Abstract
Bovine coronaviruses, like other animal coronaviruses, have a predilection for intestinal and respiratory tracts. The viruses responsible for enteric and respiratory symptoms are closely related antigenically and genetically. Only 4 bovine coronavirus isolates have been completely sequenced and thus, the information about the genetics of the virus is still limited. This article reviews the clinical syndromes associated with bovine coronavirus, including pneumonia in calves and adult cattle, calf diarrhea, and winter dysentery; diagnostic methods; prevention using vaccination; and treatment, with adjunctive immunotherapy.
Keywords: Bovine respiratory coronavirus, Bovine enteropathogenic coronavirus, Calf diarrhea, Winter dysentery
Impact of bovine respiratory disease complex
Bovine respiratory disease complex (BRDC) represents a major cause of economic loss in the beef and dairy cattle industries worldwide. In North America especially, this complex represents the leading cause of morbidity and mortality in 6- to 10-month-old beef cattle after entry into feedlots (United States Department of Agriculture, 2000).1 The financial losses are in part due to mortality, which can reach up to 69% in beef calves during first 2 months of arrival.2 Reduced growth performance and overall treatment costs (eg, metaphylactic and therapeutic use of antibiotics) for BRDC in a 1000-cattle feedlot has been estimated to be $13.90 per animal, assuming calves are slaughtered after 200 days on feed; labor and handling costs excluded.3
The BRDC is a multifactorial disease arising from a combination of environmental, host, management, viral, and bacterial factors. The disease often develops along with stressful conditions such as weaning, shipping, commingling, dietary changes, and adjustments to the feed yard environment. These conditions favor viral infections of the lower respiratory tract that may become further complicated by Mannheimia haemolytica serotype 14 and Pasteurella multocida, both commensal bacteria of the nasal cavity. Viral infections well known to play an important role in the development of BRDC include bovine herpesvirus 1 (BHV-1), bovine respiratory syncytial virus (BRSV), bovine viral diarrhea virus (BVDV), and parainfluenza virus type 3 (PI-3). Most cattle arriving at feedlot are routinely vaccinated against these viruses, which has led to a decrease in incidence of these primary pathogens. However, other viral agents continue to cause substantial losses due to BRDC (Sanjay Kapil, unpublished data, 2008). There is currently a growing body of evidence1, 4, 5, 6, 7, 8, 9 showing that bovine coronavirus (BCoV) may be involved in the development of BRDC.
Bovine coronavirus
Animal coronaviruses are divided into 3 antigenic groups: Group 1 has no hemagglutinin-esterase (HE), and important members of this group are feline infectious peritonitis and transmissible gastroenteritis virus in swine; Group 2 has HE and contains BCoV10; and Group 3 contains avian virus–like infectious bronchitis virus. There are only a few studies on molecular epidemiology of BCoV.11, 12, 13 These studies have targeted the spike gene of BCoV for phylogenetic analysis because it is the most critical surface protein that binds to the receptor N-acetyl-9-O-acetylneuraminic acid of the virus.14 The Japanese BCoV isolates (1999–2008) cluster in 4 phylogenic groups. Japanese isolates collected after 2005 were included in antigenic Group 4.11 In another study from South America, the Brazilian BCoV isolates were genetically divided into 2 groups.12 At present, there are no large-scale studies that have compared the BCoV isolates from America and other parts of the world.
Epidemiology of Bovine Coronaviruses
Bovine coronavirus is widespread in the cattle population, resulting in economic losses to the beef and dairy industry throughout the world. The virus has been detected on all continents and there is serologic incidence (>90%) that suggests most cattle become exposed to BCoV in their lifetime. In a recent study, the presence of BCoV in lungs was second in incidence after bovine herpesvirus.15 Both in beef and dairy herds, BCoV can be associated with calf diarrhea, calf respiratory disease, winter dysentery, respiratory disease in adult cattle, and combined pneumonia and diarrhea in calves and adults.16, 17 The coronavirus strains isolated from nasal secretions and lung tissues of cattle with fatal cases of pneumonia have been classified as bovine respiratory coronaviruses (BRCoV). The coronavirus strains isolated from neonatal calves and adult cattle with diarrhea are referred to as bovine enteric or enteropathogenic coronaviruses (BECoV).18 Furthermore, for clinical purposes, BECoV can be further subdivided into BCoV- induced calf diarrhea (BCoV-CD) and winter dysentery (BCoV-WD). The clinical manifestation of the disease syndromes are not solely related to the virus itself but also to host and environmental factors; for example, the immunologic status of the animal, environmental temperature, and secondary coinfections with other pathogens.19
Researchers have debated over last several years whether BCoV isolated from the gastrointestinal and respiratory tracts of affected cattle are the same virus or are dissimilar, perhaps altered in biologic, antigenic, and genetic (sequence) properties. Several publications have supported the hypothesis that enteric and respiratory BCoV may be the same virus detected at different stages of its infectious life cycle.20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Early reports28, 29 suggested antigenic and genomic similarity between isolates of BCoV from the respiratory and enteric tracts of cattle. Later studies5, 25, 30, 31 identified differences in antigenic, genomic, and culture characteristics between the 2 groups of BCoV isolates. At present, it is still unclear whether isolates of BRCoV and BECoV can be distinguished antigenically.30, 32 Specific factors associated with their respective tropism for the respiratory or digestive tracts are also undefined. The reasons for scientific uncertainties33 include that BCoV RNA genome is the longest (approximately 32,000 bases) compared with other animal viruses and is capable of further evolution. In addition, the number of complete sequences published on this virus is scarce, for example, 4 isolates comprising Mebus (U00735), Québec (AF220295), and Louisiana (NC_003045) enteric and respiratory BCoV. Due to the insufficient number of BCoV isolates sequenced, an accurate comparison of BECoV and BRCoV origin is therefore difficult to establish. Moreover, there are temporal changes and geographic differences that further confound the conclusions.34
Coronaviruses within the antigenic group 2 are known to cross between species. Beyond cattle, other domestic animals (horses, water buffalo,35 camel,36 New World camelids37), and wildlife (deer, elk38) and zoo animals (giraffe39) also have BCoV-like viruses that can infect calves because these viruses are genetically related to BCoV. The infection of small ruminants with coronavirus is less common. There is seasonal variation in the incidence of BCoV diarrhea. Stressors, including inclement weather40 and shipping, are important contributing factors that may exacerbate disease from BCoV and BRCoV infections.
Transmission and pathogenesis
Infection is primarily via feco-oral and to a lesser extent, respiratory (aerosol) routes.20, 23, 41, 42, 43 Bovine enteropathogenic coronavirus is shed in mucosal secretions from the upper respiratory tract and excretions from the gastrointestinal tract.43, 44, 45 Bovine coronavirus is ubiquitous in the cattle population and persists in adults as subclinical infections.44, 46 However, under stressful conditions adult cattle can shed BCoV in feces and nasal secretions.46 Most often, transmission of BECoV is horizontal, and occurs from carrier dam to offspring postpartum47 or from clinically or chronically infected calves housed in proximity to naïve ones. Evidence of vertical transmission has not been reported.47 In close herds, respiratory tract infections constitute a source of BCoV transmission to cows or young calves.26 Experimental inoculation of BCoV-CD and BCoV-WD strains of BCoV in adult dairy cows has been associated with development of clinical signs and viral shedding in the feces.48
Bovine coronavirus is a pneumoenteric virus that replicates in the enterocytes of the intestinal tract and the epithelium of the upper respiratory tract.34, 43 More specifically, the virus has been shown to replicate in the respiratory tract of calves, with viral antigen detected in the epithelium of the lung, trachea, and nasal turbinates.7, 23 Park and colleagues34 have suggested that BCoV infections of the respiratory tract may occur via inhalation, via monocyte-associated viremia that may originate from the intestines after ingestion of BCoV, or via cell-free viremia. In one study, peak of BCoV shedding in nasal secretions occurred at 3 days before arrival and in feces, at 3 days following entry to feedlot, under field conditions.20 Researchers have proposed that replication and shedding of BCoV in nasal secretions is first initiated through the respiratory tract (oropharynx) then spreads to the gastrointestinal tract through the swallowing of large quantities of virus with subsequent shedding in the feces.20, 49 Other reports have documented BCoV shedding in both nasal passages and feces within the same animal concurrently.20, 21, 22 Respiratory disease can be consistently reproduced experimentally in young calves using a pneumoenteric BCoV, Minnesota-1988 (MN-1988).50, 51
Coronavirus is an enveloped single-stranded RNA virus, and is not as stable in the environment as rotavirus.47 However, in the presence of organic material, these viruses may remain infectious for up to 3 days. Of note, coronaviruses can bind extremely well to clay, clay minerals, and charcoal in vitro, with an adsorption of 99%.52 These findings suggest that clay soils can concentrate BCoV on its surface, which can be clinically relevant, as the animals grazing on these types of soils could get exposed to infectious doses of BCoV.
