Abstract
Processing methods used for banking of skin for subsequent therapeutic use depend on whether the skin is to retain viability or not. For viable skin grafts, sterilisation techniques cannot be applied, however antibiotics and antimycotics may be used to disinfect the tissue with respect to bacteria and fungi. Nevertheless, strict standards are applied to avoid disease transmission from donor to recipient involving donor medical history, donor testing for viral diseases, aseptic retrieval and processing, and control of storage temperature. Cryopreservation is the preferred method for long term storage of viable skin grafts.
If viability is not required, then additional long term preservation methods may be used including deep-freezing, freeze-drying or high concentration solute preservation. All three methods work by reducing water activity.
In addition it is possible to apply certain sterilisation techiques that have been shown not to damage the tissue. It is important that sterilisation methods are validated in accordance with precise definitions of sterilisation, and for the initial levels of “bioburden” expected to be present immediately prior to application of the sterilisation method.
The application of improved and refined methodologies in accordance with defined standards has ensured improved graft performance while reducing risk to the recipient.
Introduction
Allogenic skin may be banked as viable or as nonviable grafts. This choice critically affects the way in which the skin is processed and preserved.
Viable skin allografts
Viable skin allografts may be obtained from living donors or, more frequently, from cadaveric donors. In the latter case, immediately after circulatory arrest, the skin becomes ischemic. This leads to progressive cell death over a period of days, which is accelerated at higher temperatures.1 Even if the cadaver is immediately placed into a refrigerator, the body cools at a slow rate, leading to at least some warm ischemia time. Nevertheless, viable skin can still be retrieved up to circa 24 hours after death. If the body is not quickly refrigerated, however, the skin will be subjected to a much longer warm ischemia period resulting in an accelerated deterioration in viability. Once the skin is removed from the body, however, it can be cooled rapidly by immersion into a refrigerated solution, thus immediately reducing the rate of cell death.
Reducing the risk of disease transmission
One of the major concerns with the use of skin allografts is the risk of disease transmission. A case involving the transmission of HIV from a skin donor to a recipient has been reported.2 Apart from viral diseases, there is also the possibility of transferring pathogenic bacteria or fungi and parasites on or within the skin allograft. There is now a reasonable consensus around the world as to the methods used to minimize the risks of disease transmission. Detailed standards and guidelines are issued by national organizations/associations such as the National Blood Service,3 British Association for Tissue Banking,4 and multinational organizations, for example, European Association of Tissue Banks.5 It is important that local standards be consulted because certain diseases may be confined to, or novel diseases arise in, certain parts of the world. The recent spread of West Nile virus in the United States and severe acute respiratory syndrome in China are cases in point. A brief summary of the generic approach is given below.
Screening of donor medical/behavioral history
Medical records held by hospitals, family doctors, and others must be screened for conditions that would exclude donation. This may include current systemic infection, diseases of unknown etiology, and previous infections with the possibility of current carrier status (hepatitis, malaria, etc). It may also be prudent to exclude a history of malignant disease particularly if the skin might be used on immunosuppressed patients, for example, those suffering massive burn injuries. Behavioral history may also be grounds for rejection of the donor owing to the high correlations between activities such as intravenous drug abuse and viral infections such as HIV and hepatitis.
Reducing the risk of hematogenous spread of bacteria
Tissue banking standards generally set limits on the maximum warm and cold ischemia times permitted after the death of the donor, because it is known that bacteria from the gut can be released into the vascular system postmortem and migrate to the internal organs and tissues.
Reducing the population of bacteria residing on the skin
Skin tissue possesses a mixed commensal population of bacteria and fungi that lives primarily in or around the hair and sebaceous follicles.6, 7 With the exception of Staphylococcus aureus, most of these are relatively nonpathogenic. In addition, transient populations of contaminating bacteria, however, may also be naturally present, or may result from contamination during the skin retrieval process. Even the commensal bacteria may damage the skin if subsequently allowed to grow during the skin storage period to reach massive population densities. Therefore, it is important to try to eliminate or minimize the microbial population of skin before retrieval. This is generally achieved using skin prepping techniques similar to those used on the skin of patients undergoing operations in hospital. When banking skin in a viable state, it is important to validate that the prepping agents have been adequately removed before harvesting the skin, and that any residuals are not toxic to the skin cells.
Although effective in reducing the bioburden, skin prepping is unlikely to totally eliminate bacteria. It is therefore essential to prevent proliferation of any residual bacteria after retrieval, during transportation, and during subsequent processing. Maintaining the skin at refrigerator or wet ice temperatures prevents the proliferation of most bacteria.
