mammary storage remains unclear and has never been studied in the canine species (Cervenak and Kacskovics, 2009). The drop in blood progesterone concentration at parturition induces the increase of prolactin secretion and the onset of the lactation: IgG are then released into the mammary alveoli lumen and excreted into the colostrum (Hurley and Theil, 2011). Mean IgG concentration in the canine colostrum is of about 20 g/L (Schäfer-Somi et al., 2005a; Chastant-Maillard et al., 2010; Mila et al., 2015), i.e. 2–3 times higher than in the maternal serum (between 0.9 and 6.3 times depending on the dam).
4.1.2. Variation factors of the colostrum quality
Immunological quality of the colostrum is amazingly variable between bitches: mean concentration of colostrum calculated as the mean of concentrations obtained separately from the five pairs of mammy glands of an individual varies from 3 to 69 g/L, without any influence of the breed or litter size (Mila et al., 2015). Age may have an impact, with colostrum of better immunological quality in females below six years of age than in older bitches.
In addition to this huge inter-individual difference, colostrum IgG concentration also varies for one given bitch among the different pairs of mammary glands, with a mean intra-individual coefficient of variation of 42 ± 32%. For one given bitch, the highest and the lowest IgG concentrations as assayed by mammary pairs differ in average by a factor of 5.9 (Chastant-Maillard et al., 2017). However, when numbered (M1 anterior thoracic to M5 posterior inguinal), none of the five pairs of mammary glands secreted systematically colostrum of better (or worse) immunological quality. No practical recommendation can be thus drawn for dog breeders to encourage suckling of a specific pair of mammary gland to optimize PIT. In the cow, posterior quarters produce colostrum of higher IgG concentration (Gross et al., 2016), whereas in the sow, reported results are controversial (Klobasa and Butler, 1987; Wu et al., 2010). Such a difference in colostral IgG concentration could be due to differences in vascularization between the different pairs, or to different densities in FcγRn receptors.
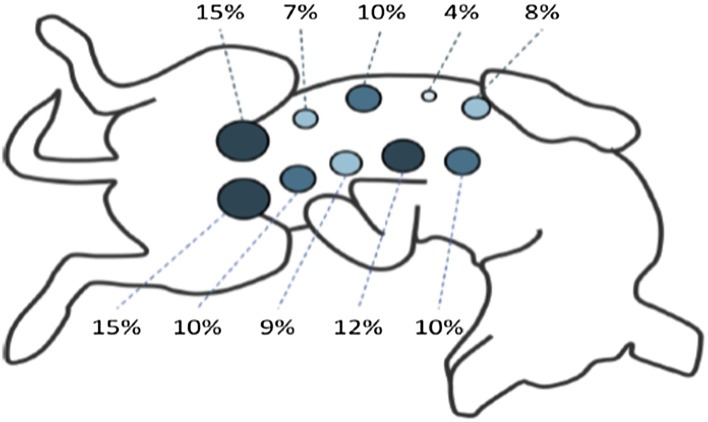
The eventual impact of such a variability in colostrum quality between teats on the quality of PIT depends on the suckling behavior of the puppy, and more precisely on the choice of the teat. Unlike piglets or kittens, puppies’ siblings do not develop any competitive behavior for teat appropriation. In average, a puppy suckles 5 ± 2 teats over the first 12 h of life (before the intestinal barrier closure), with M5 being the most often suckled (Fig. 3 ). Such multiple teat shifts contribute to erase the impact of the difference in colostral immune quality per teat. Later, data obtained during the second and third day of lactation showed that a puppy suckles an average of 2.5 ± 0.8 teats per feeding session (Arteaga et al., 2013).
Fig. 3.
Frequency of suckling (in % of suckling time) of each mammary gland during the first 24 h of life. Suckling behavior of 35 Labrador puppies followed by visual observation (Viaud, 2018).
To date, no method is available for IgG concentration evaluation in canine colostrum in kennel conditions. Refractometry, routinely used in the cow and mare, is not a reliable method of colostrum quality evaluation in the bitch (Mila et al., 2015). As no correlation between colostral and serum IgG concentrations was evidenced in the bitch (Chastant-Maillard et al., 2010; Mila et al., 2015), prediction of the colostrum quality according to maternal blood IgG level is neither possible. Evaluation of the repeatability of colostrum quality along with lactations of one given bitch would be desirable for breeding selection purposes, but also to decide which neonates would require the administration of a colostrum replacer.
Nevertheless, no correlation has been demonstrated between mean colostrum IgG concentration (mean value from 5 pairs of mammary glands per bitch) and serum IgG concentration in the newborn at two days of age in a study on 139 bitches and their 651 puppies from various breeds (Aggouni, 2016). No effect on the incidence of deficit of PIT (see below) was either evidenced. According to a theoretical approach presented in Fig. 4 , a puppy receiving an adequate volume of colostrum at an adequate time (see below) would meet the passive immune transfer requirements (serum IgG at or below 2.3 g/L) only if provided with colostrum with more than 3.4 g/L of IgG. As only one out of the 139 bitches tested by Mila et al. (2015) had colostrum below this threshold, colostrum immunological quality does not seem to be a frequent limiting factor for PIT in the canine species.
