Abstract
Chemical modification of the proteins bovine serum albumin, α-lactalbumin, β-lactoglobulin and chicken lysozyme by 3-hydroxyphthalic anhydride (3-HP) yielded compounds which exerted antiviral activity in vitro as compared with the native unmodified proteins. Of the three enveloped viruses tested, human herpes simplex virus type 1 (HSV-1), bovine parainfluenza virus type 3 and porcine respiratory corona virus, only HSV-1 proved sensitive to the 3-HP-proteins. All of the chemically modified proteins presented antiviral activity against HSV-1 when assayed before, during or after infection. However, to achieve HSV-1 inhibition, significantly higher concentrations of the modified proteins were required if present before infection as compared to during or after infection. Our results suggest that multiple mechanisms are involved in the inhibition of HSV-1 infection. Proteolytical digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme by trypsin, chymotrypsin and pepsin yielded several peptide fragments with antiherpetic activity. Chemical modification of these peptide fragments by 3-HP generated peptides with antiviral activity, however, this was almost always combined with a cytotoxic effect on the Vero cells. Overall, our results suggest that targeted chemical modification of some natural products might provide compounds effective against HSV-1 infection.
Keywords: Antiviral, Lysozyme, α-Lactalbumin, β-Lactoglobulin, 3-Hydroxyphthalic anhydride, HSV-1
1. Introduction
Current chemotherapeutic antiviral drugs have been characterised as having in many cases limited clinical efficacy, suboptimal pharmacokinetics and toxic side effects (Patick and Potts, 1998). In response to this, a number of recent studies have investigated the potential benefits of using naturally occurring compounds as therapeutic antiviral agents against herpes simplex virus type 1 (HSV-1) (Marchetti et al., 1996, Isaacs et al., 1997, Seong-Ku et al., 1999, Carlucci et al., 1999, Wachsman et al., 2000). Previous studies demonstrated the presence of antiviral agents in milk (Matthews et al., 1976, Newburg et al., 1992). One such agent, lactoferrin, was shown later to inhibit the human immunodeficiency virus (HIV-1), HSV-1 and -2, human cytomegalovirus, respiratory syncytial virus, poliovirus and rotavirus in vitro (Harmsen et al., 1995, Marchetti et al., 1996, Marchetti et al., 1998, Marchetti et al., 1999, Superti et al., 1997, Swart et al., 1996, Valenti et al., 1998, Viani et al., 1999), highlighting the importance of naturally occurring proteins as antiviral agents.
Reports of HIV-1 inhibition in vitro by polyanionic substances such as heparin and sulphated polysaccharides (Ueno and Kuno, 1987, Baba et al., 1988, Lederman et al., 1989) encouraged several groups to investigate the antiviral activity of chemically modified proteins. The introduction of negative charges into human serum albumin by chemical modification with polysaccharides, formaldehyde and aliphatic anhydrides yielded compounds with antiviral activity against HIV-1 (Jansen et al., 1991, Jansen et al., 1993, Takami et al., 1992, Kuipers et al., 1996, Kuipers et al., 1999, Swart et al., 1996, Swart et al., 1999). However, in a systematic study, Neurath et al. (1995) demonstrated aromatic anhydrides to be more effective in the conversion of natural proteins into negatively charged antiviral agents against HIV-1. The cellular CD4-receptor of lymphocytes, macrophages and monocytes was found to be the primary target for this inhibition. β-Lactoglobulin modified with 3-hydroxyphthalic anhydride (3-HP) in higher concentrations, also binds to the HIV gp120 binding site on CD4 (Neurath et al., 1996). Additionally, 3-HP-β-lactoglobulin is active against HSV-1 and -2 (Neurath et al., 1998) and Chlamydia trachomatis (Jiang et al., 1997).
We have previously shown that antimicrobial peptides could be generated by digestion of aprotinin (Pellegrini et al., 1994, Pellegrini et al., 1996), lysozyme (Pellegrini et al., 1997), α-lactalbumin (Pellegrini et al., 1999) and β-lactoglobulin (Pellegrini et al., 2001) and that proteolytic digestion of aprotinin yielded a hexapeptide with antiviral activity against HSV-1 and bovine parainfluenza virus 3 (PIV-3) (Pellegrini et al., 1994). The present study was aimed at investigating the antiviral activity of 3-HP-α-lactalbumin, 3-HP-β-lactoglobulin, 3-HP-albumin, 3-HP-lysozyme and the fragments resulting from the proteolytic digestion of the native proteins before and after chemical modification with 3-hydroxyphthalic anhydride.
2. Materials and methods
2.1. Reagents
The following proteins were used for chemical modification: bovine serum albumin (BSA, Fluka), lysozyme from chicken egg white (Fluka), α-lactalbumin (Sigma Chemical Co., St. Louis, MO) and β-lactoglobulin (Sigma). 3-Hydroxyphthalic anhydride used as the chemical reagent for the modification of the proteins was obtained from Aldrich. Nucleosil 120-10C18 for reversed phase chromatography was from Machery-Nagel. Dialysation membranes Spectra/Por® MWCO: 6000–8000 and 500 were from Spectrum Laboratories Inc. and sterile filters Millex®-GP 0.22 μm from Millipore.
2.2. Cells and viruses
The herpes simplex virus type 1 (HSV-1) was a gift from Dr. Wunderli, Institute for Medical Virology, University of Zürich. The bovine parainfluenza virus type 3 (PIV-3) and the porcine respiratory corona virus (PRCV) were available at the Institute of Virology. All three viruses were titrated by inoculation of cells with 10-fold dilutions using the endpoint dilution method of Reed and Muench (1938). Vero76 cells, used to measure the antiviral activity against HSV-1, Madin–Darby bovine kidney cells (MDBK) and Swine Testicular (ST) cells, used to measure the inhibitory effect of the chemically modified proteins against PIV-3 and PRCV, respectively, were originally purchased from ATCC. Vero76 cells and MDBK cells were grown in Eagle’s minimal essential medium (MEM) with l-glutamine (GibcoBRL) supplemented with 10% foetal calf serum (FCS, GibcoBRL), 100 U/ml penicillin and 100 μg/ml streptomycin (GibcoBRL). ST cells were cultured in the same medium containing 1% non-essential amino acids (NEA, GibcoBRL) and 1 mM sodiumpyruvate (GibcoBRL). For cell-maintenance the foetal calf serum concentration was lowered to 2% for ST cells and to 0% for Vero76 cells and MDBK cells, respectively.
