Abstract
With the intention of developing a standardised method for assessment of pathogenicity of Cryptosporidium parvum, the CPB-0 isolate was studied by propagation in 1-day-old calves followed by inoculation into specific pathogen free (SPF) piglets. The experiment was repeated. Diarrhoea and shedding of oocysts were seen in all animals infected with the CPB-0 isolate. Clinical signs included depression, inappetence, vomiting (exclusively in the piglets), and death. Histological examination at 17 and 19 days post-infection revealed parasitic stages and microscopic changes primarily restricted to colon and rectum.
The unintended presence of rotavirus in some of the experimental animals revealed an additive or synergistic effect between rotavirus and C. parvum as indicated by prolonged diarrhoea, increased oocyst shedding, decreased weight gain and elevated levels of serum haptoglobin and serum amyloid A (SAA) in piglets infected simultaneously with both pathogens. The difference in daily weight gain between infected and control animals was significant only for piglets co-infected with rotavirus. The acute phase response of haptoglobin and SAA was characterised by a large individual variation. In piglets, co-infected with rotavirus, the levels of serum haptoglobin were 3.5 and 4.6 times higher in the infected versus the controls 6 and 9 dpi, respectively (mean values: 2411 μg/ml±S.D. 2023 and 1840 μg/ml±S.D. 1697). In the controls infected with rotavirus, peak haptoglobin concentration was seen 3 dpi (mean: 1022 μg/ml±S.D. 425). Elevated levels of SAA were seen in 1 of 6 piglets infected with C. parvum, and in 5 of 6 piglets co-infected with rotavirus. Tumour necrosis factor alpha (TNFα) was undetectable in all serum samples from piglets.
The obvious advantages of the SPF pig model are the naturally acquired intestinal microflora, the development of distinct clinical signs similar to cryptosporidiosis in humans and calves, the size of the animals, and the accessibility of individuals born within a short time span. This makes the model ideal for dose–response studies, evaluation of therapeutic agents as well as for assessment of differences in the clinical response to isolates of diverse genetic background. In conclusion, it was shown that the CPB-0 isolate was pathogenic to calves and piglets at a dose of 2.5×105 oocysts, and that the clinical signs could be replicated during separate experiments. Moreover, diarrhoea, oocyst shedding, body weight changes, histological alterations, and the acute phase response of haptoglobin and SAA were identified as useful parameters for discrimination of isolate-specific differences of pathogenicity.
Keywords: Cryptosporidium parvum, Pathogenicity, Oocyst shedding, Acute phase response, Cattle–protozoa, Pig–protozoa
1. Introduction
Our knowledge about the protozoan parasite Cryptosporidium parvum has increased considerably since the 1970s when it was first discovered as a cause of diarrhoea in humans and animals (Fayer et al., 1997), and today the organism is recognised as one of the most common opportunistic parasites (Guerrant, 1997). There are at least 11 Cryptosporidium species, and new species as well as genotypes are regularly described (Fayer et al., 2000, Fayer et al., 2001). While current studies concerning the genetic structure of Cryptosporidium have been numerous, the number of biological studies has been relatively restricted. Consequently, there is an urgent need for biological studies so that the genetic variation may be correlated with clinically important characters of significance for the diagnosis, treatment and control of cryptosporidiosis.
The severity of infection in both man and animals has been found to vary depending on the specific C. parvum isolate (Pozio et al., 1992, Tzipori et al., 1994, Okhuysen et al., 1999). Moreover, differences between isolates of C. parvum have been shown at the molecular level by several techniques among those, analysis of microsatellites. Thus, a recent study demonstrated the existence of four different alleles in Danish Cryptosporidium isolates of human and bovine origin, in addition to the genetically distinct porcine genotype, which was found in Danish pigs (Enemark et al., 2002). As a consequence, the present experiments were performed, the purposes being (1) to develop an animal model for pathogenicity studies of genetically diverse isolates, (2) to evaluate and identify parameters of pathogenicity including well-known parameters such as clinical signs, oocyst shedding and histopathological changes in the intestine, (3) to study the acute phase response of tumour necrosis factor alpha (TNFα), haptoglobin and serum amyloid A (SAA), and finally (4) to characterise the ‘Copenhagen calf laboratory isolate’ with special reference to virulence.
To establish a method in which the relatively small numbers of oocysts, purified from laboratory samples, could be used, an animal infection model system was developed, i.e. Cryptosporidium isolates were propagated initially in a calf, and the pathogenicity subsequently evaluated in a group of piglets. This system enables an indication of possible host spp. related differences in pathogenicity, although the effect of cryptosporidiosis in calves cannot be statistically evaluated on this background.
As model, neonatal piglets were chosen for the following reasons: (1) they are small compared to calves, thus they can be kept in containers which prevent the spread of cryptosporidia to the environment, (2) contrary to most mice models, they develop distinct clinical signs similar to cryptosporidiosis in calves and humans, (3) using specific pathogen free (SPF) piglets, the study of cryptosporidiosis in a natural intestinal micro-flora can be undertaken without the risk of several highly pathogenic infections. Furthermore, (4) the widely disseminated SPF system, ensure that large numbers of healthy piglets are easily obtainable under Danish conditions, and finally (5) heat synchronisation in sow-herds make piglets born within a short time span obtainable in adequate numbers for statistical comparison of groups.
The clinical response to cryptosporidiosis has been shown to differ in gnotobiotic and conventionally reared piglets (Vitovec and Koudela, 1992). In addition, colostrum deprived gnotobiotic piglets are more susceptible to infections, and do not possess a normal bacterial flora (Dom and Haesebrouck, 1992). On the basis of this, animals from commercial cattle and sow herds were chosen in preference to gnotobiots, to give a more realistic course of disease. Moreover, the neonates received colostrum during the first 24 h of their lives.
Previous studies have described cryptosporidiosis in pigs caused by inoculation of bovine derived Cryptosporidium oocysts. However, many of these studies used gnotobiotic pigs, did not include examination for other pathogens or used oocysts that were not characterised by molecular techniques. These circumstances were taken into account in the present study of C. parvum using the neonate calf/SPF pig model system.
2. Materials and methods
2.1. Inoculum
C. parvum, ‘the Copenhagen calf laboratory isolate’ (CPB-0) was isolated from a Danish dairy herd in 1990, and subsequently propagated in calves approximately every third month. Pathogenicity of the isolate has been demonstrated by several fatalities in calves caused by an inoculation dose of 5×106 (data not shown), thus the dose used for propagation has recently been changed to 2.5×105. The isolate has been characterised as genotype II by analysis of the oocyst wall protein (COWP) gene, the ‘bovine genotype’ by partial sequencing of the 18S rDNA, and assigned to subgenotype C2 by sequencing of a locus containing a GAG microsatellite (Caccio et al., 2000, Enemark et al., 2002).
Oocysts were concentrated with diethyl ether, as described by Peeters and Villacorta (1995), and stored in 2.5% aqueous potassium dichromate solution at 4 °C between inoculations. Prior to inoculation the oocysts were treated with 99.9 vol.% ethanol for 10 min, washed three times by centrifugation in sterile phosphate-buffered saline (PBS, pH 7.2) and counted using a haemocytometer. A minimum of five counts was done to get an accurate inoculum size. The inoculation dose used in both calves and piglets was 2.5×105 oocysts in one ml of PBS, given orally with a syringe.
2.2. DNA extraction and genetic analysis
Molecular characterisation was carried out before and after each passage of the isolate to ensure that the isolate had not been contaminated by wild type strains of C. parvum. DNA extraction, geno- and sub-genotype assignments were performed as described previously (Enemark et al., 2002).
