Abstract
A mammalian orthoreovirus (MRV) strain was isolated from a pup with fatal diarrhea, which had a concurrent infection by canine parvovirus type 2. The reovirus isolate showed an atypical hemagglutination pattern and a retarded electrophoretic mobility of the S1 segment, which is characteristic of MRV type 3 (MRV-3). Assignment of the isolated virus to MRV-3 was confirmed by type-specific RT-PCR assays, targeting the S1 gene, and by subsequent sequence analysis of the PCR product. By phylogeny based on the S1 gene of several MRVs, the isolate fell into lineage E, along with the murine strain T3C9/61 and the bovine strains T3C18/61 and T3C31/59. Conversely, L1 sequences were found to segregate regardless of the viral type. A total of 110 fecal samples, 56 nasal and 31 ocular swabs from dogs with diarrhea or nasal/ocular discharge were tested by a nested-PCR assay specific for reoviruses, and no sample was found to contain MRV RNA, a finding that is apparently in contrast with the seroprevalence (25.77%) observed in dogs.
Keywords: Reovirus, Dog, Isolation, L1 gene, S1 gene, Sequence analysis
1. Introduction
Mammalian orthoreovirus (MRV) includes non-enveloped, double-stranded (ds) RNA viruses belonging to the genus Orthoreovirus within the family Reoviridae, which are responsible for either symptomatic or asymptomatic infections in mammals and possess a broad host range (Tyler, 2001). Their genome contains 10 dsRNA segments, which are designed as large (L, three segments), medium (M, three segments) or small (S, four segments) on the basis of the electrophoretic mobility (Nibert and Schiff, 2001). Three MRV serotypes have been recognized so far, which are distinguishable by means of the capacity of anti-reovirus sera to neutralize viral infectivity and inhibit hemagglutination (HA) (Rosen, 1960, Sabin, 1959). Neutralization and HA activities are restricted to a single reovirus gene segment, S1 (Weiner and Fields, 1977), that encodes for σ1 and σ1s proteins. The σ1 protein, a fibrous trimer located on the outer capsid of the virion (Fraser et al., 1990, Furlong et al., 1988), is responsible for viral attachment on cellular receptors (Lee et al., 1981, Weiner et al., 1980), serotype-specific neutralization (Bassel-Duby et al., 1986) and hemagglutination (Weiner et al., 1978). Analysis of the S1 gene of MRVs belonging to different serotypes has shown a strict correlation between sequence similarity and viral serotype (Cashdollar et al., 1985, Duncan et al., 1990, Nibert et al., 1990). Conversely, the other genome segments show no correlation to viral serotype, suggesting that mammalian reoviruses have evolved independently of serotype (Breun et al., 2001, Chappel et al., 1994, Goral et al., 1996, Kedl et al., 1995, Leary et al., 2002).
Mammalian reoviruses have a wide geographic distribution and can virtually infect all mammals, including humans (Tyler, 2001). In carnivores, MRV infections have been sporadically reported, although all the three serotypes have been isolated from dogs and cats (Binn et al., 1977, Csiza, 1974, Kokubu et al., 1993, Lou and Wenner, 1963, Marshall et al., 1987, Massie and Shaw, 1966, Mochizuki and Uchizono, 1993, Scott et al., 1970).
In the present study, the isolation and molecular characterization of a MRV-3 strain from a dog with diarrhea are reported.
2. Materials and methods
2.1. Clinical case
Rectal swabs from two Borzoi greyhounds (214/04-A and 214/04-B), belonging to the same litter, were submitted to our laboratory for virological investigations. The pups, 2 months of age, were affected by vomiting and diarrhea, that was bloody in pup 214/04-B. Pup 214/04-B died within 6 days from the onset of clinical signs, and it was not possible to perform necropsy. Pup 214/04-A underwent a rapid recovery. Neither respiratory nor ocular signs were reported by the owner. Both the rectal swabs resulted positive to canine parvovirus type 2 (CPV-2) by a real-time PCR assay (Decaro et al., 2005), showing titers of 1.45 × 109 and 9.51 × 108 CPV-2 DNA copies per milligram of feces for pups A and B, respectively. Both CPV strains were characterized as Glu-426 mutants (Buonavoglia et al., 2001).