Fig. 4.
Theoretical estimation of the minimal IgG concentration in colostrum for appropriate passive immune transfer.
[1] With a blood volume equivalent to 7% body weight and a hematocrit of the newborn puppy at 50%, the total volume of serum within a puppy is 3.5 milliliters for 100 g body weight (100 g x 7% x (1–hematocrit)).
[2] The minimal serum concentration to be reached by the puppy at two days of age is 2.3 g/l, representing an absorbed IgG amount of 8.05 mg (2.3×serum volume).
[3] The IgG absorption rate was 30% in average between birth and 8 h of life (Chastant-Maillard et al., 2012). An 8.05 mg absorbed amount corresponds to 26.8 mg ingested IgG.
[4] A puppy ingests 4 ml/100 g body weight and performs 2 meals over the period of intestinal permeabilty: circulating IgG are thus absorbed from 8 ml colostrum per 100 g body weight.
[5] The minimal colostrum concentration is 3.4 g/l (ingested IgG×1000/8).
4.2. Quantity of colostrum ingested
Gastric capacity in the newborn dog is estimated at four ml per 100 g of body weight, with a mean gastric emptying time of two hours. However, the actual mean volume of colostrum ingested by the newborn dog remains unknown to date. Based on calculation presented in Fig. 4, the quantity of a colostrum of medium quality (IgG concentration of 20 g/L) necessary to achieve the minimum blood IgG level (2.3 g/L) is 1.3 ml for each 100 g of newborn body weight.
4.3. Time elapsed between birth and colostrum ingestion
The delay elapsed from birth decreases both the colostrum immune quality and the intestinal potential to absorb immunoglobulins. On the maternal side, colostrum IgG concentration drops dramatically after whelping with a mean decrease of 60% (±18%) between 4 and 24 h post-partum (Albaret et al., 2016). For the neonate’s part, intestinal barrier closure is one of the main factors determining the quality of PIT. Histological description of this phenomenon, non available in the dog, is known in the bovine and porcine species. At birth, tight junctions between enterocytes as well as brush border of these cells are undeveloped. Macromolecules, including immunoglobulins, can pass through digestive mucosa and be absorbed into the lymph and later into the bloodstream. This transport seems to be non specific and most probably independent from FcγRn receptors (Cervenak and Kacskovics, 2009). Bioavailability of colostral immunoglobulins is highly due to a weak proteolytic activity of the digestive system at birth, immature digestive microbiota but also thanks to a high concentration of trypsin inhibitors in the ingested colostrum (1000 times higher than in the mature milk in the cow) (Levieux and Ollier, 1999). As early as birth, over the first hours of life intestinal permeability decreases: puppies at birth absorb in average 40% of ingested IgG, whereas absorption rate drops at 20% 4 h later, and at only 9% 12 h later. After the first 12–16 h of life, IgG absorption rate is absent (Chastant-Maillard et al., 2012).
Thus, in order to optimize PIT, the colostrum intake must take place as early as possible, and in any case during the first 8 h of life. Spontaneous suckling behavior in early life is largely unknown in the canine species. Recently, we observed during the first 24 h of life five Labrador litters with free suckling. In the 35 included puppies, the first suckling was observed between five minutes and six hours after birth, with 70% of puppies suckling for the first time during the first two hours. Over the first 12 h of life, each puppy suckled during in average 80 ± 40 min (11% of the total duration of life) in 10 sessions (Viaud, 2018). Suckling behavior seems to naturally promote an adequate PIT.
5. Long-term consequences of passive immune transfer
5.1. Systemic passive immune transfer
After intestinal barrier closure in the newborn, serum IgG concentration drops exponentially, with an IgG half-life from 8.4 to 13.4 days. Depending on their specificity, maternally derived antibodies (MDA) persist until 10–15 days of life (Gooding and Robinson, 1982; Pollock and Carmichael, 1982; Greene and Schultz, 2006; Mila et al., 2014b). In the dog, the MDA concentration falls at 1 to 3% of the initial level as early as 30 days after birth (Chappuis, 1998). However, this drop of circulating MDA tends to be accelerated in presence of pathogens in the environment or due to repeated vaccinations, by consumption within the immune response. Immune complexes formed are then secreted into the intestinal lumen through FcγRn receptors (Rath et al., 2013; Greene and Schultz, 2006). The postnatal drop in MDA seems breed-dependent, with shorter half-time in rapidly growing puppies (Chappuis, 1998). In parallel to the decrease in MDA, the newborn dog is able to produce its own antibodies since birth, with a significant increase of antibody concentration visible as early as 14–21 days of age (Fig. 1).
