Abstract
Avian infectious bronchitis virus (IBV) is a coronavirus which infects chickens and causes severe economic losses to the poultry industry worldwide. MicroRNAs (miRNAs) are important intracellular regulators and play a pivotal role in viral infections. In previous studies, we have revealed that IBV infection caused a significant down-regulation of gga-miR-30d expression in chicken kidneys. In present study, we investigated the role of gga-miR-30d in the process of IBV infection of HD11 cell line in vitro. By transfecting the mimics and inhibitor of gga-miR-30d, it was found that overexpressed gga-miR-30d inhibited IBV replication. Contrarily, low-expressed gga-miR-30d promoted IBV replication. In addition, dual-luciferase reporter assays revealed that ubiquitin-specific protease 47 (USP47), a deubiquitinase-encoding gene, was a target for gga-miR-30d. This is the first study demonstrating that miRNAs regulate IBV replication by regulating the deubiquitinating enzyme (DUBs).
Keywords: IBV, miR-gga-30d, HD11, USP47
Highlights
-
•
Gga-miR-30d, a microRNA encoded by HD11 cells, regulates IBV infection by targeting USP47.
-
•
This is the first study demonstrating that miRNAs regulate IBV replication by regulating the deubiquitinating enzyme (DUBs).
-
•
Our results highlight the importance of the ubiquitin-deubiquitinase system in virus-cell interactions.
1. Introduction
Infectious bronchitis (IB), caused by infectious bronchitis coronavirus (IBV), is a highly contagious viral disease of the chicken [1]. Since IB was first described by Schalk and Hawn in USA in 1931 [2], it has developed into one of the foremost causes of economic loss in the poultry industry, affecting the performance of both meat-type and egg-laying chickens. IBV is an enveloped, non-segmented, single-stranded, positive-sense RNA virus, classified taxonomically as a member of genus Gammacoronavirus, family Coronaviridae [3]. Initially, this pathogen replicated primarily in the respiratory tract, causing a highly contagious respiratory disease characterized by nasal discharge, snicking, rales, and tracheal ciliostasis in chickens, but later reports demonstrated that it also replicated in many other epithelial surfaces, including enteric surfaces, oviducts, and kidneys [4]. Although vaccination is the most reliable approach to control IBV, the high mutation rate and recombination events differentiate IBV into numerous genotypes and serotypes, causing poor cross-protection of vaccines [5]. Therefore, it is urgent to apply new technologies and methods to control IBV.
MicroRNAs (miRNAs) are small, non-coding RNAs 18–22 nt in length. MiRNAs usually affect gene expression by directing repressive protein complexes to the untranslated region (UTR) of target messenger RNA (mRNA) transcripts in a sequence-specific manner. Up to now, Out of over 24,000 miRNAs have been identified, including 734 mature miRNAs from Gallus gallus [6,7] and even 295 mature miRNAs encoded by viruses [Mirbase]. These huge amounts of miRNAs are implicated in almost every cellular process, including cell proliferation, differentiation, apoptosis and host-pathogen interactions [8]. Especially, in viral infections, miRNAs have been confirmed play crucial regulatory roles. For instance, miRNA-4776 was related to the survival of influenza virus [9]. MiR-3470b promoted bovine ephemeral fever virus (BEFV) replication in baby hamster Syrian kidney cells [10] and bta-miR-2361 inhibited bovine herpes virus 1 (BHV1) replication by directly targeting EGR1 gene [11]. IBV, as a virus that affects the global poultry industry, has been studied from various angles. However, few studies have reported about the miRNAs alterations in the post infection of IBV in host cell lines, such as HD11 cell line.
In previous study, by high-throughput sequencing of small RNA libraries in IBV-infected chicken kidney, we found seven highly differentially expressed miRNAs (gga-miR-30d, gga-miR-1454, gga-miR-7b, gga-miR-215-5p, gga-miR-1a-3p, gga-miR-3538 and gga-miR-2954) [12], and those miRNAs were considered to play an important role in IBV-host interactions. The significant down-regulation of gga-miR-30d caught our attention, for gga-miR-30d is a member of the miR-30 family which plays a key role in many viral infections [[13], [14], [15]]. But whether gga-miR-30d plays a role in IBV infection remains obscure.