Dogs may play a role in BCoV infection; canine respiratory coronavirus is genetically related to BCoV and has been found in kennel cough cases in Europe and the United States.53 Dogs may represent a passenger of the BCoV on farms.54
Role of bovine coronavirus in bovine respiratory disease complex
Within the last 2 decades, BRCoV has been associated, either alone or along with other respiratory pathogens, with the emergence of shipping fever pneumonia of beef cattle after transport to feedlot5 and enzootic calf pneumonia.55, 56, 57
However, there is still conflicting information in the literature regarding the true role of BCoV as a pathogen of the respiratory tract in calves7, 58 and feedlot cattle. Several investigators have shown that BCoV may represent one important pathogen involved in the development of BRDC,1, 4, 5, 6, 8, 9, 59, 60 whereas others could not detect any correlation between BCoV shedding and respiratory tract disease under field conditions.21, 32, 61 At least 3 studies failed to reproduce clinical signs of BRDC in calves after experimental inoculation with BCoV,22, 23, 62 which may be due to choice of the viral isolate used to experimentally reproduce the infection. In contrast, 2 research groups reported that BCoV can be isolated from clinically healthy cattle,24, 31 whereas others have failed to detect the presence of BCoV in feedlot calves experiencing respiratory tract disease.6, 8, 59
In 1995, it was reported that all 4 of Koch's postulates to associate BCoV with upper and lower respiratory tract disease in neonatal calves were fulfilled.45 However, these postulates do have limitations when being applied to complex diseases such as BRDC in adult cattle.9, 63 Investigators recently supported BRCoV as the primary inciting cause of the 2 epizootics of shipping fever pneumonia they investigated,4 based on Thomson's modification64 of Evans' criteria of causation.9, 65
There is evidence that BRCoV can be repeatedly isolated in the majority of calves sampled soon after feedlot entry.32, 59, 60 Cattle shedding BCoV nasally and seroconverting within the first month after entering the feedlot are at increased risk for respiratory disease60 and 1.6 times more likely to require subsequent treatment for BRDC.8 Reported rates of nasal BCoV isolation in several studies have ranged from 8.1% to 60%.8, 20, 21, 31, 60 In one report, cattle that shed BCoV in their nasal secretions during the first 28 days after feedlot arrival were 2.2 times more likely to have pulmonary lesions at slaughter compared with nonshedders.8 Although there was no statistical association between clinical signs and virus shedding, another trial reported that feedlot cattle shedding BCoV nasally were 2.7 times more likely to show respiratory signs, and those shedding BCoV fecally were 2.5 times more likely to develop diarrhea.32
Most of the calves shedding BCoV nasally at arrival have low but detectable antibody titers at arrival and typically seroconvert within the first 3 to 4 weeks after feedlot entry, suggesting that they are often infected with BRCoV at times when respiratory tract disease is likely to occur.32, 59, 61 Two published seroepidemiologic studies found that although higher antibody titers against BCoV at feedlot arrival were significantly associated with a decreased subsequent risk of treatment for BRDC within cattle groups, there was no association between evidence of recent infection (titer increase) and the incidence of BRDC.1, 61 In contrast, several investigators have shown that high antibody titers against BCoV at feedlot arrival have consistently been associated with a decrease in BCoV infection, shedding, or both, under field conditions20, 21, 32 or in experimental challenge studies.26, 62 Decreased BCoV shedding in nasal secretions and protection against BCoV infection have been associated with high serum antibody titers (geometric mean: GMT) ranging from 1600 to 2,26220, 21, 26, 32 at feed yards entry. Moreover, cattle entering feedlots with high antibody titers against BCoV appeared less likely to seroconvert to BCoV than cattle without detectable BCoV titers at arrival.1, 59, 62
Depending on the feedlot, active immunity was reported to be associated with moderate to high seroconversion (4-fold increase in BRCoV antibody titers) in the face of clinical respiratory tract infection in 58% of 814 cattle,59 61% of 604 cattle,1 91% of 85 cattle,32 and 90% in 852 cattle spread among 3 different feed yards, and in 95% of 57 cattle.21
Economic Impact of Bovine Respiratory Coronavirus
Several investigators have shown that BCoV-associated BRDC is correlated with decreased performance in feedlot cattle.1, 21, 32 According to a published report,1 shedding of BRCoV correlated with a reduction in weight gain. One study involving 837 calves in 4 feedlots from 2 states (Ohio, Texas) showed that the BCoV shedding or seroconversion status did not affect the average daily gain.8 However, shedding of BCoV in feces of 6- to 7-month-old cross-bred feedlot steers was associated with a reduced weight gain of 8.17 kg (17.9 lb) during a period of 21 days.21 In an Ohio feedlot,32 calves that seroconverted to BCoV gained 5.9 kg (13 lb) (26%) less than the nonseroconverted group during the first 21 days after arrival to the feedlot. Seroconversion to BCoV was almost associated significantly (P<.06) with reduction in weight gain but not with clinical signs. In one report involving 203 feedlot calves from New Mexico and Arkansas, animals shedding BCoV in nasal secretions, feces, or both, gained on average 8 kg (17.64 lb) less than calves that were not shedding the virus over a 35-day period following entry.20 Therefore, BCoV infections may contribute directly to economic losses in feedlot cattle by impacting weight gains or, similar to other respiratory viruses, by predisposing cattle to secondary bacterial infection.
Clinical Manifestation of Bovine Respiratory Coronavirus
Under experimental conditions, neonatal, colostrum-deprived calves inoculated with BCoV can develop respiratory distress, such as wheezing and open-mouth breathing.51 Under natural conditions, calf pneumonia caused by BRCoV can be observed in calves aged 6 to 9 months. Affected animals may develop fever,23 serous to mucopurulent nasal discharge,66 coughing, tachypnea, and dyspnea.5, 7
Respiratory illness caused by BRCoV in an Ohio feedlot was characterized by coughing and nasal discharge along with diarrhea, and was observed in 62% and 77% of cattle.32 Diagnostic investigation of 214 BRDC outbreaks in Italy was associated with an 85% morbidity rate in those due to BRCoV infection.67 The mortality rate due to BCoV infection can be high.4, 67
In another study,2 viral respiratory disease was seen in 19% of the animals and accounted for 20% of the mortality in feedlot cattle. Bovine respiratory coronavirus was detected in approximately 2% of the cases based on virus isolation in cell culture. If fluorescent antibody testing or reverse transcriptase-polymerase chain reaction (RT-PCR) were used for detection of BRCoV, the actual incidence may have been higher. The reason RT-PCR gives higher estimates of BCoV infection in lungs is because, at core body temperature, the replication of BCoV may be diminished. However, in the upper cooler parts of the respiratory tract, replication of the virus is abundant and can become the source of the virus for the lower respiratory tract.
Other clinical syndromes associated with bovine coronavirus
Bovine Enteropathogenic Coronavirus Associated with Diarrhea in Calves
Pathogenesis and pathology
Enteropathogenic bovine coronavirus is widely recognized as an important primary pathogen causing neonatal calf diarrhea (BCoV-CD).68, 69 The pathology of BCoV-CD is often more severe than that of rotavirus, resulting in a mucohemorrhagic enterocolitis.70 Infection leads to destruction of the absorptive intestinal villous epithelial cells.69, 71, 72 Virus replication is cytocidal and initially occurs throughout the length of the villi in all levels of the small intestine, eventually spreading throughout the large intestine up to the end of the large colon and rectum, causing a malabsorptive diarrhea. Large concentration of BCoV can be typically found in the spiral colon. Infected epithelial cells die, slough off, and are replaced by immature cells. Stunting and fusion of adjacent villi and atrophy of colonic ridges may be seen on microscopic examination of BCoV-infected small and large intestine, respectively.69, 71, 73, 74, 75 In case of malabsorptive diarrhea caused by BCoV, the fluid load in the gut lumen can be further exacerbated by the compensatory hyperplasia and secretions from the crypt epithelial cells.76 The absorptive and digestive capacity of the intestinal tract is therefore compromised by loss of surface area and presence of immature cells, which are unable to secrete the normal digestive enzymes.76, 77, 78 Lesions and consequences are most severe in younger animals.47
Continual enteral feeding may result in more nutrients presented to the small intestine than the damaged villi can absorb.79 Undigested nutrients are fermented in the large intestine, promoting bacterial overgrowth and production of organic acids, especially d-lactate.80 The osmotic effects of the unabsorbed nutrients draw water into the gut lumen and contribute to the diarrhea.76 Over time, if fluid losses exceed intake, extensive water (mainly from the extracellular space), sodium, chloride, potassium, and bicarbonate loss occurs.81 Dehydration and metabolic acidosis subsequently develops. The acidosis has several causes including fecal bicarbonate loss, endogenous l-lactic acid production in response to dehydration and poor tissue perfusion, and local d-lactic acid production within the gastrointestinal tract.80, 81
Epidemiology
Bovine coronavirus causes enteritis in both dairy and beef herds, with naturally occurring cases showing clinical signs of disease between 5 and 30 days of life.82, 83, 84, 85 According to the BCoV enzyme-linked immunosorbent assay (ELISA) database from Kansas State University, Manhattan, KS, 1 in 3 case of calf diarrhea in the age group of 1 to 9 weeks can be due to BCoV. Clinical disease may occur as young as 24 hours of age in colostrum-deprived calves, and as late as 5 months of age.42, 73, 85, 86 The incidence of BCoV-CD in naturally occurring outbreaks has been reported to vary from 15% to 70%.73, 87, 88
Once infected, a calf can excrete high levels of virus (eg, 1 billion virus particles per ml of feces) within 48 hours after experimental infection, and this may persist up to 14 days.50 Clinically recovered calves may continue to shed low levels of virus for weeks.50 Of note, BECoV may be detected in the feces of both diarrheic and healthy calves, with clinically diarrheic calves more commonly tested positive (incidence: 8%–69%) compared with healthy calves (incidence: 0%–24%).73, 76, 82, 85, 88, 89
BECoV has been detected intermittently at very low levels in the feces of more than 70% of clinically normal cows, despite the presence of specific antibodies in serum and feces.46, 87 In one study involving 132 cows and heifers with no previous BCoV vaccination history, all were found to have substantial levels of antibodies.90 In nonvaccinated cows, the rate of virus excretion has been reported to increase by 50% to 60% during the winter months, by 65% at parturition, and by 71% 2 weeks postpartum.44 This virus is more stable in the colder climates, due to lower ambient temperature and reduced ultraviolet light levels,47 and has been reported to cause winter dysentery in adult cattle91 especially after snow storms or sudden changes in ambient temperatures. Calves born to BCoV carrier dams have a significantly higher risk of developing BCoV diarrhea92 due to periparturient exposure from fecal contamination of the perineum, teats, and the calving area.
Economic impact
Diarrhea remains an important cause of illness and death in young beef calves. Economic losses associated with the disease include decreased performance, mortality, and the expenses of medication and labor to treat sick animals. Annually, beef cattle herds may experience death rates of 5% to 10% or greater, and sometimes up to 100% morbidity.