2.3. Experimental animals
Each series of experimental infections consisted of propagation of the C. parvum isolate in a bull calf followed inoculation, into six piglets, with three additional piglets serving as negative controls (Fig. 1 ). In the present paper we report two such series of animals infected with the CPB-0 isolate. The isolate was propagated in a 1-day-old Red Danish Dairy breed calf, from a closed dairy herd with no known health problems in new-born calves. The calf was separated from the dam immediately after birth, and brought directly to the Danish Veterinary Institute in a sterile box without having had contact with the farm environment. During the first 24 h the calf was fed 2×2 l of colostrum, which was collected from several fresh cows from the herd of origin and pooled previous to freezing at −20 °C for at least 1 week. Subsequently the calf was fed a commercial cow milk replacer (Kalvital®, Løven Agro, DK) twice daily and gradually adapted to a calf starter and hay from 10 days of age. Water, available ad libitum, was boiled, whereas feed was either frozen to −80 °C or autoclaved prior to feeding.
Fig. 1.
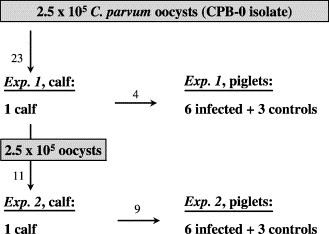
Experimental design for Experiments 1 and 2. Arrows indicate the flow as well as age of oocysts (in weeks) at inoculation.
Neonatal male piglets (Danish Landrace/Yorkshire crossbreds) were obtained from a SPF 1 herd. To prevent perinatal death the piglets were allowed to suckle their mother for 24 h before transportation to the institution in sterile boxes. The piglets were reared with a commercial sow milk replacer (Vital Somælk®, Løven Agro, DK) administered three times daily during the first 2 days, and subsequently twice daily. Food pellets were supplied from 15 days of age, and there was free access to boiled water. The piglets were inoculated with C. parvum when they were 2 days old. At 4 days of age organic iron 160 mg per piglet (Ferridex® vet., Rosco, DK) was administered intramuscularly.
Throughout episodes with diarrhoea the animals were given an oral electrolyte solution (ORS-mixture, The Royal Veterinary and Agricultural University, DK): calves 8–16 l daily via bottle, piglets ad libitum or 50–100 ml via tube 3–6 times daily, if the animals were severely dehydrated. During the first experiment, there was a delay in the administration of electrolytes, and therefore, the piglets did not receive electrolytes until 2 days after the initial signs. Experimental animals were killed by intravenously injection of a lethal dose of pentobarbital, 20 mg per ml; calves 19 days post-infection (dpi), piglets 17 dpi.
2.4. Experimental facilities and sanitary measures
The study was carried out under very strict precautions to minimise the possibility of contamination of the isolate, including totally separate facilities for infected and control groups. The piglets were kept in smooth plastic containers (137 cm×97 cm×92 cm), three animals together in each. Access to the stables was restricted to a few people who had no contact with other farm animals. Washing of hands, wearing sterile gloves, and change of clothing was obligatory prior to admittance. Between experiments the stables were cleaned mechanically with a high-pressure hose, fumigated with a potassium hydroxide, phosphor acid solution (trinol, Trinol A/S, DK), and disinfected with ammonium chloride (OO-cide®, Antec Int. Ltd., UK). Equipment was disinfected with 5% hydrogen peroxide, and frozen at −80 °C. As bedding, sterile sawdust pellets (Tørstrø, Dansk Træmel I/S, DK) and autoclaved straw was used.
2.5. Clinical examination
The experimental animals were examined at least twice daily for signs of clinical illness and the milk intake was recorded. In addition, daily faecal samples were collected directly from each animal and rectal body temperature was measured every day. Blood samples were taken from the jugular vein at irregular intervals in the calves, whereas blood samples from the piglets were taken every third day during the entire period with a single exception 9 dpi, due to severe clinical disease in one piglet. All piglets were weighed 3–4 times during the course of the experiment.
2.6. Faecal consistency and oocyst excretion
To classify the severity of diarrhoea, samples were scored on a scale from 0 to 3, as outlined in the text to Fig. 2 . Moreover, it was noted whether the faecal samples contained mucus or not. Shedding of C. parvum oocysts was analysed by a modified Ziehl-Neelsen technique (Henriksen and Pohlenz, 1981), and graded between 0 and 5 corresponding to the number of oocysts per slide (text to Fig. 2).
Fig. 2.
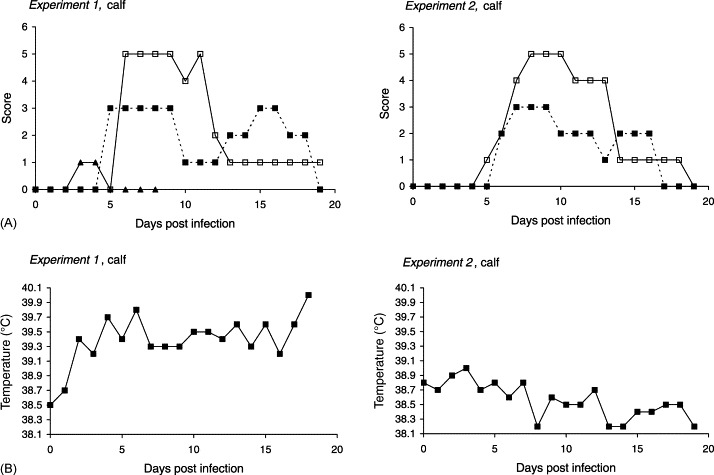
Infection dynamics of C. parvum (CPB-0 isolate) in two 1-day-old calves inoculated with 2.5×105 oocysts during separate experiments. (A) Patterns of diarrhoea, oocyst shedding, and excretion of rotavirus. Solid line (□), oocyst score: 0, no oocysts observed; 1, one or few oocysts per slide; 2, one oocyst per field of vision; 3, 2–5 oocysts per field of vision; 4, 6–10 oocysts per field of vision; 5, more than 10 oocysts per field of vision. Dotted line (■), faecal consistency (diarrhoea score): 0, normal; 1, pastose; 2, semi liquid; 3, watery diarrhoea. (▴) Detection of rotavirus: 0, not present; 1, present. (B) Body temperature.
2.7. Examination for other enteropathogens
Daily faecal samples from calves taken 0–7 dpi were analysed for co-infection with rota- and coronavirus by an enzyme linked immunosorbent assay (ELISA) (Grauballe et al., 1981). Furthermore, samples taken 0 dpi were examined by routine culture for Escherichia coli (E. coli), and a proportion of colonies screened for pilus antigens by slide agglutination. During episodes with diarrhoea additional samples were examined for Salmonella spp. by routine culture as well as for rotavirus, coronavirus, and E. coli. The same procedure, not including analysis for coronavirus, was followed in the piglets. Additionally, the presence of Isospora suis was examined 11 dpi by a modified McMaster technique (Henriksen, 1995). The occurrence of rotavirus in calves as well as in piglets was scored: 1, present; 0, not present.
2.8. Pathological examination
During post-mortem examination the gastrointestinal tract was given special attention in all test and control animals. Tissue samples, taken for each 50 cm throughout the intestines in calves, and from duodenum, jejunum (proximal, mid and distal), ileum, caecum, colon, and rectum in piglets, were fixed in 10% neutral buffered formalin within 15 min after euthanasia. Paraffin embedded tissue blocks were cut into 3 μm sections, stained by hematoxylin and eosin (H&E), and examined by light microscopy according to standard operation procedures. The degree of cryptosporidial infection as well as the range of histopathological alterations in the mucosa were given a score between 0 and 3 (Tzipori et al., 1981). For specific verification of cryptosporidia, especially during low-grade infection, immunohistochemistry was performed either as immunofluorescense (IF) detection with a FITC conjugated monoclonal antibody directed against the oocyst wall (M85, Microgen Bioproducts, UK) or as a two-step visualisation system (DAKO EnVision™, DAKO Corporation, USA), using the same antibody without FITC conjugation.
Animals dying during the experimental period were submitted to necropsy including a bacteriological examination of liver, spleen and intestines.