2.3. Protein purification
The proteins were dissolved in 0.05% trifluoroacetic acid (v/v) and purified by reversed phase chromatography on a Nucleosil 120-10C column using a linear gradient of acetonitrile (12 ml) from 0 to 70% (v/v) in 0.05% trifluoroacetic acid.
2.4. Chemical modification of the proteins
The proteins were modified with 3-hydroxyphthalic anhydride according to a procedure described by Neurath et al. (1997). Briefly, 160 mg of the purified proteins were dissolved in 5 ml of 0.1 M Na-phosphate pH 8.5. 3-Hydroxyphthalic anhydride solution was added in eight aliquots of 200 μl (125 mg/ml 3-HP dissolved in dimethylsulfoxyde) at 12 min intervals. The pH of the solution was maintained at 8.5. After 1 h incubation at 25 °C, the mixture was dialysed twice against 500 ml PBS pH 7.4. Protein concentrations were determined photometrically as described by Gill and von Hippel (1989).
2.5. Proteolytical digestion of proteins with trypsin and separation of the tryptic fragments
Fifty milligrams of the protein and 5 mg of trypsin were dissolved in 3.5 ml of 0.2 M TRA (Triethanolamine-HCl) buffer containing 20 mM CaCl2 of pH 7.8. The mixture was stirred for 6 h at 37 °C. The solution was then acidified adding 500 μl of 10% trifluoroacetic acid (v/v) and centrifuged at 50,000×g for 10 min. The supernatant of the previous step was loaded in 40 runs onto a Nucleosil 120-10C18 column which was previously equilibrated with 0.1% trifluoroacetic acid in 10% acetonitrile. One hundred microliter were loaded for every run. The column was eluted at a flow rate of 0.8 ml/min and fractions of 200 μl were collected. After collecting 10 fractions, a linear gradient from 10 to 70% acetonitrile was applied. Fractions were pooled according to the elution diagram. Each pool was divided in two samples and freeze-dried. One of the two samples was then dissolved in PBS and assayed for antiviral activity. The other sample was treated with 3-HP as described below.
2.6. Proteolytical digestion of proteins with chymotrypsin and separation of the chymotryptic fragments
Chymotryptic digestion of the proteins and the separation of the chymotryptic fragments were performed in the same experimental conditions as reported above for the tryptic digestion of the proteins. Fractions from reversed phase chromatography were pooled according to the elution diagram and treated as described above.
2.7. Proteolytical digestion of proteins with pepsin and separation of the peptic fragments
Fifty milligrams of the protein and 5 mg of pepsin were dissolved in 3.5 ml 0.1 M citrate buffer of pH 2.0. The mixture was gently stirred for 6 h at 37 °C. The enzymatic reaction was stopped by heating the sample at 80 °C for 30 min. The sample was then centrifuged at 50,000×g for 10 min. Separation of the peptic fragments was performed by reversed phase chromatography on a Nucleosil 120-10C18 column as described above. Fractions from reversed phase chromatography were pooled according to the elution diagram and treated as described in the section about the tryptic digestion of proteins.
2.8. Chemical modification of the fragments derived from the proteolytical digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme by 3-HP
Single pool samples derived from the proteolytical digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme by trypsin, chymotrypsin and pepsin were freeze-dried, and then dissolved in 2 ml 0.1 M Na-phosphate buffer of pH 8.5. Thirty-five microliter of 3-HP solution (125 mg/ml in DMSO) were added 8 times with a time interval of 12 min at room temperature. The solution was further incubated 1 h at room temperature. The pH of solution was maintained at pH 8.5 during the reaction. The samples were then dialysed with Spectra/Por® MWCO 500 against 1 l of PBS for 24 h and finally filtered by a 0.22 μm membrane. The samples were assayed for antiviral activity as described above.
2.9. Antiviral assays
2.9.1. Neutral red uptake assay
Neutral red uptake assay was performed following a similar procedure as described by Borenfreund and Puerner (1985). Briefly, 48 h after infection the maintenance medium was removed and the cells were incubated for 3 h with MEM containing 50 μg/ml neutral red (Fluka) (200 μl/well). The cells were then washed and fixed with 200 μl of 4% formaldehyde and 1% CaCl2. The neutral red was extracted by 200 μl 1% acetic acid in 50% ethanol and the absorbance was measured at 550 nm in a MR710 Dynatech Microplate®-Reader. The percent protection achieved by the compounds in the infected cells was calculated as described by Pauwels et al. (1988). Each sample was tested in triplicate.
2.9.2. Cytopathic effect inhibition assay: preincubation of cell monolayer with the test compound before virus infection
Test compounds were dissolved in MEM and incubated with confluent cell monolayers in 96-well tissue culture plates (Nunc) in increasing concentrations from 0.2 μg/ml to 2 mg/ml for 24 h at 37 °C and 5% CO2. After removal of the test compound, the cells were washed with phosphate-buffered saline (PBS) and then infected with 103 TCID50/well of HSV-1 corresponding to a multiplicity of infection (MOI) of 0.1 or 104 TCID50/well of PIV-3 and PRCV corresponding to a MOI of 1.0, respectively. After 1 h incubation the unadsorbed virus was removed, the cell monolayer was washed with PBS and further incubated in MEM until a cytophatic effect was visible (about 48 h). Uninfected cells were incubated with the test substances alone to evaluate their cytotoxicity. The inhibition of the cytopathic effect was assessed by light microscopy and measured by the neutral red uptake assay. Controls consisted of infected untreated Vero cell monolayers.
2.9.3. Cytopathic effect inhibition assay: incubation of cell monolayer with test compound and virus
The assay was performed following the protocol described above, with the exception that the test compound was added together with the virus. After an incubation time of 1 h at 37 °C and 5% CO2, the solutions containing both compound and viruses were removed, the cell monolayer was washed with PBS and further incubated in MEM for 48 h.