Clinical signs
The severity of the BECoV enteritis depends on the age of the calf at time of infection, its immunologic status, the size of the infective dose, and the virulence of the BECoV strain in question. As a general rule, the severity of the disease is increased and the incubation period is shortened in younger compared with older calves, especially those with failure of passive transfer. A yellow to blood-stained mucus-containing diarrhea initially develops, which then progresses to a profuse watery diarrhea.23, 51 When the fluid intake is insufficient to meet the losses, affected animals become clinically dehydrated, depressed, weak, and hypothermic, and their suckle reflex is loosened. The majority of calves recover, but a few may develop pyrexia, recumbency, and progression to cardiovascular collapse (from dehydration, acidosis, and associated hyperkalemia), coma, and death if the diarrhea is particularly severe and left untreated.22, 23, 69, 70, 74, 81 Some of the BECoV-infected calves may develop a pneumoenteritis syndrome in which both diarrhea and mild signs of respiratory tract disease are present.17 Affected calves shed the virus not only in their feces but also in their nasal secretions.16, 51
Differential diagnosis
Other enteropathogens, such as rotavirus, are frequently detected in feces from BECoV-infected calves.83, 88 Rotaviruses are the leading cause and coronaviruses are a major contributor of calf diarrhea; however, infections with BCoV are more severe because they affect both the small and large intestines. In most outbreaks of acute undifferentiated BCoV-CD, calves frequently shed 1, 2, or multiple agents simultaneously.85, 93 Mixed infections are typically associated with more severe disease.85 Researchers have reported that calves shedding 2 or more pathogens were 6 times more likely to develop clinical diarrhea compared with the ones that shed only one or no pathogen.84 Besides rotavirus and coronavirus, Cryptosporidium parvum, enterotoxigenic Escherichia coli, and Salmonella spp are recognized as the major infectious agents associated with diarrhea in calves.82, 84, 89, 94, 95 Without specific testing, it is usually impossible to make a definitive etiologic diagnosis solely based on clinical signs.70, 95 Signs of colitis in calves including tenesmus and presence of frank blood and mucus in the feces may be present with Salmonella spp, coronavirus, BVDV, enteropathogenic E. coli, or coccidian infection.70
Bovine Enteropathogenic Coronavirus Associated with Winter Dysentery in Adult Cattle
Etiology, pathogenesis, and pathology
During the past 2 decades, evidence has accumulated implicating BCoV as a cause of winter dysentery (BCoV-WD).63, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106 Winter dysentery is a sporadic acute, contagious hemorrhagic enterocolitis of cattle that occurs in epizootic fashion in a herd.105 The pathophysiological characteristics of BCoV infection can be attributed to lesions of the colonic mucosa.102 The intestinal lesions are comparable with those observed in calves with BECoV-induced diarrhea. Epithelial cells of colonic crypts are destroyed by viral action, leading to degeneration, necrosis of crypt epithelium, and petechial hemorrhage, without involvement of the Peyer patches. Fine streak of frank blood or large blood clots may be present in the lumen of the spiral colon, distal colon, and rectum.91, 104 Even though histologic changes have been observed predominantly in the colonic mucosa, blood from the distal duodenum was observed aborally in cattle that died of winter dysentery.104 Loss of intestinal mucosal epithelium from colonic crypts leads to transudation of extracellular fluid and blood. The respiratory tract of BCoV-WD affected animals may show hyperemia of the tracheal mucosa and localized foci of pneumonia.107
Epidemiology
In the United States, BCoV-WD is more common in the northern states; however, it has been reported throughout the world including Australia, Sweden, the United Kingdom, Israel, France, Belgium, Italy, Japan, Cuba, and Canada.40, 63, 97, 103, 104, 108 The disease occurs usually during the colder months of the year63 and often coincides with close confinement of cattle. Only seldom have reports described BCoV-WD occurring during the warmer season.34, 40, 108 The disease is characterized by a high morbidity rate ranging from 50% to 100%.63, 91 In contrast, mortality rate is usually low, typically less than 2%, with only a few reports describing case fatality associated with this virus.91, 99, 107 Winter dysentery outbreaks are predominantly seen in young postpartum adult dairy cows, which then experience a marked drop in milk production, resulting in 25% to 95% milk losses.66, 100, 104, 109, 110, 111 In the acute stage of the disease, this may last 3 to 6 days. When it persists for a few days to a week or more after the outbreak terminates, economic loss can be substantial.112
Though infrequent, BCoV-WD has been also observed in adult beef cattle97 and in 6- to 9-month-old feedlot calves.91 Although most reports indicate this to be a disease of adult cattle, in a herd outbreaks of mild diarrhea may be observed in heifers and calves as young as 4 months old.40, 113
The incubation period for BCoV-WD ranges from 2 to 8 days. In small housed herds, the incidence of diarrhea during an outbreak begins with the explosive appearance of signs in 10% to 15% of animals on the first day.113 By the second day, another 20% to 40% are affected; morbidity reaches 100% by the fourth day.104 By the end of the week the first affected animals are completely recovered, and only a small number of new cases occur.113 Within 2 to 3 weeks of the onset of diarrhea, all animals have recovered. In large herds the outbreak may be prolonged for 6 to 8 weeks.63 This scenario is typical of a herd that had not experienced an epizootic of winter dysentery during the preceding few years.
Epidemiologic studies of BCoV-WD have suggested that various host and environmental factors may contribute to the appearance of the disease.48, 63, 101, 106, 114 These factors include the age and reproductive status of the animal, with recently calved 2- to 6-year-olds being the most susceptible,112 and previous history of a BCoV disease outbreak in herds comprising more than 60 cows.114 Environmental risk factors for BCoV-WD include drop in atmospheric temperature, close confinement, poor ventilation, and using manure-handling equipment to handle feed. Although nonspecific, historical findings associated with BCoV-WD outbreak may include recent stressors (eg, inclement weather), incoming farm visitors who have had close contact with cattle, or introduction of a newly purchased animal.112
Clinical signs
Winter dysentery is an acute diarrheic disease of predominantly dairy and infrequently beef cattle characterized by an acute onset of dark brown, often hemorrhagic, watery, and commonly profuse diarrhea accompanied by some degree of anorexia and depression.63, 66, 102, 114 The diarrhea may contain a slight to copious amount of mucus. The amount of blood varies from case to case and ranges from just visible flecks or streaks to large clots, or it may be uniformly mixed into the feces.113 Pyrexia is usually not present during the diarrheal phase of the disease, although it has been reported to precede it by 24 to 48 hours107, 112 or have no consistent relationship.115 Mild to moderate signs of respiratory disease (eg, cough, nasal discharge)40, 91, 107, 116 have been inconsistently observed preceding or concurrent with the diarrhea.26, 113 As mentioned previously, milk production can be significantly reduced in affected lactating cows. A few cases may show mild colic signs while other animals appear weak.113 If the diarrhea is severe or persists longer than 1 or 2 days, dehydration and secondary polydipsia may develop. Ruminal motility is commonly reduced and intestinal borborygmi may be increased.
The odor in a barn during an outbreak of BCoV-WD has been described as musty, fetid, and unpleasantly sweet.66, 109 The period of illness in an individual is brief, and within a herd the outbreak usually lasts less than 2 weeks.113 According to most reports the shorter and less severe the diarrhea, the more rapid the recovery and return to normal condition ensues.109
Differential diagnosis
Winter dysentery is usually recognized by the clinical syndrome described here and by exclusion of other causes of acute and contagious diarrhea of adult cattle.107 Diarrhea caused by BVDV, coccidiosis, and salmonellosis must be considered in the differential diagnosis of BCoV-WD. PCR, virus isolation or immunohistochemistry (ear notch), fecal floatation, and fecal bacterial culture should be performed to rule out BVDV, coccidiosis, and salmonellosis, respectively. Specific hematologic changes that would be consistent with a diagnosis of BCoV-WD have not been reported.113 If significant dysentery persists for longer than a day, anemia may develop due to significant blood loss.
The disease syndrome is more often than not diagnosed on the basis of history of acute onset of diarrhea and dysentery affecting at least 15% of the adult cattle herd; rapid spread causing a drop in milk production of 10% or more; resulting in less than 2% fatalities.101 The rapid occurrence of multiple cases within a herd combined with spontaneous recovery over a few days and absent oral mucous membranes erosions suggests BCoV-WD.
Diagnosis of bovine coronaviruses
Because of similarity of clinical signs induced by various infectious agents, physical examination of calves or adult cattle with respiratory tract disease or diarrhea is not sufficient for diagnosis of BRCoV infection. Bovine respiratory coronavirus can only be identified through laboratory confirmation of appropriate specimens submitted. Suggested antemortem samples for BRCoV infection in calves and adult cattle include nasal swabs submitted in phosphate-buffered saline (pH 7.2) or normal saline in red-top tubes. Oropharyngeal fluid collected with a probang cup can be used to diagnose BRCoV in adult cattle. Trachea (upper one-third), and lungs can be collected at necropsy. However, the distribution of BCoV in the lungs is focal and thus, it is critical that multiple pieces are submitted for virus detection. Viral antigen has been detected in the apical and middle lung lobes of calves infected experimentally with BCoV (MN-1998) whereby 5% to 10% of the macrophages were positive for BCoV (Fig. 1 ) (Tawfik Aboellail, and Sanjay Kapil, unpublished data, 2000). Other suitable respiratory tissues include nasal turbinates, but these may be difficult to sample. Nasal turbinates and nasal glands have been found to be strongly positive for BCoV antigen using immunohistochemistry and immunofluorescence (Figs. 2 and 3 ).117 Spiral colon is the sample of choice for detection of BECoV at necropsy because the virus persists in that specific location for the longest time after oral infection.50 A fresh fecal sample collected directly from the rectum can be also included, as cattle with respiratory disease commonly shed the virus in the feces concomitantly. Approximately 2 to 5 g of fresh feces can be sent to the laboratory in wide-mouth jars. It is important to submit all tissue and fecal samples over ice-packs using overnight delivery to increase the likelihood of BCoV detection.
Fig. 1.
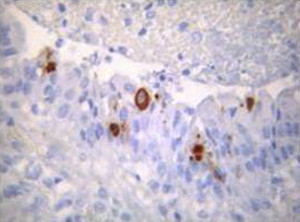
BCoV antigen in macrophages was detected by immunohistochemistry (8F2) in formalin-fixed section of lungs from an adult cow showing clinical signs of lower respiratory tract infection.
Fig. 2.