To eliminate residual bacteria and fungi in viable skin, an antibiotic/antimycotic cocktail may be used. If used at reduced temperatures (4°C), the skin may be incubated for up to 24 hours in the cocktail; however, only antibiotics that are effective at this temperature should be included. At normothermic temperature (37°C), most antibiotics can be considered; however, the exposure period should be minimized.
Refrigerator storage
Even if the skin is placed into an oxygenated nutrient-rich medium, ischemic necrosis of the tissue still occurs because the diffusion path from the tissue periphery to the central cells is extensive. Therefore, oxygen and nutrients cannot diffuse fast enough to supply the cells, and toxic metabolites cannot be removed quickly enough. This results in a central necrosis that radiates out to eventually include all of the tissue. Reducing the temperature reduces the metabolic rate of the cells and hence the nutritional demands and metabolite production. Therefore, one method that has been explored for the viable storage of skin is the use of refrigerator temperatures with or without nutrient media.
As early as 1903, Wentscher8 reported the successful storage and grafting of skin autografts at temperatures near 0°C for 14 days. Further studies by Carrel9, 10, 11 using normothermic and hypothermic storage conditions confirmed the utility of the latter. Eventually, refrigerator storage for skin autografts became the norm in burn units. The simplest technique was to fold the skin so that the cut surfaces were in apposition and then wrap the skin sandwich in tulle gras and/or saline gauze to prevent desiccation.12, 13 Using this method, autologous skin can be used after around 2 weeks of storage, although cell viability is very low at this point. Unlike autograft, allograft has already lost some viability by the time it is retrieved; therefore, maximum storage times using this method are reduced to 7 or 8 days.14 Because extensive microbiology screening, however, is required, together with scrutiny of the medical records, postmortem report, and so on, this short storage period is insufficient.
Many attempts have been made to extend the refrigerator storage period for skin. Addition of homologous serum at 10% to 33% was found to be beneficial because it provided nutrients and diluted and buffered acids produced as by-products of metabolism.15, 16 Alternatively, tissue culture media as a source of nutrients and various buffering systems have been evaluated.17, 18, 19, 20, 21 These, however, only extend slightly the period of useful storage.
Therefore, although refrigerator storage of skin has proved to be a useful method for the temporary storage of viable autografts, it is not currently suitable for allografts.
Cryopreservation
A better method for the long-term preservation of skin grafts is cryopreservation. This technique facilitates the cooling of tissues to ultralow temperatures while protecting the viability of the cells. For any cell type, there is an optimum cooling rate that produces maximal cell survival. To either side of this optimum, the survival falls away (Fig. 1 ). At cooling rates faster than the optimum, intracellular ice appears, causing the cell to die.22 In contrast, when cooling is slower than the optimum, the cells spend longer time exposed to very high salt concentrations that build up as the water is removed from solution to form extracellular ice crystals. The cells also shrink because of osmosis.22
Fig. 1.
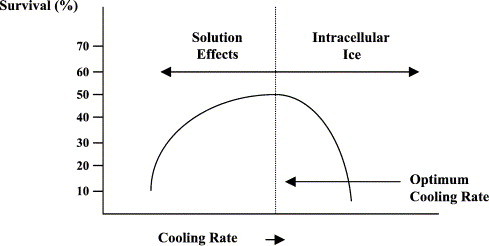
The effect of cooling rate on the survival of cells.
When there are different types of cells within a tissue, it is unlikely that each cell type will exhibit the same optimum cooling rate. Therefore, maintaining high viability for all of the cells would be impossible. This can be achieved, however, if cryoprotective chemicals are added to the cells before freezing.
Several cryoprotective chemicals have been identified since the chance discovery by Polge et al23 that glycerol was able to protect sperm cells from cryogenic damage. The general properties of cryoprotectants are that the molecules can pass through the cell membrane into the cell and be relatively nontoxic at very high multimolar concentrations. The commonest cryoprotective agents (CPAs) are glycerol and dimethyl sulfoxide.24 Cryoprotective agents are able to prevent solution effect injury. Their mode of action is probably 2-fold. First, they act as solvents for the salt, thus reducing the salt concentration that the cells are subjected to at the high subzero temperatures (where solution effects are most damaging). In addition, their presence within the cells prevents excessive shrinkage of the cells during this cooling phase.25 Therefore, in the presence of CPAs, it is possible to use very slow cooling rates that minimize intracellular ice formation while protecting the cells against solution effects. High viabilities of all cell types can thus be achieved using this slow cooling rate. For many tissues, the optimum cooling rate is around −1°C per minute when CPAs are used.