2.9. Haptoglobin level in serum
Bovine serum haptoglobin was determined by a sandwich ELISA (Godson et al., 1996) as described previously (Heegaard et al., 2000) using a pool of bovine serum as standard. The bovine serum standard was calibrated against affinity-purified bovine haptoglobin (Godson et al., 1996). The lower detection limit of the assay as defined by the linear range of the standard curve was 1.15 μg/ml. Serum samples were tested in serial dilutions of 1:100, 1:300, 1:900, resulting in a lower limit of detection of 115 μg/ml.
Porcine haptoglobin was analysed by an in-house sandwich ELISA, using an in-house monoclonal antibody directed against porcine haptoglobin (3.8/D7) as catching antibody, and biotinylated rabbit anti human haptoglobin (DAKO A0030) as detection antibody, followed by peroxidase-conjugated streptavidin (DAKO P397). As standard was used a pool of pig serum calibrated against a porcine haptoglobin standard from Saikin Kagaku Co. Ltd. (Japan). All samples were applied in duplicate and ODs were read at 490 nm subtracting 650 nm.
2.10. Serum amyloid A
The concentration of SAA in the porcine serum samples was assessed by a sandwich ELISA from Tridelta (Tridelta Development Ltd., Bray Co. Wicklow, Ireland) in accordance with the manufacturers instructions. The lower detection limit of the assay as defined by the linear range of the standard curve was 9.4 ng/ml. Serum samples were initially diluted 1:500, resulting in a lower limit of detection of 4.69 μg/ml. When concentrations exceeded the range of the assay, samples were diluted further and re-tested.
2.11. Tumour necrosis factor alpha level in serum
Duplicate serum samples from piglets were analysed by a commercial ELISA kit (Pig ELISA TNFα, Endogen, Inc., USA) according to manufactures instructions. A standard curve was generated from the OD values of increasing concentrations of recombinant porcine TNFα, and the TNFα level in the serum samples was calculated form the standard curve. The assays produced OD values in the range from 0.09 to 2.44 for standards containing 40–1500 pg/ml. Negative control preparations gave results below 0.03.
2.12. Statistical analysis
The units of analyses were the individual measures (diarrhoea and excretion of oocysts) or the difference between two measures (daily weight gain). To compare differences in the occurrence of diarrhoea between inoculated and control piglets, logistic regression of the proportion of days with diarrhoea in the experimental period was used. Logistic regression was performed by the GENMOD procedure in SAS© System for Elementary Statistical Analysis (SAS Institute, Inc., USA).
The t-tests, performed by the procedure TTEST in SAS, were used to compare differences in average daily weight gain during the experimental period between inoculated and control piglets.
Potential differences in the excretion of oocysts by inoculated piglets were tried estimated. It was assumed that the observed measurement of quantity of excreted oocysts, 0–5 on an ordinal scale, was the result of passing thresholds on an underlying latent continuous scale. By assuming a latent scale, it was possible to fit an ordinal probit model to data, where the potential difference of excretion of oocysts between the experiments was estimated. The estimate was adjusted for the effect of time relapse after inoculation by adding the linear effect as well as the squared linear effect of time to the model. Because the measures were obtained repeatedly from each individual animal, a pig-level random effect was added to the model to adjust for the dependency of measures obtained from the same individual. The model was estimated using the NLMIXED procedure in SAS. To evaluate the adequacy of the model assumptions and estimated values, the fit of the model was evaluated by inspection of plots in which predicted values of the model were superimposed on the observed values.
3. Results
3.1. Molecular characterisation of Cryptosporidium
Following passage through inoculated calves and piglets in Experiments 1 and 2, respectively, no genetic changes were observed. In other words, the isolate was characterised as genotype II by analysis of the COWP gene, the ‘bovine genotype’ by partial sequencing of the 18S rDNA, and assigned to subgenotype C2 by sequencing of a locus containing a GAG microsatellite. No other Cryptosporidium spp. or genotypes were detected.
3.2. Clinical signs and oocyst excretion
3.2.1. Calves
Experiment 1: Voluminous, watery diarrhoea was seen from 5 dpi, and continued with varying intensity until 18 dpi (Fig. 2A). During the third week of infection the faeces had high mucus content. Concurrent with incipient diarrhoea, the calf became mildly depressed and listless. Mild fever (temperatures between 39.6 and 40.0 °C) was present 4, 6 dpi, and towards the end of the experimental period (Fig. 2B). Regardless of this, the appetite was good throughout the entire period, and the calf remained relatively unaffected except from mild lethargy 9, 13 and 18 dpi. The prepatent period was 6 days with peak numbers of oocysts occurring from 6 to 12 dpi.
Experiment 2: Essentially, the clinical picture was repeated in the second experiment. Watery diarrhoea started 6 dpi simultaneously with the oocyst shedding, and continued until 16 dpi (Fig. 2A). Apathy, muscle tremor, and cold extremities were seen 8–9 dpi, but the appetite was retained throughout the period, and fever was not present (Fig. 2B).
3.2.2. Piglets
Experiment 1: The first clinical response, 2–3 days after inoculation, was vomiting in four of six experimentally infected piglets (lasting 1–3 days), succeeded by inappetence (1–8 days), depression and watery, yellow diarrhoea with clumps of mucus (1–6 days) (Table 1 , Fig. 3A ). Intermittent, dry coughing was observed 3–6 dpi in several piglets including the controls. Following the onset of vomiting and diarrhoea, subnormal temperatures (<38.8 °C) were observed in five of six piglets (Fig. 3C). A large individual variation in the clinical signs was seen between the piglets. Thus, one piglet had semi-liquid faeces for a single day succeeded by the excretion of high numbers of oocysts through several days without any clinical signs, whereas another piglet had to be euthanised 7 dpi due to dehydration and circulatory failure. The remaining piglets recovered after a period of diarrhoea, despite temperatures as low as 35.4 °C. Subnormal temperatures were also observed in controls as well as infected piglets 1 dpi, but this was normalised following adjustment of the heat lamps. Cryptosporidium oocysts in the faeces commenced from between 1 day before till 2 days after the onset of clinical illness and were shed for 12–16 days, i.e. all infected piglets, except the one that was euthanised, continued to shed oocysts after the diarrhoea had ended. Towards the end of the experiment oocysts were excreted intermittently by three piglets. Three littermates (controls) remained free from enteric signs as well as cryptosporidia throughout the study, however mild depression (0–7 dpi), and lameness on one leg (0–8 dpi) following injury during transport were seen in one piglet in this group (Table 1, Fig. 3B).
Table 1.
Infection dynamics of C. parvum (CPB-0 isolate) in piglets inoculated with 2.5×105 oocysts during repeated experiments
| Experiment 1 (n = 6)a | Experiment 2 (n = 6) | |
| Incubation period | 2.8 (range 2–4) | 2.3 (range 1–3) |
| Maximum diarrhoea score | 2.8 (range 2–3), 4 dpi | 2.7 (range 2–3), 4 dpi |
| Duration of diarrhoea (score = 2) | 3.5 days (range 1–6) | 6.0 days (range 3–7) |
| Prepatent period | 3.5 days (range 2–5) | 3.0 days (range 2–4) |
| Maximum oocyst score | 3.8, 4 dpi | 4.7, 6 dpi |
| Duration of oocyst excretionb | 12.3 days (5–16) | 14.3 days (13–16) |
| Daily weight gain (infected) | 279 g (±S.D. 77) | 247 g (±S.D. 25) |
| Daily weight gain (controls, n = 3) | 318 g (±S.D. 55) | 300 g (±S.D. 16) |
Experiment 1: mono-infection with C. parvum. Experiment 2: co-infection with rotavirus.
One piglet was euthanised 7 days post-infection because of poor health associated with diarrhoea.
Oocyst excretion was not completed at termination of the observational period.
Fig. 3.