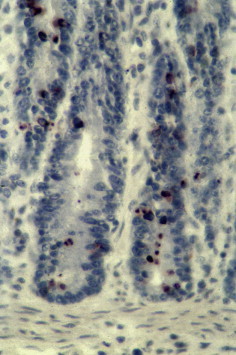
Immunohistochemistry on formalin-fixed section of the intestine submitted from a calf stained with 8F2 anti nucleoprotein antigen of BCoV. Brown staining in the crypts indicates positivity for BCoV.
Fig. 3.
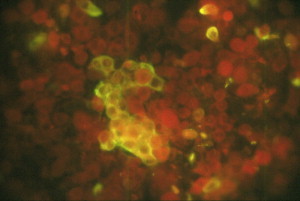
Nasal cells positive for BCoV by direct fluorescent antibody test using anti-BCoV fluorescein isothiocyanate conjugate. Swab was collected from a calf experimentally infected with a pneumoenteric BCoV (MN-1988).
Diagnostic tests of choice for BCoV are antigen-capture ELISA118 using Z3A5 monoclonal as capture antibody. Another useful diagnostic reagent is 8F2 monoclonal antibody (MoAb), which binds to nucleocapsid protein of BCoV, the most predominant protein of the virus (www.ruraltechinc.com).119 The 8F2 reacts with the viral antigen in formalin-fixed intestines (see Fig. 3) and lungs. The 8F2 MoAb reacts with a conserved epitope of the antigenic group 2 coronaviruses such as alpaca, equine, camel, and elk coronavirus. Other laboratory-based methods for detection of BCoV include hemagglutination assay using mouse erythrocytes; this method, with modification (such as slide agglutination test), can be used in developing countries and for animal-side testing. RT-PCR assays can be used for sensitive detection of BCoV in clinical samples. The targets are the conserved nucleocapsid gene for detection of the virus and spike gene for epidemiologic investigation and strain differentiation. At present, there is no commercial test available for BCoV antigen detection in the United States. However, lateral flow immunoassays (LFT) are useful cow and calf-side tests, and are available in European Union for BCoV antigen detection in the feces.120
Based on experimental infection with a pneumoenteric isolate of BRCoV, neonatal colostrum-deprived calves develop interstitial pneumonia (Fig. 4 ) and emphysema, pulmonary congestion and hemorrhage (Fig. 5 ), and edema of the interlobular septa, with the ventrolateral areas of the lungs being mainly affected.45 Most of these calves showed cryptitis in the spiral colon on histologic examination (Fig. 6 ).
Fig. 4.
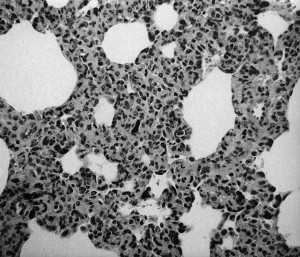
Interstitial pneumonia in a calf experimentally infected with MN-1988 pneumoenteric BCoV isolate.
Fig. 5.
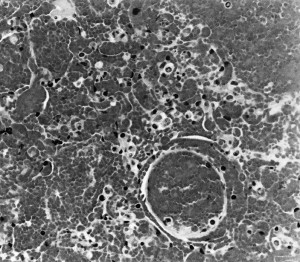
Pulmonary hemorrhages in a calf inoculated with a pneumoenteric isolate of BCoV.
Fig. 6.
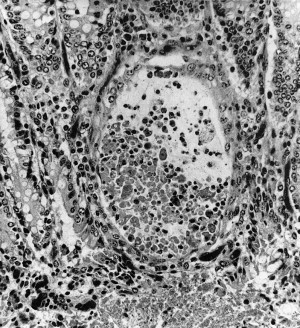
Crypt dilation after experimental infection of a calf with MN-1988 BCoV isolate.
Treatment of bovine coronavirus infection
Bovine Respiratory Coronavirus
There are no specific antiviral treatments for BCoV infection in beef or dairy cattle. However, because viral infection can predispose to development of secondary bacterial infection in the lungs and the fact that BCoV cannot be differentiated clinically from other important viral or bacterial pathogens involved in BRDC, parenteral antibiotic therapy administered early in the disease process, at sufficient dosage and duration, is recommended to prevent or limit the development of bacterial pneumonia in cattle with viral respiratory tract infection.55 The use of nonsteroidal anti-inflammatory drugs (NSAIDs) such as flunixin meglumine (Banamine), currently labeled specifically for the control of pyrexia associated with BRDC in the United States, has been shown to be useful in other viral pneumonias (eg, PI-3) by improving clinical signs and reducing lung consolidation.121 However, flunixin meglumine may not be cost-effective when used in large feedlots and can potentially lead to renal toxicity or abomasal ulceration if overdosed, used for prolonged periods of time, or used in severely dehydrated animals. Approved in the European Union for use in food animals, a single injection of the long-acting NSAID meloxicam (Metacam) administered as an adjunct therapy for BRDC in feedlot cattle resulted in substantial pharmacoeconomic benefit.122 Based on a limited number of publications123, 124 and due to its impairment of immune function, it is usually not recommended to use corticosteroids as an adjunct therapy for the treatment of undifferentiated pneumonia in feedlot cattle. Other ancillary therapies suggested for treatment of BRDC have included Vitamin C or B injection, bovine respiratory vaccine (IBR, BVDV, BRSV, PI-3) at the time of BRDC therapy, antihistamines, anthelmintics (eg, levamisole), probiotics, and oral electrolytes; however, no published data are available to support their use.123, 124
Supportive therapy represents an important component of BRDC treatment, and is aimed at relieving stress and allowing time for the sick animal to foster its own resistance.55 All sick cattle should be provided shelter to avoid adverse weather conditions and not be crowded while isolated in designated hospital pens. These cattle should be provided best-quality, highly palatable feed, and easy access to fresh drinking water and mineral mixes.
Bovine Enteropathogenic Coronavirus
Calf diarrhea
Treatment of calves suffering from BECoV enteritis is entirely supportive and should be instituted based on clinical signs and, if possible, laboratory data including blood gas analysis, to determine the extent of metabolic acidosis, blood glucose and electrolyte abnormalities, and testing for the presence of BECoV in feces. Treatment goals should follow the same general guidelines as those recommended for diarrhea caused by other enteropathogens. In brief, treatment should include correction of fluid loss and dehydration, electrolyte imbalance, acidosis, hypoglycemia, and hypothermia. This correction is achieved primarily through administration of oral electrolytes or intravenous polyionic isotonic crystalloid fluid therapy, and provision of a warm and dry environment. Selection of the type of fluids, the amount to provide, and rate and route of administration is based on the age and weight of the animal(s), severity and duration of clinical signs, level of metabolic acidosis, and whether the affected calf has a suckle reflex. The authors refer the reader to other in-depth reviews on the treatment and management of diarrhea caused by BCoV in calves.70, 93, 125, 126
Winter dysentery
Most animals with BCoV-WD recover spontaneously in a few days without specific treatment, but in some cases supportive therapy may be indicated.113 Many palliative treatments have been recommended and used over the years, including intestinal astringents, protectants, adsorbents, and antibiotics. Based on 30 years of observations, Roberts112 considered none of these treatments altered the course of the disease. Abundant provision of fresh drinking water, palatable feed, and free-choice salt is the most useful nonspecific therapy. Oral or intravenous fluid therapy may be indicated, depending on the extent of dehydration. The occasional animal with prolonged or severe dysentery may necessitate a whole blood transfusion.
Strategy for control and prevention of bovine coronavirus
There are no specific and effective methods to control or prevent disease caused by BCoV. Identification and isolation of carrier cows and calves should in theory decrease the pathogen load in the environment; however, in practice this approach may be unattainable as BCoV infections are typically widespread, even in close herds.42, 87, 92 Therefore, management procedures must emphasize enhancing passive and active immunity to prevent clinical disease, minimizing stressors, and reducing the exposure of young susceptible calves. Protection relies on continual presence of a protective antibody within the gut lumen, which is passively acquired from the dam in the form of specific neutralizing antibodies (predominantly immunoglobulin type 1 [IgG1]) ingested in the colostrum or milk.78, 90 This IgG isotype has activity against the spike protein of BCoV.
Lactogenic immunity will vary depending on the levels of maternal BECoV antibodies present in the serum and colostrum.43 Ingestion of colostrum from an immune cow prevents the shedding of BCoV in the feces, and reduces the risk of spread to other herd mates (including calves) and environmental contamination. However, when colostral antibody wanes, calves become susceptible to BCoV infections, especially the ones born to primiparous cows.46
In problem herds, the immune status of susceptible calves may be raised either via vaccination of pregnant cows to increase the level of passively acquired immunity, or via oral vaccination of newborn calves to stimulate active immunity.78 Oral administration of spray-dried bovine serum supplemented calf milk replacer (from Land-O-Lakes, Kansas City, MO) has been associated with a decrease in the severity of the clinical signs in calves experimentally inoculated with BCoV (Sanjay Kapil, unpublished data, 1999).127 This protection may vary and is dependent largely on the total amount of antibody titer against BCoV present.
Factors that can have an adverse effect on the calf's immune status include dystocia, exposure to environmental extremes such as hot, cold, wet, or windy conditions, overcrowding, excessive handling in the postnatal period, and direct exposure to pathogens.82, 128, 129 As a result, newborn calves should be placed in sheltered environments, ideally on dry bedding, while trying to keep the concentration of enteric pathogens present at a minimum. Management strategies or husbandry practices to reduce exposure of the neonate to infected fecal material or aerosol primarily include thorough cleaning and disinfection of calving or nursery areas, adequate ventilation, physical isolation of clinically affected animals,130, 131 and noncommingling between younger and older calves, as described in the Sandhills calving system.132 The authors refer the reader to other review articles for more specific biosecurity and biocontainment recommendations to minimize gastrointestinal pathogen exposure for both dairy or beef herds calving in confined areas or at pastures.70, 93, 133
Disinfection
As with any infectious disease, isolation and segregation of affected animals in quarantine, sanitation, and disinfection of footwear and equipment may help limit the spread of the disease. Bovine coronavirus is an enveloped RNA virus and is more sensitive to soaps, lipid solvents such as ether or chloroform, and common disinfectants such as formalin, phenol, and quaternary ammonium compounds, compared with nonenveloped viruses.47 Removal of organic material is critical before application of disinfectant for maximal effectiveness. Bovine coronavirus can remain infectious for up to 3 days in soil, feces, and bedding materials.