2.2. Virus isolation
The rectal swabs were homogenized in Eagle's minimal essential medium (E-MEM), treated with antibiotics and inoculated onto freshly trypsinized Crandell feline kidney (CrFK) cells. The inoculated cells were monitored by an indirect immunofluorescence (IF) assay using a CPV-2 monoclonal antibody. Cells inoculated with sample 214/04-A showed no cytopathic effect (CPE), in spite of a strong CPV-2 specific intranuclear fluorescence, whereas, unexpectedly, in the cells inoculated with sample 214/04-B a CPE was observed, that was characterized by rounding of the infected cells and deterioration of the cell monolayer, in spite of a very weak signal by the IF assay for CPV-2. In order to prevent CPV-2 replication on cell cultures, CPV-2 was selected against by using a monoclonal antibody (A4E3). There was no evidence for growth of CPV-2 in the subsequent passages, as shown by both IF and real-time PCR assays. In contrast, the CPE progressively increased, with complete destruction of the inoculated monolayers. The inoculated cells were stained with hematoxylin-eosin (HE) for detection of inclusion bodies. Negative staining electron microscopy examination was carried out on the cryolysate of the infected cell cultures showing evident CPE.
2.3. Hemagglutination assays
Two-fold dilutions in phosphate buffered saline of the isolated virus (cryolysate of the sixth passage on CrFK cells), with a titer of 104.75 TCID50/50 μl, were subjected to HA assays using 96-well microtiter plates and 1% red blood cells (RBC). Human (0, A, B and AB), cow, sheep, goat, pig, dog, cat, rabbit and mouse RBC were tested at 4 °C room temperature and 37 °C.
2.4. Polyacrylamide gel electrophoresis (PAGE)
For PAGE, dsRNA was extracted from the infected CrFK cells using the guanidine thiocyanate/Glass Milk method (Gentsch et al., 1992), run in a 10% polyacrylamide gel at constant current of 10 mA for 10 h and visualized by silver staining as previously described (Dolan et al., 1985).
2.5. PCR and RT-PCR assays
The rectal swabs from the two dogs and the sixth serial passage of the corresponding inoculated cells were subjected to PCR and RT-PCR assays for the detection of the main viral pathogens of dogs, i.e. canine adenovirus type 1 and type 2 (Hu et al., 2001), canid herpesvirus type 1 (Schulze and Baumgartner, 1998), canine coronavirus (CCoV) (Pratelli et al., 1999), feline and canine caliciviruses (Hashimoto et al., 1999, Marsilio et al., 2005) and rotaviruses (Gouvea et al., 1994). RNA for the detection of reoviruses was extracted using the guanidine thiocyanate/Glass Milk method, following the protocol described by Gentsch et al. (1992). For the detection of MRV a nested-PCR assay was performed (Leary et al., 2002), using primer pairs L1-rv5/L1-rv6 for RT-PCR and L1-rv7/L1-rv8 for nested-PCR (Table 1 ).
Table 1.
Primers used for PCR amplification and sequence analysis
| Primer | Sequence 5′ to 3′ | Target | MRV specificity | Sense | Position | Amplicon size (bp) |
|---|---|---|---|---|---|---|
| L1-rv5a | gCATCCATTgTAAATgACgAGTCTg | L1 | All types | + | 1888–1912 | 416 |
| L1-rv6a | CTTgAgATTAgCTCTAgCATCTTCTg | − | 2278–2303 | |||
| L1-rv7a | gCTAggCCgATATCgggAATgCAg | L1 | All types | + | 1930–1953 | 344 |
| L1-rv8a | gTCTCACTATTCACCTTACCAgCAg | − | 2249–2273 | |||
| S1-R1Fb | ggAgCTCgACACAgCAAATA | S1 | Type 1 | + | 958–977 | 505 |
| S1-R1Rb | gATgATTgACCCCTTgTGC | − | 1444–1462 | |||
| S1-R2Fc | CTCCCgTCACggTTAATTTg | S1 | Type 2 | + | 1047–1066 | 394 |
| S1-R2Rc | gATgAgTCgCCACTgTgC | − | 1423–1440 | |||
| S1-R3Fd | TgggACAACTTgAgACAggA | S1 | Type 3 | + | 338–357 | 326 |
| S1-R3Rd | CTgAAgTCCACCRTTTTgWA | − | 644–663 | |||
| ENT-S1-R3Fd | gCTATTggTCggATggAT | S1 | Type 3 | + | 1–18 | 1416 |
| ENT-S1-R3Rd | gATgAAATgCCCCAgTgC | − | 1399–1416 | |||
Leary et al., 2002. Primer position is referred to the L1 sequence of T1L/53 (accession: NC00_4271).