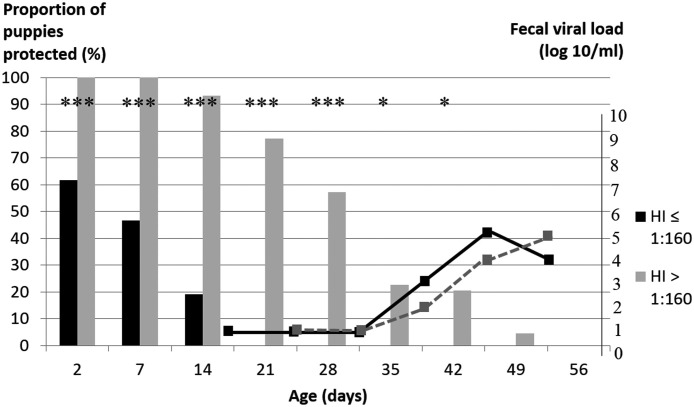
The quality of PIT at two days of age correlates with the specific immunity level and thus with health status of the neonate. In a kennel with spontaneous CPV2 circulation, puppies with CPV2-specific antibody titer above 1:160 at two days of age were longer protected against parvovirus infection and excreted CPV2 significantly later in the pre-weaning period than puppies with lower MDA levels (45 vs 38 days of age, respectively; Fig. 5 ). Mortality rates between 2 and 56 days of life were also significantly different in the two groups: 7% (3/45) and 26% (9/34), respectively (Mila et al., 2014b). The survival rate over the entire neonatal period is thus markedly influenced by the quality of specific PIT, with MDA probably preventing the infection thanks to virus neutralization (Mila et al., 2014b).
Fig. 5.
CPV2-specific antibody titers (histogram) and fecal viral loads (squares at Day39, 45, 53 of age). Puppies (n=79 from various breeds within one kennel) were classified in two groups depending on their titer at Day2 (hemagglutination inhibition): 45 puppies with HI titer > 1:160 (black), 34 puppies with HI ≤1:160 (grey). The protective titer is 1:80. Viral load was evaluated by RT PCR (significant above 102 copies/ml). NeoCare unpublished data. Methods are described by Mila et al. (2014b).
Although puppies with an adequate PIT may not exhibit any clinical signs of infection, they may still actively participate in the virus circulation by shedding virus (Elia et al., 2005). They may put at risk some vulnerable puppies (i.e. with failure of PIT), if introduced into a kennel naïve to CPV2. Indeed, MDA persistence at the time of vaccination at about 8 weeks of age is problematic to dog breeders. Although puppies are able to produce antibodies in response to natural infection or vaccination since birth (and even during the fetal life), seroconversion occurs only in the presence of very low levels of specific MDA (Gooding and Robinson, 1982; Chappuis, 1998; Toman et al., 2002; Day, 2007). A so called immunological gap appears, with remaining MDA preventing the mount of a correct response to vaccination whereas no more providing puppy with adequate immune protection (Decaro et al., 2005). Among 88 puppies vaccinated against CPV2 between 8 and 10 weeks of age, eight puppies did not develop a protective response due to the presence of MDA (Thibault et al., 2016). Taking into account the late presence of colostral MDA, a third vaccination at about 16 weeks of age was implemented in 2015 into the international recommended vaccination protocol (WSAVA, 2015; Day, 2017).
5.2. Local passive immune transfer
Beside the transfer of MDA, colostrum also ensures the acquisition of other immune compounds, suspected in the canine species based on information obtained in large animals. Colostrum -and later, milk- contributes to local digestive immunity by IgA, participating into the enteropathogens neutralization within the intestinal lumen. Except IgA, other nonspecific colostral compounds, such as lysozyme and lactoferrin, participate in the newborn immune response, even though they are considered of minor importance (Handl et al., 2009). In other domestic animals, white blood cells, such as macrophages, neutrophils and lymphocytes are other compounds of the PIT: these cells are able to cross the intestinal barrier and enhance neonatal immunity during the first month of life (Liebler-Tenorio et al., 2002; Langel et al., 2015, 2016). They release IgA locally when in contact with digestive pathogens (Wheeler et al., 2007). Globally, colostrum increases the digestive immunity thanks to the presence of non-specific antimicrobial factors, controls the development of the digestive microbiota, modulate development of Peyer’s patches and digestive epithelium, participate in the adequate immune response. Stimulation of the intestinal immune system (the most developed one of the entire organism) during the first days of life is essential to adapt the immune response and to limit infections, inflammatory diseases and allergies (Kelly and Coutts, 2000; Rogier et al., 2014). Facing the marked unexplained increase in the prevalence of dysimmune diseases and inflammatory processes (including obesity) in the dog (Sundburg et al., 2016; Banfield, 2018), the impact of systemic, but also digestive immunity established during the first days of life on the long-term health remains to be investigated.