2.9.4. Cytopathic effect inhibition assay: incubation of cell monolayer with the test compound after virus infection
The assay was carried out as reported above with the following difference: first, the cell monolayer was infected with the virus. After 1 h incubation the unadsorbed virus was removed, the cell monolayers were washed with PBS and then incubated with the test compound in MEM for 48 h.
2.9.5. Virus yield inhibition assay
Quantification of HSV-1 inhibition was performed by a virus yield inhibition assay according to the procedure reported by Gil-Fernandez et al. (1987) and Aboudy et al. (1994). Confluent cell monolayers in 96-well plates were treated with the compounds before, during and after virus infection as described above. After 24 h incubation at 37 °C, 5% CO2, the plates were frozen and thawed three times. Confluent cell monolayers, grown in 24-well plates (Nunc), were inoculated with 10-fold dilutions of the supernatants for 1 h at 37 °C and 5% CO2. After removal of the inoculum, monolayers were washed once with PBS and overlaid with MEM supplemented with 0.8% carboxy methyl cellulose (Fluka), 2% FCS, 100 U/ml penicillin and 100 μg/ml streptomycin and incubated at 37 °C and 5% CO2 for 3 days. Methylcellulose medium was then removed, monolayers were fixed with methanol, stained with 0.5% crystal violet and the plaque number was counted. Results were expressed as percentage of plaque inhibition by comparison with untreated control cell monolayers. EC50 and EC90, the concentrations needed to achieve 50 and 90% of plaque reduction compared to untreated infected cell monolayers, were determined directly from the curves obtained by plotting the inhibition of the virus yield against the concentration of the samples. Data presented are results of repeated experiments performed in triplicate.
2.10. Cytotoxicity
Confluent Vero76 cell monolayers grown in 96-well cell culture plates were incubated with two-fold serial dilutions of the test compounds—starting with a concentration of 30 μg/ml to 8 mg/ml—for 48 h at 37 °C and 5% CO2. At this time the cell viability was evaluated by neutral red uptake assay and light microscopy. The percent cell survival was calculated as the ratio of treated uninfected cells/untreated uninfected cells and expressed as a percentage. These values were plotted against the concentrations of the test compound. The concentration needed to cause 50% cytotoxicity (CC50) was determined graphically (Aysi et al., 1991). Each sample was tested in triplicate.
3. Results
3.1. Antiviral activity
3.1.1. Cytopathic effect inhibition assay
The inhibition of the cytopathic effect by the native and chemically modified proteins was evaluated before, during or after virus infection. Albumin, α-lactalbumin, β-lactoglobulin and lysozyme did not show any antiviral activity either against HSV-1 or against PIV-3 and PRCV, respectively. In contrast, the modified proteins, i.e. 3-HP-albumin, 3-HP-α-lactalbumin, 3-HP-β-lactoglobulin and 3-HP-lysozyme, showed antiviral activity against HSV-1, but not against PIV-3 and PRCV. 3-HP-β-lactoglobulin (Fig. 1 ), 3-HP-albumin and 3-HP-lysozyme (not shown) inhibited the cytopathic effect of HSV-1 in all three experiments, i.e. when present before (Fig. 1A), during (Fig. 1B), or after the infection (Fig. 1C). 3-HP-α-lactalbumin showed only a minimal inhibition when incubated with the monolayer before infection, whereas the inhibition was evident when the compound was present during or after infection (data not shown).
Fig. 1.
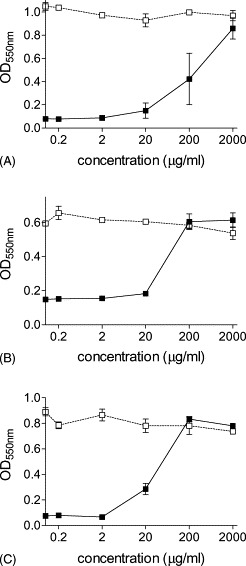
Effect of increasing concentrations of 3-HP-β-lactoglobulin on the viability of HSV-1 infected (■) and uninfected (□) Vero76 cells in the neutral red uptake assay. The multiplicity of infection was 0.1. 3-HP-β-lactoglobulin was present in the assay before (A), during (B) or after (C) HSV-1 infection. The inhibition of the cytopathic effect is characterised by high OD550 values detected in the infected Vero cells treated with 3-HP-β-lactoglobulin. The data are reported on the horizontal axis in log10 units. The data represent mean values±S.E. for at least three separate experiments.
3.1.2. Antiviral activity of the modified proteins as measured by reduction in virus yield
The antiviral activity of the modified proteins against HSV-1 was quantified by determining the virus yield 24 h post infection in treated and untreated Vero76 cells. Test compounds were present before, during or after the infection step. All modified proteins afforded potent inhibition of HSV-1 replication (Fig. 2 , Table 1 ).
Fig. 2.
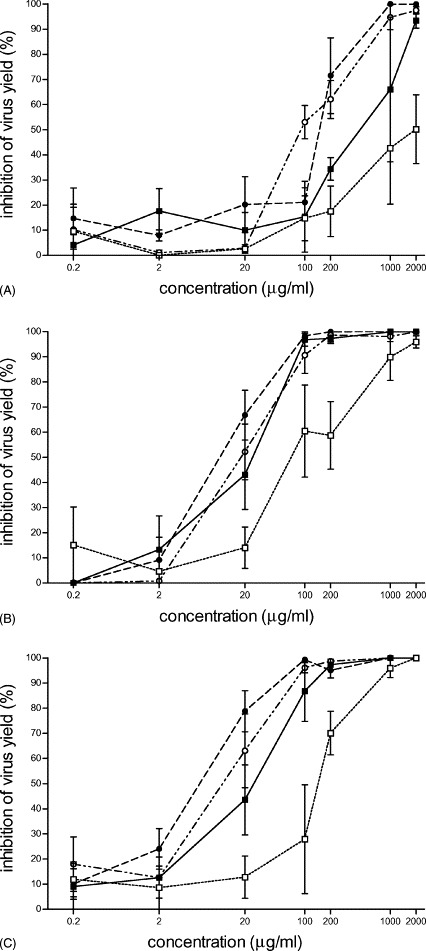
Dose–response of the inhibitory effect on the virus yield of 3-HP-α-lactalbumin (□), 3-HP-β-lactoglobulin (■), 3-HP-albumin (○) and 3-HP-lysozyme (•). The multiplicity of infection was 0.1. The inhibition of the virus yield was calculated as percentage of plaque reduction comparing treated with untreated infected cells. Chemically modified proteins were present in the assay before (A), during (B) or after (C) virus infection. The data reported on the horizontal axis are expressed in log10 units. The results represent mean values±S.E. for at least three separate experiments.