Primer position is referred to the S1 sequence of T1L/53 (accession: M35963).
Primer position is referred to the S1 sequence of T2J/55 (accession: M35964).
Primer position is referred to the S1 sequence of T3D/55 (accession: M10262).
2.6. Prediction of MRV type
As neither monoclonal antibodies nor polyclonal antisera specific to the three MRV serotypes were available in our laboratory, we developed RT-PCR assays able to differentiate the MRV serotypes, which were used to characterize the MRV strain isolated from sample 214/04-B. In the GenBank database, several S1 sequences of MRV-3 are available and this allowed us to select primers in highly conserved regions. Conversely, type 1- and type 2-specific primers were designed on the basis of the S1 sequence data of a few strains, i.e. the reference strains T1L/53 and T2J/55 for MRV-1 and MRV-2, respectively (Table 1).
2.7. Sequence analysis and phylogeny
The entire S1 gene was amplified using primers ENT-S1-R3F and ENT-S1-R3R (Table 1) binding the 5′ and 3′ ends of the S1 segment, respectively.
The PCR products obtained with primer pairs L1-rv5/L1-rv6 (L1 gene, 416 bp) and ENT-S1-R3F/ENT-S1-R3R (S1 gene, 1416 bp) were cloned and subjected to sequence analysis (Genome Express, Meylan, France). Alignments and sequence analysis were performed using the BioEdit software package (Hall, 1999). The nucleotide sequences are available in the DDBJ/EMBL/GenBank databases under accession nos. AY781188 (partial L1 gene) and AY785910 (S1 gene). The nt sequences of the amplified fragments were aligned with a selection of MRV reference strains belonging to the three serotypes (Table 2 ). Phylogenetic and molecular evolutionary analyses were conducted using MEGA3 (Kumar et al., 2004). Parsimony trees were elaborated using a heuristic algorithm and supplying statistical support by bootstrapping over 100 replicates.
Table 2.
Nucleotide identity (%) of MRV reference strains to isolate T3D/04 in the S1 and L1 segments
| MRV strain | GenBank accession no. |
nt identity (%) to isolate T3D/04 |
||
|---|---|---|---|---|
| S1 | L1 | S1 | L1 | |
| T1/human/Ohio/Lang/1953 (T1L/53) | NC00_4271 | M35963 | 13 | 90 |
| T2/human/Ohio/Jones/1955 (T2J/55) | NC00_4272 | M35964 | 3 | 77 |
| T3/human/Ohio/Dearing/1955 (T3D/55) | NC00_4282 | M10262 | 79 | 91 |
| T3/human/Washington, D.C./Abney/1957 (T3A/57) | AY007396 | L37677 | 79 | 90 |
| T3/human/Tahiti/clone 8/1960 (T3C8/60) | AY007393 | L37679 | 80 | 86 |
| T3/murine/France/clone 9/1961 (T3C9/61) | AY007384 | L37676 | 93 | 86 |
| T3/bovine/Maryland/clone 18/1961 (T3C18/61) | AY007409 | L37684 | 88 | 89 |
| T3/bovine/Maryland/clone 31/1959 (T3C31/59) | AY007383 | L37683 | 89 | 86 |
| T3/bovine/Maryland/clone 43/1960 (T3C43/60) | AY007397 | L37682 | 79 | 90 |
| T3/bovine/Maryland/clone 44/1960 (T3C44/60) | AY007417 | L37681 | 79 | 89 |
| T3/bovine/Maryland/clone 45/1960 (T3C45/60) | AY007418 | L37680 | 79 | 89 |
| T3/human/Washington, D.C./clone 84/1957 (T3C84/57) | AY007419 | L37678 | 79 | 89 |
| T3/human/Washington, D.C./clone 93/1955 (T3C93/55) | AY007420 | L37675 | 78 | 89 |
| T3/human/Colorado/1996 (T3C/96) | NA | AY302467 | 70 | NC |
NA, sequence not available; NC, nt identity not calculated.
