Abstract
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease of the central nervous system that is usually considered a monophasic disease. ADEM forms one of several categories of primary inflammatory demyelinating disorders of the central nervous system including multiple sclerosis, optic neuropathy, acute transverse myelitis, and neuromyelitis optica (Devic’s disease). Post-infectious and post-immunisation encephalomyelitis make up about three-quarters of cases, where the timing of a febrile event is associated with the onset of neurological disease. Post-vaccination ADEM has been associated with several vaccines such as rabies, diphtheria–tetanus–polio, smallpox, measles, mumps, rubella, Japanese B encephalitis, pertussis, influenza, hepatitis B, and the Hog vaccine. We review ADEM with particular emphasis on vaccination as the precipitating factor. We performed a literature search using Medline (1976–2007) with search terms including “ADEM”, “acute disseminated encephalomyelitis”, “encephalomyelitis”, “vaccination”, and “immunisation”. A patient presenting with bilateral optic neuropathies within 3 weeks of “inactivated” influenza vaccination followed by delayed onset of ADEM 3 months post-vaccination is described.
Keywords: Influenza vaccination, Optic neuropathy, Acute disseminated encephalomyelitis, Multiphasic disseminated encephalomyelitis, Corticosteroid therapy
1. Introduction
Acute disseminated encephalomyelitis (ADEM) is an inflammatory demyelinating disease of the central nervous system (CNS) that is usually considered a monophasic disease, but a relapsing variant (distinct from multiple sclerosis) is well recognised – multiphasic disseminated encephalomyelitis (MDEM).1 Post-infectious and post-immunisation encephalomyelitis make up about three-quarters of cases, where the timing of a febrile event is associated with the onset of neurological disease.2 Although the two syndromes are distinguished by their precipitant, clinically and pathologically they are very similar. ADEM forms one of several categories of primary inflammatory demyelinating disorders of the CNS. Others include multiple sclerosis (MS), optic neuropathy, acute transverse myelitis, and neuromyelitis optica (Devic’s disease).1
ADEM has an estimated annual incidence of 0.8 per 100,000 with a median age of onset of 6.5 years.2 Although ADEM can occur at any age, it is more common in children. Optic neuropathy and ADEM are rare complications associated with vaccinations.[1], [3], [4], [5] Most case reports describe patients experiencing a unilateral or bilateral optic neuropathy or ADEM, rather than simultaneous onset of both conditions. Optic neuropathy has also recurred in one patient after repeat administration of the influenza vaccination.4 The presumptive mechanism is immune-mediated demyelination although immune-complex mediated vasculopathy has also been postulated.5 Spontaneous recovery or improvement after corticosteroids has been reported for post-vaccination optic neuropathy and ADEM3 although permanent visual loss has also been described.5
We present a review of ADEM with particular emphasis on vaccination as the precipitating factor. We searched the literature using Medline (1976–2007), EMBASE (1980–2007) and PubMed (www.ncbi.nlm.nih.gov/sites/entrez) with the following search terms: “ADEM”, “acute disseminated encephalomyelitis”, “post-infectious encephalomyelitis”, “encephalomyelitis”, “encephalitis”, “multiphasic disseminated encephalomyelitis”, “vaccination”, “immunisation”, “influenza”, and “post-influenza”. We then describe a patient presenting with bilateral optic neuropathies within 3 weeks of an influenza vaccination and delayed onset of ADEM 3 months post-vaccination.
2. Review of the literature
2.1. Case definitions for ADEM
The following case definitions for ADEM have been extracted from Sejvar et al. and the Brighton Collaboration Encephalitis Working Group.6
The case definitions are structured in three different levels of diagnostic certainty.
-
a)
Level 1 of diagnostic certainty
-
i.
Demonstration of diffuse or multifocal areas of demyelination by histopathology.
OR
-
ii.Focal or multifocal findings referable to the central nervous system, including one or more of the following:
-
•Encephalopathy (see case definition for encephalitis for specification of encephalopathy),
-
•Focal cortical signs (including but not limited to: aphasia, alexia, agraphia, cortical blindness),
-
•Cranial nerve abnormality/abnormalities,
-
•Visual field defect/defects,
-
•Presence of primitive reflexes (Babinski’s sign, glabellar reflex, snout/sucking reflex),
-
•Motor weakness (either diffuse or focal; more often focal),
-
•Sensory abnormalities (either positive or negative; sensory level),
-
•Altered deep tendon reflexes (hypo- or hyperreflexia, asymmetry of reflexes), or
-
•Cerebellar dysfunction, including ataxia, dysmetria, cerebellar nystagmus,
-
•
AND
-
iii.
MRI findings displaying diffuse or multifocal white matter lesions on T2-weighted, diffusion-weighted (DWI), or fluid-attenuated inversion recovery (FLAIR) sequences (± gadolinium enhancement on T1 sequences),
AND
-
iv.
