Abstract
Recombinant antibodies and their derivatives are increasingly being used as therapeutic agents. Clinical applications of antibodies often require large amounts of highly purified molecules, sometimes for multiple treatments. The development of very efficient expression systems is essential to the full exploitation of the antibody technology. Production of recombinant protein in the milk of transgenic dairy animals is currently being tested as an alternative to plasma fractionation for the manufacture of a number of blood factors (human antithrombin, human alpha-1-antitrypsin, human serum albumin, factor IX). The ability to routinely yield mg/ml levels of antibodies and the scale-up flexibility make transgenic production an attractive alternative to mammalian cell culture as a source of large quantities of biotherapeutics. The following review examines the potential of transgenic expression for the production of recombinant therapeutic antibodies.
Keywords: Transgenic expression, Goats, Mammary gland, Antibody, Purification
1. Introduction
One of the challenges created by the biotechnology revolution is the development of methods for the economical production of highly purified proteins at large scales. Recent developments indicate that expression of recombinant proteins in the milk of transgenic animals may be particularly well suited for the production of complex polypeptides.
Since Edward Jenner first used cowpox to protect against smallpox, the use of the immune system to fight disease has proven spectacularly successful. The arrival of the hybridoma technology (Köhler and Milstein, 1975) brought a new level of therapeutic potential to the use of the immune mechanisms, with monoclonal antibodies being widely recognized as potential “magic bullets”. However, when monoclonal antibodies were found to be effective in vitro, their xenogenic (murine) nature led to the development human anti-mouse-antibody responses in patients (Borrebaeck et al., 1993; Khazaeli et al., 1994), often precluding repeat treatments. Only, after the development of approaches (recently reviewed in Vaughan et al., 1998) permitting the production of monoclonal antibodies that are (or appear) human, could the clinical benefits of these agents be fully realized.
The pace of approval of monoclonal antibodies for various conditions has recently accelerated: abciximab (Reopro™) for the prevention of acute cardiac ischemia following coronary angioplasty, rituximab (Rituxan™) for the treatment of non-Hodgkin B-cell lymphoma, trastuzumab (Herceptin™) for metastatic breast cancer, infliximab (Remicade™) for severe Crohn's disease and rheumatoid arthritis (Hall, 1995; Glaser, 1996; Sherman-Gold, 1997; Dickman, 1998; Hoyle, 1998; Webber, 1998). Since therapeutic antibodies are often developed to treat clinical indications that have a large number of patients (cancers, arthritis) and that their effective dose is generally rather large, it has been imperative to develop very efficient recombinant protein production technologies.
Mammalian cell culture has emerged as the method of choice for the production of most monoclonal antibodies currently commercialized. However, even with improvements in culture technology and fermentation scale-up, costs of purified antibodies in the range of 1000 to 2000 US$/g (at 10,000 liter fermentation scale) are not uncommon with higher costs for smaller fermentation scale (Werner, 1998). Alternative systems are being developed, with the objective of lowering capital investments and cost of goods associated with large scale production of recombinant monoclonal antibodies. These include microbial and insect cell-based (recently reviewed in Verma et al., 1998), plant-based (reviewed in Larrick et al., 1998), as well as transgenic animal-based production systems. This review will focus on the production of recombinant monoclonal antibodies in the milk of transgenic animals, summarizing the status of the technology and examining potential advantages and challenges.
2. The mammary gland expression system
The ability to modify animal genomes through microinjection technology has offered new alternatives for the manufacture of recombinant proteins. Targeting the production of human recombinant protein pharmaceuticals to the milk of transgenic farm animals (recently reviewed by Houdebine, 1995; Maga and Murray, 1995; Echelard, 1996; Clark, 1998; Meade et al., 1998) solves many of the problems associated with either microbial or animal cell expression systems. Bacteria often improperly fold complex proteins, introducing more involved and expensive processes, and both bacteria and yeast lack adequate post-translational modification. Bioreactors for cell cultures require high initial capital expenditures, use large volumes of expensive culture media, and often suffer from relatively low yields.
To express a recombinant protein in the milk of a transgenic animal (Fig. 1 ), expression vectors containing a gene encoding the protein of interest fused to milk specific regulatory elements (Fig. 2 ) are generally introduced by microinjection of a one-cell embryo, or alternatively transfected into a cell line suitable for somatic cell nuclear transfer. Following integration into the germline, the mammary gland-specific transgene is transmitted in Mendelian fashion and, if expressed, becomes a dominant genetic characteristic that will be predictably inherited by offspring of the founder animal. Often, transgenic animals will express the protein(s) of interest in gram per liter quantities depending on the mammary-specific regulatory sequences employed, the gene to be expressed, as well as the integration site of the transgene.
Fig. 1.