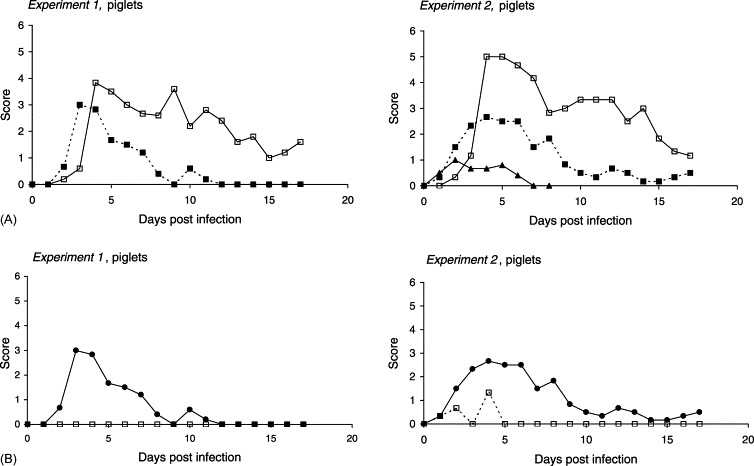
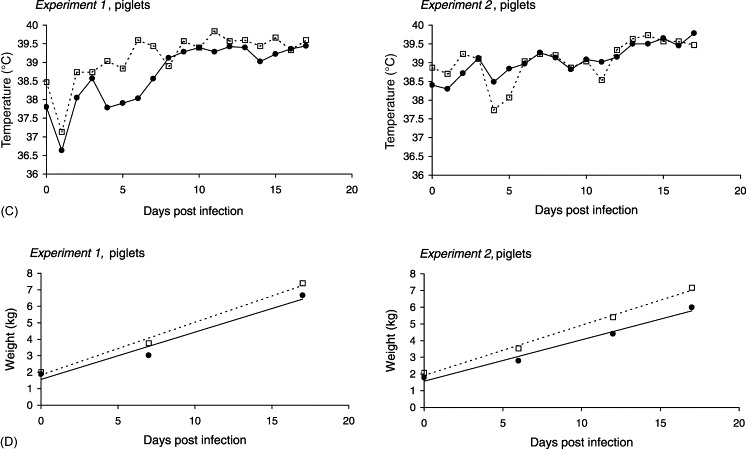
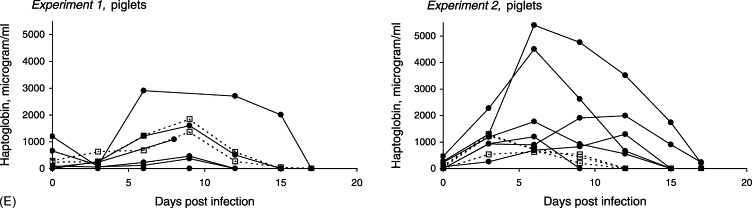
Infection dynamics of C. parvum (CPB-0 isolate) in 2-day-old piglets inoculated with 2.5×105 oocysts during separate experiments. (A) Patterns of diarrhoea, oocyst shedding, and excretion of rotavirus. Solid line (□): mean oocyst score. Dotted line (■): mean faecal consistency (diarrhoea score). (▴) Detection of rotavirus: 0, not present; 1, present. (B) Mean diarrhoea score. (•) Piglets mono-infected with C. parvum (Experiment 1); piglets experimentally infected with C. parvum and naturally infected with rotavirus (Experiment 2). (□) Uninfected controls (Experiment 1); controls naturally infected with rotavirus (Experiment 1). (C) Mean body temperature: (•) infected; (□) controls. (D) Mean body weight: (•) infected; (□) controls. (E) Individual serum haptoglobin response: (•) infected; (□) controls.
Experiment 2: At 1–3 dpi, three piglets vomited (1–2 days), and all of the six piglets developed anorexia (lasting 3–6 days) as well as diarrhoea (3–7 days). Lethargy and depression were present during periods with watery diarrhoea. Except for one piglet the onset of diarrhoea preceeded detection of oocysts in the faeces. As in the first experiment oocysts were shed intermittently by three animals towards the end of the period, and all but one excreted oocysts when they were killed 17 dpi (Table 1, Fig. 3A). Cryptosporidium oocysts were not detected in the control piglets. Nevertheless, these animals went through a short period, less than 24 h, of diarrhoea with scores between 1 and 3 (Fig. 3B) and a corresponding drop of body temperature (Fig. 3C).
The proportion of days with diarrhoea was significantly higher in the infected groups compared to the controls (P<0.0001) in both experiments. Compared to Experiment 1 the proportion of days with diarrhoea as well as the oocyst shedding was significantly higher in Experiment 2 (P=0.0007 and P=0.0341, respectively). The plots of predicted values of oocyst excretion correlated with the observed values indicating that the estimated probit model described the observed data relatively well.
The mean body weights of infected and controls, measured at different intervals throughout the study, are illustrated in Fig. 3D, whereas the mean daily weight gain of the different groups are shown in Table 1. The weight gain during the experimental period did not differ significantly between the control groups (P=0.5682). In contrast, the mean daily weight gain was larger in the controls compared to the infected animals (difference between infected and controls: 0.039 kg±0.068 S.D. (Experiment 1), 0.053 kg±0.023 S.D. (Experiment 2)), but the difference was only significant in Experiment 2 (P=0.4665, Experiment 1; P=0.0137, Experiment 2).
3.3. Examination for other enteropathogens
3.3.1. Calves
Experiment 1: A mixed culture of non-haemolytic E. coli was found during episodes with diarrhoea in addition to rotavirus, which were shed 3–4 dpi (Fig. 2A).
Experiment 2: Non-haemolytic E. coli were detected in the second calf, but no enterotoxigenic E. coli (ETEC-F5+), rota-, coronavirus or Salmonella were found.
3.3.2. Piglets
Experiment 1: Bacteriological examination of faecal samples revealed a mixed culture of non-haemolytic E. coli in a few samples from four of six piglets, but there was no histological evidence of coliform adherence to the mucosa in any of these piglets at necropsy. The majority of samples, including controls, contained no pathogenic bacteria, and neither rotavirus nor I. suis were found. By post-mortem examination of one piglet, which was euthanised prematurely, no bacteria were detected in the parenchymatous organs. A possible infectious cause to the respiratory signs was not further investigated.
Experiment 2: Non-haemolytic E. coli O8 in mixed culture was detected in one pig on one occasion 3 dpi. Virological examination of daily faecal samples from 0 to 8 dpi, demonstrated the presence of rotavirus in all but one animal, including the three controls. Excretion of rotavirus was detected from 2 to 3 dpi in the infected group (lasting 3–5 days), and from 3 to 4 dpi in the controls, that were still excreting virus 8 dpi, several days after any signs of clinical illness. Pastose stools lasting 2 days were seen in one of the controls; in another, faeces was semi-liquid for 1 day, whereas the third control piglet had watery diarrhoea at one sampling occasion without inappetence or any other clinical signs (Fig. 3B). No other pathogens were detected in samples from Experiment 2.
3.4. Pathology
3.4.1. Calves
Experiment 1: At autopsy, the only finding was moderately enlarged mesenteric lymph nodes. Histological examination of the intestine revealed no parasitic stages. However, stunting and fusion of villi, replacement of enterocytes by immature cells, and increased cellularity of lamina propria were observed focally in the distal jejunum, ileum and caecum.
Experiment 2: The mesenteric lymph nodes were moderately enlarged. Solitary cryptosporidia were observed in the distal colon and rectum. Stunting and fusion of villi, replacement of enterocytes by immature cells, increased cellularity and marked eosinophilia of lamina propria were seen in the terminal jejunum, ileum and colon.
3.4.2. Piglets
Experiment 1: Catarrhal enteritis and severe dehydration was seen in one piglet, which was euthanised 7 dpi because of poor health associated with diarrhoea. The content of the stomach and the small intestines was sparse and watery. In the remaining piglets, no gross lesions were detected neither among infected nor among controls following autopsy 17 dpi.
