Abstract
Community-acquired pneumonia (CAP) is a common cause of pediatric hospital admission. Empiric antibiotic therapy for hospitalized children with serious CAP now targets the most likely pathogen(s), including those that may demonstrate significant antibiotic resistance. Cell-free plasma next-generation sequencing (CFPNGS) was first made available for Pediatric Infectious Diseases physicians in June 1, 2017, to supplement standard-of-care diagnostic techniques. A retrospective chart review was performed for children hospitalized with CAP between June 1, 2017, and January 22, 2018, to evaluate the impact of CFPNGS. We identified 15 hospitalized children with CAP without other underlying medical conditions for whom CFPNGS was performed. CFPNGS identified a pathogen in 13 of 15 (86%) children compared with 47% for those using standard culture and PCR-based methods alone. Changes in antibiotic management were made in 7 of 15 (47%) of children as a result of CFPNGS.
Abbreviations: CAP, community-acquired pneumonia; CFPNGS, cell-free plasma next-generation sequencing; IDSA, Infectious Diseases Society of America; NGS, next-generation sequencing; MRSA, methicillin-resistant Staphylococcus aureus; HUS, hemolytic uremic syndrome; CRRT, continuous renal replacement therapy
Keywords: NGS, Pneumonia, Pediatric, Cell-free plasma sequencing, Infectious disease, Precision medicine
1. Introduction
Pediatric community-acquired pneumonia (CAP) is a leading cause of hospital admissions(Li and Tancredi, 2010, Grijalva et al., 2010, Grijalva et al., 2011) with a potentially wide range of bacterial, viral, fungal, and mycobacterial etiologies (Erlichman et al., 2017). In a large multisite study of 2638 children hospitalized with CAP, 89% had radiographic evidence of pneumonia. Standard-of-care testing identified a viral infection in 66% of patients, a bacterial infection in 8% of patients, and both a viral and bacterial infection in 7% of patients (Jain et al., 2015). The current Pediatric CAP Guidelines by the Infectious Diseases Society of America (IDSA)/Pediatric Infectious Diseases Society (PIDS) recommend blood culture and viral testing for hospitalized patients with moderate to severe community-acquired or complicated pneumonia in order to identify a pathogen(s). Recommended empiric treatment include a third-generation cephalosporin for any patient with evidence of empyema, with the addition of vancomycin or clindamycin if there are clinical, laboratory, or imaging characteristics consistent with methicillin-resistant Staphylococcus aureus (Bradley et al., 2011) (MRSA) infection.
In cases where cultures (e.g., blood, respiratory, pleural fluid) are negative, physicians rely on epidemiology and clinical features to predict the most likely infectious organism(s) and guide empiric antibiotic selection. This can be challenging as causal organisms have overlapping clinical, radiographic, and laboratory features and/or atypical presentations. Additionally, the clinical course of complicated pneumonias is often lengthy despite the presence of appropriate antimicrobial coverage (Breuer et al., 2018). In the absence of an identified pathogen, children with serious infections may be treated with broad-spectrum antibiotics for prolonged courses, due to concern for MRSA.
DNA sequencing of cell-free plasma(De Vlaminck et al., 2015, Long et al., 2016, Hong et al., 2018) allows for detection of pathogen DNA derived from both bloodstream infections and deeper body sites, including cases where there has been antibiotic pretreatment prior to cultures and in those with fastidious, difficult-to-culture organisms (Abril et al., 2016). This case series reflects an institutional experience applying cell-free sequencing technology, a new commercially available test whose application has not been extensively studied. The current retrospective series in children with CAP was performed to assess the clinical impact of this test compared with more traditional microbiologic methods.
2. Patients and methods
A retrospective chart review was approved by the Human Research Protections Program at the University of California San Diego (HRP#180245) to evaluate the use of a cell-free plasma next-generation sequencing (CFPNGS) plasma test in the management of children with infections between June 1, 2017, and January 22, 2018. Inclusion criteria included previously healthy children with CAP or complicated CAP as defined by the IDSA/PIDS guidelines, for whom CFPNGS was ordered and who were discharged prior to February 28, 2018. CFPNGS was a restricted test, ordered by pediatric infectious diseases faculty through the electronic medical record. No prospective, standardized criteria delineated when to obtain CFPNGS; rather, it was at the discretion of the pediatric infectious disease faculty. Children with chronic or complex underlying medical disease were excluded (i.e., underlying oncologic, genetic, or chronic illnesses and/or use of immunomodulatory medications). Chart review was performed using the electronic medical record and incorporated into a REDCap database (Harris et al., 2009).