Although CPAs are relatively nontoxic at low temperatures, the toxicity can become significant at higher temperatures. In addition, the rate at which CPAs enter the cells is temperature and CPA dependent, being faster at higher temperatures. Therefore, the optimum temperature and the exposure time need to be validated.
The basal medium into which the CPA is dissolved for incubation of the skin tissue should be a balanced salt solution, and many of the commercial tissue culture media have proved to be satisfactory.26 Many buffering systems used for media at normothermic temperatures, however, lose their buffering capacity at lower temperature. For cryopreservation purposes, the zwitterionic buffers HEPES and TES have been shown to work well.24, 27, 28
A cooling rate of −1°C per minute can be achieved accurately by using a controlled-rate cooling apparatus; an approximation can be achieved, however, by placing tissue in an insulated container within a deep freeze or a vapor phase of liquid nitrogen.1, 24, 26, 29 Many different protocols have been advocated for the packaging and long-term storage of skin, ranging from rolls of skin within a tube30 to the use of flat packs31, 32, 33 in metal laminated pouches. The latter are generally preferred, in that the greater surface area to volume ratio ensures more even cooling across the skin tissue, and the metal laminates are good conductors of heat. The tissue is normally packed in 2 flat pack bags (an inner and an outer bag), both of which are sterile. This facilitates aseptic handling during the thawing and clinical application of the skin tissue.
In the presence of CPA, a cooling rate of −1°C per minute will ensure survival of most of the cells within a tissue; as the cooling rate is increased; however, cell populations are sequentially adversely affected. Many cells in the body derived from leukocytes or closely related lineages are known to be exquisitely sensitive to cryogenic injury. The depletion of immunostimulatory “passenger leukocytes” was demand was demonstrated by increasing the cooling rate for pancreatic islets of Langerhans while maintaining the viability of the insulin-producing islet cells. 34 This concept of cooling rate–dependent immunomodulation was evaluated for skin tissue.35 A cooling rate of −30°C per minute was shown to maintain the viability of keratinocytes and fibroblasts while reducing the immunogenicity (as assessed by the mixed epidermal cell/lymphocyte response assay) of murine allografts by 95%. This was assumed to be due to an effect of the faster cooling rate on the major immunostimulatory cell in the skin—the Langerhans cell.
Once skin tissue has been cooled by a controlled-rate process to at least −80°C, it can then be transferred for long-term storage into the vapor phase of liquid nitrogen (below −130°C). The vapor phase is currently preferred owing to cases of virus transmission between packs of bone marrow that were stored in the liquid phase, presumably because of gross contamination of the giving set ports on the marrow bags by viruses present in the liquid nitrogen. The nitrogen had become contaminated by the rupture of a bag containing marrow from a patient subsequently shown to be positive for hepatitis B.36
Once the skin is at a temperature lower than −130°C, no further loss of cell viability is incurred. When the skin is required for grafting purposes, it should be transported to the user hospital either in a liquid nitrogen dry shipper (at temperatures below −130°C) or together with dry ice (CO2) in an insulated container (−79°C). The optimum thawing procedure is a rapid warming method. This can be achieved by plunging the packs of skin into a 37°C water bath until the tissue is just thawed (prolonged storage at 37°C in the presence of the CPA would be detrimental). The outer bag can then be disinfected and cut along the margin, and the inner bag removed aseptically to a sterile field. After aseptic opening of the inner bag, the skin is removed to a sterile kidney dish and rinsed to remove the CPA solution. Because the cells contain high concentrations of CPA, they are hyperosmotic compared with normal saline. To avoid osmotic lysis of the cells, either the saline can be added gradually or an impermeant solute such as sucrose can be added to the saline to reduce the difference in osmolarity.37
As soon as the washing procedure is completed, the skin should be applied immediately to the wound. Viability declines rapidly after thawing of the skin38 and further storage before use cannot be recommended.
Nonviable skin allografts
Because skin cell viability is not retained in nonviable allografts, this opens up many more possibilities for the preservation and sterilization of the skin tissue.
Preservation
The principle for all methods of long-term preservation is to avoid the degradative changes that may occur in the presence of water. These include microbial growth that not only would pose an infection risk but also would result in damage to the matrix. Enzymatic degradation from enzymes released from the skin cells or bacteria could cause significant damage.39 Oxidation reactions may occur; for example, lipid peroxidation is a chain reaction that results in rancidity of fats and toxicity in bone.40 Hydrolytic reactions can also damage matrix components. All of these detrimental reactions are dependent on water being present (Fig. 2 ). Therefore, the removal, immobilization, or sequestration of water is the basis for long-term preservation of tissue grafts. This can be achieved in 3 main ways:
-
1.