6. Alternatives to colostrum for passive immune transfer
In some situations, colostrum is not available to puppies, or in limited quantities. In the absence of the dam, absence of maternal behavior, insufficient colostrum production according to the litter size and puppy’s inability to suckle, an alternative to maternal colostrum should be administrated. An optimal colostrum replacer should provide puppies with an adequate energy but also immune supply, the last one making this nutritional solution a challenge for veterinary industries. Numerous colostrum replacers are potentially available, homo- or heterologous, designed from mammary secretions, blood or egg products. Only a few of them were scientifically evaluated regarding puppies’ PIT and/or health. Besides the effect on the systemic PIT, evaluation of the local digestive immune action, as well as its short and long term consequences on the health status of the supplemented animal would be of interest. Whatever the type of colostrum replacer, it should be administrated, similarly as maternal colostrum, during the first eight hours of life, via a baby bottle or a feeding tube. Although bottle feeding is a procedure closer to the physiological suckling, feeding tube allows to control the volume and the time of colostrum ingestion. Dog breeders, often reluctant to tube feeding, should be encouraged to learn this technique, in order to deal with urgent situations and be able to increase chances of appropriate PIT.
6.1. Colostrum/milk
6.1.1. Canine colostrum
Frozen canine colostrum is to date the best alternative to maternal colostrum, as practiced in bovine and equine species. The optimal time for colostrum collection from the donor bitch is a compromise between the physiological drop in colostral immune quality after parturition (50% of the initial IgG concentration as early as 24 h post-partum) and the time required for the donor’s own litter to acquire PIT (intestinal barrier closure at 12–16 h of life). The donor bitch can thus be milked during its second day of lactation. Preferably, the colostrum should be obtained from bitches housed in the same kennel, recently boosted with vaccines before whelping, and whose previous litters exhibited low mortality rates and high growth rate over the first two months of age.
Manual milking of a bitch is rather easy to proceed, but oxytocin injection may be helpful in some females (1–2 UI SC a couple of minutes before milk collection). After teats are cleaned and dried, colostrum is collected into 1–5 ml plastic tubes to be immediately stored at −20 °C. Attention must be taken concerning the hygiene conditions at the collection, as the collected secretions are intended to be later administered to highly vulnerable individuals. In human medicine, the recommended maximum duration for frozen colostrum storage is 6 months; one year in bovine and equine neonatology. Thawing using microwave oven has to be avoided, as this process destroys immune potential of antibodies. It is rather recommended to thaw colostrum at 37 °C, preferably using baby-bottle warmer or water-bath. After thawing, colostrum is administrated to the newborn dog at the dose of 1.5 ml per 100 g of body weight.
To date, colostrum banking is the best solution aiming to replace the maternal colostrum, providing the newborn with energy and immunity, but also with hormones and growth factors, even though white blood cells are destroyed.
6.1.2. Canine mammary secretions after the colostral phase
Mean IgG concentration in canine mature milk is 1–2 g/L, compared with 20 g/L in the colostrum, and far below the minimal threshold avoiding failure of PIT in puppies. No data are available in puppies, but no significant increase in serum IgG concentration has been evidenced in kittens treated with mature milk of a donor queen (Claus et al., 2006).
6.1.3. Canine mammary secretion of pseudopregnancy
IgG concentration in pseudopregnancy secretions (11.6 ± 9.9 g/L) was not significantly different from those measured in the colostrum (18.0 ± 12.0 g/L), but significantly higher than in the mature milk (2.0 ± 1.3 g/L). Similarly, as for the colostrum, IgG concentration may vary by a factor of 1.6 to 8.8 for one given bitch (Abrard et al., 2018). Immune transfer obtained in puppies after ingestion of pseudopregnancy secretions is to be evaluated to validate their interest as colostral substitutes.
6.1.4. Bovine colostrum
Bovine colostrum is an alternative source of antibodies, easily available in large quantities. However, its immune interest for the canine newborn, never evaluated, would be probably limited, as bovine colostrum is free from antibodies directed against major canine pathogens, such as antibodies targeting CPV2.
6.1.5. Milk replacers
No canine immunoglobulins are present in industrial milks, modified from bovine milk. No PIT can thus be expected from these formulas.