2.1. Sample processing
Peripheral blood samples were collected in a BD Vacutainer Plasma Preparation Tube (PPT tube, Becton, Dickson and Company, Franklin Lakes, NJ), centrifuged, and sent to the Karius laboratory (Redwood City, CA) for CFPNGS (De Vlaminck et al., 2015). Cell-free DNA was extracted from plasma with a magnetic bead-based method (Omega Biotek, Norcross, GA). DNA libraries were constructed using a modified Ovation System V2 library kit (NuGEN, San Carlos, CA). Negative buffer-only controls and positive controls consisting of healthy patient plasma with addition of known mixture of microbial DNA fragments were processed in parallel, and all samples were sequenced on the Illumina NextSeq 500.
2.2. Standard of care
Blood, urine, respiratory and pleural bacterial, fungal, and mycobacterial cultures, as well as multiplex PCR (EPlex Respiratory Pathogen Panel) for detection of viral pathogens, were obtained at the discretion of treating physicians. Samples for culture and CFPNGS were not temporally matched at acquisition. Available standard care testing data were included when obtained within 72 h of the CFPNGS. Treating physicians determined antimicrobial therapy. The pediatric infectious disease faculty assessed the effects of the CFPNGS test on the selection of antibiotic therapy, subsequent procedures, and length of stay.
2.3. Analysis pipeline
Primary sequencing output files were processed using bcl2fastq (v2.17.1.14) to generate demultiplexed sequencing reads files. Reads were filtered based on sequencing quality and trimmed based on partial or full adapter sequences. Sequence alignment of remaining reads was performed against Karius' human and synthetic-molecules references using Bowtie2 (Langmead and Salzberg, 2012). Sequencing reads that exhibited alignment against the human or synthetic molecule references were collected and filtered out from further analysis. The remaining reads were aligned against Karius' proprietary microorganism reference database using NCBI-blast (version 2.2.30). To determine whether the levels observed in the samples exceeded those expected to originate from the environment alone, a Poisson model parameterized by the estimated background abundances was applied. Only taxa that rejected this null hypothesis at high significance levels were reported and included in downstream analyses. The entire process from DNA extraction through analysis was typically completed within 28 h.
3. Results
During the 8-month review period, a total of 125 children were admitted to the hospital with CAP, 31 of whom had CFPNGS testing obtained at the discretion of the pediatric infectious disease attending physician. Of these 31 children, 2 were excluded due to a diagnosed underlying genetic disorder and 14 due to chronic comorbid conditions. Patient demographics for the remaining cohort demonstrated an average age of 4.16 years and 53% male gender (Table 1 ). The average length of hospital stay was 14 days, reflecting the fact that CFPNGS tended to be requested only in cases of complicated pneumonia. In total, 10 of the 15 children were diagnosed with complicated CAP (pneumonia with pleural effusion or empyema), and 9 required admission to the pediatric intensive care unit (PICU). Nine children were treated with thoracoscopy for parapneumonic effusion or empyema, and 6 children required surgical intervention with video-assisted thoracoscopic surgery.
Table 1.
Demographics (n = 15).
| Average age at admission (range) | 4.16 years (0.7–10.7) |
| Male (% male) | 8 (53%) |
| Length of hospital stay (range) | 14.1 days (3–29.6) |
| Preceding viral illness | 11 (73%) |
| Preceding antibiotic | 8 (53%) |
| Consolidation on CXR | 12 (80%) |
| Pleural effusion on CXR | 10 (75%) |
| ICU admission | 9 (60%) |
| Intubated | 4 (27%) |
| Chest tube placed | 9 (60%) |
| Surgical intervention | 6 (40%) |
| Hemolytic uremic syndrome requiring CRRT | 2 (13%) |
CRRT = continuous renal replacement therapy.