Deep freezing. At the freezing point of the tissue, ice nucleation occurs. As the temperature falls further, ice crystals form and existing crystals grow. Ice crystals are pure water and hence the remaining solution becomes progressively concentrated. Eventually, the point is reached at which there is too little free water left in solution to facilitate the microbial growth and the degradative chemical reactions. At this temperature, long-term preservation is achieved. In practice, storage in a −80°C deep freeze has proved satisfactory for long-term storage.
-
2.
Freeze drying. This process involves the removal of water from skin in the frozen state by sublimation, which is achieved by applying a vacuum to the tissue and condensing the removed water molecules downstream.25 Sublimation from the frozen state helps to protect molecules that would otherwise be adversely affected by high salt concentrations at higher temperatures, for example, denaturation of proteins. Drying must continue until enough water has been removed to prevent degradation reactions, which equates to less than 5% residual water as measured gravimetrically. Although accepted in most tissue banking standards, “residual water” is not identical to “water activity,” which is the most appropriate measure.41
The US Navy Tissue Bank was the first to commence large-scale freeze drying of human tissues for implantation in the early 1950s. In 1955, the use of freeze-dried skin was first reported. It was subsequently shown that freeze drying reduced the immunogenicity of skin42 without interfering with its beneficial properties.31, 32, 43, 44
-
3.
Use of high-concentration solutes. High concentrations of salts or sugars have been used for centuries for the long-term preservation of food items. The principle here is that each solute molecule is able to sequester water molecules in a hydration shell around the molecule. The water within the hydration shell is almost crystalline in nature and is unable to participate in other chemical reactions. The Euroskin Bank developed a solute preservation method using glycerol.45 The skin was incubated in successively more concentrated glycerol solutions (50%, 70%, and 85%) and maintained long term in 85% glycerol. More recently, the glycerol and water flux kinetics have been characterized and a more efficient validated protocol was proposed.46 The water activity of skin in different glycerol concentrations has been measured using a commercially available instrument.41 This study demonstrated that the 85% concentration originally chosen by the Euroskin Bank is optimal for minimizing degradative reactions.
Fig. 2.
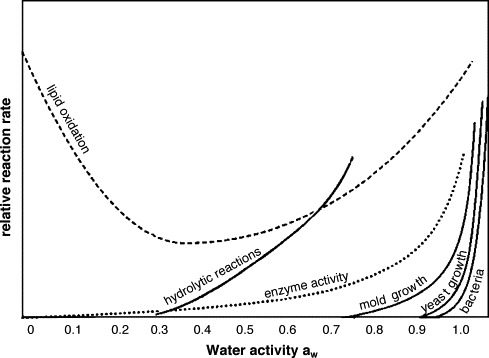
The relationship between water activity and detrimental microbial and chemical reactions. Adapted from Cell Tissue Bank 2004.
When the glycerolization technique is used to preserve the skin, it is very important that the glycerol is removed from the skin before clinical use. This is achieved by repeatedly washing the skin in physiological saline, and the process must be validated by the skin bank. At least 30 to 60 minutes are required to remove most of the glycerol.46 Failure to remove glycerol may lead to high systemic concentrations when used on open wounds. At high doses, glycerol has toxic effects on muscles leading to myonecrosis. Muscle breakdown products may result in renal failure and even death.47, 48 The LD50 for glycerol is 0.00442 ml/g.49
Sterilization of nonviable skin allografts
For viable skin allografts, it is not possible to apply a sterilization technique because sterilization methods tend to inactivate human cells at an equal or greater rate than for the bacterial cells. The best that can be achieved is a combination of serology screening for viruses and bacterial disinfection using antibiotic cocktails. For nonviable grafts, a range of sterilization techniques can be considered. Dry heat and autoclaving damage the structure of the skin tissue, including denaturation of collagen, and hence are not suitable. Techniques that have been successfully used include ethylene oxide gas50 and γ irradiation.51 Concerns and limitations of these methods, however, are beginning to appear. Ethylene oxide gas and its reaction product with chloride—ethylene chlorohydrin—are very toxic. Although acceptable levels for these compounds had been proposed,52 more recent data suggest that there is no safe level of ethylene oxide with respect to genotoxicity. There is therefore a move away from the use of this sterilant.