In present study, HD11, an avian macrophage-like cell line, was been used as an infection platform to infect IBV. In addition, HD11 cells were transfected with the gga-miR-30d mimics or inhibitor to evaluate whether gga-miR-30d expression had potential effects on IBV replication. Our results shown that gga-miR-30d was a key regulator of IBV infection. Furthermore, gga-miR-30d regulated the replication of IBV by targeting the 3′-UTR of USP47. USP47 encodes a deubiquitinating enzyme (DUB) in cells, and its role in viral infection is gradually emerging [16]. This is the first report on miRNA regulation of IBV infection by regulating the DUBs, which providing a new theoretical basis for controlling IBV.
2. Materials and methods
2.1. Cells and virus
HD11 cell line was kindly provided by Prof. Xin-An Jiao, Yang Zhou University. The cells were been cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, USA), 100 IU/mL penicillin and 100 μg/mL streptomycin sulfate. IBV Beaudette strain (genbank:DQ001339) was kindly gift from Prof. Ding-xiang Liu, Nanyang Technological University.
2.2. Virus infection and virus titration
HD11 cells were seeded at a density of 60–70% per well in six-well plates. Then the cells were infected with IBV Beaudette (Multiplicity of Infection, MOI = 10) and incubated in 5% CO2 at 37 °C for 1 h. Following the incubation, the cells were rinsed with phosphate buffered saline (PBS), and cultured in 37 °C incubator with DMEM supplemented with 2% FBS (5% CO2). Controls were the cells that were mock infected. Viral titers were measured by determining the 50% tissue culture infectious dose (TCID50) according to the Reed-Muench calculation method [17].
2.3. RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was prepared from HD11 cells by Trizol reagent (Invitrogen, USA) according to the manufacturer's protocol, and 1 μg of total RNA was used for cDNA synthesis by reverse transcription, using a PrimeScript™ RT reagent Kit (TaKaRa, Japan). Relative qRT-PCR was performed to analysis the expression level of predicted target genes, gga-miR-30d and quantification of virus in HD11 cells and fold changes were calculated using the 2-ΔΔCt method. Primers used for qPCR as shown in Supplementary Table 1 . The specific stem loop primers (5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTTCCA-3′) for gga-miR-30d were synthesized at Ribobio (Guangzhou, China), using U6 as reference gene. The primers for the predicted target genes and IBV N gene were synthesized at Tsingke (Beijing, China), using β-actin as reference gene. The SYBR green Ex Taq premix for qPCR was purchased from TaKaRa.
2.4. Gga-miR-30d mimics and inhibitor transfection
MiRNA mimics and inhibitor are small, chemically modified double-stranded RNAs that mimic or inhibit endogenous miRNAs. Their negative controls are random sequence miRNA mimic molecules that have been extensively tested in cell lines and tissues and validated to not produce identifiable effects on known miRNAs function. In our study, all miRNA mimics, inhibitor and their negative controls (miR-30d NC mimics, miR-30d NC inhibitor) were synthesized at Ribobio and diluted to 50 nM. HD11 cells were transfected with miRNA-30d inhibitor oligonucleotide (complementary strand to miRNAs 5′-CTTCCAGTCGGGGATGTTTACA-3′), miRNA-30d mimics oligonucleotide (corresponding to the miRNA sequence 5′- TGTAAACATCCCCGACTGGAAG-3′) or their negative controls by using the lipofectamine 2000 (Invitrogen, USA) according to the protocol provided by the supplier. Cells after 24 h of transfection were infected with IBV at a MOI of 10 for another 36 h. After that the cells were harvested and total RNA was isolated to subsequent studies.
2.5. Gga-miR-30d target genes prediction and validation
To investigate the function and the underlying mechanism of gga-miR-30d in IBV infection, online software platforms miRDB and TargetScan-Vet were used to predict the target genes of gga-miR-30d. After obtaining the predicted target genes, gga-miR-30d mimics were transfected into HD11 cells. Then, the expression level of the target genes were quantified by qRT-PCR.