Parasitic stages and microscopic lesions were absent in the controls as well as in two infected piglets. With the exception of duodenum, Cryptosporidium stages were seen throughout the entire intestine of the one piglet that died prematurely, in addition to catarrhal enteritis and severe mucosal damage (Fig. 4 ). In the remaining piglets, infection was restricted to colon and rectum. Corresponding to the severity of the clinical signs, stunting and fusion of villi, and replacement of enterocytes by immature cells were present in two piglets.
Fig. 4.
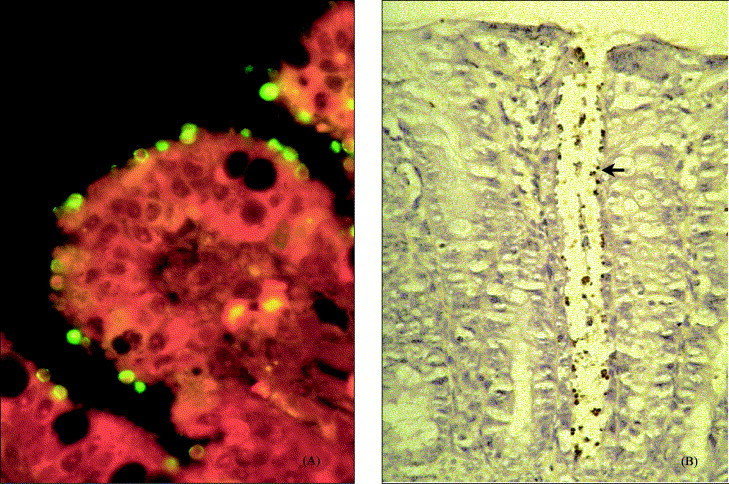
Histopathological slides (7 days post-infection) from a 9-day-old piglet experimentally infected with C. parvum (CPB-0 isolate). (A) Ileum mucosa showing stunting of villi, and numerous Cryptosporidium stages covering the surface (immunofluroescens staining; original magnification: 400×). (B) Colonic mucosa showing Cryptosporidium stages (arrow) confined to the crypt (immunohistochemistry; original magnification: 200×).
Experiment 2: Following necropsy, no macroscopic lesions were observed. Histological examination revealed low numbers of cryptosporidia in addition to lesions, characterised by replacement of enterocytes by immature cells in the colon and rectum of all experimentally infected piglets. Furthermore, stunting and fusion of villi as well as replacement of enterocytes by immature cells were observed in the jejunum of one control piglet despite normal consistency of faeces and the absence of macropic changes.
3.5. Haptoglobin level in serum
3.5.1. Calves
During the first experiment in which co-infection with rotavirus was detected, elevated levels of haptoglobin were observed 4 and 6 dpi (1427 and 553 μg/ml, respectively) with maximum concentration preceding the onset of diarrhoea. In contrast, no haptoglobin response was detectable in the second calf through the 19 days of evaluation, despite distinct clinical signs.
3.5.2. Piglets
Due to clinical illness (lameness and depression), the acute phase response was not evaluated in one of the controls. In the remaining controls of the first experiment elevated levels of haptoglobin were seen 3–12 dpi (Fig. 3E). A notable difference between the individual responses was observed among the infected animals according to the degree of clinical signs. Piglets least affected by cryptosporidiosis, showed no or very weak haptoglobin response, whereas those piglets that were most severely affected displayed distinctly elevated concentrations of haptoglobin 6–15 dpi. The highest concentrations were observed in the piglet, which also had the lowest growth rate and the most pronounced degree of mucosal changes following autopsy (with the exception of the piglet that died prematurely because of diarrhoea).
Elevated levels of haptoglobin were seen 3–9 dpi in the controls of Experiment 2, with peak values 3 dpi (mean: 1022 μg/ml±S.D. 425) corresponding to the mild diarrhoea caused by rotavirus infection (Fig. 3B and E). Consistent with the more protracted course of the diarrhoea, the haptoglobin response in the infected group was more pronounced compared to the controls as well as principals of Experiment 1, with elevated concentrations in all animals 3–15 dpi, peaking 6 dpi. On days 6 and 9 post-infection, the mean haptoglobin levels in serum were 3.5 and 4.6 times higher in the infected animals versus the controls (mean values: 2411 μg/ml±S.D. 2023 and 1840 μg/ml±S.D. 1697, respectively). One piglet, which was clinically less affected than the rest of the group (shortest period of watery diarrhoea, highest weight gain), also showed the weakest haptoglobin response.
3.6. Serum amyloid A
Of all animals from Experiment 1 only one piglet demonstrated a SAA response 3–6 dpi (145 and 280 μg/ml, respectively) in agreement with the haptoglobin response, the clinical signs and the mucosal changes. Following repetition of the study, SAA levels in the controls were normal despite rotavirus infection, whereas five of six piglets infected with C. parvum had elevated levels (peak concentrations: 219–345 μg/ml) at one or more sampling occasions between 3 and 9 dpi. The piglet showing the weakest haptoglobin response had SAA concentrations within normal limits throughout the period (data not shown).
3.7. TNFα level in serum
TNFα was undetectable, i.e. <40 pg/ml in all serum samples from piglets.
4. Discussion
We have developed a two-stage infection model that allows propagation of Cryptosporidium oocysts, and evaluation of pathogenicity. The experimental animals were obtained from commercial herds, artificially reared and brought up in isolation. SPF-piglets (born by normal farrowing with a naturally acquired intestinal microflora) were chosen because it is a well-known fact that a normal intestinal microflora exerts a barrier effect against enteropathogens, thus influencing the course of clinical disease (Hudault et al., 2001). Our model increases the risk of co-infection with other potentially pathogenic organisms, which makes the interpretation of the results more complicated. The advantage however, is that the model is less expensive than a gnotobiotic model, and allows the study of Cryptosporidium in a more natural setting of other micro-organisms.
Of the different parameters analysed in the present study the clinical response, i.e. the degree of diarrhoea and the body weight changes were the most obvious indicators of virulence. The histopathological changes in the gut in addition to changes in serum haptoglobin and SAA levels were also correlated with cryptosporidiosis, and showed promising qualities as indicators of pathogenicity. In the piglets, peak concentrations of oocysts in the faeces were correlated with diarrhoea in 11 of 12 animals, but oocyst shedding continued several days after termination of the diarrhoea. Thus, in accordance with results from calves (Jenkins et al., 1993), the detection of oocysts in faeces was not necessarily correlated with clinical cryptosporidiosis.
To be able to detect differences in the pathogenicity between isolates, a relatively low number 2.5×105 of oocysts was used in the present study, the aim being not to produce clinical signs in all cases unless a rather pathogenic isolate was used as inoculum. Other investigators have used doses of 106 to 107 C. parvum oocysts to induce clinical signs in experimentally infected piglets and calves (Tzipori et al., 1994, Fayer et al., 1998). In a study by Vitovec and Koudela (1992) self-limiting, watery diarrhoea lasting 2 days was produced in conventionally reared piglets infected with 5×106 oocysts, but no clinical signs were seen in conventionally reared piglets inoculated with 2.5×105 oocysts. In gnotobiotic piglets infected with 5×106 oocysts the clinical signs were more severe including death, and oocysts were still detectable in the faeces at termination of the experiment 16 dpi. In spite of the comparatively low inoculation dose used in our study, the pattern of oocyst shedding was similar to what was found by Tzipori et al. (1994) in piglets inoculated with 107 oocysts at 2 days of age. The clinical signs were consistent with previous descriptions of anorexia, vomiting, watery diarrhoea, dehydration and death in neonatal, experimentally infected piglets (Tzipori et al., 1981, Tzipori et al., 1994, Moon et al., 1982, Vitovec and Koudela, 1992). In contrast, no signs of clinical illness were seen in either new-born or weaned miniature piglets repeatedly inoculated with 106 to 107 C. parvum oocysts (Arnault et al., 1994). Neither were any clinical manifestations observed in experimentally infected piglets older than 15 days (Tzipori et al., 1982), and isolation studies as well as epidemiological surveys have shown that oocyst shedding was not statistically associated with diarrhoea in naturally infected pigs from conventional farms (Sandford, 1987, Quilez et al., 1996).