Questions have also been raised about the effectiveness of γ irradiation. Many small viruses and spore-forming bacteria are fairly resistant to γ irradiation. The high doses that would be required to inactivate HIV in a window-period donor (more than 80 kGy)53 would cause extensive damage to the tissue matrix including collagen denaturation. Therefore, a current consideration is whether “sterilization” can be achieved instead by using combinations of microbial inactivation procedures. To understand how this might be achieved will require an understanding of the concept of sterilization.
When a given microbial inactivation procedure is applied to a particular situation, the rate of microbial inactivation usually approximates that shown in Fig. 3 ; that is, an increasing amount of inactivation is required for the fewer and fewer surviving microorganisms. Although the line becomes vanishingly close to the x-axis, it never crosses it; thus, it is asymptotic. Therefore, it is never possible to guarantee the absence of microorganisms. Sterility is a statistical phenomenon.
Fig. 3.
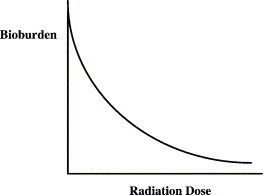
The relationship between dose of sterilant and microbial inactivation.
If a log10 scale is used for the microbial population density, then the inactivation curve approximates a straight line. From this straight line, the D value can be calculated. This is the sterilization dose required to reduce the microbial density by 1 log10 (90%) (Fig. 4 ). Where the line crosses the x-axis indicates there is an average of less than 1 viable organism per item being sterilized. Again, this is a statistical phenomenon so that when 10−1 is reached, this indicates 1 viable microbe for every 10 items being treated. The Food and Drug Administration has recently indicated that to claim sterility of a banked tissue product, enough sterilization dose should be applied to reach the 10−6 level, that is, 1 viable microbe per 1 million tissue items sterilized. Because it is not possible to destructively culture 1 million tissue grafts, the value is obtained by extrapolation of the line in Fig. 5 to the 10−6 level using the D value concept. This is referred to as the 10−6 sterility assurance level (SAL). To reach the 10−6 SAL level from the x-axis clearly requires 6 D values. Additional D values, however, are required to move from the original bioburden to the x-axis. If the initial bioburden is low, for example, only 10 microbes per graft, then log10 of 10 = 1, that is, 1 additional D value is required, giving a total of 7 to reach a SAL of 10−6. If, on the other hand, there are 1 million microbes per graft, then log10 of 106 = 6, that is, 6 additional D values are required, giving a total of 12.
Fig. 4.
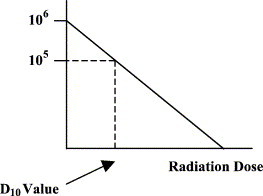
Calculation of the D value from the log10 plot.
Fig. 5.
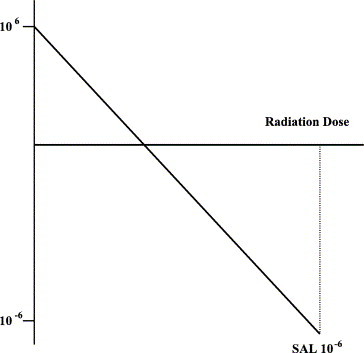
Deriving the 10−6 SAL by extrapolation.
In a study of ethylene oxide sterilization of bone grafts,54 the D value was shown to be 8 minutes exposure to the gas. Therefore, in this case, 7 D values equate to 56 minutes of exposure, whereas 12 D values require 96 minutes of exposure.
With γ irradiation, the D value for Clostridium species is around 3 kGy.55 Taking again bioburdens ranging from 101 to 106 bacteria per graft (requiring a total of 7 and 12 D values, respectively, to reach the 10−6 SAL), the respective irradiation doses required would be 21 and 36 kGy. Irradiation doses above circa 25 kGy cause collagen denaturation. Therefore, in the latter case, rather than relying on irradiation to achieve all of the required 1012 reduction, it would be preferable to use an alternative method to reduce the initial bioburden, for example, to circa 101 and then irradiation for the final reduction step.
The author has recently evaluated the use of peracetic acid for sterilization of skin grafts.56 A major advantage of this chemical agent is that its breakdown products—acetate, water, and oxygen—are nontoxic. Peracetic acid–treated skin has been shown to exhibit biocompatible properties in vitro and to be compatible with the high-concentration glycerol preservation technique. Whether peracetic acid alone can achieve a SAL of 10−6 is currently under investigation.