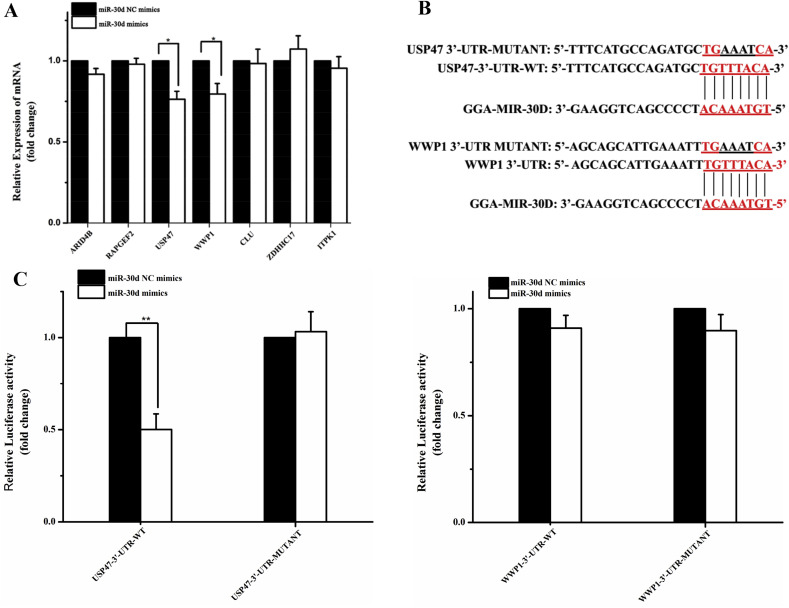
The psiCHECK™-2 Vector (Promega, USA) was used to dual-luciferase reporter assay in this study. Firstly, the 3′-UTR of predicted genes were cloned into psiCHECK™-2 Vector by using designed primers (Supplementary Table 1) and restriction enzymes NotI and XhoI (TaKaRa, Japan). Then, the constructed plasmids were co-transfected with gga-miR-30d mimics into HD11 cells by using lipofectamine 2000. The negative control was the predicted genes sequence with designated mutations at seed site (Fig. 3 B ). Post-transfected 36 h, the luciferase activity was measured using the dual-luciferase reporter assay system (Promega, USA) according to the manufacturer's guidelines. Finally, Rluc (Renilla Luciferase)/Fluc (Firefly Luciferase) relative activities were been calculated. All measurements were taken in triplicate. The results are presented as the mean ± SD.
Fig. 3.
USP47 is one of the targets of gga-miR-30d. (A)The relative expression of the seven predicted genes in HD11 cells after transfected with miR-30d mimics for 24 h, measured by qRT-PCR, using β-acting as the reference gene. All data were representative of three independent experiments and presented as means ± SD.*p < 0.05, **p < 0.01 (B) The details of the molecular interactions between miR-30d and its targets. The seed sequence of gga-miR-30d is underlined and marked with a red font. The mutant sequence is USP47 3′-UTR or WWP1 3′-UTR with indicted mutated at seed sequence. (C) MiR-30d mimics or miR-30d NC mimics were co-transfected with indicated reporter constructs in HD11 cells. Firefly and Renilla luciferase activity levels were measured at 36 h post-transfection. The activity of firefly luciferase was normalized to that of Renilla luciferase.
2.6. Proliferation curve of IBV in HD11 and indirect fluorescent immunity (IFA)
The cells inoculated with the virus were harvested at every 6 h. The proliferation curve was drawn by measuring the titers of IBV by TCID50. The post-infected 24 h Cells was used for IFA as described previously [18]. Infectious bronchitis virus nucleoprotein monoclonal antibody was purchased from Novus Biologicals (USA). Alexa Fluor 488-labeled Goat Anti-Mouse IgG (H + L) was purchased from Beyotime (China).
2.7. Statistical analysis
Statistical significance was determined using the conventional Student's t-test. All assays were run in triplicate. The data are shown as the mean ± SD, and a p value < 0.05 was considered statistically significant (*p < 0.05; **p < 0.01).