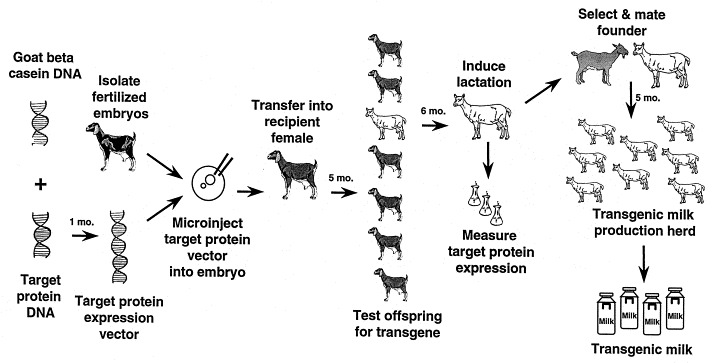
Schematic representation of the transgenic production process. The coding region of the protein to be expressed is linked to mammary gland specific regulatory elements. The resulting transgene is introduced by pronuclear microinjection into embryos of the selected species (alternatively, somatic cell nuclear transfer using cell lines transfected with the transgene can be used as method to create transgenic sheeps, goats or cattle). Embryos are then transferred to the oviduct (or the uterus) of a surrogate mother and carried to term. Transgenic offspring are identified and, when mature, are either bred or hormonally induced to lactate. Expression level of the target protein in the milk of transgenic animals is determined and a suitable founder line is chosen for the generation of the production herd.
Fig. 2.
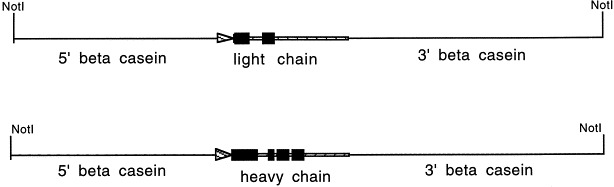
Transgenes for milk expression of antibodies. The gene of interest replaces the coding region of caprine β-casein, a milk specific gene. The promoter region (6.2 kb) linked to the coding regions of either light or heavy chains immunoglobulin, followed by untranslated caprine beta casein 3′ sequences and downstream elements (7.2 kb). Black boxes indicate the exons of the light and heavy chain of hBR96-2 (IgG1). Striped boxes indicate genomic introns. Arrows indicate direction of transcription.
3. Milk-specific transgenes
Transgenes targeting the expression of recombinant proteins to the mammary gland are usually chimeric, being derived from the fusion of the gene encoding the protein(s) of interest with mammary-specific regulatory sequences (Fig. 2). Regulatory sequences from several milk-specific genes have been isolated and used first in transgenic mice, then in large animals. Most of this work has been carried out with: the ovine β-lactoglobulin gene, rodent (mouse, rat and rabbit) whey acid protein (WAP) genes, bovine α-lactalbumin and α-s1-casein genes, as well as the caprine β-casein gene (reviewed in Maga and Murray, 1995; Echelard, 1996; Meade et al., 1998). According to the regulatory sequences employed, variable levels of expression for the protein of interest were observed. For example, using the human tissue plasminogen activator (tPA) cDNA only low level (μg/ml) expression was observed with transgenic rabbits and mice when using a construct containing 1.6 kb of bovine α-s1-casein 5′-flanking sequences (Riego et al., 1993), whereas the human tPA cDNA under the control of caprine β-casein sequences was expressed at high levels in transgenic goat's milk (>1 mg/ml; Ebert et al., 1991, Ebert et al., 1994). Recombinant protein expression will vary according to the nature and size of the mammary gland regulatory elements as well as the nature (cDNA or genomic) and sequence of the expressed gene. Usually genomic transgenes are expressed at higher levels than cDNA transgenes (Whitelaw et al., 1991; Hurwitz et al., 1994). However, systematic studies comparing side by side the expression of cDNA and genomic antibody transgenes in milk have not been published.
4. Species selection
Several animal species have been used for production of recombinant proteins in milk (Table 1 ). The choice of animal to employ for transgenic expression is determined by the amount of protein needed as well as reproductive characteristics. Transgenic mice represent an ideal system to test expression constructs prior or concomitant to the generation of larger founder transgenic animal. Fortunately, the milk promoter sequences from all mammals tested are capable of expression in the mouse mammary gland. This allows the relatively inexpensive and rapid testing and optimization of the transgene constructs. If expression of the transgene by the mammary gland is deleterious to the health of the animal, it will be often evident in the mouse model (Devinoy et al., 1994; Korhonen et al., 1997). However, the very limited murine milk yield restricts expression of recombinant proteins to milligram quantities.
Table 1.
Reproductive and lactational characteristics of animal species commonly used for expression of recombinant proteins in milk
| Species | Reproductive age (months) | Length of gestation (months) | Average number of offspring | Average % of transgenic birth | Average yield per natural lactation (l) |
|---|---|---|---|---|---|
| Mouse | 1 | 0.75 | 10 | 10–25 | 0.0015 |
| Rabbit | 6 | 1 | 8 | 5–15 | 1.5 |
| Pig | 8 | 4 | 9 | 5–15 | 120 |
| Sheep | 8 | 5 | 2 | 3–5 | 300–400 |
| Goat | 8 | 5 | 2 | 3–5 | 600–800 |
| Cattle | 15 | 9 | 1 | 0.5–3 | 10,000 |
Rabbits have been used for the production of gram amounts of proteins (Massoud et al., 1991; Brem et al., 1994; Korhonen et al., 1997; Stromqvist et al., 1997; Coulibaly et al., 1999). However, their labor-intensive milking and high husbandry costs preclude using rabbits for the production of kilograms of therapeutic antibodies. To this end, dairy goats are ideal. The average milk output per doe is 600 to 800 liters per 300-day lactation, although yields greater than 1000 liters per lactation have been recorded. Recombinant antibody concentration in milk in the range between 1 and 5 g/l has been reproducibly achieved by this group. In these conditions, herds of transgenic goats of manageable size could easily yield 1 to 300 kg of purified product per year. This represents the low to middle range of the high-volume protein category that would be required for the majority of therapeutic antibodies currently under development.