As regards the assessment of pathogenicity in calves, an evaluation based on observations in a single animal can only be indicative since a number of studies have shown noticeable individual variation in the severity of the clinical signs (Fayer et al., 1985, Fayer et al., 1998). Lethargy, inappetence and watery diarrhoea, the most prominent signs of calves infected with the CPB-0 isolate, have been described in numerous studies (Fayer et al., 1997). Nevertheless, comparison is difficult since several studies do not exclude the presence of other enteropathogens, and factors such as age at inoculation, inoculation dose, host breed, management, etc. varies between studies. Moreover, basic genetic information about the inoculum has been lacking. Thus, reports of natural infections and most experimental infections do not describe isolates by name or number.
The higher body temperature observed during the first calf experiment might have been the result of co-infection with rotavirus but it could also have been caused by natural individual variation between the calves. However, there seemed to be a tendency towards slightly earlier occurrence of diarrhoea in addition to severe diarrhoea for a prolonged period in the case of co-infection with rotavirus compared to mono-infection. This tendency was verified by Experiment 2 (inoculation of piglets co-infected with rotavirus) in which the duration of the diarrhoea was significantly longer than in piglets exclusively infected with cryptosporidia (Experiment 1).
Comparison of the pathogenic effect between isolates is only practicable on the assumption that the experimental conditions can be repeated, thus the controls of each experiment should not be significantly different. In the present study, no difference regarding growth was seen between the controls in the two experiments, moreover, the clinical signs were mild and of short duration in spite of rotavirus infection in one of the groups (Experiment 2). The fact that the difference between infected and controls was only significant in Experiment 2 was probably influenced by the presence of rotavirus in this group, the relatively low number of experimental animals in the study, and a large individual variation particularly among infected piglets in the first experiment.
There is only limited information available about the significance of the individual pathogens in combined infections. Rotaviruses have been described as important enteric pathogens in neonates of many species including calves and piglets. Under experimental conditions in gnotobiotic and colostrum-deprived pigs rotaviruses are able to produce severe gastroenteritis and villous atrophy. They are frequently detected in pigs with diarrhoea but subclinical infections are equally common (Paul and Stevenson, 1992). There is some evidence that combined infections with viruses, E. coli and Cryptosporidium spp. are frequent (Liebler et al., 1992), and that the presence of other pathogenic agents seems to enhance the development of clinical cryptosporidiosis (Fayer et al., 1985, Naciri, 1989). Han et al. (1995) confirmed this in a study in which groups of 10-day-old piglets were given either C. parvum, porcine rotavirus or both pathogens together. In accordance with our results, diarrhoea was most severe, faecal oocyst counts higher and the intestinal lesions more pronounced in piglets given both pathogens, suggesting an additive or synergistic effect between these pathogens. The current finding of elevated levels of haptoglobin and SAA in combined infections compared with mono-infected piglets, either with rotavirus or C. parvum, intimate a more severe outcome of concurrent infection with these organisms. The present results additionally suggest that the virulence of rotavirus in the absence of other pathogens can be negligible in conventional piglets even if they are infected at a very young age. Inoculation studies in which the serogroup of the virus is known and the dose of each pathogen is regulated are needed though to further elucidate the interaction between the organisms.
In the study by Tzipori et al. (1994), it was found that weight gain or loss in mice was not a consistent measure of infection or the impact of treatment. This is contrary to our results showing a larger weight gain in the control groups of both experiments compared to infected animals. Yet, daily registration of the body weight gain and evaluation earlier in the course of infection, e.g. 10 dpi might be a better indicator of pathogenicity based on the observation of an apparent tendency towards compensatory growth in the infected groups following termination of the diarrhoea.
Fever as a sign of cryptosporidiosis seems not to be a good marker based on the present findings. On the contrary a temperature drop was observed in both the calf (Experiment 2) and the piglets correlated with maximum diarrhoea and oocyst shedding.
Histopathological changes in the gut during the acute stages of infection have been well described in piglets. Additionally, it has been demonstrated that during the course of infection the mucosal changes move from the proximal to the distal part of the GI tract (Tzipori et al., 1982, Tzipori et al., 1994, Vitovec and Koudela, 1992). However, it has been shown that piglets killed 11 to 17 dpi had neither histological lesions nor any evidence of Cryptosporidium infection (Tzipori et al., 1981, Vitovec and Koudela, 1992). In the present study, moderate histopathological alterations in addition to developmental stages of C. parvum were seen predominantly in the colon and rectum of all experimentally infected animals except for two piglets, suggesting that histological changes even 17–19 dpi might be useful indicators of pathogenicity. Histological changes seen in the jejunum of one of the controls could probably be attributed to infection with rotavirus, which was demonstrated by ELISA. In the calves, no differences were observed between the two trials as regards the mucosal changes, despite the presence of rotavirus during the first study.
The effects of cytokines on cryptosporidiosis have been studied in different models including immunocompromised human patients (Flanigan et al., 1992, Gomez Morales et al., 1996), mice models (Ungar et al., 1991, Urban et al., 1996), and calves (de Graaf and Peeters, 1997, Fayer et al., 1998). Although our current understanding of the immune response to C. parvum is limited, several studies have shown a key role for interferon-γ (IFNγ) primarily in mediating innate resistance to C. parvum but also in the resolution of infection (reviewed by Theodos, 1998). In the present study we chose to focus on the dynamics of the cytokine TNFα and acute phase proteins, which have not previously been studied in pigs infected with cryptosporidia.
Although TNFα has been shown to be involved in the protective immunity to many pathogenic organisms (Vassalli, 1992), studies of the role for TNFα in the immune response to C. parvum have failed to demonstrate any significance (McDonald and Bancroft, 1994, Wyatt et al., 1997). Other experiments revealed that human intestinal cells produce TNFα following C. parvum infection (Seydel et al., 1998), but the expression of this cytokine did not correlate with enteric signs (Robinson et al., 2001). A recent study of C. parvum infected gamma interferon knockout mice showed that injection of TNFα significantly reduced oocyst shedding, suggestive of participation in the control of parasite development (Lacroix et al., 2001). In the present study, there was a lack of any detectable systemic TNFα response, suggesting that the role of TNFα, if any, might be restricted to a local response in the intestine, and therefore not suitable as a humoral indicator of C. parvum pathogenicity in piglets.
In contrast, the SPF piglet model seems to be relevant for the study of haptoglobin and SAA responses, as illustrated by the distinct reactions in those piglets most severely affected clinically. Haptoglobin and SAA are major acute phase proteins in most species studied (Mackiewicz et al., 1993), and well-known general indicators of inflammation, trauma and other pathological conditions (Kushner and Rzewnicki, 1994, Baumann and Gauldie, 1994). Serum haptoglobin has been shown to be highly correlated with the inoculation dose as well as with strain virulence in, e.g. mice and pigs inoculated with Toxoplasma gondii (Jensen et al., 1998, Jungersen et al., 1999), and therefore, potentially attractive as a measure of pathogenicity in hosts infected with other protozoa such as C. parvum. In the present study, increased serum levels of haptoglobin and SAA were observed during the first as well as the second experiment, i.e. irrespective of concurrent rotavirus infection. However, the responses were more dramatic in piglets infected with both pathogens as an indication of more severe enteric damage. In the infected piglets of Experiment 2, haptoglobin reached maximum concentrations up to above 5000 μg/ml while SAA increased up to 280 μg/ml. No SAA response was observed in the controls of Experiment 2, but a haptoglobin response with peak values 3 dpi (mean 1022 μg/ml) was found to be correlated to the mild diarrhoea caused by rotavirus. In Experiment 1, serum concentrations of haptoglobin peaked around 9 dpi in infected plus controls. In the light of this observation, it is concluded that the respiratory signs seen possibly as a result of cooling influenced the acute phase response. Therefore, the acute phase response to C. parvum cannot be evaluated based on this experiment. Nevertheless, there was a correlation to the clinical signs, as manifested by the clear response in one piglet, which also showed elevated levels of SAA, indicating that a measurable SAA response could only be induced by severe enteric signs. Thus, this acute phase protein might be a valuable tool to point out the most severely affected individuals.