3. Results
3.1. Measurement of IBV growth in HD11 cells
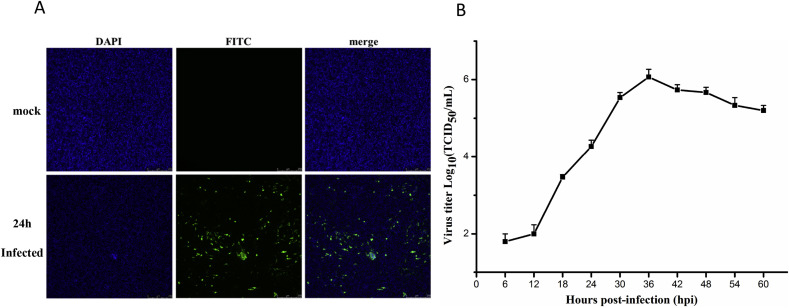
Macrophages play essential roles in both innate and adaptive immune responses. Recent reports indicate that HD11, a chicken macrophage-like cell line, is susceptible to IBV Beaudette strain [21]. To study the role of gga-miR-30d in chicken cell innate immunity, HD11 was been used as the infection platform. The cells were infected with IBV at a MOI of 10 and harvested at indicated time points (0, 6, 12, 18, 24, 30, 36, 42, 48, 54 and 60 h). IFA and virus titers were performed to analyze the status of IBV in HD11 cells. As shown in Fig. 1B. IBV titers began to increase rapidly after 12 h of infection, and at about 36 h after infection, the virus titers reached the maximum (106.067 TCID50/mL). IBV Beaudette strain replication in HD11 cells was also studied by performing an immunofluorescence assay. IFA (Fig. 1A) revealed a strong green fluorescence signal observed in cells after 24 h of infection. No fluorescence signal was observed in the control group. This indicates that HD11 as a chicken source cell line is a suitable platform for IBV proliferation, at least for IBV Beaudette strain.
Fig. 1.
The characteristics of IBV replication in HD11 cells (A) Cells were fixed at 24 h, and virally infected cells were visualized by immunofluorescence (IF) staining of IBV N protein (green). Cell nuclei were visualized by DAPI staining. (B) Proliferation curve of IBV in HD11 cells. Virus titers were measured by TCID50 at every 6 h after infection. Each measurement was performed in triplicate and the error bars represent the standard deviation. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. IBV infection down-regulated gga-mir-30d expression
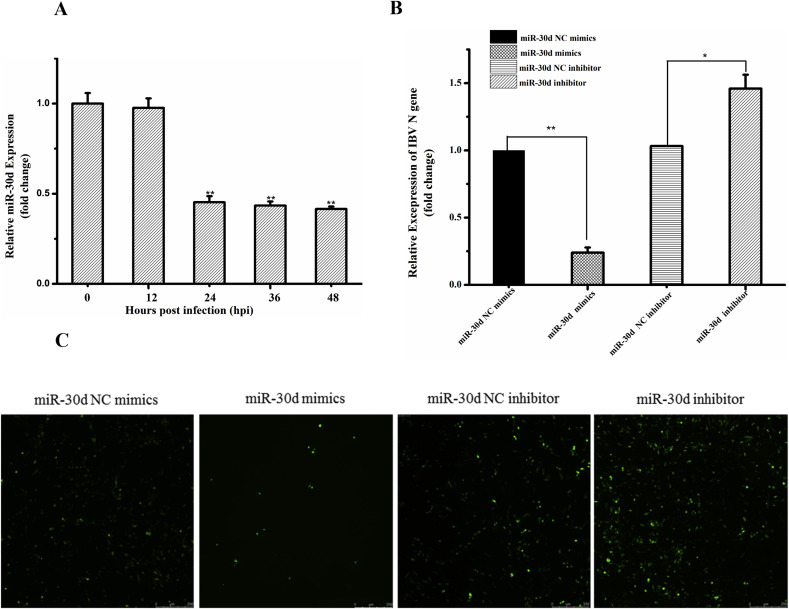
In previous experiments, we found that IBV infection significantly down-regulated the expression level of gga-miR-30d in tissues [12]. To determine whether there is influence on gga-miR-30d expression following IBV infection in HD11 cell line, relative qRT-PCR was performed to analysis the expressions of gga-miR-30d in post-infected HD11 cells at indicted time points (0 h, 12 h, 24 h, 36 h, 48 h). In the same situation, the expression level of gga-miR-30d in mock infected cells serve as controls. As shown in Fig. 2A, the expression level of gga-miR-30d began to decrease after 12 h of viral infection. When infected for 24 h, the expression level of gga-miR-30d down-regulated about two fold compared with the control. Little change was observed at 0–12 h after infection. Coincidentally, IBV started to replicate a lot at about 12 h after infection (Fig. 1B), and the expression level of gga-miR-30d in the cells began to down-regulate in the same time period. This means that the down-regulation of gga-miR-30d may be related to the replication of IBV, and gga-miR-30d may play a role in IBV replication.