Bovine Coronavirus Vaccines
Vaccines for bovine enteropathogenic coronavirus
There are 2 types of vaccines commercially available for the prevention of enteric disease caused by BCoV in neonatal calves. One is a killed vaccine (Scour-Guard 3(K), Pfizer Animal Health, NY; Guardian, Intervet/Schering-Plough Animal Health, NE; Scour-Bos, Novartis Animal Health, IA) administered to late pregnant cows for passive maternal immunization of their calves. The other is a modified-live BCoV (Calf-Guard, Pfizer Animal Health, NY) administered orally to calves at birth to provide active immunization. However, in a field study conducted in Austria, the efficacy of such immunization was questioned.134 Passive immunization via colostrum has been found to be a reliable mode of vaccinating calves against BCoV, even though antibodies present locally within the gastrointestinal tract, an open-ended system, are typically short lived. Immunizing the pregnant cattle to produce a high colostral IgG1 level will protect the newborn calf against BCoV infection only if there is transfer of passive colostral antibodies within 24 hours after birth. Calves immunized after 24 hours of age may not be protected.
Vaccines differ in injection site reactions, and this can be an important consideration before use. In a preliminary study, the attenuated respiratory strain of the virus was found to be safe for intramuscular administration in cattle.135 In another trial, about 50% of cows vaccinated with inactivated BCoV and bacterial component vaccine became lame and developed myositis.136
Antigenic variation among bovine enteropathogenic and respiratory coronaviruses
Previous reports have demonstrated that BCoV replicates in the upper respiratory tract of gnotobiotic calves following experimental inoculation.23, 41 Moreover, Hasoksuz and colleagues31 reported that most (9 of 10) BRCoV strains isolated from the nasal passages of cattle entering feedlots in Ohio were similar to reference strains of BECoV previously isolated from calves with diarrhea and adult cattle with winter dysentery. This finding suggests that BRCoV and BECoV are antigenically related and cross-reactive, therefore vaccines developed to protect against enteric strains may also protect against respiratory tract strains of BCoV if administered intranasally.31, 60, 62 However, BECoV isolates are antigenically classified among 3 subgroups 1–3 using a polyclonal antiserum against the Mebus strain of BCoV. Antigenic Group 3 is the predominant BECoV presently circulating in the United States.137 Recent calf diarrhea isolates of BECoV are not blocked in hemagglutination-inhibition test by polyclonal antiserum prepared against antigenic Group 1 (Mebus isolate of BECoV). From the list of commercially available BECoV vaccines listed previously, only one (Guardian, Intervet/Schering-Plough Animal Health, NE) contains both antigenic variants of BECoV.
Vaccine for bovine respiratory coronaviruses
Modern vaccination programs using multivalent killed or modified-live viral vaccines and attenuated bacterial antigen have been associated with a decrease in BRDC incidence and prevalence; however, none of the commercially available vaccines are labeled for prevention of BRCoV. Protection of the respiratory tract from BRCoV has not been well studied except for one trial60 in which extralabel use of the modified-live coronavirus and rotavirus vaccine in feedlot calves administered intranasally significantly decreased the subsequent rate of treatment for respiratory disease in cattle that had relatively low serum antibody titers against BCoV at arrival.60 More specifically, there was an overall reduction of 26% among vaccinated calves treated for BRDC on entry to the feedlot. Furthermore, BCoV vaccinated calves with detectable intranasal BCoV had a 36% reduction in treatment for BRDC, and those with low antibody titers at arrival had 42% reduction, compared with unvaccinated calves. Although the 26% and 36% reduction was not statistically significant, it was considered clinically significant. The association between antibody titers against BCoV at feedlot arrival seems to correlate with protection from respiratory tract infection, and may promote higher weight gains1 and reduce virus shedding in nasal secretions.138 In one study, feedlot cattle that had BCoV antibody titers of greater than 1600 did not shed BCoV fecally or nasally.32
In the future, BCoV vaccines may be needed to decrease the economic losses due to BCoV infection in feedlot cattle. It has been suggested recently that feedlot calves should be vaccinated against BCoV at least 3 weeks before shipping to induce a serum IgG GMT of 1860 or more, to provide protection from BCoV infection and its direct or combined effects with other pathogens of BRDC.20 Plummer and colleagues60 suggested that development of an intranasally administered vaccine against BCoV, either by itself or combined with BHV-1, may have an added or synergistic effect in reducing incidence and subsequent treatment for BRDC in feedlot cattle.
Summary
Bovine coronavirus is widespread in the cattle industry and is associated with significant economic losses. Clinical syndromes associated with BCoV (calf diarrhea, pneumonia in calves and adult cattle, winter dysentery, and combined pneumonia and diarrhea in young and adult cattle) are due to the virus tropism for the intestinal tract, nasal passages, proximal trachea, and lungs.
Only 4 BCoV isolates have been completely sequenced and thus, the information about the genetics of the virus is still limited. The coronaviruses responsible for enteric and respiratory syndromes in adult cattle and calves are closely related antigenically and genetically, and can be reproduced experimentally. From a vaccination point of view, the enteropathogenic and respiratory BCoV have not been clearly differentiated; however, they do cross-react to a great extent.
It is likely that BCoV work synergistically with other pathogens of the bovine respiratory tract in combination with various stressors to allow bacterial or viral colonization of the lungs, thus leading to development of BRDC. In calves, lack of colostral immunity is an important predisposing factor in the development of most infectious diseases, including diarrhea and respiratory tract infection caused by BCoV. Inclement weather is an important environmental stressor contributing to outbreaks of winter dysentery in dairy cattle.
There is a growing body of evidence in support of a causal relationship between BCoV and BRDC by predisposing or directly inducing lower respiratory tract disease, leading to poor growth performance in feedlot cattle and calves. This issue must be considered in disease prevention or preconditioning programs in the near future. The emergence of the respiratory tract infections caused by BRCoV in the cattle industry warrants timed, active immunization with appropriate antigens (antigenic Groups 1 and 3) to prevent this infection in stocker and feedlot cattle. Current strategies to prevent BCoV-induced enteritis in calves consist in vaccinating the late pregnant cows to provide passive colostral protection.
References
- 1.Martin S.W., Nagy E., Shewen P.E. The association of titers to bovine coronavirus with treatment for bovine respiratory disease and weight gain in feedlot calves. Can J Vet Res. 1998;62(4):257–261. [PMC free article] [PubMed] [Google Scholar]
- 2.Gagea M.I., Bateman K.G., Dreumel T.V. Diseases and pathogens associated with mortality in Ontario feedlots. J Vet Diagn Invest. 2006;18(1):18–28. doi: 10.1177/104063870601800104. [DOI] [PubMed] [Google Scholar]
- 3.Snowder G.D., Van Vleck L.D., Cundiff L.V. Bovine respiratory disease in feedlot cattle: environmental, genetic, and economic factors. J Anim Sci. 2006;84:1999–2008. doi: 10.2527/jas.2006-046. [DOI] [PubMed] [Google Scholar]
- 4.Storz J., Purdy C.W., Lin X. Isolation of respiratory coronaviruses, other cytocidal viruses and Pasteurella spp. from cattle involved in two natural outbreaks of shipping fever. J Am Vet Med Assoc. 2000;216(10):1599–1604. doi: 10.2460/javma.2000.216.1599. [DOI] [PubMed] [Google Scholar]
- 5.Storz J., Stine L., Liem A. Coronavirus isolations from nasal swabs samples in cattle with signs of respiratory tract disease after shipping. J Am Vet Med Assoc. 1996;208(9):1452–1455. [PubMed] [Google Scholar]
- 6.Lin X.Q., O eilly K.L., Storz J. Antibody responses to respiratory coronavirus infections of cattle during shipping fever pathogenesis. Arch Virol. 2000;145(11):2335–2349. doi: 10.1007/s007050070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McNulty M.S., Bryson D.G., Allan G.M. Coronavirus infection of the bovine respiratory tract. Vet Microbiol. 1984;9(5):425–434. doi: 10.1016/0378-1135(84)90063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lathrop S.L., Wittum T.E., Brock K.V. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am J Vet Res. 2000;61(9):1062–1066. doi: 10.2460/ajvr.2000.61.1062. [DOI] [PubMed] [Google Scholar]
- 9.Storz J., Lin X., Purdy C.W. Coronavirus and Pasteurella infections in bovine shipping fever pneumonia and Evans' criteria for causation. J Clin Microbiol. 2000;38(9):3291–3298. doi: 10.1128/jcm.38.9.3291-3298.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasoksuz M., Vlasova A., Saif L.J. Detection of group 2a coronaviruses with emphasis on bovine and wild ruminant strains. Virus isolation and detection of antibody, antigen, and nucleic acid. Methods Mol Biol. 2008;454:43–59. doi: 10.1007/978-1-59745-181-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanno T., Kamivoshi T., Ishihara R. Phylogenic studies of bovine coronaviruses isolated in Japan. J Vet Med Sci. 2009;71(1):83–86. doi: 10.1292/jvms.71.83. [DOI] [PubMed] [Google Scholar]
- 12.Takiuchi E., Alfieri A.F., Alfieri A.A. Molecular analysis of the bovine coronavirus S1 gene by direct sequencing of diarrheic fecal specimens. Braz J Med Biol Res. 2008;41(4):277–282. doi: 10.1590/s0100-879x2008000400004. [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Hägglund S., Hakhverdan M. Molecular epidemiology of bovine coronavirus on the basis of comparative analysis of the S gene. J Clin Microbiol. 2006;44(3):957–960. doi: 10.1128/JCM.44.3.957-960.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popova R., Zhang X. The spike but not the hemagglutinin/esterase protein of bovine coronavirus is necessary and sufficient for viral infection. Virology. 2002;294(1):222–236. doi: 10.1006/viro.2001.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kapil S, Lamm CG, McVey DS, et al. Detection of bovine respiratory coronavirus in beef cattle. In: Proceedings of the 27th annual meeting of the American Society of Virologists. Cornell University, Ithaca, NY, July 15, 2008. p. 9–1.