2.8. Sample collection and screening for MRV RNA and MRV-specific antibodies
A total of 110 fecal samples, 56 nasal swabs and 31 ocular swabs were subjected to nested-PCR for detection of MRV (Leary et al., 2002). The fecal samples were collected from dogs with diarrhea and included 22 samples previously tested positive to CCoV by a real-time RT-PCR assay (Decaro et al., 2004), 19 samples previously tested positive to CPV-2 by a real-time PCR assay (Decaro et al., 2005), 7 samples positive both to CCoV and CPV-2 and 62 samples negative to CCoV and CPV-2. In addition, 96 serum samples collected from dogs of different ages and different geographic origin were tested for antibodies to the MRV isolate by a virus neutralization (VN) assay, using CrFK cells and 100 TCID50/50 μl of the virus. At day 30 after the onset of clinical signs, a serum sample was also collected from pup 214/04-A.
3. Results
3.1. Isolation and virological characterization of the MRV strain
An MRV strain was isolated on CrFK cells from the rectal swab of pup 214/04-B. The CPE was initially characterized by rounding of the infected cells and, after a better adaptation to in vitro growth, by complete destruction of the cell monolayer. At the sixth passage on CrFK cells, the isolate reached a titer of 104.75 TCID50/50 μl, although it tested negative by IF and real-time PCR assays for CPV-2. EM of the cryolysate of this passage demonstrated the presence of icosahedral non-enveloped viral particles characteristic of reoviruses (Fig. 1 ). By HE staining, perinuclear intracytoplasmic inclusions typical of MRV were detected in the infected cells (Fig. 2 ). The MRV strain showed an atypical HA pattern, as it displayed HA activity (1:64) using pig RBC. Interestingly, no HA was observed using human type 0 RBC, a pattern that is usually showed by all mammalian reoviruses (Tyler, 2001). Low HA titers (1:4) were obtained using rabbit RBC. Unlike other MRV-3 strains (Dermody et al., 1990a, Lerner et al., 1963), the isolate showed a weak HA activity to bovine RBC and only using high amounts of virus.
Fig. 1.
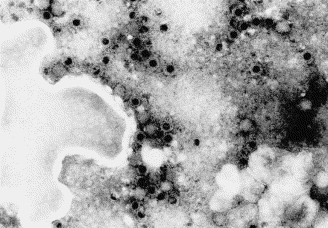
Reovirus-like particles from CrFK cells infected with strain T3D/04. The viral preparation was negative-stained with sodium phosphotungstate and observed by EM (25,000×).
Fig. 2.
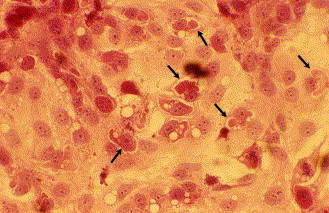
Perinuclear inclusion bodies (arrows) in the cytoplasm of CrFK cells infected with strain T3D/04. HE staining 400×.
3.2. Molecular characterization of the MRV strain
By PAGE and subsequent silver staining, the dsRNA purified from the infected cells showed an electrophoretic pattern typical of MRV, although co-migration of the L2 and L3 segments was observed (Fig. 3 ). The largest of the S-class gene segments (S1) migrated slowly, which is a characteristic of MRV-3 strains (Hrdy et al., 1979).
Fig. 3.
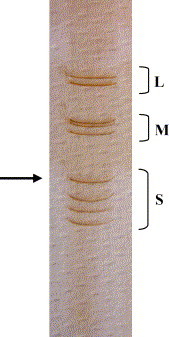
Electrophoretic analysis of T3D/04 dsRNA in a silver-stained polyacrylamide gel. The arrow indicates the position of the serotype-determining S1 segment.
RT-PCR and nested-PCR specific for MRV, carried out on a conserved region of the L1 gene, yielded products of the expected size (416 and 344 bp, respectively) only for specimen 214/04-B and for the corresponding inoculated CrFK cells, while no amplification was obtained from sample 214/04-A and the corresponding inoculated cells (Fig. 4 ). By the type-specific RT-PCR assays the isolate was recognized as MRV-3 (Fig. 5 ), and therefore designated T3/canine/Italy/Decaro/2004 (T3D/04), according to the conventional system used to identify MRV strains. Sequencing of the amplicon generated by primer pair S1-R3F/S1-R3R confirmed the characterization obtained by the RT-PCR genotyping assay, and the isolate was definitively assigned to MRV-3.
Fig. 4.