Moreover, the time from initiating microinjection to full lactation (gestation+growth to sexual maturity+gestation) is 16 to 18 months for goats. For cattle, it is approximately 3 years. Thus, the shorter caprine interval permits expansion of the production herds within the time frame needed for regulatory approval of the transgenically produced therapeutic proteins. Finally, there is a much lower incidence of scrapie in goats (only 7 cases reported by the United States Department of Agriculture through 1998) relative to sheep (1117 US cases through 1992; Wineland et al., 1998) which have an almost similar reproductive performance but lower lactation output.
5. Introduction of transgenes in the germline
To date, the method most frequently employed to produce transgenic animals has been pronuclear microinjection (exhaustively reviewed, among others, by Pinkert, 1994; Houdebine, 1996). Techniques allowing the direct microinjection of foreign genes into murine pronuclei were adapted to gene transfer into the pronuclei of rabbits, pigs and ruminants (Hammer et al., 1985; Bondioli et al., 1991; Ebert et al., 1991). Generally, fertilized eggs are flushed from the oviduct of superovulated donors, microinjected with a few hundred copies of the transgene, transferred to the uterus or oviduct of pseudopregnant recipients (following more or less extensive culture in vitro), and developed to term. In cattle, one cell stage embryos generated from in vitro matured and in vitro fertilized slaughterhouse-derived oocytes can also be used.
While successful and widely used, the microinjection approach has had limited efficiency. Transgene integration into the genome of founder animals is low, varying from less than 0.1% (cattle) to 5% (mice) of the microinjected embryos producing transgenic offspring. Moreover, the frequent generation of founder animals carrying the transgene in only a subset of their cells (mosaics) following pronuclear microinjection (Wilkie et al., 1986; Burdon and Wall, 1992; Whitelaw et al., 1993) can complicate the expansion of transgenic herds by reducing the frequency of germline transmission of the transgene(s).
The recent discovery that differentiated somatic cells can function as karyoplast donors for nuclear transfer has provided a wide range of possibilities for germline modification in sheep (Campbell et al., 1996; Wilmut et al., 1997), cattle (Cibelli et al., 1998) and goat (Baguisi et al., 1999). The use of recombinant somatic cell lines for nuclear transfer allows the introduction of transgenes by traditional transfection methods. It has the potential to increase the efficiency of transgenic animal production and overcome the problem of founder mosaicism. However, transfected somatic cell nuclear transfer has not been used very extensively yet and its efficacy in the various ruminant species is undetermined.
In addition to successful transgenic founder production, somatic cell nuclear transfer could allow for the selection of the appropriate transgenic cell line before the generation of cloned transgenic embryos. This would be particularly important for the transgenic production of recombinant monoclonal antibodies in milk where heavy chain and light chain transgenes should be expressed in the same secretory cells of the mammary epithelium at equivalent levels. It is also important that transgenes expressing each protein are co-integrated in the same locus to avoid segregation of heavy chain and light chain transgenes during herd propagation (Fig. 3 ).
Fig. 3.
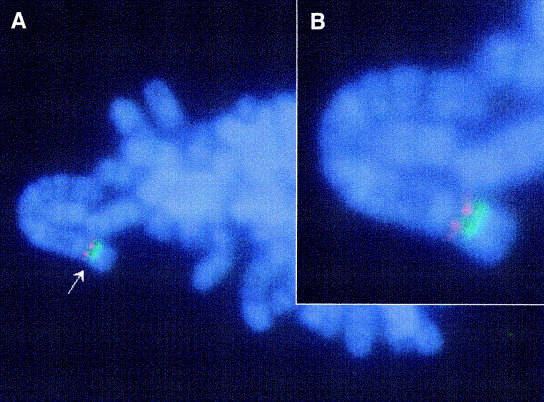
Fluorescence in situ hybridization (FISH) of blood metaphase spread form a transgenic goat carrying heavy and light chain antibody transgenes co integrated in the same chromosome (A) 150×; (B) 300×. The heavy chain-specific signal is red, the light chain-specific signal is green.
6. Expression of recombinant antibodies in milk
There are several reports of expression of antibodies in the milk of transgenic animals (summarized in Table 2 ). In one case (Limonta et al., 1995) an anti-CD6 mouse/human chimeric antibody (IgG1) was expressed at levels up to 0.4 mg/ml in the milk of mice carrying two transgenes respectively linking rabbit WAP regulatory sequences to genomic light chain and heavy chain sequences.
Table 2.