In conclusion, the infection dynamics of C. parvum (CPB-0 isolate) was described in one calf (Experiment 2) and a group of piglets (Experiment 1) in which no other enteropathogens were detected. Further, it was shown that this particular isolate was pathogenic to calves as well as piglets even at a relatively low dose, and that the clinical signs could be replicated during separate experiments. Moreover, diarrhoea, oocyst shedding, body weight changes, histological alterations, and the acute phase response of haptoglobin and SAA were identified as useful parameters of pathogenicity. These results demonstrate that clinical cryptosporidiosis can be induced in SPF piglets with a naturally acquired intestinal microflora, and thus should be included as a causative agent of diarrhoea in young pigs.
Diarrhoea and shedding of oocysts were present in animals infected with the CPB-0 isolate independent of simultaneous infection with rotavirus. In addition, the unintended presence of rotavirus in some of the experimental animals revealed an additive or synergistic effect between rotavirus and C. parvum as demonstrated by the prolonged diarrhoea, increased oocyst shedding, decreased weight gain and elevated levels of serum haptoglobin and SAA in piglets infected simultaneously with both pathogens.
The obvious advantages of the SPF pig model are the naturally acquired intestinal microflora, the development of distinct clinical signs similar to cryptosporidiosis in humans and calves, the size of the animals, and the accessibility of individuals born within a short time span. This makes the model ideal for dose–response studies, evaluation of therapeutic agents as well as for assessment of differences in the clinical response to isolates of diverse genetic background.
Acknowledgements
The authors would like to thank John Pedersen and his staff for vigorous disinfection of the stables, and Cynthia D. Juel, Annie Ravn, Ulla L. Andreasen, Heidi G. Pedersen and Tine Petersen for skilled technical assistance. This work was carried out as part of the Danish research programme: Food quality with a focus on food safety (FØSI00-SVS-8).
Footnotes
Tested free from Sarcoptes scabiei, Mycoplasma hyopneumoniae, Actinobacillus pleuropneumoniae, toxin positive Pasteurella multocida, Brachyspira hyodysenteriae, and porcine respiratory and reproductive syndrome (PRRS) virus.
References
- Arnault I, Répérant J.M, Naciri M. Humoral antibody response and oocyst shedding after experimental infection of histocompatible new-born and weaned piglets with Cryptosporidium parvum. Vet. Res. 1994;25:371–383. [PubMed] [Google Scholar]
- Baumann H, Gauldie J. The acute phase response. Immunol. Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Cacciò S, Homan W, Camilli R, Traldi G, Kortbeek T, Pozio E. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology. 2000;120:237–244. doi: 10.1017/s0031182099005508. [DOI] [PubMed] [Google Scholar]
- de Graaf D.C, Peeters J. Specific interferon-gamma IgA and IgM responses after experimental infection of neonatal calves with Cryptosporidium parvum. Int. J. Parasitol. 1997;27:131–134. doi: 10.1016/s0020-7519(96)00167-1. [DOI] [PubMed] [Google Scholar]
- Dom P, Haesebrouck F. Comparative virulence of NAD-dependent and NAD-independent Actinobacillus pleuropneumoniae strains. J. Vet. Med. 1992;39:303–306. doi: 10.1111/j.1439-0450.1992.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Enemark H.L, Ahrens P, Juel C.D, Petersen E, Petersen R.F, Andersen J.S, Lind P, Thamsborg S.M. Molecular characterization of Danish Cryptosporidium parvum isolates. Parasitology. 2002;125:331–341. doi: 10.1017/s0031182002002226. [DOI] [PubMed] [Google Scholar]
- Fayer R, Ernst J.V, Miller R.G, Leek R.G. Factors contributing to clinical illness in calves experimentally infected with a bovine isolate of Cryptosporidium. Proc. Helminthol. Soc. Wash. 1985;52:64–70. [Google Scholar]
- Fayer, R., Speer, C.A., Dubey, J.P., 1997. The general biology of Cryptosporidium. In: Fayer, R. (Ed.), Cryptosporidium and Cryptosporidiosis. CRC Press, Boca Raton, pp. 1–41.
- Fayer R, Gasbarre L, Pasquali P, Canals A, Almeria S, Zarlenga D. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int. J. Parasitol. 1998;28:49–56. doi: 10.1016/s0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Fayer R, Morgan U, Upton S.J. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 2000;30:1305–1322. doi: 10.1016/s0020-7519(00)00135-1. [DOI] [PubMed] [Google Scholar]
- Fayer R, Trout J.M, Xiao L, Morgan U.M, Lal A.A, Dubey J.P. Cryptosporidium canis n. sp. from domestic dogs. J. Parasitol. 2001;87:1415–1422. doi: 10.1645/0022-3395(2001)087[1415:CCNSFD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Flanigan T, Whalen C, Turner J, Soave R, Toerner J, Havlir D, Kotler D. Cryptosporidium infection and CD4 counts. Ann. Intern. Med. 1992;116:840–842. doi: 10.7326/0003-4819-116-10-840. [DOI] [PubMed] [Google Scholar]
- Godson D.L, Campos M, Attah-Poku S.K, Redmond M.J, Cordeiro D.M, Sethi M.S, Harland R.J, Babiuk L.A. Serum haptoglobin as an indicator of the acute phase response in bovine respiratory disease. Vet. Immunol. Immunopathol. 1996;51:277–292. doi: 10.1016/0165-2427(95)05520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez Morales M.A, Ausiello C.M, Guarino A, Urbani F, Spanuolo M.I, Pignata C, Pozio E. Severe protracted intestinal cryptosporidiosis associated with interferon γ deficiency: pediatric case report. Int. J. Parasitol. 1996;22:848–850. doi: 10.1093/clinids/22.5.848. [DOI] [PubMed] [Google Scholar]
- Grauballe P.C, Vestergaard B.F, Meyling A, Genner J. Optimized enzyme-linked immunosorbent assay for detection of human and bovine rotavirus in stools: comparison with electron-microscopy, immunoelectro-osmophoresis, and fluorescent antibody techniques. J. Med. Virol. 1981;7:29–40. doi: 10.1002/jmv.1890070104. [DOI] [PubMed] [Google Scholar]
- Guerrant R.L. Cryptosporidiosis: an emerging, highly infectious threat. Emerg. Infect. Dis. 1997;3:51–57. doi: 10.3201/eid0301.970106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D.U, Kang M.I, Park N.Y, Wee S.H. Pathogenesis of enteritis induced by Cryptosporidium parvum alone and combined with porcine rotavirus in piglets. Korean J. Vet. Res. 1995;35:149–158. [Google Scholar]
- Heegaard P.M.H, Godson D.L, Toussaint M.J, Toornehooj K, Larsen L.E, Viuff B, Rønsholt L. The acute phase response of haptoglobin and serum amyloid A (SAA) in cattle undergoing experimental infection with bovine respiratory syncytial virus. Vet. Immunol. Immunopathol. 2000;77:151–159. doi: 10.1016/S0165-2427(00)00226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen, S.A., 1995. Eimeria and Isospora. In: Eckert, J., Braun, R., Shirley, M.W., Coudert, P. (Eds.), Biotechnology—Guidelines on Techniques in Coccidiosis Research. Office for Official Publications of the European Communities, Luxembourg, pp. 74–78.