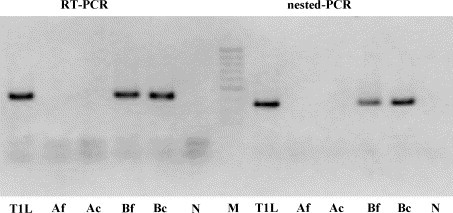
PCR assays carried out on fecal samples of pups 214/04-A and 214/04-B and inoculated cells. Amplification of the L1 fragment by RT-PCR (416 bp) and nested-PCR (344 bp). T1L (strain T1L/53); Af (sample 214/04-A, feces); Ac (sample 214/04-A, inoculated cells); Bf (sample 214/04-B, feces); Bc (sample 214/04-B, inoculated cells); N (negative fecal sample); M (marker GeneRuler 50 bp DNA Ladder, MBI Fermentas GmbH, Germany).
Fig. 5.
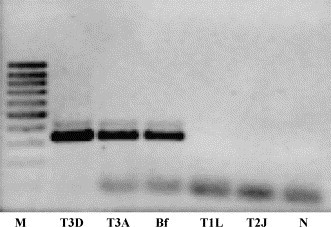
Strain characterization by the type 3-specific RT-PCR assay targeting the S1 segment (primer pair S1-R3F/S1-R3R, PCR product of 326 bp). M (marker GeneRuler 100 bp DNA Ladder, MBI Fermentas GmbH, Germany); T3D (strain T3D/55); T3A (strain T3A/57); Bf (sample 214/04-B, feces); T1L (strain T1L/53); T2J (strain T2J/55); N (negative fecal sample).
3.3. Sequence analysis and phylogeny
Comparison of S1 and partial L1 nt sequences of strain T3D/04 with the sequences retrieved from the databases is reported in Table 2.
The L1 fragment displayed the highest nt sequence identity (91%) to the reference strain T3D/55. Phylogeny showed that the L1 gene sequences segregate into lineages irrespective of their serotype (Fig. 6a), confirming the results of a previous study (Leary et al., 2002).
Fig. 6.
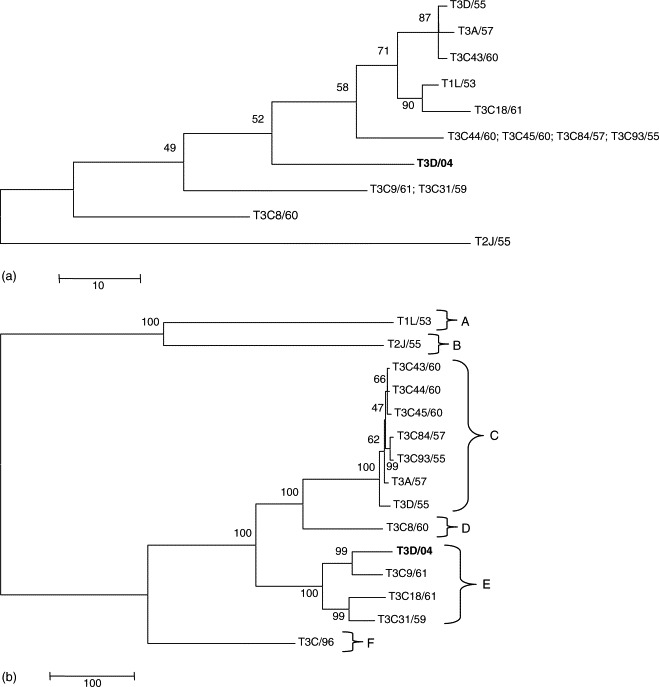
Maximum parsimony trees based on partial L1 (a) and S1 (b) nucleotide sequences of MRV strains. Accession numbers of the strains used for phylogeny are reported in Table 2. Trees are unrooted and drawn to scale. Bootstrap values were calculated and are indicated at each node.
The S1 gene of strain T3D/04 revealed the closest nt sequence identity to the murine strain T3C9/61 (93%). Strain T3D/04 exhibited 79% positional identity to reference strain T3D/55 and 70% nt identity to the novel human strain T3C/96, recently isolated from a child with meningitis (Tyler et al., 2004). By phylogenetic analysis, a sixth lineage, represented by the type 3 highly divergent T3C/96 strain, was added to the five lineages previously reported (Dermody et al., 1990b) with isolate T3D/04 falling into lineage E along with the murine strain T3C9/61 and the bovine strains T3C18/61 and T3C31/59 (Fig. 6b).
3.4. Screening of dog samples for MRV RNA and MRV-specific antibodies
By nested-PCR, MRV RNA was not detected in any of the 197 canine samples analyzed, suggesting that reoviruses are not widespread in the sampled dog population.