Monophasic pattern to illness (absence of relapse within a minimum of 3 months of symptomatic nadir).
b) Level 2 of diagnostic certainty
-
i.
Focal or multifocal findings referable to the central nervous system (as outlined in the Level 1 of diagnostic certainty section),
AND
-
ii.
Magnetic resonance imaging (MRI) findings (as outlined in the Level 1 of diagnostic certainty section),
AND
-
iii.
Insufficient follow-up time achieved to document absence of relapse within a minimum period of 3 months following symptomatic nadir.
c) Level 3 of diagnostic certainty
-
i.
Focal or multifocal findings referable to the central nervous system (as outlined in the Level 1 of diagnostic certainty section),
d) Exclusion criteria for all levels of diagnostic certainty
-
i.
Presence of a clear alternative acute infectious or other diagnosis for illness,
-
ii.
Recurrence or relapse of illness at any point following a 3 month period of clinical improvement from symptomatic nadir, or
-
iii.
If known, MRI findings or histopathologic data inconsistent with the diagnosis of ADEM.
2.2. Post-vaccination encephalomyelitis
The initially termed “neuroparalytic accidents” gained recognition in 1853 after the widespread introduction of Jenner’s smallpox (actually cowpox) vaccine, and in 1885 with Pasteur’s rabies vaccine.1 Post-vaccination ADEM is associated with several vaccines including those for rabies, diphtheria–tetanus–polio, smallpox, measles, mumps, rubella, Japanese B encephalitis, pertussis, influenza, hepatitis B, and the Hog vaccine.[1], [7], [8], [9], [10] For most vaccines, incidence rates are as low as 0.1 to 0.2 per 100,000 vaccinated individuals.2 ADEM following immunisation seems to occur significantly more frequently after primary vaccination as compared to revaccination.11 Post-vaccination encephalomyelitis accounts for less than 5% of present cases of ADEM.1 It should be emphasized to parents, patients, health care providers, and all others concerned with immunization safety, that encephalomyelitis or ADEM – or any other adverse event – that follows administration of an inactivated component or live vaccine may be temporally associated with, but is not necessarily the result of, administration of a vaccine.6 Furthermore, the time interval from immunisation to the onset of the clinical event is not considered part of the definition itself.
Sejvar et al. reviewed the neurological adverse events associated with the smallpox vaccination in the USA between 2002 and 2004. They found 214 such occurrences with only 3 of these suspected of being ADEM,12 giving an estimated rate of 5 cases per million vaccines. Reported rates vary from 1 in 4000 to 1 in 80,000 after primary vaccination, and from 1 in 50,000 to 1 in 450,000 after revaccination.13
The reported incidence of ADEM following the Japanese encephalitis vaccination varies significantly depending on the population.[2], [14], [15] Between 1996 and 1998, the Japanese reported a rate of 0.2 per 100,000 as compared to a more frequent occurrence of 1 in 50,000 to 75,000 in Denmark.15 Meanwhile there were no such events reported in the USA after administering more than 813,000 doses of Japanese encephalitis vaccine between 1993 and 1998.15 In contrast, Menge et al. quoted an incidence rate as high as 1 in 600.2
The association between the influenza vaccination and ADEM has only come to light in the recent years, and hence there have been no large population studies and no estimated incidence rates. A 14-year-old female developed ADEM 2 weeks after an influenza vaccination,16 while 2 adult males, aged 62 and 70, were diagnosed with ADEM and transverse myelitis with acute motor axonal neuropathy respectively within 1 week of vaccination.17 In a review of adverse events associated with intranasal influenza vaccine between 2003 and 2005 in the USA, 10 neurological events were reported of the total 460 adverse events,18 which included 2 people with Guillain-Barre syndrome, 1 with Bell’s palsy, one ADEM, one febrile convulsion, and 3 non-serious reports of dystonic tongue, tongue paraesthesia and ataxia/vertigo. Nakayama and Onoda found 3 cases of ADEM and nine of Guillain-Barre among 38.02 million doses of influenza vaccine administered between 1994 and 2004 by the Kitasato Institute, Japan.19 Giant cell arteritis has also been associated recently with the influenza vaccination.15 All the influenza vaccines available in Australia are either split virion or subunit vaccines prepared from purified inactivated influenza virus cultivated in embryonated hens’ eggs. The safety and efficacy of split virion and subunit vaccines are generally considered to be equivalent.20
Initially, post-vaccination ADEM was thought to be caused by the vaccine’s viral component but it was later recognised that it could also be related to contamination with CNS tissue in which the vaccine was propagated.[1], [2] For example, the anti-rabies vaccine had been cultured from rabbit, sheep or goat brain, and the Japanese encephalitis vaccine from murine brain.[2], [21] This theory has been highlighted by a significant drop in post-vaccination ADEM incidence rates after the development of vaccines based on recombinant proteins rather than from in-vivo infected tissue.[1], [2] Results from experimental allergic encephalomyelitis (EAE) also support this concept. In EAE, a disease that clinically and pathologically resembles ADEM, inflammation is produced when an experimental animal is inoculated with myelin or myelin antigens.1 High-affinity antibodies directed against myelin-basic protein (MBP) have been identified in ADEM patients vaccinated with Semple strain rabies, but not MS patients.2 However, this does not explain the origin and cause of post-vaccination ADEM due to smallpox and several other vaccines that are prepared without the involvement of neural tissues.1
The most common vaccinations associated with ADEM are the non-neural measles, mumps and rubella vaccines. The incidence of 1–2 per million for live measles vaccine is less than the reported 1 in 1000 incidence of post-infectious ADEM following infection with the measles virus itself.1 Arguably, with both the virus and the vaccine as causes, vaccination dramatically reduces the incidence of ADEM.