Table 1.
Antiviral activity of 3-HP-modified proteins against HSV-1, as monitored by the cytopathic effect assay
| 3-HP proteins | CC50a (mg/ml) | EC50b (μg/ml) |
EC90c (μg/ml) |
SI |
||||||
| A | B | C | A | B | C | A | B | C | ||
| 3-HP-α-lactalbumin | >8 | nd | 65 | 160 | nd | 1000 | 670 | nd | >123 | >50 |
| 3-HP-β-lactoglobulin | >8 | 465 | 30 | 30 | 1800 | 80 | 120 | >17 | >267 | >267 |
| 3-HP-albumin | >8 | 100 | 18 | 14 | 830 | 100 | 75 | >80 | >444 | >570 |
| 3-HP-lysozyme | 5 | 170 | 14 | 6 | 590 | 65 | 50 | 29 | 357 | 833 |
SI: selectivity index (SI=CC50/EC50). A: 3-HP compounds were present before HSV-1 infection of the cell monolayer; B: 3-HP compounds were present during HSV-1 infection of the cell monolayer; C: 3-HP compounds were present after HSV-1 infection of the cell monolayer; nd: not determined.
CC50: concentration of the compound which reduces cell viability by 50%.
EC50: concentration of the compound which reduces the yield of HSV-1 by 50%.
EC90: concentration of the compound which reduces the yield of HSV-1 by 90%.
3.1.2.1. Presence of the modified proteins before HSV-1 infection
In attempts to find out whether pre-treatment of the cells with the modified proteins resulted in an inhibition of virus yield, cultures were incubated for 24 h with the test compounds which were then removed before infection. Pre-incubation of cells with 3-HP-albumin and 3-HP-lysozyme inhibited almost completely the virus yield at a concentration of 1 mg/ml (Fig. 2A). 3-HP-β-lactoglobulin reduced the virus yield almost completely at a concentration of 2 mg/ml. 3-HP-α-lactalbumin inhibited the HSV-1 yield by 50% only when assayed at this concentration.
The EC50 value for 3-HP-α-lactalbumin was not determined because it did not yield a complete inhibition of the virus multiplication, when assayed at a concentration of 2 mg/ml. The EC50 values determined for the 3-HP-proteins ranged between 100 μg/ml for 3-HP-albumin and 465 μg/ml for 3-HP-β-lactoglobulin, whereas EC90 values ranged between 590 μg/ml for 3-HP-lysozyme and 1800 μg/ml for 3-HP-β-lactoglobulin (Table 1).
3.1.2.2. Presence of the modified proteins during the infection
All compounds proved to be potent inhibitors of HSV-1 multiplication. The titration curves of the antiviral activity of the compounds investigated (Fig. 2B) showed a similar slope. 3-HP-α-lactalbumin was less effective at low concentrations compared to the other 3-HP-proteins; however, when assayed at a concentration of 2 mg/ml, all 3-HP-proteins inhibited completely the activity of HSV-1. 3-HP-albumin, 3-HP-β-lactoglobulin, and 3-HP-lysozyme caused a distinct reduction of the virus yield already at a concentration of 20 μg/ml. 3-HP-lysozyme, although toxic to the Vero cells, was the most potent inhibitor with an EC50 value of 14 μg/ml, whereas 3-HP-α-lactalbumin was the weakest inhibitor with an EC50 of 65 μg/ml. The EC90 values ranged between 65 μg/ml for 3-HP-lysozyme and 1000 μg/ml for 3-HP-α-lactalbumin (Table 1).
3.1.2.3. Presence of the modified proteins after viral infection
In order to evaluate the antiviral activity after viral adsorption, 3-HP-proteins were incubated with the infected cell monolayer 1 h post infection for 24 h. Results are reported in Table 1 and shown in Fig. 2C. All the 3-HP-proteins strongly inhibited viral multiplication when assayed at a concentration of 2 mg/ml. 3-HP-albumin, 3-HP-β-lactoglobulin and 3-HP-lysozyme inhibited the virus production already at concentrations of 20 μg/ml. 3-HP-lysozyme was the most potent inhibitor with an EC50 value of 6 μg/ml, whereas 3-HP-α-lactalbumin was the weakest inhibitor with an EC50 value of 160 μg/ml. The EC90 values ranged between 50 μg/ml for 3-HP-lysozyme and 670 μg/ml for 3-HP-α-lactalbumin (Table 1).
3.2. Cytotoxicity
Of all the 3-HP-proteins investigated for antiviral activity only 3-HP-lysozyme showed a cytotoxic effect on Vero76 cells after 48 h incubation. The CC50 value of 3-HP-lysozyme determined by neutral red uptake assay was 5 mg/ml, whereas the other compounds did not cause any cytotoxic effects at a concentration up to 8 mg/ml (Table 1). The selectivity index values ranged between 50 for 3-HP-α-lactalbumin and 833 for lysozyme.
3.3. Antiviral activity of the peptide fragments derived from the proteolytic digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme
Antiviral activity determinations of the pools derived from the proteolytic digestion of the proteins albumin, α-lactalbumin, β-lactoglobulin and lysozyme tested by neutral red uptake assay, were performed against HSV-1 only.
3.3.1. Antiviral activity of the tryptic fragments
Proteolytic digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme by trypsin resulted in a mixture of several fragments that could be partially separated by reversed phase chromatography. The elution diagrams of the reversed phase chromatography of the digested proteins with the pooled fractions are shown in Fig. 3 . The antiviral tests were performed assaying the pools before, during or after viral infection of the cell monolayers.
Fig. 3.