Antibody production in the milk of transgenic animals
| Regulatory sequences | Antibody expressed | Animal | Protein level (mg/ml) | References |
|---|---|---|---|---|
| Rabbit WAP (6.3 kb 5′, 8.3 kb 3′) | anti-CD6 mouse/human chimeric IgG1 | Mice | 0.4 | Limonta et al., 1995 |
| Mouse WAP (2.3 kb 5′, 1.6 kb 3′) | anti-TGEV mouse/human chimeric IgG1 | Mice | 5 | Castilla et al., 1998a, Castilla et al., 1998b |
| Ovine β-lactoglobulin. (4.3 kb 5′, 1.9 kb 3′) | anti-TGEV mouse/porcine chimeric IgGA | Mice | 6 | Sola et al., 1998 |
| Caprineβ-casein (6.2 kb 5′, 7.2 kb 3′) | Chimeric mouse/human anti-human transferrin rec. IgG1 fused to angiogenin | Mice | 0.8 | Newton et al., this issue |
| Caprineβ-casein (6.2 kb 5′, 7.2 kb 3′) | Single chain anti-human transferrin rec. IgG1 fused to angiogenin | Mice | 1.6 | Newton et al., submitted |
| Caprineβ-casein (6.2 kb 5′, 7.2 kb 3′) | chimeric human/mouse anti-Lewis Y IgG1 | Mice | 4 | Gavin et al., 1997; Pollock et al., unpublished |
| Caprineβ-casein (6.2 kb 5′, 7.2 kb 3′) | chimeric human/mouse anti-Lewis Y IgG1 | Goats | 14 | Gavin et al., 1997; Pollock et al., unpublished |
Even higher expression (up to 5 mg/ml) was achieved in mice generated by co-microinjection of transgenes linking either ovine β-lactoglobulin (Simons et al., 1987) or murine whey acid protein (Shamay et al., 1991) regulatory sequences to cDNAs encoding either chimeric mouse/human or chimeric porcine/human light chain and heavy chain anti-TGEV (transmissible gastroenteritis coronavirus) IgG genes (Castilla et al., 1998a, Castilla et al., 1998b; Sola et al., 1998). Interestingly, similar expression levels (up to 6 mg/ml) were observed with the expression of the same anti-TGEV variable region linked to an IgA isotype (Castilla et al., 1998a, Castilla et al., 1998b). In both cases, milk produced antibodies were found to be functional in a coronavirus neutralization assay.
In another example, two versions of angiogenin fused to E6, an antibody directed against the human transferrin receptor (Hoogenboom et al., 1990) were expressed in transgenic mouse milk. The human/mouse chimeric IgG1 version (Newton et al., this issue) was expressed up to 0.8 mg/ml. The single-chain version (Newton et al., submitted; Susanna Rybak, personal communication) was expressed up to 1.6 mg/ml. The single-chain version was expressed in milk at levels 3000-fold greater than when produced in Escherichia coli, and was superior in functional assays (stability, antigen binding, protein synthesis inhibition, cytotoxicity) to the material purified from inclusion bodies. The IgG1 version was produced at levels ∼160,000-fold higher than was produced in a myeloma cell culture system, and retained all biological properties (Newton et al., this issue; submitted; Susanna Rybak, personal communication). These data illustrate the potential of the mammary gland expression system for the production of immunoglobulin-enzyme fusion proteins.
In our group, transgenic mice and goats have been generated for the expression of seven different monoclonal antibodies (Gavin et al., 1997; Pollock et al., unpublished). High-level expression in transgenic mice was observed in all cases. In projects where goat expression data is already available, levels of recombinant antibodies were equivalent or higher to that observed in the corresponding transgenic mouse models (see characteristic examples in Fig. 4 Fig. 5 ). All the expression constructs for these studies employed the caprine β-casein regulatory elements (DiTullio et al., 1992; Roberts et al., 1992) linked to either genomic or cDNA genes coding for the heavy and light chain sequences.
Fig. 4.
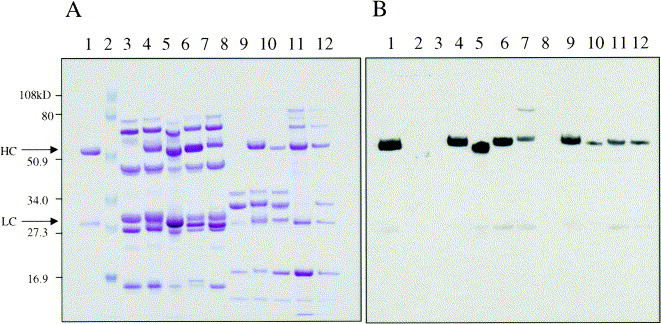
SDS-PAGE and Western analysis of several antibodies produced in mice and goats. Two 10–18% SDS-PAGE gels were electrophoresed in parallel under reducing conditions. (A) Lanes were loaded with 0.2 μl of either mouse or goat milk and stained with Coomassie Brilliant Blue R250 (Sigma). (B) Lanes were loaded with 0.1 μl of either mouse or goat milk. Antibodies were detected with affinity purified HRP-goat anti-human IgG (Cappel) and an Enhanced Chemiluminescence Kit (Amersham). This antibody recognizes the heavy chain more efficiently than the light chain. Lane 1. 10 mg/ml of standard antibody (in water). Lane 2. Pre-stained molecular weight markers (Biorad low-range, lot 77813). Lane 3. Negative mouse milk. Lane 4. Negative mouse milk spiked with 10 mg/ml of antibody standard. Lane 5. hBR96-2 a humanized IgG1 produced in mouse milk. Lane 6. Human IgG1 produced in mouse milk. Lane 7. Humanized IgG4 produced in mouse milk. Lane 8. Negative goat milk. Lane 9. Negative goat milk spiked with 10 mg/ml of standard antibody. Lane 10. hBR96-2 a humanized IgG1 produced in goat milk. Lane 11. Human IgG1 produced in goat milk, hormonally induced lactation. Lane 12. Human IgG1 produced in goat milk, natural lactation.
Fig. 5.
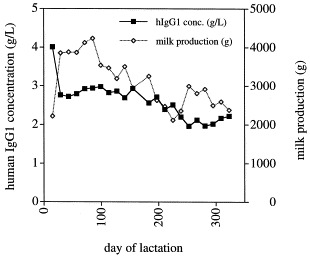
Milk and antibody production during the first natural lactation of a transgenic goat.