2.3. Post-infectious encephalomyelitis
Post-infectious ADEM is associated with a preceding or concomitant infection that is most commonly viral. Measles, mumps, rubella, varicella-zoster, Epstein-Barr virus (EBV), cytomegalovirus (CMV), herpes simplex virus, hepatitis A or B, Coxsackie virus, influenza A or B, human immunodeficiency virus (HIV), human T-cell lymphotropic virus-1 (HTLV-1), human herpes virus 6, vaccinia, Rocky Mountain spotted fever, and human corona virus have been implicated as causing post-infectious ADEM.[1], [7], [22] Bacterial infections are also associated with post-infectious forms of ADEM, most commonly Mycoplasma pneumoniae. 7 Other culprit pathogens include Borrelia, Campylobacter, Leptospira, Chlamydia, Legionella, and group A beta-haemolytic streptococci.[1], [7]
2.4. Other causes of ADEM
Reports of ADEM following solid organ transplantation are rare, and include one in which EBV was identified as the pathogen.[22], [23] It is unclear, however, whether the overall incidence of ADEM is higher in recipients of organ transplantation than in the general population.
ADEM has also been described as a paraneoplastic disorder in some cases of leukaemia and non-Hodgkin’s lymphoma.22
2.5. Multiphasic disseminated encephalomyelitis
Recurrent ADEM where episodes differ clinically is termed multiphasic disseminated encephalomyelitis (MDEM). In some cases of ADEM, the premature cessation or tapering of therapy may lead to symptom recurrence. Hence, the monophasic nature of ADEM is defined as a lack of recurrence (within 3 months) in the absence of treatment or while on appropriate treatment; and relapse that occurs during cessation or tapering of treatment should be considered as belonging to one monophasic episode.6 The following criteria apply when differentiating MS and MDEM:24
-
a)
Altered mental state, relapses <5 months apart, rapidly evolving deficits and swift, complete recovery favour MDEM. Diplopia and asymmetrical deficits mainly resemble MS.
-
b)
The number, morphology and distribution of lesions on MRI, with lesions >1 cm or involving the cortical ribbon or thalamus, or located infratentorially, and the later disappearance of T2 abnormalities, being distinctive of ADEM. The subsequent development of new lesions on MRI is quite typical of MS.
-
c)
Marked cerebrospinal fluid (CSF) pleocytosis and a normal IgG index are typical for ADEM and would be highly unusual in MS.
-
d)
Bilateral prolonged visual evoked potentials (VEPs) with no history of optic neuritis occurs commonly in MS, but rarely in ADEM.
2.6. Pathogenesis of ADEM
2.6.1. Molecular mimicry
Antigenic epitopes, comprising of delicate structural or partial amino-acid sequence homologies, are shared between an inoculated pathogen or vaccine, and a host CNS protein.2 As a result, the pathogen is not recognised as “foreign” for elimination, nor “self” for immune tolerance. At the inoculation site the pathogen is initially processed by T cell activation and cross activation of antigen-specific B cells. These autoreactive cells can enter the CNS during immune surveillance and by chance, may encounter the homologous myelin protein. The local reactivation by antigen presenting cells subsequently culminates in a destructive autoimmune process in the CNS.2 Much research has focused on T cell mediated autoimmune response to myelin autoantigens, such as MBP, proteolipid protein and myelin oligodendrocyte glycoprotein, which can induce ADEM.23 Some studies have suggested a role for B cells and antibodies to gangliosides such as GM1 and GD1a, while others have identified T helper 2 cells reactive to MBP, which were found in the peripheral blood of ADEM patients.23
2.6.2. The re-infectious aetiology
The re-infectious aetiology theory postulates that CNS demyelination occurs as a possible result of direct neurotoxicity of a neurotropic virus, and that vaccination with an attenuated virus strain may cause problems only if administered during a preceding infection, in which previously primed virus-specific cytotoxic T cells are reactivated.2
2.6.3. The post-infectious aetiology
The disruption of the blood-brain barrier sustained after direct CNS infection with a neurotropic virus may subsequently result in the leakage of CNS autoantigens into the systemic circulation. These autoantigens are then processed in the systemic lymphatic organs leading to a breakdown in tolerance and emergence of a self-reactive and encephalitogenic T cell response.2 Studies on patients who developed ADEM following anti-rabies vaccination suggest that MBP may be encephalitogenic in this scenario.7
2.6.4. Immuno-inflammatory model
The immuno-inflammatory model combines the concept of molecular mimicry with the inflammatory cascade process.2 A “first hit” is experienced after an antecedent infection with a virus that expresses determinants allowing molecular mimicry. This need not be clinically eventful or significant. A second infection with an unrelated virus results in sufficient reactivation of the primed autoreactive T cells to eventuate in demyelination of the CNS. This constitutes the “second hit”.