Application of a sterilization step will significantly reduce the risks of cross-infection and therefore will support the use of the nonviable skin for non–life-threatening wounds such as ulcers. There is evidence that repeated application of skin grafts to ulcers can reduce the bacterial load and help to condition the wound bed to stimulate healing or prepare the wound for an autograft.57, 58, 59
Conclusions
Skin allografts continue to be an important therapeutic tool. The development of surgical skin banking in individual burn units has largely given way to large regional or national skin or multitissue banks. This has facilitated rigorous tissue processing and scientific validation of processing procedures used. Increasing regulation and accreditation of skin banks by governmental and intergovernmental agencies have further strengthened skin processing and banking standards, thus ensuring high levels of safety and efficacy.
References
- 1.Lawrence J.C. Storage and skin metabolism. Br J Plast Surg. 1972;25:449–453. doi: 10.1016/s0007-1226(72)80091-2. [DOI] [PubMed] [Google Scholar]
- 2.Clarke J.A. HIV transmission and skin grafts. Lancet. 1987;i:983. doi: 10.1016/s0140-6736(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 3.James V., editor. Guidelines for the blood transfusion services in the United Kingdom. HMSO; Norwich: 2002. pp. 205–223. [Google Scholar]
- 4.British Association for Tissue Banking. General standards and technical guidelines. Available from: www.batb.org.uk/standards.htm [Accessed 2004].
- 5.European Association of Tissue Banks. Standards. Available from: www.eatb.de/html/standards.htm. [Accessed 2004].
- 6.Holland K.T., Kearney J.N. Microbiology of skin. In: Skerrow D., Skerrow C.J., editors. Methods in skin research. Wiley; Chichester: 1985. pp. 433–474. [Google Scholar]
- 7.Kearney J.N., Harnby D., Gowland G. The follicular distribution and abundance of resident bacteria on human skin. J Gen Microbiol. 1984;130:797–801. doi: 10.1099/00221287-130-4-797. [DOI] [PubMed] [Google Scholar]
- 8.Wentscher J. Ein weiter beitrag zur uberlebensfahigkeit der meschlichen epidermiszellen. Deutsch Z Chir. 1903;70:21–44. [Google Scholar]
- 9.Carrel A. Rejuvenation of cultures of tissues. JAMA. 1911;57:1611. doi: 10.1001/jama.250.8.1085. [DOI] [PubMed] [Google Scholar]
- 10.Carrel A. The preservation of tissues and its application in surgery. JAMA. 1912;59:523–527. [Google Scholar]
- 11.Carrel A. On the permanent life of tissues outside the organism. J Exp Med. 1912;15:516–528. doi: 10.1084/jem.15.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown J.B., Fryer M.P., Zaydon T.J. A skin bank for postmortem homografts. Surg Gynecol Obstet. 1955;101:401–412. [PubMed] [Google Scholar]
- 13.O'Neill J.A., Grosfeld J.L., Boles E.T. The extended use of skin homografts. Arch Surg. 1969;99:263–268. doi: 10.1001/archsurg.1969.01340140135020. [DOI] [PubMed] [Google Scholar]
- 14.Wachtel T.L., Ninnemann J.L., Fisher J.C. Viability of frozen allografts. Am J Surg. 1979;138:783–787. doi: 10.1016/0002-9610(79)90296-4. [DOI] [PubMed] [Google Scholar]
- 15.Marrangoni A.G. An experimental study on refrigerated skin grafts stored in 10% homologous serum. Surg Forum. 1951;1:425–434. doi: 10.1097/00006534-195012000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Allgower M., Blocker T.G. Viability of skin in relation to various methods of storage. Tex Rep Biol Med. 1952;10:3–21. [PubMed] [Google Scholar]
- 17.Rosenquist M.D., Cram A.E., Kealey G.P. Skin preservation at 4°C: a species comparison. Cryobiology. 1988;25:31–37. doi: 10.1016/0011-2240(88)90017-x. [DOI] [PubMed] [Google Scholar]
- 18.Hurst L.N., Lindsay W.K., Lee J. Viability of skin grafts stored in various media. Can J Surg. 1973;16:206–209. [PubMed] [Google Scholar]
- 19.Cram A.E., Domayer M.A. Short-term preservation of human autografts. J Trauma. 1983;23:872–873. doi: 10.1097/00005373-198310000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Cram A.E., Domayer M.A., Shelby J. Human skin storage techniques: a study utilizing a nude mouse recipient. J Trauma. 1983;23:924–926. [PubMed] [Google Scholar]