- 16.Carman P.S., Hazlett M.J. Bovine coronavirus infection in Ontario, 1990-1991. Can Vet J. 1992;33(12):812–814. [PMC free article] [PubMed] [Google Scholar]
- 17.Storz J. Respiratory disease of cattle associated with coronavirus infections. In: Howard J.L., Smith R.A., editors. Current veterinary therapy: food animal practice 4. WB Saunders; Philadelphia: 1998. pp. 291–293. [Google Scholar]
- 18.Lin X.Q., O'Reilly K.L., Storz J. Antibody responses of cattle with respiratory coronavirus infections during pathogenesis of shipping fever pneumonia are lower with antigens of enteric strains than with those of a respiratory strain. Clin Diagn Lab Immunol. 2002;9(5):1010–1013. doi: 10.1128/CDLI.9.5.1010-1013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsunemitsu H., Saif L.J. Antigenic and biologic comparisons of bovine coronavirus derived from neonatal calf diarrhea and winter dysentery of adult cattle. Arch Virol. 1995;140(7):1303–1311. doi: 10.1007/BF01322757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas C.J., Hoet A.E., Sreevatsan S. Transmission of bovine coronavirus and serologic responses in feedlot calves under field conditions. Am J Vet Res. 2006;67(8):1412–1420. doi: 10.2460/ajvr.67.8.1412. [DOI] [PubMed] [Google Scholar]
- 21.Cho K.O., Hoet A.E., Loerch S.C. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route. Am J Vet Res. 2001;62(9):1436–1441. doi: 10.2460/ajvr.2001.62.1436. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds D.J., Debney T.G., Hall G.A. Studies on the relationship between coronaviruses from the intestinal and respiratory tracts in calves. Arch Virol. 1985;85(1–2):71–83. doi: 10.1007/BF01317007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saif L.J., Redman D.R., Moorhead P.D. Experimentally induced coronavirus infections in calves: viral replication in the respiratory and intestinal tracts. Am J Vet Res. 1986;47(7):1426–1432. [PubMed] [Google Scholar]
- 24.Tsunemitsu H., Yonemichi H., Hirai T. Isolation of bovine coronavirus from feces and nasal swabs of calves with diarrhea. J Vet Med Sci. 1991;53(3):433–437. doi: 10.1292/jvms.53.433. [DOI] [PubMed] [Google Scholar]
- 25.Hasoksuz M., Lathrop S., Al-dubaib M.A. Antigenic variation among bovine enteric coronaviruses (BEVC) and bovine respiratory coronaviruses (BRCV) detected using monoclonal antibodies. Arch Virol. 1999;144(12):2441–2447. doi: 10.1007/s007050050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Kanawati Z.R., Tsunemitsu H., Smith D.R. Infection and cross-protection studies of winter dysentery and calf diarrhea bovine coronavirus strains in colostrum-deprived and gnotobiotic calves. Am J Vet Res. 1996;57(1):48–53. [PubMed] [Google Scholar]
- 27.Hasoksuz M., Sreevatsan S., Cho K.O. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 2002;84(1–2):101–109. doi: 10.1016/S0168-1702(02)00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds D.J. Coronavirus replication in the intestinal and respiratory tracts during infection of calves. Ann Rech Vet. 1983;14(4):445–446. [PubMed] [Google Scholar]
- 29.Zhang X., Herbst W., Kousoulas K.G. Comparison of the S genes and the biological properties of respiratory and enteropathogenic bovine coronaviruses. Arch Virol. 1994;134(3–4):421–426. doi: 10.1007/BF01310579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelinas A.-M., Boutin M., Sasseville A.M.-J. Bovine coronaviruses associated with enteric and respiratory diseases in Canadian dairy cattle display different reactivities to anti-HE monoclonal antibodies and distinct amino acid changes in their HE, S and ns4.9 protein. Virus Res. 2001;76(1):43–57. doi: 10.1016/S0168-1702(01)00243-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasoksuz M., Lathrop S.L., Gadfiled K.L. Isolation of bovine respiratory coronaviruses from feedlot cattle and comparison of their biological and antigenic properties with bovine enteric coronaviruses. Am J Vet Res. 1999;60(10):1227–1233. [PubMed] [Google Scholar]
- 32.Hasoksuz M., Hoet A.E., Loerch S.C. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle at an Ohio feedlot. J Vet Diagn Invest. 2002;14(4):308–313. doi: 10.1177/104063870201400406. [DOI] [PubMed] [Google Scholar]
- 33.Kanno T., Hatama S., Ishihara R. Molecular analysis of the S glycoprotein gene of bovine coronaviruses isolated in Japan from 1999-2006. J Gen Virol. 2007;88(Pt 4):1218–1224. doi: 10.1099/vir.0.82635-0. [DOI] [PubMed] [Google Scholar]
- 34.Park S.J., Kim G.Y., Choy H.E. Dual enteric and respiratory tropisms of winter dysentery bovine coronavirus in calves. Arch Virol. 2007;152(10):1885–1900. doi: 10.1007/s00705-007-1005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decaro N., Martella V., Elia G. Biological and genetic analysis of a bovine-like coronavirus isolated from water buffalo (Bubalus bubalis) calves. Virology. 2008;370(1):213–222. doi: 10.1016/j.virol.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wunschman A., Frank R., Pomeroy K. Enteric coronavirus infection in a juvenile dromadery (Camelus dromaderius) J Vet Diagn Invest. 2002;14(5):441–444. doi: 10.1177/104063870201400518. [DOI] [PubMed] [Google Scholar]
- 37.Genova S.G., Streeter R.N., Simpson K.M. Detection of an antigenic group 2 coronavirus in an adult alpaca with enteritis. Clin Vaccine Immunol. 2008;15(10):1629–1632. doi: 10.1128/CVI.00232-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majhdi F., Minocha H.C., Kapil S. Isolation and characterization of a coronavirus from elk calves with diarrhea. J Clin Microbiol. 1997;35(11):2937–2942. doi: 10.1128/jcm.35.11.2937-2942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasoksuz M., Alekseev K., Vlasova A. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J Virol. 2007;81(10):4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Decaro N., Mari V., Desario C. Severe outbreak of bovine coronavirus infection in dairy cattle during the warmer season. Vet Microbiol. 2008;126(1–3):30–39. doi: 10.1016/j.vetmic.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saif L.J. Development of nasal, fecal and serum isotype-specific antibodies in calves challenged with bovine coronavirus or rotavirus. Vet Immunol Immunopathol. 1987;17(1–4):425–439. doi: 10.1016/0165-2427(87)90159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heckert R.A., Saif L.J., Hoblet K.H. A longitudinal study of bovine coronavirus enteric and respiratory infections in dairy calves in two herds in Ohio. Vet Microbiol. 1990;22(2–3):187–201. doi: 10.1016/0378-1135(90)90106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heckert R.A., Saif L.J., Myers G.W. Epidemiologic factors and isotype-specific antibody responses in serum and mucosal secretions of dairy calves with bovine coronavirus respiratory tract and enteric tract infections. Am J Vet Res. 1991;52(6):845–851. [PubMed] [Google Scholar]
- 44.Collins J.K., Riegel C.A., Olson J.D. Shedding of enteric coronavirus in adult cattle. Am J Vet Res. 1987;48(3):361–365. [PubMed] [Google Scholar]
- 45.Kapil S., Goyal S.M. Bovine coronavirus-associated respiratory disease. Comp Cont Educ Pract Vet. 1995;17(9):1179–1181. [Google Scholar]
- 46.Crouch C.F., Bielefeldt Ohmann H., Watts T.C. Chronic shedding of bovine enteric coronavirus antigen-antibody complexes by clinically normal cows. J Gen Virol. 1985;66(Pt 7):1489–1500. doi: 10.1099/0022-1317-66-7-1489. [DOI] [PubMed] [Google Scholar]
- 47.Evermann J.F., Benfield D.A. Coronaviral infections. In: Williams E.S., Barber I.K., editors. Infectious diseases of wild mammals. 3rd edition. Iowa State University Press; Ames (IA): 2001. pp. 245–253. [Google Scholar]
- 48.Tsunemitsu H., Smith D.R., Saif L.J. Experimental inoculation of adult dairy cows with bovine coronavirus and detection of coronavirus in feces by RT-PCR. Arch Virol. 1999;144(1):167–175. doi: 10.1007/s007050050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saif L.J., Smith K.L. Enteric viral infections of calves and passive immunity. J Dairy Sci. 1985;68(1):206–228. doi: 10.3168/jds.S0022-0302(85)80813-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kapil S., Trent A.M., Goyal S.M. Excretion and persistence of bovine coronavirus in neonatal calves. Arch Virol. 1990;115(1–2):127–132. doi: 10.1007/BF01310629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kapil S., Pomeroy K.A., Goyal S.M. Experimental infection with a virulent pneumoenteric isolate of bovine coronavirus. J Vet Diagn Invest. 1991;3(1):88–89. doi: 10.1177/104063879100300123. [DOI] [PubMed] [Google Scholar]
- 52.Clark K.J., Sarr A.B., Grant P.G. In vitro studies on the use of clay, clay minerals and charcoal to adsorb bovine rotavirus and bovine coronavirus. Vet Microbiol. 1998;63(2–4):137–146. doi: 10.1016/S0378-1135(98)00241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorusso A., Desario C., Mari V. Molecular characterization of a canine respiratory coronavirus strain detected in Italy. Virus Res. 2009;141(1):96–100. doi: 10.1016/j.virusres.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaneshima T., Hohdatsu T., Hagino R. The infectivity and pathogenicity of a group 2 bovine coronavirus in pups. J Vet Med Sci. 2007;69(3):301–303. doi: 10.1292/jvms.69.301. [DOI] [PubMed] [Google Scholar]
- 55.Woolums A.R., Ames T.R., Baker J.C. The bronchopneumonias (respiratory disease complex of cattle, sheep, and goats) In: Smith B.P., editor. Large animal internal medicine. 4th edition. Mosby Elsevier; St. Louis (MO): 2009. pp. 602–643. [Google Scholar]
- 56.Thomas L.H., Gourlay R.N., Stott E.J. A search for new microorganisms in calf pneumonia by the inoculation of gnotobiotic calves. Res Vet Sci. 1982;33(2):170–182. doi: 10.1016/S0034-5288(18)32331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Busato A., Steiner L., Tontis A. Frequency and etiology of calf losses and calf diseases in cow-calf farms. I. Methods of data collection, calf mortality, and calf morbidity. Dtsch Tierarztl Wochenschr. 1997;104(4):131–135. [PubMed] [Google Scholar]
- 58.Ganaba R., Belanger D., Dea S. A seroepidemiological study of the importance in cow-calf pairs of respiratory and enteric viruses in beef operations from northwestern Quebec. Can J Vet Res. 1995;59(1):26–33. [PMC free article] [PubMed] [Google Scholar]
- 59.Lathrop S.L., Wittum T.E., Loerch S.C. Antibody titers against bovine coronavirus and shedding of the virus via the respiratory tract in feedlot cattle. Am J Vet Res. 2000;61(9):1057–1061. doi: 10.2460/ajvr.2000.61.1057. [DOI] [PubMed] [Google Scholar]
- 60.Plummer P.J., Rohrbach B.W., Daugherty R.A. Effect of intranasal vaccination against bovine enteric coronavirus on the occurrence of respiratory tract disease in a commercial backgrounding feedlot. J Am Vet Med Assoc. 2004;225(5):726–731. doi: 10.2460/javma.2004.225.726. [DOI] [PubMed] [Google Scholar]
- 61.O'Connor A., Martin S.W., Nagy E. The relationship between the occurrence of undifferentiated bovine respiratory disease and titer changes to bovine coronavirus and bovine viral diarrhea virus in 3 Ontario feedlots. Can J Vet Res. 2001;65(3):137–142. [PMC free article] [PubMed] [Google Scholar]
- 62.Cho K.O., Hasoksuz M., Nielsen P.R. Cross-protection studies between respiratory and calf diarrhea and winter dysentery coronavirus strains in calves and RT-PCR and nested PCR for their detection. Arch Virol. 2001;146(12):2401–2409. doi: 10.1007/s007050170011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saif L.J. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: an enigma resolved? Cornell Vet. 1990;80(4):303–311. [PubMed] [Google Scholar]
- 64.Thomson R.G. A perspective on respiratory disease in feedlot cattle. Can Vet J. 1980;21(6):181–185. [PMC free article] [PubMed] [Google Scholar]
- 65.Evans A.S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976;49(9):175–195. [PMC free article] [PubMed] [Google Scholar]
- 66.Traven M., Naslund K., Linde N. Experimental reproduction of winter dysentery in lactating cows using BCV-comparison with BCV infection in milk-fed calves. Vet Microbiol. 2001;81(2):127–151. doi: 10.1016/S0378-1135(01)00337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavirani S, Galvani G, Taddei S, et al. Involvement of coronavirus in acute bovine respiratory disease (BRD) of cattle. In: Proceedings of the X International Symposium of Veterinary Laboratory Diagnosticians. Salsomaggiore (PR), Italy, July 4–7, 2001. p. 395–6.