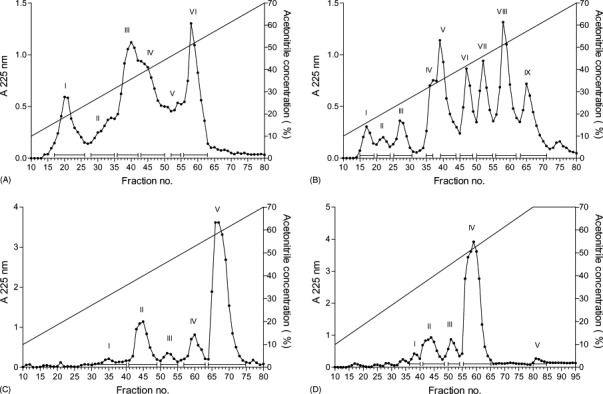
Reversed phase chromatography on a Nucleosil 120-10C18 column of the peptide fragments derived from the tryptic digestion of: albumin (A); α-lactalbumin (B); β-lactoglobulin (C); lysozyme (D).
Pre-incubation of the pools, derived from the tryptic digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme, with Vero cells before virus infection did not afford an inhibition of the cytopathic effect. When the pools were simultaneously incubated with the virus, an inhibition of the cytopathic effect was observed only with pool VI derived from albumin (70% cell protection) and pool IX from α-lactalbumin (90% cell protection). However, these pools were highly cytotoxic because only 10% of Vero cells survived upon a 48-h incubation. Antiviral activity could be observed for almost all the pools when they were incubated after virus infection. Only pools I and V derived from the tryptic digestion of albumin did not show any antiviral activity, whereas pools II–IV and VI weakly inhibited HSV-1 multiplication. Pool VI which had the property to inhibit the cytopathic effect of HSV-1, when assayed in presence of virus and after viral infection, was strongly cytotoxic.
Pools II, III and VIII resulting from the digestion of α-lactalbumin by trypsin were moderately active against HSV-1 and cytotoxic for Vero cells. All the other pools were ineffective in inhibiting the cytopathic effect of HSV-1.
Antiviral activity from the β-lactoglobulin fragments could be detected only in pools II and III, and were linked to high grade of toxicity for Vero cells.
Pools I–IV derived from the digestion of lysozyme with trypsin were not active against HSV-1.
3.3.2. Antiviral activity of the chymotryptic fragments
Albumin, α-lactalbumin, β-lactoglobulin and lysozyme digested by chymotrypsin were fractionated by reversed phase chromatography on a Nucleosil 120-10C18 (not shown). Antiviral activity against HSV-1 could be revealed only when the pools were added after virus infection of the cell monolayer. The pools assayed either before or during virus infection did not show any antiherpetic activity.
Antiviral activity of fragments derived from the albumin digestion by chymotrypsin could be observed in two pools; however, they were toxic for Vero cells. Almost all pools obtained from the chymotryptic digestion of α-lactalbumin inhibited the viral replication only partially and showed moderate cytotoxic effect on Vero cells. Similar results were obtained from the chymotryptic fragments of β-lactoglobulin. Three of the seven pools derived from the chymotryptic digestion of lysozyme showed antiherpetic activity linked with a moderate cytotoxicity for Vero cells. The other pools did not show any antiviral activity.
3.3.3. Antiviral activity of the peptic fragments
Peptic digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme resulted in several peptide fragments which could be resolved by reversed phase chromatography (not shown). All pools showed antiviral activity only when added after viral infection of the Vero cells monolayer. They failed to inhibit the cytopathic effect when present in the assay before or during virus infection. Only two of the four pools obtained from the peptic digestion of albumin inhibited weakly the viral replication when added after viral infection; however, they showed at the same time a moderate cytotoxic effect on Vero cells.
Inhibitory activity of the fragments generated by peptic digestion of α-lactalbumin against HSV-1 was present in almost all the pools. Only one of them lacked antiviral activity. The active pools were slightly cytotoxic towards Vero cells.
All the pools obtained from the peptic digestion of β-lactoglobulin showed HSV-1 inhibition. However, they all were cytotoxic for the Vero cells.
Only one pool obtained by digestion of lysozyme by pepsin presented a weak antiherpetic activity, combined, however, with cytotoxicity.
3.4. Antiviral activity of the peptide fragments derived from the proteolytic digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme after chemical modification by 3-HP
The compounds present in the pools derived from the proteolytic digestion of the proteins were chemically modified by 3-hydroxyphthalic anhydride as described in Section 2. The antiviral activity of the chemically modified pools was investigated against HSV-1 only.
No chemically modified pools derived from the digestion of albumin, α-lactalbumin, β-lactoglobulin and lysozyme with trypsin, chymotrypsin or pepsin showed any antiviral activity when incubated with the cell monolayer before virus infection.
Almost all the pools were ineffective or only weakly active when present during virus infection. In the case of 3-HP-modified pools derived from tryptic digestion cell protection ranged from 15 to 40%. Among the 3-HP-pools derived from chymotryptic digestion a weak antiherpetic activity was only detected in two pools derived from α-lactalbumin. All 3-HP-pools derived from peptic digestion were devoid of any antiherpetic effect (data not shown).
Most of the pools showed an antiherpetic effect when assayed after virus infection. In the case of 3-HP-pools derived from tryptic digestion cell protection ranged from 10 to 50% (pool IV and pool IX from α-lactalbumin, respectively). However, all the pools with some inhibitory effect toward HSV-1 were also toxic for the Vero cells.
All 3-HP-pools derived from chymotryptic digestion of lysozyme inhibited the cytophatic effect of HSV-1. In contrast, the 3-HP-pools derived from chymotrypsin digestion of the other proteins were either inactive or only weakly active against HSV-1. The cell protection ranged from 10 to 60%. However, all pools were toxic for Vero cells (data not shown).
The 3-HP-pools derived from peptic digestion of albumin and lysozyme showed only weak or no antiviral activity at all. Cell protection by pools from α-lactalbumin and β-lactoglobulin ranged from 10 to 30% (data not shown).
4. Discussion
The present study shows that 3-HP-modified proteins strongly inhibited the multiplication of HSV-1, and thus confirms that the introduction of hydrophobic and negatively charged residues in the polypeptide chain is a useful procedure to confer antiviral activity to a protein.
However, the fact that 3-HP-modified proteins failed to inhibit PIV-3 and PRCV, both enveloped viruses like HSV-1, indicates that unspecific damage of the virus envelope, caused by hydrophobic and electronegative interaction between the 3-HP-proteins and envelope proteins, is unlikely. The native proteins and the chemical compound 3-HP alone did not show any antiviral activity against the virus investigated, indicating that the inhibition of the cytopathic effect of HSV-1 was a peculiar property of the modified proteins.