Fig. 2.
Interaction between gga-miR-30d and replication of IBV. (A) Relative quantitation RT-PCR analysis of endogenous miR-30d in HD11 cells infected with IBV (MOI of 10) for 48 h. All data were representative of three independent experiments and presented as means ± SD.*p < 0.05, **p < 0.01 (B) IBV genome N mRNA levels were determined by relative quantitative RT-PCR assay and fold changes were calculated using the 2-ΔΔCt method after transfected with miR-30d mimics, inhibitor and their negative controls. (C) Under the same processing conditions as Fig. 2B, Virally infected cells were visualized by immunofluorescence staining of IBV N protein (green) to further confirm the results. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Overexpression of gga-miR-30d inhibited IBV replication and low expression of gga-miR-30d promoted IBV replication
To further determine the interactions between IBV replication and gga-miR-30d expression, the mimics, inhibitor and their negative controls were designed and transfected into HD11 cells, respectively. 24 h after transfection, the cells were infected with IBV for additional 36 h. The replication level of IBV was determined by qRT-PCR. As shown in Fig. 2B. IBV is significantly reduced (p < 0.01) after transfected the mimics compared to the negative control. Conversely, it was increased significantly compared to the negative control (P < 0.05) after transfected with inhibitor. To further confirm the results, IFA was performed. As shown in Fig. 2C, Weak fluorescence was observed after transfection of mimics, but strong fluorescence was observed after transfection of inhibitor compared with the mock transfection. Those data showed that the expression level of gga-miR-30d was negatively correlated with the level of IBV replication in HD11 cells. Overexpression of gga-miR-30d inhibited IBV replication, low expression of gga-miR-30d promoted IBV replication. This suggests that gga-miR-30d is a key regulator of IBV replication in HD11 cells.
3.4. Gga-miR-30d regulates USP47 expression by directly targeting its 3′UTR
To investigate the molecular mechanism underlying gga-miR-30d-mediated regulation of IBV replication, the targets of gga-miR-30d in HD11 cells were predicted by bioinformatics software (TargetScan and miRDB). Combining our previous experimental data [12], seven potential target genes were screened out (ARID4B, RAPGEF2, USP47, WWP1, CUL2, ZDHHC17, ITPK1). Then miR-30d mimics were transfected into HD11 cells for 24 h, and the expression of predicted genes were detected by qPCR respectively. Controls were the cells transfected with miR-30d NC mimics. As shown in Fig. 3A, the expression levels of USP47 and WWP1 were significantly decreased, but little change was observed in other five genes.
To further determine the targets of gga-miR-30d, a dual luciferase reporter system psiCHECK™-2 was employed in this experiment. The 3′-UTR of USP47 and WWP1 were cloned into psiCHECK™-2 vector, respectively (USP47-3′-UTR-WT, WWP1-3′-UTR-WT). Controls were the USP47 and WWP1 with designated mutations in their seed sequence (USP47 3′-UTR-MUTANT, WWP1-3′-UTR-MUTANT, Fig. 3B). Then the constructed plasmid were co-transfected into HD11 cells together with miR-30d mimics or miR-30d NC mimics, respectively. 36 h after transfected, Firefly and Renilla luciferase activity levels were measured separately. As shown in Fig. 3C, significant decreased luciferase signal was observed following co-transfection of miR-30d mimics and USP47-3′-UTR-WT reporter plasmid, whereas no obviously inhibited luciferase activity was obtained following other treatments. This means that gga-miR-30d combined directly with the 3′-UTR of USP47, and USP47 was a target of gga-miR-30d in HD11 cells.