6.2. Canine serum or plasma
Despite containing antibodies directed against canine pathogens, canine blood products display IgG concentration at about three times lower of that of the canine colostrum. In case of oral administration of canine serum to colostrum-deprived newborn puppies, the increase in their blood IgG concentration was insignificant compared with that achieved after ingestion of maternal colostrum and below the threshold defining failure in PIT (Poffenbarger et al., 1991; Bouchard et al., 1992; IgG threshold at 2.3 g/L from Mila et al., 2014a). Oral administration of the canine plasma during the first eight hours after birth to puppies with free access to the dam and the colostrum did not allow to reduce the proportion of puppies at deficit of PIT (Mila et al., 2014a), but a tendency for a lower morbidity rate in supplemented puppies was observed (Mila et al., 2017a). Only parenteral administration, with associated risks, was suggested to allow PIT: a subcutaneous injection of 2 ml or 4 mL/100 g body weight at birth allowed to reach mean IgG concentrations of respectively 2.6 and 4.5 g/l (Bouchard et al., 1992). However, some severe lesions have been reported after such a treatment: “impressive subcutaneous pockets of liquid” according to Bouchard et al. (1992) until large (>10 cm) skin necrotic zones in our own experience.
No study was conducted in puppies with administration of heterospecific serum for PIT acquisition. In kittens, equine serum and purified equine antibodies given orally transferred no significant passive immunity (Crawford et al., 2003). Besides the effects on systemic immunity, early supplementation (before the intestinal barrier closure) with canine plasma was associated to an increased diversity of microbial digestive communities, whose long term consequences remain to be explored (Mila et al., 2017a).
6.3. Hyperimmune egg powder
Hens vaccinated against canine antigens produce antibodies targeting those antigens. These antibodies (called IgY, for yolk immunoglobulins) are secreted in the egg yolk at high concentrations and can thus be easily available, in large amounts and in a non invasive way. Benefits of CPV2-specific and E. coli-specific IgY on the canine neonate have been recently demonstrated, as large breed puppies receiving IgY orally once before the intestinal barrier closure presented greater growth rate over the neonatal period (D0-D21) than controls: 824 ± 349 g in IgY supplemented puppies vs 662 ± 334 g in controls, n=334 puppies (Mila et al., 2017a). Such colostrum alternative containing CPV2-specific and E. coli-specific IgY is available for dog breeders (PuppyProTech, Royal Canin, Aimargues, France). IgY supplementation is a strategy validated in other species (but after intestinal barrier closure; Diraviyam et al., 2014) and promising for the canine newborns since IgY directed against numerous canine pathogenic bacteria (as for example Salmonella), viruses (canine coronavirus CCoV, canine herpesvirus CHV1 …) and parasites (Giardia) could be produced. IgY half-life in the puppy’s bloodstream is unknown to date, but probably short, as the half-life of IgY administrated to piglets at 10 h of life was of 1.85 h versus 12 days for homologous IgG (Yokoyama et al., 1993). Nevertheless, the effect of IgY administration in the newborn probably relies not only on passive immune transfer, and its role on the digestive microbiota and digestive immune competences are to be explored.
7. Conclusions
Systemic passive immune transfer in puppies depends mainly on the time elapsed between birth and colostrum ingestion rather than on the immune quality of colostrum. While PIT is recognized as essential for neonatal health in other species, such as bovine, equine and porcine, it is often neglected in the canine. Scientific knowledge in this area is to be transferred to breeders, encouraging colostrum banking and early colostrum ingestion. Controlled suckling in canine newborns could contribute to decrease neonatal mortality rate, improving financial situation in kennels and animal welfare.
Funding
Authors research on passive immune transfer in puppies was partially supported by Merial (Lyon, France) and Royal Canin R&D (Aimargues, France).
Declaration of Competing Interest
Authors patented antibodies against various pathogens administered before 24 h of age or between 24 h and up to 90 days of age in the dog to improve dog health (W02015004181). They designed and scientifically evaluated PuppyProTech - colostral alternative (Royal Canin, Aimargues, France).
Acknowledgments
Authors want to thank all breeders and all veterinary students who participated in improvement of knowledge about passive immune transfer in the dog, and particularly Charlotte Aggouni, Amélie Albaret, Morgane Delebarre, Leslie Garrier, Milène Gonnier, Barbara Hanse, Morgane Mantelli, Maelys Martin, Cynthia Olivier, Laurène Plante, Chloé Robic, Lisa Rossig, Camille Viaud.
Contributor Information
Sylvie Chastant, Email: s.chastant@envt.fr.
Hanna Mila, Email: h.mila@envt.fr.
References
- Abrard M., Ronsin P., Mila H., Chastant-Maillard S. Potential of mammary secretions during pseudopregnancy as colostral substitutes. Proceeding of the 21rd congress, European Veterinary Society for Small Animal Reproduction; Venise, Italy. 22-23 June; 2018. p 100. [Google Scholar]
- Aggouni C. Université Paul-Sabatier; Thèse Doct Vét, Toulouse: 2016. Étude de la qualité immunologique et énergétique du colostrum de la chienne: impact sur la santé du chiot. 2016. 94 p. [Google Scholar]
- Albaret A., Mila H., Grellet A., Chastant-Maillard S. Pattern of immunoglobulin G concentration in canine colostrum and milk during the lactation. Proceeding of the 8th International Symposium on Canine and Feline Reproduction; Paris, France. 22-25 June; 2016. p 93. [Google Scholar]
- Arteaga L., Rödel H.G., Elizalde M.T., Gonzalez D., Hudson R. The pattern of nipple use before weaning among littermates of the domestic dog. Ethology. 2013;119:12–19. [Google Scholar]
- Banfield Pet Hospital: State of Pet Health, 2018. https://www.banfield.com/state-of-pet-health.