However, antibodies to MRV were detected in 25 (25.77%) out of the 97 sera tested by VN. Antibody titers ranging from 1:2 to 1:16 were detected in 20 dogs, whereas VN antibody titers >1:32 were found in five animals, with the highest titer (1:256) in the recovered pup 214/04-A.
4. Discussion
The present study represents the first molecular characterization of a reovirus strain isolated from a dog. To date, there are a few reports on the epidemiology of reoviruses in dogs, although all the three MRV serotypes have been isolated in such species (Binn et al., 1977, Kokubu et al., 1993, Lou and Wenner, 1963, Massie and Shaw, 1966). MRV-1 strains have been recovered from dogs with pneumonia (Lou and Wenner, 1963) or enteritis (Appel, 1987), in association with either canine distemper virus or CPV-2. MRV-2 and MRV-3 have been isolated from dogs with upper respiratory tract disease (Binn et al., 1977) and diarrhea (Kokubu et al., 1993), respectively. However, to our knowledge, none of the isolates was characterized at the molecular level and no sequence data is available in the databases. As in other mammalians, the pathogenetic role of reoviruses in dogs is still unclear, since attempts to reproduce a specific disease in germ- and disease-free dogs resulted in contrasting findings (Appel, 1987, Holzinger and Griesemer, 1966). Nevertheless, it has been suggested that MRV infection may act in synergism with other pathogens, aggravating the course of concomitant infections (Appel, 1987). Herewith, we described a double infection by MRV and CPV-2 in a dog died from severe enteritis. MRV was not detected in the CPV-2 infected dog that survived, even if MRV-specific antibodies were detectable 4 weeks after the onset of the disease, indicating that the dog had been exposed to MRV-3 infection as well.
Strain T3D/04 displayed an atypical HA pattern, as it was able to agglutinate only pig RBC. Unlike other MRV strains (Tyler, 2001), this isolate did not agglutinate human type 0 RBC and unlike other MRV-3 strains (Dermody et al., 1990a, Lerner et al., 1963), HA was weak using bovine RBC.
Reoviruses possess a wide host range and naturally occurring MRV infections have been described in cattle, sheep, pigs, horses, dogs, cats, mice, as well as in human and non-human primates (Tyler, 2001). In mice and in non-human primates, reovirus infections can affect the respiratory, gastrointestinal and nervous systems (Tyler, 2001). In humans, mammalian reoviruses are mainly responsible for respiratory and/or enteric disease. However, MRV-3 infection has been associated to a case of meningitis in a child (Tyler et al., 2004). The ability to induce neurological disease is a characteristic of MRV-3 and a neurovirulent MRV-3 strain has been isolated from a cat with ataxia (Csiza, 1974). Because of the apparent lack of species barriers, MRVs may potentially spread from animals to humans and vice versa. From this perspective, further studies would help obtaining a more in-depth understanding of the ecology of reoviruses.
Acknowledgements
We are grateful to Dr. Michele Muscillo (Istituto Superiore di Sanità, Rome, Italy) for providing the reference MRV strains and Dr. Colin R. Parrish (Cornell University, Ithaca, NY, USA) for providing monoclonal antibodies to CPV-2.