4. Discussion
IBV is not easily adapted to cell culture. Almost all IBV field isolates can only be propagated in embryonated chicken eggs or transiently proliferated in primary chicken embryo kidney cells [19]. However, the Beaudette strain, a cell-adapted strain, replicates efficiently in various cultured mammalian cell lines, such as Vero, H1299, HepG2, Hep3B and Huh7 [20]. Those cells are derived from mammals, which impose certain restrictions on IBV as an avian virus. HD11, a chicken cell line, have recently been found to be susceptible to IBV Beaudette strain [21]. In addition, HD11 is a chicken macrophage-like cell line. There is lack of lymph nodes and resident macrophages in avian respiratory tract, thus requiring an infux of phagocytic cells to initiate the defense against infectious agents [22]. Therefore macrophages play an important role in avian innate immunity. Studying the interaction between IBV and avian macrophage HD11 will help to better understand avian innate immunity.
In previous study, we used HD11 as an infection platform to study the changes in intracellular miRNAs after IBV infection, and found that miR-146a-5p could promote replication of IBV by targeting IRAK2 and TNFRSF18 [23]. In present study, we found another key miRNA gga-miR-30d regulating IBV replication by targeting USP47.
Gga-miR-30d is a miRNA encoded by chicken chromosome 2 and is a member of the miR-30 family. MiR-30 family plays an important role in immune response, especially in the process of viral infection. It was reported miR-30a could inhibit the replication of EV71 (intestinal virus type 71) by regulating the autophagy activity of cells [13]. MiR-30b and miR-30c inhibited the replication and proliferation of hepatitis C virus (HCV) in Huh 7.5 cells [14]. Ssc-miR-30d-R-1 was potential therapeutic agent for controlling Porcine reproductive and respiratory syndrome virus (PRRSV) infection [15]. In this study, we found overexpressed gga-miR-30d would inhibit the replication of IBV. Whereas low expressed would promote the replication of IBV in HD11 cells. This finding may deal with IBV infections.
Deubiquitinating enzymes (DUBs) are a kind of important intracellular enzymes, play different roles in almost every cellular process [24]. Not surprisingly, viruses inevitably coopt DUBs to accomplish their own life cycle, from entry into host cells to the release of progeny viral particles [25]. Hijacking of the ubiquitin-deubiquitinase system by viruses continues to emerge as a central theme around the viral life cycle. USP47, a member of the DUBs family, was recently identified as a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase β [26]. Besides, USP47 also plays an important role in innate immunity. It has been reported that P22077, a selective ubiquitin-specific protease 7/47 (USP7/USP47) inhibitor, inhibited the proliferation of HIV virus by inhibiting the function of USP47 [27].
Many viruses can up-regulate the activity of DUBs to evade host antiviral immune response and promote virus replication [28]. In our experiment, a similar phenomenon has been observed. Gga-miR-30d is a key factor regulating the expression of USP47 by binding to its 3 ′-UTR. Overexpressed gga-miR-30d down-regulated the expression of USP47, resulted in inhibiting the replication of IBV. Down-regulation of gga-miR-30d increased the activity of USP47 and promoted the replication of IBV. However, how does USP47 regulate IBV replication remains to be further explored. In a word, it may be a common strategy for virus infection to use the UDBs to resist the immune response of host and enhance its replication.
In addition to the DUBs encoded by the cells, many viruses also can encode DUBs or deubiquitinating-like proteins to promote their own replication. Coronavirus, for instance MHV-A59, SARS-CoV, HCoV–NL63 [[29], [30], [31]], encode the protein with deubiquitinating activity to promote viral infection. Similarly, as one of Coronavirus, it was reported that IBV-encoded PLP-TM functions as a DUB enzyme, which significant reduced the levels of ubiquitin (Ub)-, K48-, and K63-conjugated proteins in the early stage of viral infection [32]. By degrading polyubiquitin chains associated with ubiquitin proteins, IBV PLP-TM may prevent the activation of host antiviral signaling pathways to escapes host antiviral immune system. In short, both the cell-encoded DUBs and virus-encoded DUBs play a key regulatory role in viral infection. These results further highlight the importance of the ubiquitin-deubiquitinase system in virus-cell interactions and cellular innate immunity.
In summary, gga-miR-30d, a microRNA encoded by HD11 cells, regulates IBV infection by targeting USP47. Our study provides a theoretical basis for the prevention of IBV and provides new experimental evidence for the important role of DUBs in virus infection.