2.6.5. Genetic predisposition
Berkovic et al. noticed that many of the children who experienced vaccination-induced encephalopathy had a similar clinical course to that seen in severe myoclonic epilepsy in infancy (SMEI), which is an epileptic encephalopathy associated with prolonged febrile seizures, intractable myoclonus and other seizures and psychomotor decline.[25], [26] The authors identified mutations in the SCN1A gene in 11 of 14 patients with suspected vaccine encephalopathy. This leads to the question of whether the SCN1A mutation was a predisposing factor waiting to be triggered by fever or other stresses. More than 50% of SMEI patients experienced their first seizure after DPT vaccination.25 Similarly, patients who have a certain underlying genetic predisposition may be more prone to developing ADEM post-vaccination.
2.7. Pathology
There is a distinct histopathological pattern of ADEM lesions of perivenous inflammation surrounding small vessels in both the CNS grey and white matter.2 Most of these lesions seem of similar age, and are infiltrated by lymphocytes, macrophages and to a lesser degree, neutrophils. Perivascular oedema, endothelial swelling and vascular endothelial infiltrations are additional findings. Demyelination may not be evident in hyperacute or acute lesions, but may develop later in the lesion evolution in a pathognomonic “sleeve like” fashion where it is confined to the hypercellular areas. There is only a small degree of axonal damage.
2.8. Clinical features
Depending on the inciting agent, the onset of symptoms may vary slightly: from 1 to 14 days with non-neural vaccines, less than 1 week after the appearance of a rash in exanthematous illnesses, and 1 to 3 weeks after rabies vaccination.1 Symptom onset is usually rapid with progression over hours to a peak in days, and the neurological features vary with both focal and non-focal elements. No pathognomonic clinical features are seen in ADEM, but a combination of altered consciousness or behaviour and multifocal neurological deficits especially if closely timed to an infection or immunisation, should alert the clinician to its possible diagnosis.2 Certain series show that spinal cord dysfunction is more prevalent in adults than in children.27 Seizures are seen more in post-infectious disease but are also associated with vaccination.28 Encephalopathy, fever, seizures, and meningism are very rare in MS, which is one of the most important differential diagnoses.[1], [29]
Focal presentation in ADEM is rather heterogeneous and depends upon the location and the degree of demyelination within the CNS. Multifocal presentations may be combinations of pyramidal and cerebellar signs, which are common, as are cranial neuropathies, which include bilateral optic neuropathies (more common in ADEM than in MS).1 About 25% of patients present with a paraparesis or quadriparesis with urinary dysfunction when myelitis is predominant. Interestingly, in these cases the peripheral nervous system is also involved more frequently in the post-infectious form30 but it has also been reported after vaccination, especially following rabies vaccination, with radicular complaints being the most frequent manifestation.1
2.9. Investigations
In general, the diagnosis of post-vaccination ADEM is made on clinical grounds with the guidance of MRI after the exclusion of an acute infective condition by lumbar puncture and other microbiological and serological tests.
2.9.1. Cerebrospinous fluid
Lumbar puncture typically reveals a lymphocytic pleocytosis and raised protein levels both greater than those seen in MS.1 A CSF oligoclonal band is less common in ADEM than in MS and when present (in 0–58% of cases reported), is usually transient, indicating that a disease-causing antigen is only transiently expressed within or outside the CNS.2
2.9.2. Radiology
CT scans of the brain in ADEM can be normal but when abnormal, usually shows non-specific, low attenuation subcortical white matter lesions that may or may not enhance. In cases of acute haemorrhagic encephalomyelitis, CT scans may reveal haemorrhage and oedema associated structural changes.1
MRI is considered the imaging modality of choice. It can be normal at initial presentation and delays between 5 and 14 days from symptom onset to MRI abnormalities may occur.[1], [2] Cerebral lesions are usually disseminated but solitary lesions occur in about 10% to 30% of cases.31 Lesion patterns often seen in ADEM include widespread, multifocal or extensive white matter lesions and lesions in the deep grey matter (the thalamus and basal ganglia) with the lesion load greater than 50% of the total white matter volume.2 Although there is no pathognomic MRI appearance for ADEM, in one study cortical involvement or lesions in the basal ganglia were present exclusively in patients with ADEM as compared to MS.29 Certain authors suggested that the diagnostic hallmark of ADEM is the demonstration of scattered, focal or multifocal (disseminated) areas of inflammation and demyelination within cerebral subcortical and deep cortical white matter, while grey matter involvement is also seen (particularly in the thalamus).6 It was previously claimed that, all lesions should enhance equally following gadolinium contrast since all lesions should be active and of the same age. Newer studies, however, shown that lesions in ADEM may evolve over several weeks and consequently only some lesions may be enhanced, or there may also be no enhancement.29 Follow-up MRI scans after a minimum interval of 6 months is recommended to establish or confirm the diagnosis of ADEM at which time there should be a resolution, partial or complete, of old lesions and no new lesions.[1], [2] The appearance of new lesions is strongly suggestive of MS.