References
- Appel M. Reovirus. In: Appel M., editor. Virus Infections of Carnivores. Elsevier; New York: 1987. pp. 95–96. [Google Scholar]
- Bassel-Duby R., Spriggs D.R., Tyler K.L., Fields B.N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J. Virol. 1986;60:64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binn L.N., Marchwicki R.H., Keenan K.P., Strano A.J., Engler W.R. Recovery of reovirus type 2 from an immature dog with respiratory tract disease. Am. J. Vet. Res. 1977;38:927–929. [PubMed] [Google Scholar]
- Breun L.A., Broering T.J., McCutcheon A.M., Harrison S.J., Luongo C.L., Nibert M.L. Mammalian reovirus L2 gene and λ2 core spike protein sequences and whole-genome comparison of reoviruses type 1 Lang, type 2 Jones, and type 3 Dearing. Virology. 2001;287:333–348. doi: 10.1006/viro.2001.1052. [DOI] [PubMed] [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L.E. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Cashdollar L.W., Chmelo R.A., Wiener J.R., Joklik W.K. The sequence of the S1 genes of the three serotypes of reovirus. Proc. Natl. Acad. Sci. U.S.A. 1985;82:24–28. doi: 10.1073/pnas.82.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappel J.D., Goral M.I., Rodgers S.E., dePamphilis C.W., Dermody T.S. Sequence diversity within the reovirus S2 gene: reovirus genes reassort in nature, and their termini are predicted to form a panhandle motif. J. Virol. 1994;68:750–756. doi: 10.1128/jvi.68.2.750-756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csiza C.K. Characterization and serotyping of three feline reovirus isolates. Infect. Immun. 1974;9:159–166. doi: 10.1128/iai.9.1.159-166.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J. Virol. Methods. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody T.S., Nibert M.L., Bassel-Duby R., Fields B.N. A sigma 1 region important for serotype 3 reovirus strains. J. Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermody T.S., Nibert M.L., Bassel-Duby R., Fields B.N. Sequence diversity in S1 genes and S1 translation products of 11 serotype reovirus strains. J. Virol. 1990;64:4842–4850. doi: 10.1128/jvi.64.10.4842-4850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K.T., Twist E.M., Horton-Slight P., Forrer C., Bell L.M., Jr., Plotkin S.A., Clark H.F. Epidemiology of rotavirus electropherotypes determined by a simplified diagnostic technique with RNA analysis. J. Clin. Microbiol. 1985;21:753–758. doi: 10.1128/jcm.21.5.753-758.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., Horne D., Cashdollar L.W., Joklik W.K., Lee P.W.K. Identification of conserved domains in the cell attachment proteins of the three serotypes of reovirus. Virology. 1990;174:339–409. doi: 10.1016/0042-6822(90)90093-7. [DOI] [PubMed] [Google Scholar]
- Fraser R.D., Furlong D.B., Trus B.L., Nibert M.L., Fields B.N., Steven A.C. Molecular structure of the cell-attachment protein of reovirus: correlation of computer-processed electron micrographs with sequence-based predictions. J. Virol. 1990;64:2990–3000. doi: 10.1128/jvi.64.6.2990-3000.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong D.B., Nibert M.L., Fields B.N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch J.R., Glass R.I., Woods P., Gouvea V., Gorziglia M., Flores J., Das B.K., Bhan M.K. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 1992;30:1365–1375. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goral M.I., Mochow-Grundy M., Dermody T.S. Sequence diversity within the reovirus S3 gene: reoviruses evolve independently of host species, geographic locale, and date of isolation. Virology. 1996;216:265–271. doi: 10.1006/viro.1996.0059. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky M.do C. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Hashimoto M., Roerink F., Tohya Y., Mochizuki M. Genetic analysis of the RNA polymerase gene of caliciviruses from dogs and cats. J. Vet. Med. Sci. 1999;61:603–608. doi: 10.1292/jvms.61.603. [DOI] [PubMed] [Google Scholar]
- Holzinger E.A., Griesemer R.A. Effects of reovirus type 1 on germfree and disease-free dogs. Am. J. Epidemiol. 1966;84:426–430. doi: 10.1093/oxfordjournals.aje.a120655. [DOI] [PubMed] [Google Scholar]
- Hrdy D.B., Rosen L., Fields B.N. Polymorphism of the migration of double-stranded RNA genome segments of reovirus isolates from humans, cattle, and mice. J. Virol. 1979;31:104–111. doi: 10.1128/jvi.31.1.104-111.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R.L., Huang G., Qiu W., Zhong Z.H., Xia X.Z., Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet. Res. Commun. 2001;25:77–84. doi: 10.1023/a:1006417203856. [DOI] [PubMed] [Google Scholar]