Funding
This research was supported by National Key R&D Program of China (2017YFD0500703), the Program of Main Livestock Standardized Breeding Technology Research and Demonstration (2016NYZ0052), the Major Science and Technology Special Program of Sichuan Province (2018NZDZX0006), and the Earmarked Fund for Modern Agroindustry Technology Research System (CARS-41-K09).
CRediT authorship contribution statement
Hao Li: Writing - original draft. Jianan Li: Writing - original draft. Yaru Zhai: Conceptualization, Methodology. Lan Zhang: Conceptualization, Methodology. Pengfei Cui: Software. Lan Feng: Software. Wenjun Yan: Software. Xue Fu: Writing - review & editing. Yiming Tian: Writing - review & editing. Hongning Wang: Supervision. Xin Yang: Supervision.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micpath.2020.103998.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41(3):239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- 2.Hawn S.A.F.M.C. An apparently new respiratory disease of baby chicks. J. Am. Vet. Med. Assoc. 1931:78. [Google Scholar]
- 3.Awad F., Chhabra R., Baylis M., Ganapathy K. An overview of infectious bronchitis virus in chickens. World Poultry Sci. J. 2014;70(2):375–383. [Google Scholar]
- 4.Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38(2):281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- 5.He K., Li M., Wei P., Mo M.L., Wei T.C., Li K.R. Complete genome sequence of an infectious bronchitis virus chimera between cocirculating heterotypic strains. J. Virol. 2012;86(24):13887–13888. doi: 10.1128/JVI.02722-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao Y., Charlesworth J., Nair V., Watson M. MicroRNA expression profiles in avian haemopoietic cells. Front. Genet. 2013;4:153. doi: 10.3389/fgene.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Y., Nair V. Role of virus-encoded microRNAs in Avian viral diseases. Viruses. 2014;6(3):1379–1394. doi: 10.3390/v6031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier A., Sagan S.M. The diverse roles of microRNAs at the host-virus interface. Viruses-Basel. 2018;10(8) doi: 10.3390/v10080440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Othumpangat S., Bryan N.B., Beezhold D.H., Noti J.D. Upregulation of miRNA-4776 in influenza virus infected bronchial epithelial cells is associated with downregulation of NFKBIB and increased viral survival. Viruses-Basel. 2017;9(5) doi: 10.3390/v9050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou P.L., Wang H.M., Zhao G.M., Hu G.X., Xia X.Z., He H.B. MiR-3470b promotes bovine ephemeral fever virus replication via directly targeting mitochondrial antiviral signaling protein (MAVS) in baby hamster Syrian kidney cells. BMC Microbiol. 2018;18 doi: 10.1186/s12866-018-1366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou P.L., Zhao M., He W.Q., He H.B., Wang H.M. Cellular microRNA bta-miR-2361 inhibits bovine herpesvirus 1 replication by directly targeting EGR1 gene. Vet. Microbiol. 2019;233:174–183. doi: 10.1016/j.vetmic.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Yang X., Gao W.Q., Liu H., Li J.A., Chen D.Y., Yuan F., Zhang Z.K., Wang H.N. MicroRNA transcriptome analysis in chicken kidneys in response to differing virulent infectious bronchitis virus infections. Arch. Virol. 2017;162(11):3397–3405. doi: 10.1007/s00705-017-3502-2. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y.X., Xu W.T., Chen D.Y., Feng C.H., Zhang L., Wang X.H., Lv X.W., Zheng N., Jin Y., Wu Z.W. Enterovirus 71 induces autophagy by regulating has-miR-30a expression to promote viral replication. Antivir. Res. 2015;124:43–53. doi: 10.1016/j.antiviral.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X.Z., Daucher M., Armistead D., Russell R., Kottilil S. MicroRNA expression profiling in HCV-infected human hepatoma cells identifies potential anti-viral targets induced by interferon-alpha. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0055733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C.M., Zhang Y.Y., Luo J., Ding H., Liu S.L., Amer S., Xie L., Lyv W., Su W., Li M., Sun Q.M., Dai J.Y., He H.X. Identification of miRNomes reveals ssc-miR-30d-R_1 as a potential therapeutic target for PRRS viral infection. Sci Rep-Uk. 2016;6 doi: 10.1038/srep24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran A.T., Rahim M.N., Ranadheera C., Kroeker A., Cortens J.P., Opanubi K.J., Wilkins J.A., Coombs K.M. Knockdown of specific host factors protects against influenza virus-induced cell death. Cell Death Dis. 2013;4:e769. doi: 10.1038/cddis.2013.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheppard M., McHenry E.W. The physiological properties of ascorbic acid: effects upon water balance and upon body composition of Guinea-pigs. Biochem. J. 1939;33(5):655–657. doi: 10.1042/bj0330655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H., Yuan X., Sun Y.J., Mao X., Meng C.C., Tan L., Song C.P., Qiu X.S., Ding C., Liao Y. Infectious bronchitis virus entry mainly depends on clathrin mediated endocytosis and requires classical endosomal/lysosomal system. Virology. 2019;528:118–136. doi: 10.1016/j.virol.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo H.C., Huang M., Yuan Q., Wei Y.Q., Gao Y., Mao L.J., Gu L.J., Tang Y.W., Zhong Y.X., Liu D.X., Sun S.Q. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tay F.P.L., Huang M., Wang L., Yamada Y., Liu D.X. Characterization of cellular furin content as a potential factor determining the susceptibility of cultured human and animal cells to coronavirus infectious bronchitis virus infection. Virology. 2012;433(2):421–430. doi: 10.1016/j.virol.2012.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X.X., Tian Y.M., Guan R., Gao W.Q., Yang X., Zhou L., Wang H.N. Infectious bronchitis virus infection induces apoptosis during replication in chicken macrophage HD11 cells. Viruses-Basel. 2017;9(8) doi: 10.3390/v9080198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fung T.S., Liu D.X. Coronavirus infection, ER stress, apoptosis and innate immunity. Front. Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H., Yang X., Zhang Z.K., Zou W.C., Wang H.N. miR-146a-5p promotes replication of infectious bronchitis virus by targeting IRAK2 and TNFRSF18. Microb. Pathog. 2018;120:32–36. doi: 10.1016/j.micpath.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isaacson M.K., Ploegh H.L. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5(6):559–570. doi: 10.1016/j.chom.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Z., Shi W. Manipulation of viral infection by deubiquitinating enzymes: new players in host-virus interactions. Future Microbiol. 2016;11:1435–1446. doi: 10.2217/fmb-2016-0091. [DOI] [PubMed] [Google Scholar]
- 26.Parsons J.L., Dianova I.I., Khoronenkova S.V., Edelmann M.J., Kessler B.M., Dianov G.L. USP47 is a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase beta. Mol. Cell. 2011;41(5):609–615. doi: 10.1016/j.molcel.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Setz C., Friedrich M., Rauch P., Fraedrich K., Matthaei A., Traxdorf M., Schubert U. Inhibitors of deubiquitinating enzymes block HIV-1 replication and augment the presentation of gag-derived MHC-I epitopes. Viruses. 2017;9(8) doi: 10.3390/v9080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey-Elkin B.A., Knaap R.C.M., Kikkert M., Mark B.L. Structure and function of viral deubiquitinating enzymes. J. Mol. Biol. 2017;429(22):3441–3470. doi: 10.1016/j.jmb.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng D., Chen G., Guo B., Cheng G., Tang H. PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res. 2008;18(11):1105–1113. doi: 10.1038/cr.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frieman M., Ratia K., Johnston R.E., Mesecar A.D., Baric R.S. Severe acute respiratory syndrome coronavirus papain-like protease ubiquitin-like domain and catalytic domain regulate antagonism of IRF3 and NF-kappaB signaling. J. Virol. 2009;83(13):6689–6705. doi: 10.1128/JVI.02220-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PloS One. 2012;7(2) doi: 10.1371/journal.pone.0030802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu L., Zhang X., Wu T., Wang Y., Meng J., Liu Q., Niu X., Wu Y. The papain-like protease of avian infectious bronchitis virus has deubiquitinating activity. Arch. Virol. 2017;162(7):1943–1950. doi: 10.1007/s00705-017-3328-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.