- Bouchard G., Plata-Madrid H., Youngquist R.S., Buening G.M., Ganjam V.K., Krause G.F., Allen G.K., Paine A.L. Absorption of an alternate source of immunoglobulin in pups. Am. J. Vet. Res. 1992;53:230–233. [PubMed] [Google Scholar]
- Cabrera R.A., Lin X., Campbell J.M., Moeser A.J., Odle J. Influence of birth order, birth weight, colostrum and serum immunoglobulin G on neonatal piglet survival. J. Anim. Sci. Biotechnol. 2012;3:42. doi: 10.1186/2049-1891-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center S., Randolph J.F., ManWarren T., Slater M. Effect of colostrum ingestion on gamma-glutamyltransferase and alkaline phosphatase activities in neonatal pups. Am. J. Vet. Res. 1991;52:499–504. [PubMed] [Google Scholar]
- Cervenak J., Kacskovics I. The neonatal Fc receptor plays a crucial role in the metabolism of IgG in livestock animals. Vet. Immunol. Immunop. 2009;128:171–177. doi: 10.1016/j.vetimm.2008.10.300. [DOI] [PubMed] [Google Scholar]
- Chappuis G. Neonatal immunity and immunisation in early age: lessons from veterinary medicine. Vaccine. 1998;16:1468–1472. doi: 10.1016/S0264-410X(98)00110-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastant-Maillard S., Aggouni C., Albaret A., Fournier A., Mila H. Canine and feline colostrum. Reprod. Domest. Anim. 2017;52:148–152. doi: 10.1111/rda.12830. [DOI] [PubMed] [Google Scholar]
- Chastant-Maillard S., Freyburger L., Marcheteau E., Thoumire S., Ravier J.F., Reynaud K. Timing of the intestinal barrier closure in puppies. Reprod. Domest. Anim. 2012;47:190–193. doi: 10.1111/rda.12008. [DOI] [PubMed] [Google Scholar]
- Chastant-Maillard S., Marcheteau E., Freyburger L., Fontbonne A., Bergamo P., Ravier J.F., Reynaud K. Identification and quantification of immunoglobulins in canine colostrum - quantification of colostral transfer. Proceedings of 7th congress European Veterinary Society for Small Animal Reproduction; Louvain La Neuve, Belgium. 14-15 May; 2010. p107. [Google Scholar]
- Claus M.A., Levy J.K., MacDonald K., Tucker S.J., Crawford C. Immunoglobulin concentrations in feline colostrum and milk, and the requirement of colostrum for passive transfer of immunity to neonatal kittens. J. Feline Med. Surg. 2006;8:184–191. doi: 10.1016/j.jfms.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford P.C., Hanel R.M., Levy J.K. Evaluation of treatment of colostrum-deprived kittens with equine IgG. Am. J. Vet. Res. 2003;64:969–975. doi: 10.2460/ajvr.2003.64.969. [DOI] [PubMed] [Google Scholar]
- Day M.J. Immune system development in the dog and cat. J. Comp. Pathol. 2007;137:S10–S15. doi: 10.1016/j.jcpa.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Day M.J. Small animal vaccination: a practical guide for vets in the UK. In Pract. 2017;39:110–118. [Google Scholar]
- Decaro N., Campolo M., Desario C., Elia G., Martella V., Lorusso E., Buonavoglia C. Maternally-derived antibodies in pups and protection from canine parvovirus infection. Biologicals. 2005;33:261–267. doi: 10.1016/j.biologicals.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Diraviyam T., Bin Z., Yuan W., Ruediger S., Antonysamy M., Xiaoying Z. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elia G., Cavalli A., Cirone F., Lorusso E., Camero M., Buonavoglia D., Tempesta M. Antibody levels and protection to canine parvovirus type 2. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:320–322. doi: 10.1111/j.1439-0450.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J.H., Baker J.A., Burgher J., Robson D., Gilman B. The immune response of dogs to distemper virus. Cornell Vet. 1958;48:103–125. [PubMed] [Google Scholar]
- Gooding G.E., Robinson W.F. Maternal antibody, vaccination and reproductive failure in dogs with parvovirus infection. Aust. Vet. J. 1982;59:170–174. doi: 10.1111/j.1751-0813.1982.tb15997.x. [DOI] [PubMed] [Google Scholar]
- Greene C.E., Schultz R.D. Immunoprophylaxis. In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. 3rd ed. Saunders; Saint Louis: 2006. pp. 1069–1119. [Google Scholar]