2.10. Treatment
There is a lack of controlled clinical trials and no proven standard treatment for post-vaccination and other causes of ADEM. Most treatment options are based on empirical and observational evidence. Once ADEM is diagnosed and an acute infectious aetiology excluded, treatment should be instituted as soon as possible. Present treatments are centred on immunosuppression and immunomodulation. The options include corticosteroids, plasma exchange, and intravenous immunoglobulin (IVIg).
2.10.1. Corticosteroids
Corticosteroid therapy is widely accepted as first line therapy for ADEM. The recommended treatment regime is intravenous methylprednisolone 1g daily with a cumulative dose of 3g to 5g followed by a 1 month to 2 month oral prednisolone taper. If there are relapses (as seen in MDEM), they frequently occur shortly after ADEM.[1], [2]
2.10.2. Plasma exchange
Plasma exchange is recommended in patients who respond poorly to intravenous corticosteroids. The usual course involves 7 exchanges over 14 days with improvements frequently seen after the first plasma exchange.1 Plasma exchange is used because serum antibodies directed against MBP and galactocerebroside are found in patients with post-rabies inoculation ADEM, as well as intrathecal synthesis of these antibodies.1
2.10.3. Intravenous immunoglobulin
IVIg is reserved for ADEM that fails to respond to corticosteroid treatment and where plasma exchange is contraindicated or difficult to access. IVIg may be preferred to plasma exchange in cases of post-vaccination encephalomyelitis.1 The use of IVIg has proven effective with particular subgroups of patients showing both CNS and peripheral nervous system (PNS) involvement and some authors have proposed that in patients with evidence of polyradiculopathy, IVIg should be considered as first line therapy.27
2.10.4. Others
With failure of the above treatment modalities, several other therapies have been tried and used with anecdotal success. These include intravenous cyclophosphamide and mitoxantrone.[1], [2] Miravalle and Roos discussed the administration of antivaccinia gamma globulin at the time of smallpox vaccination to prevent the complication of post-vaccination ADEM, but it was not effective once the complication had already occurred.32 It is also advisable to avoid immunisation for at least 6 months after the diagnosis of ADEM as relapse into MDEM has occurred following routine vaccinations.1
2.11. Prognosis
Full recovery, and in some cases spontaneous improvement, is the expected outcome in most cases and is seen in about 50% to 75% with a higher proportion (between 70% and 90%) if minor residual deficits are considered.[1], [2] The average time to recovery ranges from 1 to 6 months.1 Suggested predictors of poor outcome include older age, female gender, degree of functional impairment at clinical onset, CSF protein level, spinal cord involvement, PNS damage, and poor response to corticosteroids.[2], [27] Functional impairment following ADEM may be assessed by the Scripps neurological rating scale (SNRS), which uses a standardised neurological examination of mentation and mood, visual acuity, eye movements and lower cranial nerves, motor and sensory function, reflexes and Babinski sign, gait and cerebellar function and bladder and bowel function (SNRS score range –10 to 100; the higher the score the better the level of function).33 In a study of 60 patients with post-infectious inflammatory disorders, Marchioni et al.27 showed that a mean onset SNRS of 60.3 was associated with a favourable prognosis while a mean SNS of 37.4 was associated with a poorer outcome. The same study also demonstrated that lower mean CSF albumin and IgG concentrations of 68.3 mg/dL and 8.1 mg/dL, respectively, were associated with a favourable prognosis whereas mean concentrations of 106.5 mg/dL and 19.1 mg/dL, respectively, were associated with a poor outcome. In a population of 40 adult ADEM patients studied by Schwarz et al., 35% developed clinically definite MS over a mean observational period of 38 months.29 However, preceding infection or vaccination was not a prerequisite in their initial diagnoses of ADEM.