In one example, hBR96-2, a humanized IgG1 version of the BR96 anti-LewisY monoclonal antibody developed at Bristol Myers Squibb for anti-cancer applications (Rosok et al., 1996; Tolcher et al., 1999) was expressed in mice and goats. Transgenic animals were generated by co-microinjection of the milk expression constructs (Fig. 2). Twenty double transgenic mouse lines were generated, carrying both the heavy and light chain transgenes. Antibody expression up to 4 mg/ml was detected in transgenic mouse milk. A total of five transgenic goat lines was also generated by co-microinjection (Gavin et al., 1997), three of which carried both transgenes (one male and two female founders). Expression up to 14 g/l has been recorded in the milk of one of the female founders. High-level expression (5 g/l) was also observed from female offspring of the male founder. The other female founder only produced low-levels (0.1 g/l) of the antibody (D. Pollock et al., in preparation). It was also shown that the milk produced hBR96-2 antibody has retained full antigen binding properties (Perry Fell, personal communication).
7. Purification of recombinant human antibodies produced in goat milk
The published literature contains a wealth of information concerning the purification of monoclonal antibodies (Prior, 1991; Ransohoff and Levine, 1991; Brooks et al., 1992; Graf et al., 1994; Page and Thorpe, 1998).
The challenge of monoclonal antibody purification from cell culture is that high volumes of the conditioned culture media needs to be processed due to lower expression level (usually in the mg/l range). Typical contaminants in serum free media are cell debris, lipids, host DNA, host cell proteins, viruses and pyrogens. Other major impurities include bovine albumin, transferrin, bovine IgG in serum supplemented cultures.
Monoclonal antibodies produced in the milk of transgenic goats at the relatively modest level of 2 g/l represent 7% of the total protein. As the antibody is expressed as a whey protein, standard dairy procedures can be adapted to the initial separation step to achieve a casein-free, fat-free and lactose-free protein concentrate in which the antibody is at 30–60% purity, depending on the expression level. Fat may be separated from milk using standard centrifugal procedures or by membrane filtration. Membrane filtration can be used to eliminate most of the caseins and small molecules. The clarified whey is excellent starting material for chromatographic purification. Alternatively, either whole milk or the whey fraction can be subject to direct capture using expanded or fluidized bed techniques.
The purification of a recombinant human or humanized antibody from milk involves the separation of endogenous whey proteins from the antibody. The techniques employed vary with the type and class of antibody expressed. They include ion exchange chromatography, hydrophobic interaction chromatography and affinity chromatography (Protein A or Protein G). Affinity supports from different manufacturers vary and conditions may be found that do separate IgGs of different species. Goat milk contains 0.3–0.5 g/l of endogenous caprine Ig which can be separated from the recombinant human or humanized IgG. In our hands (Joseph Kutzko et al., unpublished) using a combination of chromatographic steps, human IgG was successfully purified from the clarified milk of a transgenic goat (Fig. 6 ). A >3 Log10 reduction of goat IgG is achieved in a single step at a theoretical purity of 99.9% with a yield of 65% for the unoptimized process.
Fig. 6.
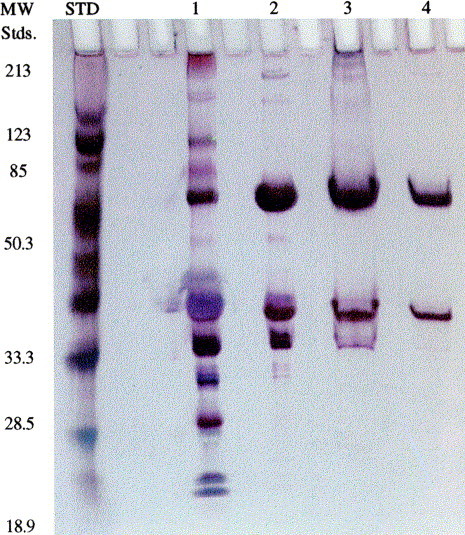
Silver stained SDS-PAGE of transgenic goat milk samples at different stages of an antibody purification process: Lane 1. Milk sample containing a human IgG1. Lane 2. Protein A eluate. Lane 3. CM HyperD column eluate (BioSepra Inc., Marlboro, MA). Lane 4. Methyl HyperD column eluate (BioSepra Inc., Marlboro, MA).
8. Conclusion
It is possible to achieve high-level expression of active recombinant immunoglobulins and immunoglobulin-fusions in the milk of transgenic animals. Transgenic expression delivers the advantages of mammalian cells such as a sophisticated refolding machinery and glycosylation. Other features of transgenic productions are scale-up flexibility since herd size can be increased (or decreased) rapidly and inexpensively, as well as the relative low-cost and low complexity of the raw product manufacturing facilities (farms) as compared to more traditional GMP cell culture facilities.
Further work is underway to fully evaluate the applicability of transgenically produced antibodies to therapeutic needs, in particular, studies of the functionality and pharmacokinetics of the milk-derived recombinant antibodies. It is expected that glycosylation structure of caprine mammary gland-derived antibodies will not be identical to CHO cell-derived material. However, there is very little evidence suggesting that differences in glycosylation resulting from expression in different mammalian host cells significantly influence the behavior of recombinant antibodies (Bebbington, 1995). In all cases, where it was examined, antigen binding of milk-expressed antibodies was found to be similar to equivalent mammalian cell culture derived molecules.