- Gross J.J., Schüpbach-Regula G., Bruckmaier R.M. Colostrum immunoglobulin concentration in mammary quarters is repeatable in consecutive lactations of dairy cows. J. Anim. Sci. 2016;94:1755–1760. doi: 10.2527/jas.2016-0362. [DOI] [PubMed] [Google Scholar]
- Handl S., Wehr U., Zentek J., Krammer-Lukas S. Histological and immunohistochemical evaluation of duodenal and colonic biopsies after oral bovine lactoferrin supplementation in beagle puppies. J. Anim. Physiol. Anim. Nutr. 2009;93:76–82. doi: 10.1111/j.1439-0396.2007.00781.x. [DOI] [PubMed] [Google Scholar]
- Hurley W.L., Theil P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–474. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D., Coutts A.G.P. Early nutrition and the development of immune function in the neonate. Proc. Nutr. Soc. India. 2000;5:177–185. doi: 10.1017/s0029665100000197. [DOI] [PubMed] [Google Scholar]
- Klobasa F., Butler J.E. Absolute and relative concentrations of immunoglobulins G, M and A, and albumin in the lacteal secretions of sows of different lactation numbers. Am. J. Vet. Res. 1987;48:176–182. [PubMed] [Google Scholar]
- Langel S.N., Wark W.A., Garst S.N., James R.E., McGilliard M.L., Petersson-Wolfe C.S., Kanevsky-Mullarky I. Effect of feeding whole compared with cell-free colostrum on calf immune status: the neonatal period. J. Dairy Sci. 2015;98:3729–3740. doi: 10.3168/jds.2014-8422. [DOI] [PubMed] [Google Scholar]
- Langel S.N., Wark W.A., Garst S.N., James R.E., McGilliard M.L., Petersson-Wolfe C.S., Kanevsky-Mullarky I. Effect of feeding whole compared with cell-free colostrum on calf immune status: vaccination response. J. Dairy Sci. 2016;99:3979–3994. doi: 10.3168/jds.2015-9892. [DOI] [PubMed] [Google Scholar]
- Levieux D., Ollier A. Bovine immunoglobulin G, -lactalbumin and serum albumin in colostrum and milk during the early post partum period. J. Dairy Res. 1999;66:421–430. doi: 10.1017/s0022029999003581. [DOI] [PubMed] [Google Scholar]
- Liebler-Tenorio E.M., Riedel-Caspari G., Pohlenz J.F. Uptake of colostral leukocytes in the intestinal tract of newborn calves. Vet. Immunol. Immunop. 2002;85:33–40. doi: 10.1016/s0165-2427(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Liepman R.S., Dembek K.A., Slovis N.M., Reed S.M., Toribio R.E. Validation of IgG cut-off values and their association with survival in neonatal foals. Equine Vet. J. 2015;47:526–530. doi: 10.1111/evj.12428. [DOI] [PubMed] [Google Scholar]
- Mila H., Feugier A., Grellet A., Anne J., Gonnier M., Martin M., Rossig L., Chastant-Maillard S. Inadequate passive immune transfer in puppies: definition, risk factors and prevention in a large multi-breed kennel. Prev. Vet. Med. 2014;116:209–213. doi: 10.1016/j.prevetmed.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Mila H., Grellet A., Desario C., Feugier A., Decaro A., Buoavoglia C., Chastant-Maillard S. Protection against canine parvovirus type 2 infection in puppies by colostrum-derived antibodies. J. Nutr. Sci. 2014;3:1–4. doi: 10.1017/jns.2014.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mila H., Feugier A., Grellet A., Anne J., Gonnier M., Martin M., Rossig L., Chastant-Maillard S. Immunoglobulin G concentration in canine colostrum: evaluation and variability. J. Reprod. Immunol. 2015;112:24–28. doi: 10.1016/j.jri.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Mila H., Grellet A., Mariani C., Feugier A., Guard B., Suchodolski J., Steiner J., Chastant-Maillard S. Natural and artificial hyperimmune solutions: impact on health in puppies. Reprod. Domest. Anim. 2017;52:163–169. doi: 10.1111/rda.12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mila H., Grellet A., Mantelli M., Mariani C., Feugier A., Chastant-Maillard S. Indirect detection of passive immune transfer in puppies. Proceeding of the 20th International Congress of the European Veterinary Society for Small Animal Reproduction; Vienna, Austria. 29 June - 1 July; 2017. p 35. [Google Scholar]
- Mila H., Grellet A., Feugier A., Decaro N., Mariani C., Chastant-Maillard S. General and type 2 parvovirus-specific passive immune transfer in puppies – evaluation by early growth. Reprod. Domest. Anim. 2018;53:96–102. doi: 10.1111/rda.13334. [DOI] [PubMed] [Google Scholar]