As indicated in Table 2, only 1 of 15 (6.7%) patients had a positive blood culture at the time of admission. This culture yielded Streptococcus pneumoniae, and CFPNGS was also positive in this case. Pneumococci were identified in 8 additional cases only by CFPNGS (Fig. 1, Table 2). Respiratory cultures were obtained only in intubated children as endotracheal aspirates and yielded putative causal organisms in 33% of the study population (7 positive cultures from 5 patients). Of the respiratory samples, only 1 of the 3 patient cultures was concordant with the CFPNGS diagnostic test (patient 1 identified S. pneumoniae from the ETT and blood cultures as well as by CFPNGS). The 2 other patients with positive respiratory cultures were patient 10 (identified Pseudomonas aeruginosa from sputum and had negative CFPNGS) and patient 3 (isolated methicillin-susceptible Staphylococcus aureus [MSSA] from ETT culture and S. pneumoniae detected by CFPNGS). Three patients had negative respiratory cultures despite a positive CFPNGS test: patient 13 identified Fusobacterium nucleatum, Epstein–Barr virus, and Parviomonas micra detected on CFPNGS; patient 5 had S. pneumoniae detected; and patient 15 had Streptococcus pyogenes, Actinomyces graevenitzii, Veillonella dispar, and Actinomyces odontolyticus detected on CFPNGS. Seven patients did not have respiratory cultures obtained. Abscess fluid cultures obtained during placement of chest tube thoracostomies were positive in only 1 of 9 children: Patient 11 isolated Streptococcus intermedius from culture which was also detected by CFPNGS, CFPNGS also detected Aggregatibacter segnis and Epstein–Barr virus (Supplementary Table 1). A combination of conventional culture methods yielded a bacterial diagnosis in only 27% of children; with inclusion of PCR testing for viral etiologies, only 40% of children had an organism identified. Of note, the viral etiologies detected on a respiratory viral screen were not detected by CFPNGS due to the fact that RNA viruses are not detected this CFPNGS test. Patient 2 had coronavirus detected from a nasopharyngeal swab and S. pneumoniae by CFPNGS, and patient 1 had human metapneumovirus detected from a nasopharyngeal swab and S. pneumoniae from CFPNGS. Traditional testing yielded a result on average 31.4 h (range 2–42.8 h) after the sample was obtained, whereas CFPNGS yielded a result on average 98.1 h (range 48–245.3 h) after the sample was obtained. The use of CFPNGS independently identified a plausible bacterial pathogen in 86% of children (13 of 15), with 60% (9 of 16) being positive for S. pneumoniae (Table 1). The results of CFPNGS led to a change in antibiotic management in 7 of 15 children (47%).
Table 2.
Culture results.a
| Patient | Age (y) | Diagnosis | Culture results | Time to culture result (h) | Organisms detected by CFPNGS | Time to CFPNGS result (h) | Change in antibiotic management due to CFPNGS result |
|---|---|---|---|---|---|---|---|
| 11 | 0.9 | Empyema | Blood culture = Streptococcus pneumoniae; ETT culture = Streptococcus pneumoniae, Moraxella catarrhalis; Respiratory Viral Panel = Human metapneumovirus | 20; 42.8; 3.5 | Streptococcus pneumoniae | 90 | none |
| 10 | 1.5 | Abscess | Respiratory Viral Panel = Coronavirus | 5.5 | Streptococcus pneumoniae | 48.6 | Clindamycin discontinued for ceftriaxone monotherapy |
| 13 | 2.4 | Empyema | ETT culture = MSSA | 26.7 | Streptococcus pneumoniae | 245.3 | Clindamycin discontinued for cefazolin monotherapy |
| 2 | 1 | Empyema | Negative | NA | Streptococcus pneumoniae | 48 | Clindamycin discontinued for ceftriaxone monotherapy |
| 5 | 4.5 | Empyema | Negative | NA | Streptococcus pneumoniae | 103.8 | Ceftaroline narrowed to ceftriaxone |
| 8 | 3.6 | Empyema | Negative | NA | Streptococcus pneumoniae | 113.7 | Ceftaroline narrowed to ceftriaxone |
| 7 | 2 | Empyema | Negative | NA | Streptococcus pneumoniae | 102.7 | none |
| 15 | 2.7 | Pneumonia | Negative | NA | Streptococcus pneumoniae | 60.1 | Cefepime and clindamycin narrowed to ampicillin |