3-HP-proteins were able not only to inhibit the initiation and the spread of infection but they also had a protective effect on the cells rendering them resistant to virus infection. However, when the cells were preincubated with the 3-HP-proteins, a clear reduction of the viral multiplication could only be detected with higher concentrations than when assayed during or after virus infection. The most effective 3-HP-protein to prevent viral infection of the cell was 3-HP-albumin, whereas the least effective was 3-HP-α-lactalbumin. For the latter protein, while it did not inhibit completely HSV-1 at the maximal concentration used in the assay, the EC50 and EC90 values were not determined.
All the 3-HP-proteins inhibited efficiently the infection of Vero cells when present during infection with HSV-1, that is at the stages of adsorption and the penetration of the virion particles into the cells. Several viral glycoproteins such as gB, gC, gD and the corresponding receptors present in the cell membrane are responsible for adsorption and penetration (WuDunn and Spear, 1989, Herold et al., 1991, Shieh et al., 1992, Trybala et al., 1994, Marchetti et al., 1996, Cocchi et al., 2001). This might imply that the antiviral activity of the 3-HP-proteins, when present during infection, is based on an interaction of the compounds with such viral glycoproteins. This is supported by the fact that the binding of anti-gC monoclonal antibodies to HSV-1 is inhibited by 3-HP-β-lactoglobulin ( Neurath et al., 1998).
Virus replication was, in addition, efficiently inhibited after infection of the cells. This might, on one hand, be due to the cell damage caused by viral multiplication that allows the compound to penetrate into the cell. Such a mechanism has been postulated for cystatin C, (Björk et al., 1990). On the other hand, the binding of the modified proteins to the viral envelope glycoproteins could be taken as a basis to explain an inhibition at the intercellular or extracellular level. In fact 3-HP-β-lactoglobulin binds to the glycoprotein E (Neurath et al., 1998), which mediates, together with gI, the direct cell-to-cell transmission of HSV-1 (Dingwell et al., 1994, Weeks et al., 1997). The blockage of gE would inhibit the viral spread on the intercellular level. The binding of the modified proteins to glycoproteins gB, gC and/or gD of the virions, which are released from cells after a first replication cycle, could give an explanation for an extracellular inhibition.
Negative charge and hydrophobic interaction between antiviral compounds and virus envelope proteins have been suggested as a possible active principle for antiviral activity (Neurath et al., 1995). Chemical targets of the 3-HP are the positively charged lysine residues of the polypeptide chain, which, after chemical reaction with 3-HP, becomes negatively charged. 3-HP-albumin, with its 59 negatively charged 3-HP-lysine residues, was the protein with the highest antiviral activity. 3-HP-α-lactalbumin possessing 12 modified lysine residues was five times less active than 3-HP-β-lactoglobulin, which has 15 lysine residues, when assayed after viral infection of Vero cells. Lysozyme having half of the lysine residues of 3-HP-α-lactalbumin had a higher antiviral activity in comparison to 3-HP-α-lactalbumin, nevertheless, it was strongly toxic for the Vero cells.
As a first screening for antiviral compounds, we examined the peptide fragments obtained from the proteolytic digestion of the proteins. Although the single peptides were not isolated, our results show that peptide sequences with antiviral activity are present in the proteins investigated. Many of the pools tested were able to limit the virus spread after infection of Vero cells by HSV-1. However, most of the pools were toxic to different extents for Vero cells when incubated for 48 h.
The importance of the presence of 3-HP-lysine in the peptide sequence for antiherpetic activity, as reported by Neurath et al. (1998), has been confirmed extensively in the present study. We, therefore, wanted to know whether the chemical modification of the lysine residues by 3-HP would lead to an increase in the antiviral activity of peptide fragments generated by proteolytic digestion. 3-HP-proteins and 3-HP-peptides are instable and precipitate at an acidic pH. This makes their purification by reversed phase chromatography impossible, since this procedure is performed at an acidic pH. Thus, we decided to not directly digest the 3-HP-proteins, but to chemically modify peptide fragments present in the pools. Most of the 3-HP modified pools showed antiherpetic activity when present after the infection of the Vero cells and several of them were able to block the multiplication of the virus. However, all the 3-HP-pools examined were toxic for Vero cells to different extent. Since at the present stage of our research the single peptides of the pools with antiherpetic activity have not been isolated, it is not possible to say with certainty whether antiviral activity and toxic effects are due to the same molecule or to two separate entities.
In conclusion, our results confirm that the modification of the lysine residues by 3-HP is a useful technology to develop compounds with antiherpetic activity, and they show, moreover, that peptides with antiherpetic activity can be generated through proteolytic digestion of proteins. Short peptides with antiviral activity are of particular interest because synthetic peptides have a low production cost and a reduced antigenicity, which consequently limits possible hypersensitivity.
Acknowledgements
We are grateful to Mrs Eva Loepfe for her kind and skilful assistance and collaboration during the initial phase of this work.