Acknowledgements
The authors thank Dr. Susanna M. Rybak for sharing her submitted manuscripts on antitransferrin receptor antibodies expression in milk and Dr. Perry Fell for sharing unpublished data.
References
- Baguisi A, Behboodi E, Melican D.T, Pollock J.S, Destrempes M.M, Cammuso C, Williams J.L, Nims S.D, Porter C.A, Midura P, Palacios M.J, Ayres S.L, Denniston R.S, Hayes M.L, Ziomek C.A, Meade H.M, Godke R.A, Gavin W.G, Overström E.W, Echelard Y. Production of transgenic goats by somatic cell nuclear transfer. Nat. Biotechnol. 1999;17:456. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- Bebbington, C., 1995. Expression of antibody genes in mammalian cells. In: Zola, H. (Ed.), Monoclonal Antibodies. The Second Generation. Bios Scientific Publishers, Oxford.
- Bondioli K.R, Biery K.A, Hill K.G, Jones K.B, De Mayo F.J. Production of transgenic cattle by pronuclear injection. Biotechnology. 1991;16:265. [PubMed] [Google Scholar]
- Borrebaeck C.K, Malmborg A.C, Ohlin M. Does endogenous glycosylation prevent the use of mouse monoclonal antibodies. Immunol. Today. 1993;14:477. doi: 10.1016/0167-5699(93)90259-n. [DOI] [PubMed] [Google Scholar]
- Brem G, Hartl P, Besenfelder U, Wolf E, Zinovieva N, Pfaller R. Expression of synthetic cDNA sequences encoding human insulin-like growth factor-1 (IGF-1) in the mammary gland of transgenic rabbits. Gene. 1994;149:351. doi: 10.1016/0378-1119(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Brooks D.A, Bradford T.M, Hopwood J.J. An improved method for the purification of IgG monoclonal antibodies from culture supernatants. J. Immunol. Methods. 1992;155:129. doi: 10.1016/0022-1759(92)90279-3. [DOI] [PubMed] [Google Scholar]
- Burdon T.G, Wall R.J. Fate of microinjected genes in preimplantation mouse embryos. Mol. Reprod. Dev. 1992;33:436. doi: 10.1002/mrd.1080330410. [DOI] [PubMed] [Google Scholar]
- Campbell K.H.S, McWhir J, Ritchie W.A, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- Castilla J, Sola I, Pintado B, Sanchez-Morgado J.M, Enjuanes L. Lactogenic immunity in transgenic mice producing recombinant antibodies neutralizing coronavirus. Adv. Exp. Med. Biol. 1998;440:675. doi: 10.1007/978-1-4615-5331-1_87. [DOI] [PubMed] [Google Scholar]
- Castilla J, Pintado B, Sola I, Sanchez-Morgado J.M, Enjuanes L. Engineering passive immunity in transgenic mice secreting virus-neutralizing antibodies in milk. Nat. Biotechnol. 1998;16:349. doi: 10.1038/nbt0498-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli J.B, Stice S.L, Golueke P.J, Kane J.J, Jerry J, Blackwell C, Ponce de Leon F.A, Robl J.M. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998;280:1256. doi: 10.1126/science.280.5367.1256. [DOI] [PubMed] [Google Scholar]
- Clark A.J. The mammary gland as a bioreactor: Expression, processing, and production of recombinant proteins. J. Mammary Gland Biol. Neopl. 1998;3:337. doi: 10.1023/a:1018723712996. [DOI] [PubMed] [Google Scholar]
- Coulibaly S, Besenfelder U, Fleischmann M, Zinovieva N, Grossmann A, Wozny M, Bartke I, Togel M, Muller M, Brem G. Human nerve growth factor beta (hNGF-beta): mammary gland specific expression and production in transgenic rabbits. FEBS Lett. 1999;444:111. doi: 10.1016/s0014-5793(98)01728-1. [DOI] [PubMed] [Google Scholar]
- Devinoy E, Thepot D, Stinnakre M.G, Fontaine M.L, Grabowski H, Puissant C, Pavirani A, Houdebine L.M. High level production of human growth hormone in the milk of transgenic mice: the upstream region of the rabbit whey acidic protein (WAP) gene targets transgene expression to the mammary gland. Transgenic Res. 1994;3:79. doi: 10.1007/BF01974085. [DOI] [PubMed] [Google Scholar]
- Dickman S. Antibodies stage a comeback in cancer treatment. Science. 1998;280:1196. doi: 10.1126/science.280.5367.1196. [DOI] [PubMed] [Google Scholar]
- DiTullio P, Cheng S.H, Marshall J, Gregory R.J, Ebert K.M, Meade H.M, Smith A.E. Production of cystic fibrosis transmembrane conductance regulator in the milk of transgenic mice. Bio/Technology. 1992;10:74. doi: 10.1038/nbt0192-74. [DOI] [PubMed] [Google Scholar]
- Ebert K.M, Selgrath J.P, DiTullio P, Denman J, Smith T.E, Memon M.A, Schindler J.E, Monastersky G.M, Vitale J.A, Gordon K. Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: Generation of transgenic goats and analysis of expression. Bio/Technology. 1991;9:835. doi: 10.1038/nbt0991-835. [DOI] [PubMed] [Google Scholar]