| 14 | 5.1 | Pneumonia | Cultures not obtained | NA | Streptococcus pneumoniae | 69.8 | none |
| 1 | 10.7 | Pneumonia | Sputum culture = Pseudomonas aeruginosa | 24.1 | No organisms detected | 100.3 | none |
| 6 | 6.4 | Abscess | Pulmonary abscess culture = Streptococcus intermedius | 43.4 | Streptococcus intermedius, Aggregatibacter segnis, Epstein–Barr virus | 115.5 | none |
| 12 | 9.1 | Abscess | Respiratory viral panel = Coronavirus | 2 | No organisms detected | 159.7 | none |
| 3 | 10.7 | Pneumonia | Negative | NA | Fusobacterium nucleatum, Epstein–Barr virus, Parvimonas micra | 54.7 | Ceftriaxone discontinued for clindamycin monotherapy |
| 4 | 0.7 | Pneumonia | Negative | NA | Pseudomonas aeruginosa, Burkholderia multivorans, Human adenovirus C | 104.9 | none |
| 9 | 1.1 | Empyema | Negative | Streptococcus pyogenes, Actinomyces graevenitzii, Veillonella dispar, Actinomyces odontolyticus | 54 | none | |
| bacterial dx = 4/15; Viral dx = 3/15 | Avg Cx = 31.4 | bacterial dx = 13/15 | Avg = 98.1 | 47% change in antibiotic management | |||
| Avg RVP = 3.6 | viral dx = 3/15 |
D = diagnostic yield of testing obtained within 72 h of when the CFPNGS was obtained, which identified bacterial infections in 4 of 15 patients and viral infections in 3 of 15 patients as compared to CFPNGS testing which yielded bacterial etiology in 13 of 15 patients and viral infections in 3 of 15 patients. The CFPGNS diagnosis positive patients led to a change of management in 7 of 15 patients (47%).
Time for CFPNGS was from when blood was drawn until faxed result was scanned into electronic medical record including laboratory processing, shipping, time at Karius Inc., and time to get result into EMR after result faxed in.
Fig. 1.
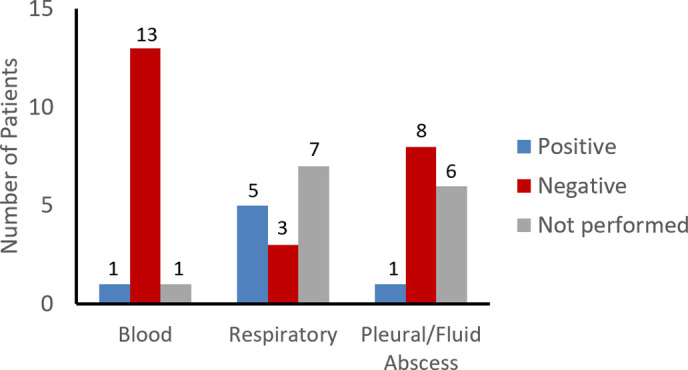
Culture results from the 15 patients for blood, respiratory, and pleural fluid/abscess cultures.
4. Discussion
In this retrospective study, CFPNGS identified 13 of 15 children to have bacterial infectious etiologies that could potentially explain acute pneumonia, as opposed to only 4 of 15 by traditional culture methods. Since the introduction of the 7-valent Pneumococcal Conjugate Vaccine (PCV7), followed by PCV13, there has been a decrease in pediatric hospital admissions for pneumococcal pneumonia by 26–61%, but an increase in staphylococcal pneumonia and unspecified empyema by 1.89- to 4.08-fold (Grijalva et al., 2010). Although there is an increase in staphylococcal pneumonia, there may be a decrease in MRSA (Hultén et al., 2018). Prior to the introduction of the pneumococcal conjugate vaccine and the emergence of community-acquired MRSA, many physicians felt confident in the management of CAP based on a presumed diagnosis of pneumococcal or streptococcal pneumonia in children admitted with CAP. However, given the changing epidemiological landscape of pediatric CAP, many physicians are now less confident in the diagnosis of fully susceptible S. pneumoniae in the absence of a positive culture. Therefore, empiric antibiotic coverage has broadened to include both S. aureus and multidrug-resistant pneumococcus. A consensus guideline provided recommendations for the treatment of children with severe CAP (or complicated CAP), but a paucity of strong prospective data did not support a high level of evidence to tailor treatment for hospitalized children with culture-negative CAP (Fontela et al., 2017). Lastly, the high prevalence in CAP patients who had antibiotic exposure (53% in our cohort) prior to relevant culture samples, including blood cultures, further reduces the sensitivity of cultures for pathogen detection using conventional methods. The use of CFPNGS has helped address this issue by identifying the infectious etiology in children, even when standard microbiologic cultures are negative. However, the lack of rigorous data regarding sensitivity and specificity of CFPNGS has left clinicians with minimal information in determining who would benefit from CFPNGS.