3. Illustrative case report
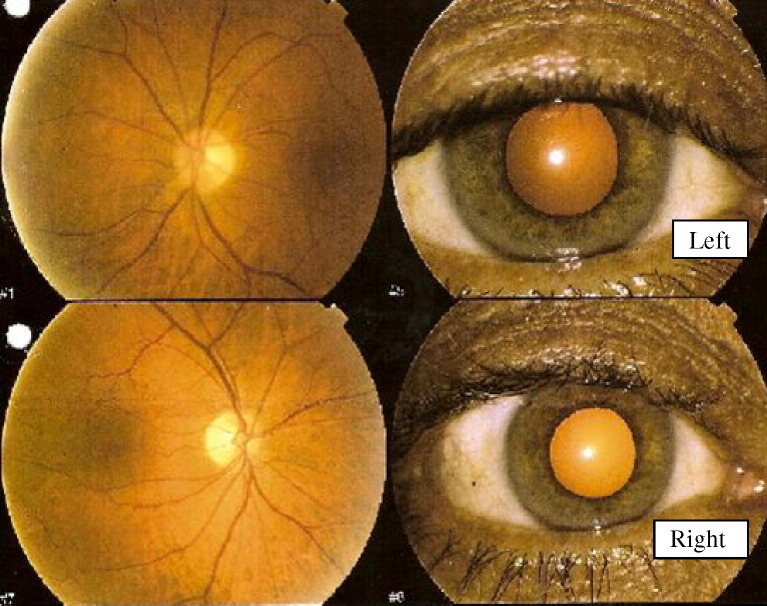
A 61-year-old male presented in early July 2005 with a 3-week history of bilateral visual blurring, worse in the right eye, and bilateral pain on eye movement. He had received the inactivated influenza vaccine (Fluvax) 3 weeks prior to symptom onset. His past medical history was unremarkable and he was not receiving regular medication. On examination, his visual acuity was 6/15 in the right eye and 6/6 in the left eye. He demonstrated an afferent pupillary defect on the right. Fundoscopy revealed pallor of both optic discs, more so on the right (Fig. 1 ). The remainder of his neurological and general medical examination was otherwise unremarkable.
Fig. 1.
Images of the patient’s optic nerves (see Illustrative case report) taken about 4 weeks after his influenza vaccination showing bilateral optic atrophy, more marked in the right eye (lower image).
He underwent an initial brain MRI, which was within normal limits. His visual evoked potentials were significantly prolonged at 140 msec bilaterally (N < 110 msec). A clinical diagnosis of bilateral optic neuropathies complicating influenza vaccination was made. No specific therapy was instituted. Over the next 2 months, his visual symptoms persisted although he was still able to drive and attend work.
In late September 2005, he presented with a 1 week history of increasing daytime somnolence, fluctuating alertness and orientation consistent with delirium. His clinical examination demonstrated hypersomnolence and mild disorientation to time and place. His visual acuity was 6/18 in both eyes. The rest of his neurological examination was normal. Investigations revealed normal full blood count (FBC), erythrocyte sedimentation rate (ESR) and biochemistry. A CT brain scan was normal. An initial lumbar puncture found a CSF protein concentration of 0.71 g/L (Normal < 0.40) and glucose of 3.1 mmol/L (Normal = 2.4–5.4) with 24 × 106 white blood cells per μL (87% mononuclears). CSF cell surface markers were negative for clonal T cells. Herpes simplex and tuberculosis polymerase chain reaction (PCR) and cryptococcal antigen were negative.
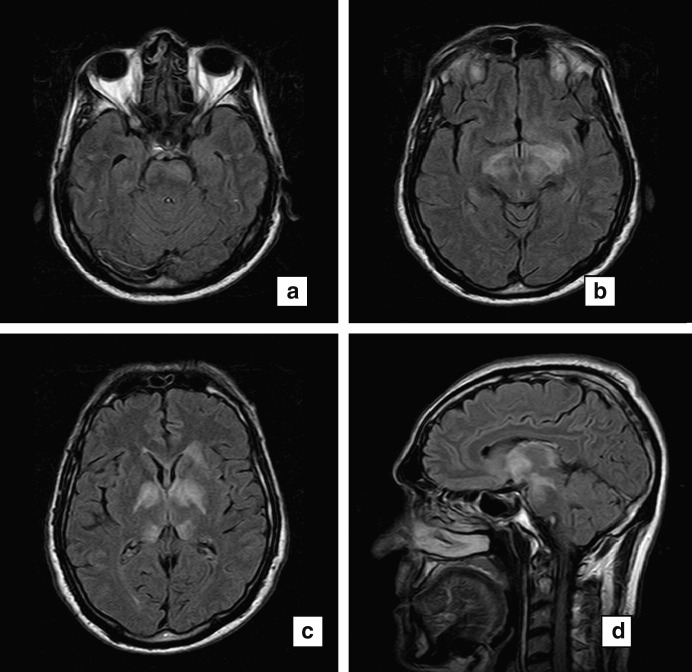
An MRI brain scan revealed fairly symmetric signal abnormality involving the central grey matter predominantly (Fig. 2 a–d). Signal change extended rostrally to the frontal periventricular white matter and caudally to the left pons. There was no gadolinium contrast enhancement and fat-saturated T1-weighted sequences of the orbits showed no abnormal enhancement. Further investigations including vitamin B12 and folate levels, antinuclear antibodies, antineutrophil cytoplasmic antibodies (ANCA), serum angiotensin-converting enzyme, serum electrophoresis, HIV serology, IgM serology for EBV, CMV and Australian encephalitis, mycoplasma serology, Venereal Disease Research Laboratory test (VDRL), antineuronal antibodies and CT scans of the chest, abdomen and pelvis were normal. The patient was diagnosed with ADEM and treated with high dose intravenous steroids consisting of 1g methylprednisolone daily for 5 days followed by oral tapering steroids. His orientation and alertness returned to normal within 2 weeks. On follow-up review 2 months later, he had fully recovered apart from visual acuity of 6/12 bilaterally and bilateral optic disc pallor. A repeat MRI of the brain was significantly improved. He remained clinically well at his last visit in March 2006.