- 68.Lavazza A., Boldini M., Lombardi G. Identification of rotavirus and coronavirus in neonatal diarrhoea of calves submitted to the Brescia laboratory in 1991-1992. Atti della Societa Italiana di Buiatria. 1993;25:249–258. [Google Scholar]
- 69.Mebus C.A., Stair E.L., Rhodes M.B. Pathology of neonatal calf diarrhea induced by a coronavirus-like agent. Vet Pathol. 1973;10(1):45–64. doi: 10.1177/030098587301000105. [DOI] [PubMed] [Google Scholar]
- 70.Gunn A.A., Naylor J.A., House J.K. Diarrhea. In: Smith B.P., editor. Large animal internal medicine. 4th edition. Mosby Elsevier; St. Louis (MO): 2009. pp. 340–363. [Google Scholar]
- 71.Mebus C.A., Newman L.E., Stair E.L. Scanning electron, light, and immunofluorescent microscopy of intestine of gnotobiotic calf infected with calf diarrheal coronavirus. Am J Vet Res. 1975;36(12):1719–1725. [PubMed] [Google Scholar]
- 72.Mebus C.A., Stair E.L., Rhodes M.B. Neonatal calf diarrhea: propagation, attenuation, and characteristics of a coronavirus-like agent. Am J Vet Res. 1973;34(2):145–150. [PubMed] [Google Scholar]
- 73.Langpap T.J., Bergeland M.E., Reed D.E. Coronaviral enteritis of young calves: virologic and pathologic findings in naturally occurring infections. Am J Vet Res. 1979;40(10):1476–1478. [PubMed] [Google Scholar]
- 74.Bridger J.C., Woode G.N., Meyling A. Isolation of coronavirus from neonatal calf diarrhea in great Britain and Denmark. Vet Microbiol. 1978;3(2):101–113. [Google Scholar]
- 75.Babiuk L.A., Sabara M., Hudson G.R. Rotavirus and coronavirus infections in animals. Prog Vet Microbiol Immunol. 1985;1:80–120. [PubMed] [Google Scholar]
- 76.Moon H.W. Mechanisms in the pathogenesis of diarrhea: a review. J Am Vet Med Assoc. 1978;172(4):443–448. [PubMed] [Google Scholar]
- 77.Woode G.N., Smith G.N., Dennis M.J. Intestinal damage in rotavirus infected calves assessed by D-xylose malabsorption. Vet Rec. 1978;102(15):340–341. doi: 10.1136/vr.102.15.340-a. [DOI] [PubMed] [Google Scholar]
- 78.Clark M.A. Bovine coronavirus. Br Vet J. 1993;149(1):51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nappert G., Hamilton D., Petrie L. Determination of lactose and xylose malabsorption in preruminant diarrheic calves. Can J Vet Res. 1993;57(3):152–158. [PMC free article] [PubMed] [Google Scholar]
- 80.Ewaschuk J.B., Naylor J.M., Palmer R. D-Lactate production and excretion in diarrheic calves. J Vet Intern Med. 2004;18(5):744–747. doi: 10.1892/0891-6640(2004)18<744:dpaeid>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 81.Lewis L.D., Phillips R.W. Pathophysiologic changes due to coronavirus-induced diarrhea in the calf. J Am Vet Med Assoc. 1978;173(5 Pt 2):636–642. [PubMed] [Google Scholar]
- 82.Bendali F., Bichet H., Schelcher F. Pattern of diarrhoea in newborn beef calves in south-west France. Vet Res. 1999;30(1):61–74. [PubMed] [Google Scholar]
- 83.Durham P.J.K., Farquharson B.C., Stevenson B.J. Rotavirus and coronavirus associated diarrhoea in calves. N Z Vet J. 1979;27(12):271–272. doi: 10.1080/00480169.1979.34669. [DOI] [PubMed] [Google Scholar]
- 84.Hoet A.E., Smiley J., Thomas C. Association of enteric shedding of bovine torovirus (Breda virus) and other enteropathogens with diarrhea in veal calves. Am J Vet Res. 2003;64(4):485–490. doi: 10.2460/ajvr.2003.64.485. [DOI] [PubMed] [Google Scholar]
- 85.Reynolds D.J., Morgan J.H., Chanter N. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986;119(2):34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- 86.Mostl K., Burki F. Incidence of diarrhea and of rotavirus- and coronavirus-shedding in calves, whose dams had been vaccinated with an experimental oil-adjuvanted vaccine containing bovine rotavirus and bovine coronavirus. Zentralbl Veterinarmed B. 1988;35(3):186–196. doi: 10.1111/j.1439-0450.1988.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 87.Crouch C.F., Acres S.D. Prevalence of rotavirus and coronavirus antigens in the feces of normal cows. Can J Comp Med. 1984;48(3):340–342. [PMC free article] [PubMed] [Google Scholar]
- 88.Marsolais G., Assaf R., Montpetit C. Diagnosis of viral agents associated with neonatal calf diarrhea. Can J Comp Med. 1978;42(2):168–171. [PMC free article] [PubMed] [Google Scholar]
- 89.Snodgrass D.R., Terzolo H.R., Sherwood D. Aetiology of diarrhea in young calves. Vet Rec. 1986;119(2):31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- 90.Crouch C.F., Oliver S., Hearle D.C. Lactogenic immunity following vaccination of cattle with bovine coronavirus. Vaccine. 2000;19(2-3):189–196. doi: 10.1016/S0264-410X(00)00177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cho K.O., Halbur P.G., Bruna J.D. Detection and isolation of coronavirus from feces of three herds of feedlot cattle during outbreaks of winter dysentery-like diseases. J Am Vet Med Assoc. 2000;217(8):1191–1194. doi: 10.2460/javma.2000.217.1191. [DOI] [PubMed] [Google Scholar]
- 92.Bulgin M.S., Ward A., Barrett D.P. Detection of rotavirus and coronavirus shedding in two beef cow herds in Idaho. Can Vet J. 1989;30(3):235–239. [PMC free article] [PubMed] [Google Scholar]
- 93.Barrington G.M., Gay J.M., Evermann J.F. Biosecurity for neonatal gastrointestinal diseases. Vet Clin North Am Food Anim Pract. 2002;18(1):7–34. doi: 10.1016/S0749-0720(02)00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moon H.W., McClurkin A.W., Isaacson R.E. Pathogenic relationships of rotavirus, Escherichia coli, and other agents in mixed infections in calves. J Am Vet Med Assoc. 1978;173(5 Pt 2):577–583. [PubMed] [Google Scholar]
- 95.Acres S.D., Saunders J.R., Radostits O.M. Acute undifferentiated neonatal diarrhea of beef calves: the prevalence of enterotoxigenic E. coli, reo-like (rota) virus and other enteropathogens in cow-calf herds. Can Vet J. 1977;18(5):113–121. [PMC free article] [PubMed] [Google Scholar]
- 96.Benfield D.A., Saif L.J. Cell culture propagation of a coronavirus isolated from cows with winter dysentery. J Clin Microbiol. 1990;28(6):1454–1457. doi: 10.1128/jcm.28.6.1454-1457.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Espinasse J., Viso M., Laval A. Winter dysentery: a coronavirus-like agent in the faeces of beef and dairy cattle with diarrhoea. Vet Rec. 1982;110(16):385. doi: 10.1136/vr.110.16.385. [DOI] [PubMed] [Google Scholar]
- 98.Broes A., Van Opdenbosch E., Wellemans G. Isolement d'un coronavirus chez des bovins atteints d'enterite hemorragique hivernale (winter dysentery) en Belgique [Isolation of a coronavirus from Belgian cattle with winter haemorrhagic enteritis] Ann Med Vet. 1984;128(4):299–303. [Google Scholar]
- 99.Natsuaki S., Goto K., Nakamura K. Fatal winter dysentery with severe anemia in an adult cow. J Vet Med Sci. 2007;69(9):957–960. doi: 10.1292/jvms.69.957. [DOI] [PubMed] [Google Scholar]
- 100.Saif L.J., Redman D.R., Brock K.V. Winter dysentery in adult dairy cattle: detection of coronavirus in the faeces. Vet Rec. 1988;123(11):300–301. doi: 10.1136/vr.123.11.300. [DOI] [PubMed] [Google Scholar]
- 101.Smith D.R., Fedorka-Cray P.J., Mohan R. Epidemiologic herd-level assessment of causative agents and risk factors for winter dysentery in dairy cattle. Am J Vet Res. 1998;59(8):994–1001. [PubMed] [Google Scholar]
- 102.van Kruiningen H.J., Khairallah L.H., Sasseville V.G. Calfhood coronavirus enterocolitis: a clue to the etiology of winter dysentery. Vet Pathol. 1987;24(6):564–567. doi: 10.1177/030098588702400616. [DOI] [PubMed] [Google Scholar]
- 103.Takahashi E., Inaba Y., Sato K. Epizootic diarrhoea of adult cattle associated with a coronavirus-like agent. Vet Microbiol. 1980;5(2):151–154. [Google Scholar]