- Ebert K.M, DiTullio P, Barry C.A, Schindler J.E, Ayres S.L, Smith T.E, Pellerin L.J, Meade H.M, Denman J, Roberts B. Induction of human tissue plasminogen activator in the mammary gland of transgenic goats. Bio/Technology. 1994;12:699. doi: 10.1038/nbt0794-699. [DOI] [PubMed] [Google Scholar]
- Echelard Y. Recombinant protein production in the milk of transgenic animals. Curr. Opin. Biotechnol. 1996;7:53. doi: 10.1016/s0958-1669(96)80058-9. [DOI] [PubMed] [Google Scholar]
- Gavin W.G, Pollock D, Fell P, Yelton D, Cammuso C, Harrington M, Lewis-Williams J, Midura P, Oliver A, Smith T.E, Wilburn B, Echelard Y, Meade H. Expression of the antibody hBR96 in the milk of transgenic mice and production of hBR96 transgenic goats. Theriogenology. 1997;47:214. [Google Scholar]
- Glaser V. Can ReoPro repolish tarnished monoclonal therapeutic? Nat. Biotechnol. 1996;14:1216. doi: 10.1038/nbt1096-1216b. [DOI] [PubMed] [Google Scholar]
- Graf H, Rabaud J.N, Egly J.M. Ion exchange resins for the purification of monoclonal antibodies from animal cell culture. Bioseparation. 1994;4:7. [PubMed] [Google Scholar]
- Hall S.S. Monoclonal antibodies at age 20: promise at last? Science. 1995;270:915. doi: 10.1126/science.270.5238.915. [DOI] [PubMed] [Google Scholar]
- Hammer R.E, Pursel V.G, Rexroad C.E, Jr., Wall R.J, Bolt D.J, Ebert K.M, Palmiter R.D, Brinster R.L. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Hoogenboom H.R, Raus J.C.M, Volckaert G. Cloning and expression of a chimeric antibody directed against the human transferrin receptor. J. Immunol. 1990;144:3211. [PubMed] [Google Scholar]
- Houdebine L.M. The production of pharmaceutical proteins from the milk of transgenic animals. J. Biotechnol. 1995;34:269. doi: 10.1016/0168-1656(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Houdebine, L.M. (Ed.), 1996. Transgenic Animals — Generation and Use, Harwood Academic Publishers, Amsterdam.
- Hoyle R. Genentech is poised for an anti-cancer breakthrough. Nat. Biotechnol. 1998;16:887. doi: 10.1038/nbt1098-887. [DOI] [PubMed] [Google Scholar]
- Hurwitz D.R, Nathan M, Barash I, Ilan N, Shani M. Specific combinations of human serum albumin introns direct high level expression of albumin in transfected COS cells and in the milk of transgenic mice. Transgenic Res. 1994;3:365. doi: 10.1007/BF01976768. [DOI] [PubMed] [Google Scholar]
- Khazaeli M.B, Conry R.M, LoBuglio A.F. Human immune response to monoclonal antibodies. J. Immunother. 1994;15:42. doi: 10.1097/00002371-199401000-00006. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Korhonen V.P, Tolvanen M, Hyttinen J.M, Uusi-Oukari M, Sinervirta R, Alhonen L, Jauhiainen M, Janne O.A, Janne J. Expression of bovine beta-lactoglobulin/human erythropoietin fusion protein in the milk of transgenic mice and rabbits. Eur. J. Biochem. 1997;245:482. doi: 10.1111/j.1432-1033.1997.00482.x. [DOI] [PubMed] [Google Scholar]
- Larrick J.W, Yu L, Chen J, Jaiswal S, Wycoff K. Production of antibodies in transgenic plants. Res. Immunol. 1998;149:603. doi: 10.1016/s0923-2494(98)80013-8. [DOI] [PubMed] [Google Scholar]
- Limonta J, Pedraza A, Rodriguez A, Freyre F.M, Barral A.M, Castro F.O, Lleonart R, Gracia C.A, Gavilondo J.V, de la Fuente J. Production of active anti-CD6 mouse/human chimeric antibodies in the milk of transgenic mice. Immunotechnology. 1995;1:107. doi: 10.1016/1380-2933(95)00010-0. [DOI] [PubMed] [Google Scholar]
- Maga E.A, Murray J.D. Mammary gland expression of transgenes and the potential for altering the properties of milk. Bio/Technology. 1995;13:1452. doi: 10.1038/nbt1295-1452. [DOI] [PubMed] [Google Scholar]
- Massoud M, Bischoff R, Dalemans W, Pointu H, Attal J, Schultz H, Clesse D, Stinnakre M.G, Pavirani A, Houdebine L.M. Expression of active recombinant human alpha 1-antitrypsin in transgenic rabbits. J. Biotechnol. 1991;18:193. doi: 10.1016/0168-1656(91)90247-s. [DOI] [PubMed] [Google Scholar]
- Meade, H.M., Echelard, Y., Ziomek, C.A., Young, M.W., Harvey, M., Cole, E.S., Groet, S., Smith, T.E., Curling, J.M., 1998. Expression of recombinant proteins in the milk of transgenic animals. In: Fernandez, J.M., Hoeffler, J.P. (Eds.), Gene Expression Systems: Using Nature For The Art of Expression, Academic Press, San Diego.