Fig. 2.
MRI (a–c) Axial fluid attenuated inversion recovery (FLAIR) images and (d) sagittal FLAIR images performed in October 2005 about 3 months post-influenza vaccination showing involvement of central grey matter including (a) the left pons, (b) centromedial thalami, and (c) right and left globus pallidus.
The patient’s clinical presentation was most likely due to post-influenza vaccination optic neuritis and encephalomyelitis. A patient with a similar biphasic presentation followed an anti-rabies vaccination34 has been reported in a 45-year-old male who presented with transverse myelitis 14 days after anti-rabies vaccination and developed bilateral optic neuritis 1 month later.
References
- 1.Bennetto L., Scolding N. Inflammatory/post-infectious encephalomyelitis. J Neurol Neurosurg Psychiatry. 2004;75(Suppl. 1):i22–i28. doi: 10.1136/jnnp.2003.034256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menge T., Kiesseier B.C., Nessler S. Acute disseminated encephalomyelitis: an acute hit against the brain. Curr Opin Neurol. 2007;20:247–254. doi: 10.1097/WCO.0b013e3280f31b45. [DOI] [PubMed] [Google Scholar]
- 3.Ray C.L., Dreizin I.J. Bilateral optic neuropathy associated with influenza vaccination. J Neuroophthalmol. 1996;16:182–184. [PubMed] [Google Scholar]
- 4.Hull T.P., Bates J.H. Optic neuritis after influenza vaccination. Am J Ophthalmol. 1997;124:703–704. doi: 10.1016/s0002-9394(14)70918-3. [DOI] [PubMed] [Google Scholar]
- 5.Kawasaki A., Purvin V.A., Tang R. Bilateral anterior ischemic optic neuropathy following influenza vaccination. J Neuroophthalmol. 1998;18:56–59. [PubMed] [Google Scholar]
- 6.Sejvar J.J., Kohl K.S., Bilynsky R. Encephalitis, myelitis, and acute disseminated encephalomyelitis (ADEM): case definitions and guidelines for collection, analysis, and presentation of immunisation safety data. Vaccine. 2007;25:5771–5792. doi: 10.1016/j.vaccine.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 7.Murthy J.M. Acute disseminated encephalomyelitis. Neurol India. 2002;50:238–243. [PubMed] [Google Scholar]
- 8.Koplan J.P., Schoenbaum S.C., Weinstein M.C. Pertussis vaccine – an analysis of benefits, risks and costs. N Engl J Med. 1979;301:906–911. doi: 10.1056/NEJM197910253011703. [DOI] [PubMed] [Google Scholar]
- 9.Dodick D.W., Silber M.H., Noseworthy J.H. Acute disseminated encephalomyelitis after accidental injection of a hog vaccine: successful treatment with plasmapheresis. Mayo Clin Proc. 1998;73:1193–1195. doi: 10.4065/73.12.1193. [DOI] [PubMed] [Google Scholar]
- 10.Tourbah A., Gout O., Liblau R. Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS? Neurology. 1999;53:396–401. doi: 10.1212/wnl.53.2.396. [DOI] [PubMed] [Google Scholar]
- 11.Booss J., Davis L.E. Smallpox and smallpox vaccination: neurological implications. Neurology. 2003;60:1241–1245. doi: 10.1212/01.wnl.0000063319.64515.6b. [DOI] [PubMed] [Google Scholar]
- 12.Sejvar J.J., Labutta R.J., Chapman L.E. Neurologic adverse events associated with smallpox vaccination in the United States, 2002–2004. JAMA. 2005;294:2744–2750. doi: 10.1001/jama.294.21.2744. [DOI] [PubMed] [Google Scholar]
- 13.Johnson R.T. Smallpox: the threat of bioterrorism and the risk of the vaccine. Neurology. 2003;60:1228–1229. doi: 10.1212/wnl.60.8.1228. [DOI] [PubMed] [Google Scholar]
- 14.Plesner A., Arlien-Soborg P., Herning M. Neurological complications and Japanese encephalitis vaccination. Lancet. 1996;348:202–203. doi: 10.1016/s0140-6736(05)66156-9. [DOI] [PubMed] [Google Scholar]