- 104.Kruiningenvan H.J., Hiestand L., Hill D.L. Winter dysentery in dairy cattle: recent findings. Comp Cont Educ Pract Vet. 1985;7(10):S591–S599. S598–9. [Google Scholar]
- 105.Tsunemitsu H., El-Kanawati Z.R., Smith D.R. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J Clin Microbiol. 1995;33(12):3264–3269. doi: 10.1128/jcm.33.12.3264-3269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith D.R., Fedorka-Cray P.J., Mohan R. Evaluation of cow-level risk factors for the development of winter dysentery in dairy cattle. Am J Vet Res. 1998;59(8):986–993. [PubMed] [Google Scholar]
- 107.MacPherson L.W. Bovine virus enteritis (winter dysentery) Can J Comp Med Vet Sci. 1957;21(6):184–192. [PMC free article] [PubMed] [Google Scholar]
- 108.Barrera Valle M., Rodriguez Batista E., Betancourt Martell A. Short communication. First report in Cuba of bovine coronavirus detection in a winter dysentery outbreak. Spanish J Agr Res. 2006;4(3):221–224. [Google Scholar]
- 109.Campbell S.G., Cookingham C.A. The enigma of winter dysentery. Cornell Vet. 1978;68(4):423–441. [PubMed] [Google Scholar]
- 110.Fleetwood A.J., Edwards S., Foxell P.W. Winter dysentery in adult dairy cattle. Vet Rec. 1989;125(22):553–554. doi: 10.1136/vr.125.22.553. [DOI] [PubMed] [Google Scholar]
- 111.Durham P.J., Hassard L.E., Armstrong K.R. Coronavirus-associated diarrhea (winter dysentery) in adult cattle. Can Vet J. 1989;30(10):825–827. [PMC free article] [PubMed] [Google Scholar]
- 112.Roberts S.J. Winter dysentery in dairy cattle. Cornell Vet. 1957;47(3):372–388. [PubMed] [Google Scholar]
- 113.Guard C.L., Fecteau G. Winter dysentery in cattle. In: Smith B.P., editor. Large animal internal medicine. 4th edition. Mosby Elsevier; St. Louis (MO): 2009. pp. 876–877. [Google Scholar]
- 114.White M.E., Schukken Hein Y., Tanksley B. Space-time clustering of, and risk factors for, farmer-diagnosed winter dysentery in dairy cattle. Can Vet J. 1989;30(12):948–951. [PMC free article] [PubMed] [Google Scholar]
- 115.Kahrs R.F., Scott F.W., Hillman R.B. Epidemiologic observations on bovine winter dysentery. Bovine Practitioner. 1973;8:36–39. [Google Scholar]
- 116.Traven M., Silvan A., Larsson B. Experimental infection with bovine coronavirus (BCV) in lactating cows: clinical disease, viral excretion, interferon-alpha and antibody response. Bovine Pract. 1995;29:64–65. [Google Scholar]
- 117.Dar A.M., Kapil S., Goyal S.M. Comparison of immunohistochemistry, electron microscopy, and direct fluorescent antibody test for the detection of bovine coronavirus. J Vet Diagn Invest. 1998;10(2):152–157. doi: 10.1177/104063879801000206. [DOI] [PubMed] [Google Scholar]
- 118.Schoenthaler S.L., Kapil S. Development and applications of a bovine coronavirus antigen detection enzyme-linked immunoassay. Clin Diagn Lab Immunol. 1999;6(1):130–132. doi: 10.1128/cdli.6.1.130-132.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Daginakatte G.C., Chard-Bergstrom C., Andrews G.A. Production, characterization, and uses of monoclonal antibodies against recombinant nucleoprotein of elk coronavirus. Clin Diagn Lab Immunol. 1999;6(3):341–344. doi: 10.1128/cdli.6.3.341-344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luginbuhl A., Reitt K., Metzler A. Field study of the prevalence and diagnosis of diarrhea-causing agents in the newborn calf in a Swiss veterinary practice area. Schweiz Arch Tierheilkd. 2005;147(6):245–252. doi: 10.1024/0036-7281.147.6.245. [DOI] [PubMed] [Google Scholar]
- 121.Selman I.E., Allan E.M., Gibbs H.A. Effect of anti-prostaglandin therapy in experimental parainfluenza type 3 pneumonia in weaned, conventional calves. Vet Rec. 1984;115(5):101–105. doi: 10.1136/vr.115.5.101. [DOI] [PubMed] [Google Scholar]
- 122.Friton G, Cajal C, Leemann R, et al. Pharmaco-economic benefit of meloxicam (Metacam) in the treatment of respiratory disease in feedlot cattle. In: Proceedings of the 23rd World Buiatrics Conference. Quebec, Canada, July 11–16, 2004.
- 123.Apley M. Ancillary therapy in food animal infectious disease with a focus on steroids and NSAIDS in bovine respiratory disease and toxic mastitis: what should (and shouldn't we be doing?). In: Proceedings of the Ontario Veterinary Medical Association (OVMA). Ontario; 2008. p. 203–10.
- 124.Apley M. Ancillary therapy of bovine respiratory disease. Vet Clin North Am Food Anim Pract. 1997;13(3):575–592. doi: 10.1016/s0749-0720(15)30314-5. [DOI] [PubMed] [Google Scholar]
- 125.Naylor J.M. Oral electrolyte therapy. Vet Clin North Am Food Anim Pract. 1999;15(3):487–504. doi: 10.1016/s0749-0720(15)30160-2. [DOI] [PubMed] [Google Scholar]
- 126.Berchtold J. Intravenous fluid therapy of calves. Vet Clin North Am Food Anim Pract. 1999;15(3):505–531. doi: 10.1016/S0749-0720(15)30161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arthington J.D., Jaynes C.A., Tyler H.D. The use of bovine serum protein as an oral support therapy following coronavirus challenge in calves. J Dairy Sci. 2002;85(5):1249–1254. doi: 10.3168/jds.S0022-0302(02)74189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bendali F., Sanaa M., Bichet H. Risk factors associated with diarrhoea in newborn calves. Vet Res. 1999;30(5):509–522. [PubMed] [Google Scholar]
- 129.Kasari T.R., Wikse S.E. Perinatal mortality in beef herds. Vet Clin North Am Food Anim Pract. 1994;10(1):1–185. doi: 10.1016/s0749-0720(15)30594-6. [DOI] [PubMed] [Google Scholar]
- 130.Heath S.E. Neonatal diarrhea in calves: investigation of herd management practices. Comp Cont Educ Pract Vet. 1992;14(3):385–393. [Google Scholar]
- 131.Heath S.E. Neonatal diarrhea in calves: diagnosis and intervention in problem herds. Comp Cont Educ Pract Vet. 1992;14(7):995–1002. [Google Scholar]
- 132.Smith DR, Grotelueschen DM, Knott T, et al. Prevention of neonatal diarrhea with the Sandhills calving system. In: Proceedings of the 37th Annual Conference of American Association of Bovine Practitioners. Forth Worth, TX, September 23–25, 2004. p. 23–5.
- 133.Pence M., Robbe S., Thomson J. Reducing the incidence of neonatal calf diarrhea through evidence-based management. Comp Cont Educ Pract Vet. 2001;23:S73–S75. [Google Scholar]
- 134.de Leeuw P.W., Tiessink J.W. Laboratory experiments on oral vaccination of calves against rotavirus or coronavirus induced diarrhea. Zentralbl Veterinarmed B. 1985;31(1):55–64. doi: 10.1111/j.1439-0450.1985.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 135.Decaro N., Campolo M., Mari V. A candidate modified-live bovine coronavirus vaccine: safety and immunogenicity evaluation. New Microbiol. 2009;32(1):109–113. [PubMed] [Google Scholar]
- 136.O'Toole D., Steadman L., Raisbeck M. Myositis, lameness, and recumbency after use of water-in-oil adjuvanted vaccines in near-term beef cattle. J Vet Diagn Invest. 2005;17(1):23–31. doi: 10.1177/104063870501700106. [DOI] [PubMed] [Google Scholar]
- 137.Kapil S., Richardson K.L., Maag T.R. Characterization of bovine coronavirus isolates from eight different states in the USA. Vet Microbiol. 1999;67:221–230. doi: 10.1016/S0378-1135(99)00042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin X., O'Reilly K.L., Burrel M.L. Infectivity-neutralizing and hemagglutinin-inhibiting antibody responses to respiratory coronavirus infections of cattle in pathogenesis of shipping fever pneumonia. Clin Diagn Lab Immunol. 2001;8(2):357–362. doi: 10.1128/CDLI.8.2.357-362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