References
- Aboudy Y., Mendelson E., Shalit I., Besalle R., Fridkin M. Activity of two synthetic amphiphilic peptides and magainin-2 against herpes simplex virus types 1 and 2. Int. J. Pep. Protein Res. 1994;43:573–582. doi: 10.1111/j.1399-3011.1994.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Aysi N., Gupta S.V., Qualtiere L. Modified tetrazolium-based colorimetric method for determining the activities of anti-HIV compounds. J. Virol. Methods. 1991;33:335–344. doi: 10.1016/0166-0934(91)90033-v. [DOI] [PubMed] [Google Scholar]
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. USA. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk L., Grubb A., Kjellen L. Cystatin C, a human proteinase inhibitor, blocks replication of herpes simplex virus. J. Virol. 1990;64:941–943. doi: 10.1128/jvi.64.2.941-943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenfreund E., Puerner J.A. Toxicity determined in vitro by morphological alterations and neutral red adsorption. Toxicol. Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Carlucci M., Ciancia M., Matulewicz M., Cerezo A., Damonte E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999;43:93–102. doi: 10.1016/s0166-3542(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Cocchi F., Lopez M., Dubreuil P., Campadelli Fiume G., Menotti L. Chimeric nectin 1-poliovirus receptor molecules identify a nectin 1 region functional in herpes simplex virus entry. J. Virol. 2001;75:7987–7994. doi: 10.1128/JVI.75.17.7987-7994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwell K.S., Brunetti C.R., Hendricks R.L., Tang Q., Tang M., Rainbow A.J., Johnson D.C. Herpes simplex virus glycoprotein E and I facilitate cell-to-cell spread in vivo and across junctions of cultured cells. J. Virol. 1994;68:834–845. doi: 10.1128/jvi.68.2.834-845.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Fernandez G., Perez S., Vilas P., Perez C., de las Heras F.G., Garcia Gancedo A. Antiviral activity of uridine 5′-diphosphate glucose analogues against some enveloped viruses in cell culture. Antivir. Res. 1987;8:299–310. doi: 10.1016/s0166-3542(87)80007-4. [DOI] [PubMed] [Google Scholar]
- Gill S.F., von Hippel P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989;182:319–326. doi: 10.1016/0003-2697(89)90602-7. [DOI] [PubMed] [Google Scholar]
- Harmsen M.C., Swart P.J., de Béthune M.P., Pauwels R., De Clercq E., The T.H., Meijer D.K. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficency virus and human cytomegalovirus replication in vitro. J. Infect. Dis. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- Herold B.C., WuDunn D., Soltys N., Spear P.G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs C.E., Kascsak R., Pullarkat R.K., Xu W., Schneidman K. Inhibition of herpes simplex virus replication by retinoic acid. Antivir. Res. 1997;33:117–127. doi: 10.1016/s0166-3542(96)01009-1. [DOI] [PubMed] [Google Scholar]
- Jansen R.W., Molema G., Pauwels R., Schols D., De Clercq E., Meijer D.K.F. Potent in vitro anti-human immunodeficiency virus-1 activity of modified human serum albumins. Mol. Pharmacol. 1991;39:818–823. [PubMed] [Google Scholar]
- Jansen R.W., Schols D., Pauwels R., De Clercq E., Meijer D.K.F. Novel negatively charged, human serum albumins display potent and selective in vitro anti-human immunodeficiency virus type 1 activity. Mol. Pharmacol. 1993;44:1003–1007. [PubMed] [Google Scholar]
- Jiang, S., Li, Y.-Y., Lin, K., Strick, N., Neurath, R.A., George, K.S., Choudhury, S., Esmaeli-Azad, B., 1997. Virucidal and antibacterial activities of 3-HP-β-LG. In: Vaccines 97. Molecular Approaches to the Control of Infection Diseases, Cold Spring Harbor, Laboratory Press, Inc., pp. 327–330.
- Kuipers M.E., Huisman J.G., Swart P.J., de Béthune M.P., Pauwels R., Schuitemaker H., De Clercq E., Meijer D.K.F. Mechanism of anti-HIV activity of negatively charged albumins: biomolecular interaction with the HIV-1 envelope protein gp120. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1996;11:419–429. doi: 10.1097/00042560-199604150-00001. [DOI] [PubMed] [Google Scholar]
- Kuipers M.E., van de Berg M., Swart P.J., Laman J.D., Meijer D.K.F., Koppelman M.H.G., Huisman H. Mechanism of anti-HIV activity of succinylated human serum albumin. Biochem. Pharmacol. 1999;57:889–898. doi: 10.1016/s0006-2952(98)00369-4. [DOI] [PubMed] [Google Scholar]
- Lederman S., Gulick R., Chess R. Dextran sulfate and heparin interact with CD4 molecules to inhibit the binding of coat protein (gp120) of HIV. J. Immunol. 1989;143:1149–1154. [PubMed] [Google Scholar]
- Marchetti M., Longhi C., Conte M.P., Pisani S., Valenti P., Seganti L. Lactoferrin inhibits herpes simplex virus type 1 adsorption to Vero cells. Antivir. Res. 1996;29:221–231. doi: 10.1016/0166-3542(95)00840-3. [DOI] [PubMed] [Google Scholar]
- Marchetti M., Pisani S., Antonini G., Valenti P., Seganti L., Orsi N. Metal complexes of bovine lactoferrin inhibit in vitro replication of herpes simplex virus types 1 and 2. Biometals. 1998;11:89–94. doi: 10.1023/a:1009217709851. [DOI] [PubMed] [Google Scholar]
- Marchetti M., Superti F., Ammendiola M.G., Rossi P., Valenti P., Seganti L. Inhibition of poliovirus type 1 infection by iron-, manganese- and zinc-saturated lactoferrin. Med. Microbiol. Immunol. 1999;187:199–204. doi: 10.1007/s004300050093. [DOI] [PubMed] [Google Scholar]
- Matthews T.H.J., Lawrence M.K., Nair C.D.G., Tyrell D.A.J. Antiviral activity in milk of possible clinical importance. Lancet. 1976;ii:1378–1389. doi: 10.1016/s0140-6736(76)91922-x. [DOI] [PubMed] [Google Scholar]
- Neurath A.R., Debnath A.K., Strick N., Li Y.Y., Lin K., Jiang S. Blocking of CD4 cell receptors for the human immunodeficiency virus type 1 (HIV-1) by chemically modified bovine milk proteins: potential for AIDS prophylaxis. J. Mol. Recognit. 1995;8:304–316. doi: 10.1002/jmr.300080504. [DOI] [PubMed] [Google Scholar]