Similar to previous studies (Lin et al., 2013, Krenke et al., 2016), we found the yield of blood cultures and pleural effusion cultures to be low, possibly secondary to antibiotic pretreatment. It is interesting to note that there was not complete concordance between CFPNGS and traditional culture/viral PCR (see Table 2). This is an important challenge for the application of a novel technology. Since this CFPNGS test does not detect RNA viruses, there was not concordance with CFPNGS and respiratory PCR testing. Also, local sampling in traditional ETT cultures could explain why only 1 of 3 respiratory cultures were in agreement with the CFPNGS since the respiratory sample was collected from the upper airway and may not represent infection in the lung. Based upon the detection of an organism (often Streptococcus pneumoniae) consistent with the clinical presentation and with the site of infection in an area of high blood flow (the lung) leading to increased shedding of pathogen DNA into the blood, it seems reasonable to suggest that CFPNGS may better reflect the nature of a pulmonary infection than a sample obtained from the upper airway. Further rigorous studies are required to delineate the accuracy of CFPNGS versus traditional cultures. CFPNGS may also present a challenge for infectious disease specialists as organisms that are not common pathogens, such as Parvimonas micra and Veillonella dispar, are identified in critically ill patients in combination with other potentially pathogenic bacteria or in isolation. Does this CFPNGS signal suggest co-infection, bacterial presence in another part of the body (e.g., oral flora from a patient with gingivitis), or pathogenicity that is perhaps more common than we had previously thought? As this technology becomes more broadly accepted, many of these questions will have to be carefully examined.
As a direct result of CFPNGS, antibiotic selection was narrowed in 7 children (47% of the studied population) to beta-lactams rather than continuing lincosamides or glycopeptides. Treating infectious diseases clinicians believed that, with identification of a plausible organism, broad coverage was no longer needed. Importantly, none of the children treated with narrowed antimicrobial therapy based on CFPNGS results were readmitted for late complications of CAP that could be attributed to inappropriate antibiotic therapy. It should also be noted, however, that the negative predictive value of this test has not been fully characterized.
This review addresses proof of concept of the clinical application of CFPNGS in CAP in children. It does not compare CFPNGS to other established diagnostic tests such as pathogen-specific bacterial real-time PCR; however, such prospective, comparative studies would be helpful to further differentiate the role of CFPNGS in the context of other available tests in clinical practice.
This retrospective study has numerous limitations to the broad applicability of these findings. First, the study is limited in scope given the small patient numbers and the uncontrolled nature of the decision to consult the infectious diseases service and to have the consultant order the CFPNGS test, although these data reflect a real-world experience. Children with CAP selected for CFPNGS evaluation represented a more severely ill patient population, as demonstrated by a high rate of PICU admission, prolonged hospitalization, and the predominance of complicated CAP with empyema and/or parapneumonic effusion. The average time to CFPNGS result was 4 days, although it is possible that this time may decrease as efficiencies in transport, processing, and analysis improve. Overall, these data suggest that CFPNGS in conjunction with standard culture techniques may significantly increase diagnostic yield and facilitate antibiotic selection in severe CAP.
The following are the supplementary data related to this article.
Culture results and duration of antibiotic therapy.
Footnotes
Funding source: No funding was secured for this study.
Financial disclosure: DKH is an employee of Karius, Inc. The other authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: DKH is an employee of Karius, Inc. The other authors have no conflicts of interest to disclose.