- 21.Cram A.E., Domayer M.A., Scupham R. Preservation of human skin: a study of two media using the athymic (nude) mouse model. J Trauma. 1985;25:128–130. [PubMed] [Google Scholar]
- 22.Farrant J. General observations on cell preservation. In: Ashwood-Smith M.J., Farrant J., editors. Low temperature preservation in medicine and biology. Pitman Medical; Tunbridge Wells: 1980. pp. 1–18. [Google Scholar]
- 23.Polge C., Smith A.U., Parkes A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164:666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- 24.Kearney J.N., Wheldon L.A., Gowland G. Effects of cryobiological variables on the survival of skin using a defined murine model. Cryobiology. 1990;27:164–170. doi: 10.1016/0011-2240(90)90008-r. [DOI] [PubMed] [Google Scholar]
- 25.Kearney J.N. Wound healing. In: Settle J.A.D., editor. Principles and practice of burns management. Livingstone; London: 1996. pp. 187–195. [Google Scholar]
- 26.Kearney J.N. Cryopreservation of cultured skin cells. Burns. 1991;17:380–383. doi: 10.1016/s0305-4179(05)80070-1. [DOI] [PubMed] [Google Scholar]
- 27.Pegg D.E. Perfusion technology. In: Karow A.M., Pegg D.E., editors. Organ preservation for transplantation. Marcel Dekker; New York: 1981. pp. 477–495. [Google Scholar]
- 28.Taylor M.J., Hunt C.J. A new preservation solution for storage of corneas at low temperatures. Curr Eye Res. 1985;4:963–973. doi: 10.3109/02713689509000003. [DOI] [PubMed] [Google Scholar]
- 29.May S.R., Roberts D.P. Development of a passive device for freezing large amounts of transplantable skin at one time in a −70°C mechanical refrigerator. Cryobiology. 1988;25:186–196. doi: 10.1016/0011-2240(88)90025-9. [DOI] [PubMed] [Google Scholar]
- 30.Ninnemann J.L., Fisher M.D., Frank H.A. Clinical skin banking: a simplified system for processing, storage and retrieval of human allografts. J Trauma. 1978;18:723–725. [PubMed] [Google Scholar]
- 31.Cochrane T.D., Watson J. The long-term storage of skin. Br J Hosp Med. 1971;5:473–476. [Google Scholar]
- 32.Hackett M.E.J. Preparation, storage and use of homograft. Br J Hosp Med. 1975;13:272–284. [Google Scholar]
- 33.Blondet R., Gibert-Thevenin M.A., Pierre A. Skin preservation by programmed freezing. Br J Plast Surg. 1982;35:530–536. doi: 10.1016/0007-1226(82)90058-3. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M.J., Banks H.L. Function of lymphocytes and macrophages after cryopreservation by procedures for pancreatic islets: potential for reducing tissue immunogenicity. Cryobiology. 1988;25:1–17. doi: 10.1016/0011-2240(88)90014-4. [DOI] [PubMed] [Google Scholar]
- 35.Ingham E., Matthews B.J., Kearney J.N. Modulation of the immunogenicity of murine skin by variation of the cryopreservation protocol. Cryobiology. 1991;28:563. [Google Scholar]
- 36.Tedder R.S., Zuckerman M.A., Goldstone A.H. Hepatitis B transmission from contaminated cryopreservation tank. Lancet. 1995;346:137–140. doi: 10.1016/s0140-6736(95)91207-x. [DOI] [PubMed] [Google Scholar]
- 37.Pegg D.E., Hunt C.J., Fong L.P. Osmotic properties of the rabbit corneal endothelium and their relevance to cryopreservation. Cell Biophys. 1987;10:169–189. doi: 10.1007/BF02797398. [DOI] [PubMed] [Google Scholar]
- 38.Kearney J.N. Evaluation of proteinase inhibitors and free radical inhibitors/scavengers in reducing post-thaw viability loss of cryopreserved skin. Burns. 1998;24:507–512. doi: 10.1016/s0305-4179(98)00068-0. [DOI] [PubMed] [Google Scholar]
- 39.May S.R., Wainwright J.F. Integrated study of the structural and metabolic degeneration of skin during 4°C storage in nutrient medium. Cryobiology. 1985;22:18–34. doi: 10.1016/0011-2240(85)90004-5. [DOI] [PubMed] [Google Scholar]
- 40.Moreau M.F., Gallois Y., Baslé M.F. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials. 2000;21:369–376. doi: 10.1016/s0142-9612(99)00193-3. [DOI] [PubMed] [Google Scholar]