- Neurath A.R., Jiang S., Strick N., Lin K., Li Y.Y., Debnath A.K. Bovine β-lactoglobulin modified by 3-hydroxyphthalic anhydride blocks the CD4 cell receptor for HIV. Nat. Med. 1996;2:230–234. doi: 10.1038/nm0296-230. [DOI] [PubMed] [Google Scholar]
- Neurath A.R., Debnath A.K., Strick N., Li Y.Y., Lin K., Jiang S. 3-Hydroxyphtaloyl-β-lactoglobulin. I. Optimization of production and comparison with other compounds considered for chemoprophylaxis of mucosally transmitted human immunodeficiency virus type 1. Antivir. Chem. Chemother. 1997;8:131–139. [Google Scholar]
- Neurath A.R., Strick N., Li Y.Y. 3-Hydroxyphthaloyl-β-lactoglobulin. III Antiviral activity against herpesviruses. Antivir. Chem. Chemother. 1998;9:177–184. doi: 10.1177/095632029800900209. [DOI] [PubMed] [Google Scholar]
- Newburg D.S., Viscidi R.P., Ruff A., Yolken R.H. A human milk factor inhibits binding of human immunodeficiency virus to the CD4 receptor. Pediatr. Res. 1992;31:22–28. doi: 10.1203/00006450-199201000-00004. [DOI] [PubMed] [Google Scholar]
- Patick A.K., Potts K.E. Protease inhibitors as antiviral agents. Clin. Microbiol. Rev. 1998;11:614–627. doi: 10.1128/cmr.11.4.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Pellegrini A., Thomas U., Franchini M., Stöckli M., Klauser S., Hunziker P., von Fellenberg R. Identification of an aprotinin antiviral domain. FEBS Lett. 1994;344:261–265. doi: 10.1016/0014-5793(94)00396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini A., Thomas U., Bramaz N., Klauser S., Hunziker P., von Fellenberg R. Identification and isolation of the bactericidal domains in the proteinase inhibitor aprotinin. Biochem. Biophys. Res. Commun. 1996;222:559–565. doi: 10.1006/bbrc.1996.0783. [DOI] [PubMed] [Google Scholar]
- Pellegrini A., Thomas U., Bramaz N., Klauser S., Hunziker P., von Fellenberg R. Identification and isolation of a bactericidal domain in chicken egg white lysozyme. J. Appl. Microbiol. 1997;82:372–378. doi: 10.1046/j.1365-2672.1997.00372.x. [DOI] [PubMed] [Google Scholar]
- Pellegrini A., Thomas U., Bramaz N., Hunziker P., von Fellenberg R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta. 1999;1426:439–448. doi: 10.1016/s0304-4165(98)00165-2. [DOI] [PubMed] [Google Scholar]
- Pellegrini A., Dettling C., Thomas U., Hunziker P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta. 2001;1526:131–140. doi: 10.1016/s0304-4165(01)00116-7. [DOI] [PubMed] [Google Scholar]
- Reed L., Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Seong-Ku E., Young-So K., Chong-Kil L., Seong-Sun H. Antiherpetic activities of various protein bound polysaccharides isolated from Ganoderma lucidum. J. Ethnopharm. 1999;68:175–181. doi: 10.1016/s0378-8741(99)00086-0. [DOI] [PubMed] [Google Scholar]
- Shieh M.T., WuDunn D., Montgomery R.I., Esko J.D., Spear P.G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Superti F., Ammendolia M.G., Valenti P., Seganti L. Antirotaviral activity of milk proteins: lactoferrin prevents rotavirus infection in the enterocyte-like cell line HT-29. Med. Microbiol. Immunol. 1997;186:83–91. doi: 10.1007/s004300050049. [DOI] [PubMed] [Google Scholar]
- Swart P.J., Kuipers E.M., Smit C., Pauwels R., de Béthune M.P., De Clercq E., Meijer D., Huismann J.G. Antiviral effects of milk proteins: acylation results in polyanionic compounds with potent activity against human immunodeficiency virus type 1 and 2 in vitro. AIDS Res. Hum. Retroviruses. 1996;12:769–775. doi: 10.1089/aid.1996.12.769. [DOI] [PubMed] [Google Scholar]
- Swart P.J., Harmsen M.C., Kuipers M.E., Van Dijk A.A., Van der Strate W.A., Van Berkel P.H.C., Nuijens J.H., Smit C., Witvrouw M., De Clercq E., de Béthune M.-P., Pauwels R., Meijer D.K.F. Charge modification of plasma and milk proteins results in antiviral active compounds. J. Pept. Sci. 1999;5:563–576. doi: 10.1002/(SICI)1099-1387(199912)5:12<563::AID-PSC226>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Takami M., Sone T., Mizumoto K., Kino K., Tsunoo H. Maleylated human serum albumin inhibits HIV-1 infection in vitro. Biochim. Biophys. Acta. 1992;1180:180–186. doi: 10.1016/0925-4439(92)90066-v. [DOI] [PubMed] [Google Scholar]
- Trybala E., Bergstrom T., Svennerholm B., Jeansson S., Glorioso J.C., Olofsson S. Localization of a functional site on herpes simplex virus 1 glycoprotein C involved in binding to cell surface heparan sulphate. J. Gen. Virol. 1994;75:743–752. doi: 10.1099/0022-1317-75-4-743. [DOI] [PubMed] [Google Scholar]
- Ueno R., Kuno S. Dextran sulfate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet. 1987;13:1379. doi: 10.1016/s0140-6736(87)90681-7. [DOI] [PubMed] [Google Scholar]
- Valenti, P., Marchetti, M., Superti, F., Amendiola, M.G., Puddu, P., Gessani, S., Borghi, P., Belardelli, F., Antonini, G., Seganti, L., 1998. Antiviral activity of lactoferrin. In: Spik, G., Legrand, D., Mazurier, J., Pierce, A., Perraudin, J.P. (Eds.), Advances in Lactoferrin Research, Plenum Press, New York, pp. 199–203. [DOI] [PubMed]
- Viani R.M., Gutteberg T.J., Lathey J.L., Spector S.A. Lactoferrin inhibits HIV-1 replication in vitro and exhibits synergy when combined with zidovudine. AIDS. 1999;13:1273–1285. doi: 10.1097/00002030-199907090-00018. [DOI] [PubMed] [Google Scholar]
- Wachsman M.B., Lopez E., Ramirez J., Galagovsky L.R., Coto C.E. Antiviral effect of brassinosteroids against herpes virus and arena viruses. Antivir. Chem. Chemother. 2000;11:71–77. doi: 10.1177/095632020001100107. [DOI] [PubMed] [Google Scholar]
- Weeks B.S., Sundaresan P., Nagashunmugam T., Kang E., Friedman H.M. The herpes simplex virus-1 glycoprotein E (gE) mediates IgG binding and cell-to-cell spread through distinct gE domains. Biochem. Biophys. Res. Communn. 1997;235:31–35. doi: 10.1006/bbrc.1997.6720. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