- 15.Piyasirisilp S., Hemachudha T. Neurological adverse events associated with vaccination. Curr Opin Neurol. 2002;15:333–338. doi: 10.1097/00019052-200206000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Cheong J.H., Bak K.H., Kim C.H. Acute disseminated encephalomyelitis associated with influenza vaccination. J Korean Neurosurg Soc. 2004;35:223–225. [Google Scholar]
- 17.Nakamura N., Nokura K., Zettsu T. Neurologic complications associated with influenza vaccination: two adult cases. Intern Med. 2003;42:191–194. doi: 10.2169/internalmedicine.42.191. [DOI] [PubMed] [Google Scholar]
- 18.Izurieta H.S., Haber P., Wise R.P. Adverse events reported following live, cold adapted, intranasal influenza vaccine. JAMA. 2005;294:2720–2725. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T., Onoda K. Vaccine adverse events reported in post-marketing study of the Kitasato Institute from 1994 to 2004. Vaccine. 2007;25:570–576. doi: 10.1016/j.vaccine.2006.05.130. [DOI] [PubMed] [Google Scholar]
- 20.Immunisation Working Party of the NHMRC. The Australian Immunisation Handbook. 9th ed. Canberra: Australian Government Publishing Service, 2007:162.
- 21.Gupta V., Bandyopadhyay S., Bapuraj J.R. A. Bilateral optic neuritis complicating rabies vaccination. Retina. 2004;24:179–181. doi: 10.1097/00006982-200402000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Madan S., Aneja S., Tripathi R.P. Acute disseminated encephalitis – a case series. Indian Pediatr. 2005;42:367–371. [PubMed] [Google Scholar]
- 23.Stuv O., Zamvil S.S. Pathogenesis, diagnosis, and treatment of acute disseminated encephalomyelitis. Curr Opin Neurol. 1999;12:395–401. doi: 10.1097/00019052-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Bonev V.I., Myburgh C.J., Gledhill R.F. Relapsing-remitting demyelinating illness: multiple sclerosis, multiphasic disseminated encephalomyelitis, or an intermediate entity? Eur J Neurol. 2002;9:177–185. doi: 10.1046/j.1468-1331.2002.0354a.x. [DOI] [PubMed] [Google Scholar]
- 25.Sell E., Minassian B.A. Demystifying vaccination-associated encephalopathy. Lancet Neurol. 2006;5:465–466. doi: 10.1016/S1474-4422(06)70452-5. [DOI] [PubMed] [Google Scholar]
- 26.Berkovic S.F., Harkin L., McMahon J.M. De-novo mutations of the sodium channel gene SCN1a in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 27.Marchioni E., Ravaglia S., Piccolo G. Postinfectious inflammatory disorders: subgroups based on prospective follow-up. Neurology. 2005;65:1057–1065. doi: 10.1212/01.wnl.0000179302.93960.ad. [DOI] [PubMed] [Google Scholar]
- 28.Hamidon B.B., Raymond A.A. Acute disseminated encephalomyelitis (ADEM) presenting with seizures secondary to anti-tetanus toxin vaccination. Med J Malaysia. 2003;58:780–782. [PubMed] [Google Scholar]
- 29.Schwarz S., Mohr A., Knauth M. Acute disseminated encephalomyelitis: a follow-up study of 40 patients. Neurology. 2001;56:1313–1318. doi: 10.1212/wnl.56.10.1313. [DOI] [PubMed] [Google Scholar]
- 30.Kashyap R., Jaret P., Mahajan S. Post-vaccinal encephalitis after semple type anti-rabies vaccine. JIACM. 2004;5:281–283. [Google Scholar]
- 31.Harloff A., Rauer S., Hofer M. Fulminant acute disseminated encephalomyelitis mimicking acute bacterial meningoencephalitis. Eur J Neurol. 2005;12:67–69. doi: 10.1111/j.1468-1331.2004.01015.x. [DOI] [PubMed] [Google Scholar]
- 32.Miravelle A., Roos K. Encephalitis complicating smallpox vaccination. Arch Neurol. 2003;60:925–928. doi: 10.1001/archneur.60.7.925. [DOI] [PubMed] [Google Scholar]
- 33.Sipe J.C., Knobler R.L., Braheny S.L. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984;34:1368–1372. doi: 10.1212/wnl.34.10.1368. [DOI] [PubMed] [Google Scholar]
- 34.Kulkarni V., Nadgir D., Tapiawala S. Biphasic demyelination of the nervous system following anti-rabies vaccination. Neurol India. 2004;52:106–108. [PubMed] [Google Scholar]