- 41.Ross A., Kearney J.N. The measurement of water activity in allogeneic skin grafts preserved using high concentration glycerol or propylene glycol. Cell Tissue Bank. 2004;5:37–44. doi: 10.1023/b:catb.0000022284.53499.59. [DOI] [PubMed] [Google Scholar]
- 42.Abbott W.M., Hembree J.S. Absence of antigenicity in freeze-dried skin allografts. Cryobiology. 1970;6:416–418. doi: 10.1016/s0011-2240(70)80099-2. [DOI] [PubMed] [Google Scholar]
- 43.Young J.M., Hyatt G.W. Stored skin homografts in extensively burned patients. Arch Surg. 1960;80:208–213. doi: 10.1001/archsurg.1960.01290190028006. [DOI] [PubMed] [Google Scholar]
- 44.Chambler K., Sachs A. The use of etox lyophilised skin in burns. Br J Plast Surg. 1969;52:210–215. doi: 10.1016/s0007-1226(69)80109-8. [DOI] [PubMed] [Google Scholar]
- 45.Kreis R.W., Vloemans A.F.P.M., Hoekstra M.J. The use of nonviable glycerol-preserved cadaver skin combined with widely expanded autografts in the treatment of extensive third-degree burns. J Trauma. 1989;29:51–54. doi: 10.1097/00005373-198901000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Huang Q., Pegg D.E., Kearney J.N. An improved glycerol banking method used in the preservation of nonviable skin allografts. Cell Tissue Bank. 2004;5:3–21. doi: 10.1023/b:catb.0000022234.02322.13. [DOI] [PubMed] [Google Scholar]
- 47.Backenroth R. Glycerol induced acute renal failure attenuates subsequent HgCl2-associated nephrotoxicity: correlation of renal function and morphology. Ren Fail. 1998;20:15–26. doi: 10.3109/08860229809045086. [DOI] [PubMed] [Google Scholar]
- 48.Zurovsky Y. Models of glycerol-induced acute renal failure in rats. J Basic Clin Physiol Pharmacol. 1993;4:213–228. doi: 10.1515/jbcpp.1993.4.3.213. [DOI] [PubMed] [Google Scholar]
- 49.Uche E.M., Arowolo R.O., Akinyemi J.O. Toxic effects of glycerol in Swiss albino rats. Res Commun Chem Pathol Pharmacol. 1987;56:125–128. [PubMed] [Google Scholar]
- 50.Kearney J.N., Franklin U.C., Aguirregoicoa V. Evaluation of ethylene oxide sterilization of tissue implants. J Hosp Infect. 1989;13:71–80. doi: 10.1016/0195-6701(89)90097-2. [DOI] [PubMed] [Google Scholar]
- 51.Dziedzic-Goclawska A., Stachowicz W. Sterilisation of tissue allografts. In: Phillips G.O., von Versen R., Strong D.M., editors. vol. 1. World Scientific Publishing; Singapore: 1987. pp. 261–321. (Advances in tissue banking). [Google Scholar]
- 52.Prolo D.J., Pedrotti P.W., White D.H. Ethylene oxide sterilisation of bone, dura mater and fascia lata for human transplantation. Neurosurgery. 1980;6:529–539. doi: 10.1227/00006123-198005000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Campbell D.G., Li P. Sterilization of HIV with irradiation: relevance to infected bone allografts. ANZ J Surg. 1999;69:517–521. doi: 10.1046/j.1440-1622.1999.01615.x. [DOI] [PubMed] [Google Scholar]
- 54.Kearney J.N., Bojar R., Holland K.T. Ethylene oxide sterilisation of allogenic bone implants. Clin Mater. 1993;12:129–135. doi: 10.1016/0267-6605(93)90063-d. [DOI] [PubMed] [Google Scholar]
- 55.Hansen J.M., Shaffer H.L. Sterilization and preservation by radiation sterilization. In: Block S.S., editor. Disinfection, sterilization and preservation. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 729–746. [Google Scholar]
- 56.Lomas R.J., Cruse-Sawyer J.E., Simpson C. Assessment of the biological properties of human split skin allografts disinfected with peracetic acid and preserved in glycerol. Burns. 2003;29:515–525. doi: 10.1016/s0305-4179(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 57.Rundle J.S.H., Elton R.A., Cameron S.H. Porcine dermis in varicose ulcers—a clinical trial. Vasa. 1981;10:246–248. [PubMed] [Google Scholar]
- 58.Ersek R.A., Lorio J. The most indolent ulcers of the skin treated with porcine xenografts and silver ions. Surg Gynecol Obstet. 1984;158:431–432. [PubMed] [Google Scholar]
- 59.Morris P.J., Bondoc C., Burke J.F. The use of frequently changed skin allografts to promote healing in the nonhealing infected ulcer. Surgery. 1966;60:13–19. [Google Scholar]