References
- Abril M.K., Barnett A.S., Wegermann K., Fountain E., Strand A., Heyman B.M. Diagn Open Forum Infect Dis. 2016;3(3):ofw144. doi: 10.1093/ofid/ofw144. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J.S., Byington C.L., Shah S.S., Alverson B., Carter E.R., Harrison C. Executive summary: the management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):617–630. doi: 10.1093/cid/cir625. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer O., Picard E., Benabu N., Erlichman I., Reiter J., Tsabari R. Predictors of Prolonged Hospitalizations in Pediatric Complicated Pneumonia. Chest. 2018;153(1):172–180. doi: 10.1016/j.chest.2017.09.021. (Jan) [DOI] [PubMed] [Google Scholar]
- De Vlaminck I., Martin L., Kertesz M., Patel K., Kowarsky M., Strehl C. Noninvasive monitoring of infection and rejection after lung transplantation. Proc Natl Acad Sci U S A. 2015;112(43):13336–13341. doi: 10.1073/pnas.1517494112. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlichman I., Breuer O., Shoseyov D., Cohen-Cymberknoh M., Koplewitz B., Averbuch D. Complicated community acquired pneumonia in childhood: Different types, clinical course, and outcome. Pediatr Pulmonol. 2017;52(2):247–254. doi: 10.1002/ppul.23523. (Feb) [DOI] [PubMed] [Google Scholar]
- Fontela P.S., Quach C., Karim M.E., Willson D.F., Gilfoyle E., McNally J.D. Determinants of Antibiotic Tailoring in Pediatric Intensive Care: A National Survey. Pediatr Crit Care Med. 2017;18(9):e395–e405. doi: 10.1097/PCC.0000000000001238. (Sep) [DOI] [PubMed] [Google Scholar]
- Grijalva C.G., Nuorti J.P., Zhu Y., Griffin M.R. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin Infect Dis. 2010;50:805–813. doi: 10.1086/650573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grijalva C.G., Zhu Y., Nuorti J.P., Griffin M.R. Emergence of parapneumonic empyema in the USA. Thorax. 2011;66:663–668. doi: 10.1136/thx.2010.156406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D.K., Blauwkamp T.A., Kertesz M., Bercovici S., Truong C., Banaei N. Liquid biopsy for infectious diseases: sequencing of cell-free plasma to detect pathogen DNA in patients with invasive fungal disease. Diagn Microbiol Infect Dis. 2018;92:210–213. doi: 10.1016/j.diagmicrobio.2018.06.009. [DOI] [PubMed] [Google Scholar]
- Hultén K.G., Mason E.O., Lamberth L.B., Forbes A.R., Revell P.A., Kaplan S.L. Analysis of invasive community-acquired methicillin-susceptible Staphylococcus aureus infections during a period of declining community acquired methicillin-resistant Staphylococcus aureus infections at a large children's hospital. Pediatr Infect Dis J. 2018;37:235–241. doi: 10.1097/INF.0000000000001753. [DOI] [PubMed] [Google Scholar]
- Jain S., Williams D.J., Arnold S.R., Ampofo K., Bramley A.M., Reed C. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835–845. doi: 10.1056/NEJMoa1405870. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenke K., Sadowy E., Podsiadły E., Hryniewicz W., Demkow U., Kulus M. Etiology of parapneumonic effusion and pleural empyema in children. The role of conventional and molecular microbiological tests. Respir Med. 2016;116:28–33. doi: 10.1016/j.rmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S.T., Tancredi D.J. Empyema hospitalizations increased in US children despite pneumococcal conjugate vaccine. Pediatrics. 2010;125:26–33. doi: 10.1542/peds.2009-0184. [DOI] [PubMed] [Google Scholar]
- Lin T.Y., Hwang K.P., Liu C.C., Tang R.B., Lin C.Y., Gilbert G.L. Etiology of empyema thoracis and parapneumonic pleural effusion in Taiwanese children and adolescents younger than 18 years of age. Pediatr Infect Dis J. 2013;32(4):419–421. doi: 10.1097/INF.0b013e31828637b1. (Apr) [DOI] [PubMed] [Google Scholar]
- Long Y., Zhang Y., Gong Y., Sun R., Su L., Lin X. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch Med Res. 2016;47(5):365–371. doi: 10.1016/j.arcmed.2016.08.004. (Jul) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Culture results and duration of antibiotic therapy.