- Henriksen S.A, Pohlenz J.F.L. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet. Scand. 1981;22:594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudault S, Guignot J, Servin A.L. Escherichia coli strains colonising the gastrointestinal tract protect germfree mice against Salmonella typhimurium infection. Gut. 2001;49:47–55. doi: 10.1136/gut.49.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins M.C, Fayer R, Tilley M, Upton S.J. Cloning and expression of a cDNA encoding epitopes shared by 15- and 60-kilodalton proteins of Cryptosporidium parvum sporozoites. Infect. Immun. 1993;61:2377–2382. doi: 10.1128/iai.61.6.2377-2382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L, Heegaard P.M.H, Lind P. A study of virulence parameters for Toxoplasma gondii infections in mice. Parasitol. Res. 1998;84:382–387. doi: 10.1007/s004360050414. [DOI] [PubMed] [Google Scholar]
- Jungersen G, Jensen L, Riber U, Heegaard P.M.H, Petersen E, Poulsen J.S.D, Bille-Hansen V, Lind P. Pathogenicity of selected Toxoplasma gondii isolates in young pigs. Int. J. Parasitol. 1999;29:1307–1319. doi: 10.1016/s0020-7519(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D.L. The acute phase response: general aspects. Ballière’s Clin. Rheumatol. 1994;8:513–530. doi: 10.1016/s0950-3579(05)80113-x. [DOI] [PubMed] [Google Scholar]
- Lacroix S, Manacassola R, Naciri M, Laurent F. Cryptosporidium parvum-specific mucosal immune response in C57BL/6 neonatal and gamma interferon-deficient mice: role of tumour necrosis factor alpha in protection. Infect. Immun. 2001;69:1635–1642. doi: 10.1128/IAI.69.3.1635-1642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler, E.M., Pohlenz, J.F., Whipp, S.C., 1992. Digestive system. In: Leman, A.D., Straw, B.E., Mengeling, W.L., D’Allaire, S., Taylor, D.J. (Eds.), Diseases of Swine. Iowa State University Press, Iowa, pp. 12–20.
- Mackiewicz, A., Kushner, I., Baumann, H. (Eds.), 1993. Acute phase proteins molecular biology, biochemistry and clinical applications. CRC Press, Boca Raton, FL.
- McDonald V, Bancroft G. Mechanisms of innate and acquired resistance to Cryptosporidium parvum infection in SCID mice. Parasite Immunol. 1994;16:315–320. doi: 10.1111/j.1365-3024.1994.tb00354.x. [DOI] [PubMed] [Google Scholar]
- Moon H.W, Schwartz A, Welch M.J, McCann P.P, Runnels P.L. Experimental fecal transmission of human cryptosporidia to pigs, and attempted treatment with an Ornithine Decarboxylase inhibitor. Vet. Pathol. 1982;19:700–707. doi: 10.1177/030098588201900615. [DOI] [PubMed] [Google Scholar]
- Naciri, M., 1989. Animal and human cryptosporidiosis: opportunist infections? Pathogenicity of the genus Cryptosporidium. In: Yvore, P. (Ed.), Coccidia and Intestinal Coccidiomorphs. Proceedings of the 5th International Coccidiosis Conference. INRA Publ., pp. 51–63.
- Okhuysen P.C, Chappell C.L, Crabb J.H, Sterling C.R, DuPont H.L. Virulence of three distinct Cryptosporidium parvum isolates for healthy adults. J. Infect. Dis. 1999;180:1275–1281. doi: 10.1086/315033. [DOI] [PubMed] [Google Scholar]
- Paul, P.S., Stevenson, G.W., 1992. Rotavirus and reovirus. In: Leman, A.D., Straw, B.E., Mengeling, W.L., D’Allaire, S. (Eds.), Diseases of Swine. Iowa State University Press, Iowa, pp. 331–348.
- Peeters, J.E., Villacorta, I., 1995. Cryptosporidium. In: Eckert, J., Braun, R., Shirley, M.W., Coudert, P. (Eds.), Biotechnology—Guidelines on Techniques in Coccidiosis Research. Office for Official Publications of the European Communities, Luxembourg, pp. 202–240.
- Pozio E, Morales M.A.G, Barbieri M, La Rosa G. Cryptosporidium: different behaviour in calves of isolates of human origin. Trans. R. Soc. Trop. Med. Hyg. 1992;86:636–638. doi: 10.1016/0035-9203(92)90165-9. [DOI] [PubMed] [Google Scholar]
- Quilez J, Area-Mazás E, Sanchez-Acedo C, del Cacho E, Clavel A. Comparison of oocyst shedding and the serum immune response to Cryptosporidium parvum in cattle and pigs. Parasitol. Res. 1996;82:529–534. doi: 10.1007/s004360050157. [DOI] [PubMed] [Google Scholar]
- Robinson P, Okhuysen P.C, Chappell C.L, Lewis D.E, Shahab I, Janecki A, White A.C., Jr. Expression of tumour necrosis factor alpha and interleukin 1beta in jejuna of volunteers after experimental challenge with Cryptosporidium parvum correlates with exposure but not with symptoms. Infect. Immun. 2001;69:1172–1174. doi: 10.1128/IAI.69.2.1172-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandford S.E. Enteric cryptosporidial infection in pigs: 184 cases (1981–1985) JAVMA. 1987;190:695–698. [PubMed] [Google Scholar]
- Seydel K.B, Zhang T, Champion G.A, Fichtenbaum C, Swanson P.E, Tzipori S, Griffiths J.K, Stanley S.L, Zhang T.H. Cryptosporidium parvum infection of human intestinal xenografts in SCID mice induces production of human tumour necrosis fractor alpha and interleukin-8. Infect. Immun. 1998;66:2379–2382. doi: 10.1128/iai.66.5.2379-2382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodos C.M. Innate and cell-mediated immune responses to Cryptosporidium parvum. Adv. Parasitol. 1998;40:88–119. doi: 10.1016/s0065-308x(08)60118-9. [DOI] [PubMed] [Google Scholar]
- Tzipori S, McCartney E, Lawson G.H.K, Rowland A.C, Campbell I. Experimental infection of piglets with Cryptosporidium. Res. Vet. Sci. 1981;31:358–368. [PubMed] [Google Scholar]
- Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model system for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin. Diagn. Lab. Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzipori S, Smith M, Makin T, Halpin C. Enterocolitis in piglets caused by Cryptosporidium sp. purified from calf faeces. Vet. Parasitol. 1982;11:121–126. doi: 10.1016/0304-4017(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Ungar B.L.P, Kao T, Burris J.A, Finkelman F.D. Cryptosporidium infection in an adult mouse model: independent roles for IFN-γ and CD4+ T lymphocytes in protective immunity. J. Immunol. 1991;147:1014–1022. [PubMed] [Google Scholar]
- Urban J.F, Fayer R, Chen S, Gause W.C, Gately M.K, Finkelman F.D. IL-12 protects immunocompetent and immunodeficient neonatal mice against infection with Cryptosporidium parvum. J. Immunol. 1996;156:263–268. [PubMed] [Google Scholar]
- Vassalli P. The pathophysiology of tumour necrosis factors. Annu. Rev. Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- Vitovec J, Koudela B. Pathogenesis of intestinal cryptosporidiosis in conventional and gnotobiotic piglets. Vet. Parasitol. 1992;43:25–36. doi: 10.1016/0304-4017(92)90045-b. [DOI] [PubMed] [Google Scholar]
- Wyatt C.R, Brackett E.J, Perryman L.E, Rice-Ficht A.C, Brown W.C, O’Rourke K.I. Activation of intestinal intraepihelial T lymphocytes in calves infected with Cryptosporidium parvum. Infect. Immun. 1997;65:185–190. doi: 10.1128/iai.65.1.185-190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