- Newton, D.L., Pollock, D., DiTullio, P., Echelard, Y., Harvey, M., Wilburn, B., Williams, J., Hoogenboom, H.R., Raus, J.C.M., Meade, H.M., Rybak, S.M. Antitransferrin receptor antibody-RNase fusion protein expressed in the mammary gland of transgenic mice. J. Immunol. Methods, this issue. [DOI] [PubMed]
- Page M, Thorpe R. Purification of monoclonal antibodies. Methods Mol. Biol. 1998;80:113. doi: 10.1007/978-1-59259-257-9_11. [DOI] [PubMed] [Google Scholar]
- Pinkert, C.A. (Ed.), 1994. Transgenic Animal Technology: A Laboratory Handbook. Academic Press, San Diego, CA. [DOI] [PubMed]
- Prior C.P. Large-scale process purification of clinical product from animal cell cultures. Biotechnology. 1991;17:445. doi: 10.1016/b978-0-409-90123-8.50023-9. [DOI] [PubMed] [Google Scholar]
- Ransohoff T.C, Levine H.L. Purification of monoclonal antibodies. Bioproc. Technol. 1991;12:213. [PubMed] [Google Scholar]
- Riego E, Limonta J, Aguilar A, Perez A, de Armas R, Solano R, Ramos B, Castro F.O, de la Fuente J. Production of transgenic mice and rabbits that carry and express the human tissue plasminogen activator cDNA under the control of a bovine alpha s1 casein promoter. Theriogenology. 1993;39:1173. doi: 10.1016/0093-691x(93)90015-w. [DOI] [PubMed] [Google Scholar]
- Roberts B, DiTullio P, Vitale J, Hehir K, Gordon K. Cloning of the goat beta-casein gene and expression in transgenic mice. Gene. 1992;121:255. doi: 10.1016/0378-1119(92)90129-d. [DOI] [PubMed] [Google Scholar]
- Rosok M.J, Yelton D.E, Harris L.J, Bajorath J, Hellstrom K.E, Hellstrom I, Cruz G.A, Kristensson K, Lin H, Huse W.D, Glaser S.M. A combinatorial library strategy for the rapid humanization of anticarcinoma BR96 Fab. J. Biol. Chem. 1996;271:22611. doi: 10.1074/jbc.271.37.22611. [DOI] [PubMed] [Google Scholar]
- Shamay A, Solinas S, Pursel V.G, McKnight R.A, Alexander L, Beattie C, Hennighausen L, Wall R.J. Production of the mouse whey acidic protein in transgenic pigs during lactation. J. Anim. Sci. 1991;69:4552. doi: 10.2527/1991.69114552x. [DOI] [PubMed] [Google Scholar]
- Sherman-Gold R. Monoclonal antibodies: the evolution from '80s magic bullets to mature mainstream applications as clinical therapeutics. Gen. Eng. News. 1997;17:4. [Google Scholar]
- Simons J.P, McClenaghan M, Clark A.J. Alteration of the quality of milk by expression of sheep β-lactoglobulin in transgenic mice. Nature. 1987;328:530. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Sola I, Castilla J, Pintado B, Sanchez-Morgado J.M, Whitelaw C.B, Clark A.J, Enjuanes L. Transgenic mice secreting coronavirus neutralizing antibodies into the milk. J. Virol. 1998;72:3762. doi: 10.1128/jvi.72.5.3762-3772.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromqvist M, Houdebine L.M, Andersson J.O, Edlund A, Johansson T, Viglietta C, Puissant C, Hansson L. Recombinant human extracellular superoxide dismutase produced in milk of transgenic rabbits. Transgenic Res. 1997;6:271. doi: 10.1023/a:1018406611380. [DOI] [PubMed] [Google Scholar]
- Tolcher A.W, Sugarman S, Gelmon K.A, Cohen R, Saleh M, Isaacs C, Young L, Healey D, Onetto N, Slichenmyer W. Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J. Clin. Oncol. 1999;17:478. doi: 10.1200/JCO.1999.17.2.478. [DOI] [PubMed] [Google Scholar]
- Vaughan T.J, Osbourn J.K, Tempest P.R. Human antibodies by design. Nat. Biotechnol. 1998;16:535. doi: 10.1038/nbt0698-535. [DOI] [PubMed] [Google Scholar]
- Verma R, Boleti E, George A.J.T. Antibody engineering: comparison of bacterial yeast, insect and mammalian expression systems. J. Immunol. Methods. 1998;216:165. doi: 10.1016/s0022-1759(98)00077-5. [DOI] [PubMed] [Google Scholar]
- Webber D. Lymphoma Mabs rivalry continues. Nat. Biotechnol. 1998;16:1000. doi: 10.1038/3441. [DOI] [PubMed] [Google Scholar]
- Werner R.G. Innovative and economic potential of mammalian cell culture. Arzneim. Forsch. (Drug Res.) 1998;48:423. [PubMed] [Google Scholar]
- Whitelaw C.B.A, Archibald A.L, Harris S, McClenaghan M, Simons J.P, Clark A.J. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991;1:3. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- Whitelaw C.B.A, Springbett A.J, Webster J, Clark A.J. The majority of G0 transgenic mice are derived from mosaic embryos. Transgenic Res. 1993;2:29. doi: 10.1007/BF01977678. [DOI] [PubMed] [Google Scholar]
- Wilkie T.M, Brinster R.L, Palmiter R.D. Germline mosaicism and somatic in transgenic mice. Dev. Biol. 1986;118:9. doi: 10.1016/0012-1606(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke A.E, McWhir J, Kind A.J, Campbell K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Wineland N.E, Detwiler L.A, Salman M.D. Epidemiologic analysis of reported scrapie in sheep in the United States: 1117 cases (1947–1992) J. Am. Vet. Med. Assoc. 1998;212:713. [PubMed] [Google Scholar]