- Kedl R., Schmechel S., Schiff L. Comparative sequence analysis of the reovirus S4 genes from 13 serotype 1 and serotype 3 field isolates. J. Virol. 1995;69:552–559. doi: 10.1128/jvi.69.1.552-559.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokubu T., Takahashi T., Takamura K., Yasuda H., Hiramatsu K., Nakai M. Isolation of a reovirus type 3 from dogs with diarrhea. J. Vet. Med. Sci. 1993;55:453–454. doi: 10.1292/jvms.55.453. [DOI] [PubMed] [Google Scholar]
- Kumar S., Tamura K., Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 2004;5:150–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- Leary P.L., Erker J.C., Chalmers M.L., Cruz A.T., Wetzel J.D., Desai S.M., Mushahwar I.K., Dermody T.S. Detection of mammalian reovirus RNA by using reverse transcription-PCR: sequence diversity within the λ3-encoding L1 gene. J. Clin. Microbiol. 2002;40:1368–1375. doi: 10.1128/JCM.40.4.1368-1375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.W., Hayes E.C., Joklik W.K. Protein σ1 is the reovirus cell attachment protein. Virology. 1981;108:156–163. doi: 10.1016/0042-6822(81)90535-3. [DOI] [PubMed] [Google Scholar]
- Lerner A.M., Cherry J.D., Finland M. Hemagglutination with reoviruses. Virology. 1963;19:58–65. doi: 10.1016/0042-6822(63)90024-2. [DOI] [PubMed] [Google Scholar]
- Lou T.Y., Wenner H.A. Natural and experimental infection of dogs with reovirus type 1: pathogenecity of the strain for other animals. Am. J. Hyg. 1963;77:293–304. [Google Scholar]
- Marshall J.A., Kennett M.L., Rodger S.M., Studdert M.J., Thompson W.L., Gust I.D. Virus and virus-like particles in the faeces of cats with and without diarrhea. Aust. Vet. J. 1987;64:100–105. doi: 10.1111/j.1751-0813.1987.tb09638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsilio F., Di Martino B., Decaro N., Buonavoglia C. Nested PCR for the diagnosis of calicivirus infections in the cat. Vet. Microbiol. 2005;105:1–7. doi: 10.1016/j.vetmic.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Massie E.L., Shaw E.D. Reovirus type 1 in laboratory dogs. Am. J. Vet. Res. 1966;27:783–787. [PubMed] [Google Scholar]
- Mochizuki M., Uchizono S. Experimental infections of feline reovirus serotype 2 isolates. J. Vet. Med. Sci. 1993;55:469–470. doi: 10.1292/jvms.55.469. [DOI] [PubMed] [Google Scholar]
- Nibert M.L., Dermody T.S., Fields B.N. Structure of the reovirus cell-attachment protein: a model for the domain organization of σ1. J. Virol. 1990;64:2976–2989. doi: 10.1128/jvi.64.6.2976-2989.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M.L., Schiff L.A. Reoviruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1679–1728. [Google Scholar]
- Pratelli A., Tempesta M., Greco G., Martella V., Buonavoglia C. Development of a nested PCR assay for the detection of canine coronavirus. J. Virol. Methods. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen L. Serologic groupings of reovirus by hemagglutination inhibition. Am. J. Hyg. 1960;71:242–249. doi: 10.1093/oxfordjournals.aje.a120107. [DOI] [PubMed] [Google Scholar]
- Sabin A.B. Reoviruses. Science. 1959;130:1389–1397. [Google Scholar]
- Schulze C., Baumgartner W. Nested polymerase chain reaction and in situ hybridization for diagnosis of canine herpesvirus infection in puppies. Vet. Pathol. 1998;35:209–217. doi: 10.1177/030098589803500306. [DOI] [PubMed] [Google Scholar]
- Scott F.W., Kahn D.E., Gillespie J.H. Feline reovirus: isolation, characterization, and pathogenicity of a feline reovirus. Am. J. Vet. Res. 1970;31:11–20. [PubMed] [Google Scholar]
- Tyler K.L. Mammalian reoviruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1729–1745. [Google Scholar]
- Tyler K.L., Barton E.S., Ibach M.L., Robinson C., Campbell J.A., O’Donnel S.M., Valyi-Nagy T., Clarke P., Wetzel J.D., Dermody T.S. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 2004;189:1664–1675. doi: 10.1086/383129. [DOI] [PubMed] [Google Scholar]
- Weiner H.L., Ault K.A., Fields B.N. Interaction of reovirus with cell surface receptors. I. Murine and human lymphocutes have a receptor for the emagglutinin of reovirus type 3. J. Immunol. 1980;124:2143–2148. [PubMed] [Google Scholar]
- Weiner H.L., Fields B.N. Neutralization of reovirus: the gene responsible for the neutralization antigen. J. Exp. Med. 1977;146:1305–1310. doi: 10.1084/jem.146.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H.L., Raming R.F., Mustoe T.A., Fields B.N. Identification of the gene coding for the hemagglutinin of reovirus. Virology. 1978;86:581–584. doi: 10.1016/0042-6822(78)90099-5. [DOI] [PubMed] [Google Scholar]