- Mugnier A., Brévaux J., Mila H., Lyazrhi F., Mariani C., Adib-Lesaux A., Chastant-Maillard S., Grellet A. Low birth weight as a risk factor for early neonatal puppy mortality. Proceeding of the 21rd congress, European Veterinary Society for Small Animal Reproduction; Venise, Italy. 22-23 June; 2018. p 127. [Google Scholar]
- Poffenbarger E.M., Olson P.N., Chandler M.L., Seim H.B., Varman M. Use of adult dog serum as a substitute for colostrum in the neonatal dog. Am. J. Vet. Res. 1991;52:1221–1224. [PubMed] [Google Scholar]
- Pollock R.V., Carmichael L.E. Maternally derived immunity to canine parvovirus infection: transfer, decline, and interference with vaccination. J. Am. Vet. Med. Assoc. 1982;180:37–42. [PubMed] [Google Scholar]
- Rath T., Kuo T.T., Baker K., Qiao S.W., Kobayashi K., Yoshida M., Roopenian D., Fiebiger E., Lencer W.I., Blumberg R.S. The immunologic functions of the neonatal Fc receptor for IgG. J. Clin. Immunol. 2013;33:S9–17. doi: 10.1007/s10875-012-9768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogier E.W., Frantz A.L., Bruno M.E.C., Wedlund L., Cohen D.A., Stromberg A.J., Kaetzel C.S. Lessons from mother: long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes. 2014;5:663–668. doi: 10.4161/19490976.2014.969984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon H., Berri M., Gerdts V., Meurens F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009;33:384–393. doi: 10.1016/j.dci.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Schäfer-Somi S., Bär-Schadler S., Aurich J.E. Immunoglobulins in nasal secretions of dog puppies from birth to six weeks of age. Res. Vet. Sci. 2005;78:143–150. doi: 10.1016/j.rvsc.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Schäfer-Somi S., Bär-Schadler S., Aurich J.E. Proteinuria and immunoglobulinuria in neonatal dogs. Vet. Rec. 2005;157:378–382. doi: 10.1136/vr.157.13.378. [DOI] [PubMed] [Google Scholar]
- Sundburg C.R., Belanger J.M., Bannasch D.L., Famula T.R., Oberbauer A.M. Gonadectomy effects on the risk of immune disorders in the dog: a retrospective study. BMC Vet. Res. 2016;12:278. doi: 10.1186/s12917-016-0911-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault J.C., Bouvet J., Cupillard L., Guigal P.M. Evaluation of the impact of residual maternally-derived antibodies against canine parvovirus on the efficacy of a standard primary vaccination protocol. J. Vet. Intern. Med. 2016;30:413. [Google Scholar]
- Toman M., Faldyna M., Knotigova P., Pokorova D., Sinkora J. Postnatal development of leukocyte subset composition and activity in dogs. Vet. Immunol. Immunop. 2002;87:321–326. doi: 10.1016/s0165-2427(02)00058-2. [DOI] [PubMed] [Google Scholar]
- Viaud C. Université Paul-Sabatier; Thèse Doct Vet Toulouse. Toulouse: 2018. Le comportement de tétée du chiot et son implication dans le transfert passif d’immunité. 93 p. [Google Scholar]
- Weaver D.M., Tyler J.W., VanMetre D.C., Hostetler D.E., Barrington G.M. Passive transfer of colostral immunoglobulins in calves. J. Vet. Intern. Med. 2000;14:569–577. doi: 10.1892/0891-6640(2000)014<0569:ptocii>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Wheeler T.T., Hodgkinson A.J., Prosser C.G., David S.R. Immune components of colostrum and milk—a historical perspective. J. Mamm. Gland Biol. Neoplasia. 2007;12:237–247. doi: 10.1007/s10911-007-9051-7. [DOI] [PubMed] [Google Scholar]
- WSAVA . 2015. Guidelines for the Vaccination of Dogs and Cats.https://www.wsava.org/WSAVA/media/PDF_old/WSAVA-Vaccination-Guidelines-2015-Full-Version.pdf [Google Scholar]
- Wu W.Z., Wang X.Q., Wu G.Y., Kim S.W., Chen F., Wang J.J. Differential composition of proteomes in sow colostrum and milk from anterior and posterior mammary glands. J. Anim. Sci. 2010;88:2657–2664. doi: 10.2527/jas.2010-2972. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Peralta R.C., Sendo S., Ikemori Y., Kodama Y. Detection of passage and absorption of chicken egg yolk immunoglobulins in the gastrointestinal tract of pigs by use of enzyme-linked immunosorbent assay and fluorescent antibody testing. Am. J. Vet. Res. 1993;54:867–872. [PubMed] [Google Scholar]