Abstract
Binding of baicalein, wogonin and baicalin to fish sperm DNA was studied by using ethidium bromide dye as a fluorescence probe. To study the binding mechanism, the absorption, fluorescence, melting temperature and viscosity measurement were carried out. The experimental results indicated that the planar of flavonoids intercalated to the DNA helix. When bound to DNA, flavonoids showed hyperchromic and blue shift in the absorption spectra and fluorescence quenching (>50%) in the fluorescence spectra. Furthermore, the intercalative binding was consistent with the red shift in the position of λmax in the fluorescence spectra. It was also found that ionic strength had little or no effect on the binding of flavonoids and DNA. Stern–Volmer plots at 25 and 37 °C showed that the quenching of fluorescence by flavonoids was a combined quenching process. The binding site number n, apparent binding constant KA at 25 and 37 °C, and the corresponding thermodynamic parameters ΔG, ΔH, ΔS at 25 °C were obtained. The interaction of flavonoid–metal complexes with DNA was also studied by spectral methods, and the results suggested that the complexes intercalated into DNA.
Keywords: Baicalein, Wogonin, Baicalin, DNA, Ethidium bromide, Fluorescence quenching
1. Introduction
In recent years, fluorescent dyes [1], [2], [3], metal complexes [4], [5] and drugs [6], [7], [8], [9] have been widely employed in the research of nucleic acid. Because the intracellular target for a wide range of anticancer and antibiotic drugs is DNA, the binding studies of small molecules with DNA are very useful in understanding the drug–DNA interactions, designing new and promising drugs for clinical use and developing sensitive chemical probes of nucleic acid structure. Although DNA has a natural fluorescence, the intensity is so weak that the direct use of the fluorescence emission of DNA is limited to study its properties [10]. Ethidium bromide (EB), acridine orange, methylene blue and similar fluorescent compounds are normally used to probe DNA structure in drug–DNA and protein–DNA interactions [11]. The binding of these pigments to double-stranded DNA greatly enhances their fluorescence intensity and lifetime [12], [13], [14]. There is a higher affinity of EB with DNA duplex because EB provides greater accessibility for DNA binding rather than acridine orange and methylene blue [15]. Thus, EB was selected as the probe in this paper.
Flavonoids are naturally occurring compounds found in fruit, vegetables, and plant-derived beverages, such as wines, tea, cocoa, and fruit juices [16]. This family of polyphenolic compounds is an important constituent of the human diet, where the average intake is in the range of 50–800 mg/day [17]. Variation in the two benzene rings (A and B) of the basic structure gives rise to flavonols, flavones, catechins, flavanones, anthocyanidins and isoflavones. Scutellaria baicalensis Georgi is one of the most common flavones present in nature. It is also one commonly used herbal medicine in China and other East Asian countries, and it has been officially listed in the Chinese Pharmacopoeia for a long time [18]. Baicalein, wogonin and baicalin (Fig. 1 (a–c)) are the main active components in Scutellaria baicalensis Georgi [19]. These components possess anti-allergic [20], antioxidant [21], [22], anti-HIV [23], [24], anti-inflammatory [25], anti-tumor [26], [27], [28], anxiolytic [29], anti-hepatitis B virus [30], antigenotoxic activity [31], and anti-SARS coronavirus effects [32].
Fig. 1.
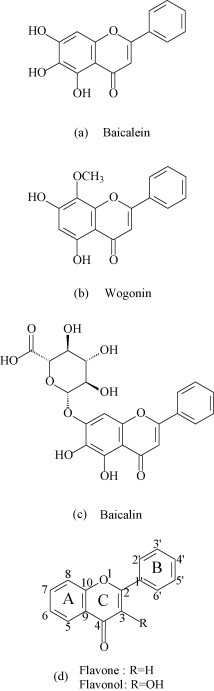
Molecular structure of (a) baicalein, (b) wogonin, (c) baicalin and (d) flavone and flavonol.
The chemical structures of flavones and flavonols are presented in Fig. 1(d). Studies show that flavonols can bind DNA by intercalation and the planarity of the chromophore is limiting factors in the interaction [33], [34], [35]. In this paper, the mechanism of flavonoids–DNA interaction was studied by absorption, fluorescence, melting temperature and viscosity measurement. Flavonoids form the stable compound with various metal cations, which have demonstrable antibacterial properties and anti-tumor activity [36], [37]. It is obvious that a better knowledge of the interaction between flavonoid–metal complexes and DNA may permit a better understanding of the biological and pharmaceutical properties of flavonoids in presence of metals. So the interaction of metal complexes with DNA was investigated by spectral methods.
2. Experimental
2.1. Reagents and materials
Highly polymerized deoxyribonucleic acid (DNA) from fish sperm and EB were commercially purchased from Sigma and used without further purification. The stock solution of DNA and EB were prepared by directly dissolving the DNA and EB in doubly distilled water and stored at −4 °C in the dark, and the concentration of DNA and EB in solution were determined spectrophotometrically using extinction coefficients of ɛ 260 (25 °C) = 6600 L mol−1 cm−1 [38] and ɛ 480 (25 °C) = 5600 L mol−1 cm−1, respectively. Purity of the final DNA preparation was checked by monitoring the absorption spectrum and the ratio of the absorbance at 260–280 nm. The solution gave a ratio of A 260/A 280 > 1.8, indicating that DNA was sufficiently free from protein [38].
The stock solutions of baicalein, wogonin and baicalin purchased from China Drug Biological Product Qualifying Institute were prepared in ethanol. The CuCl2, MgCl2 and AlCl3 used in the experiment were obtained from Tianjin Chemical Reagents Company. The inorganic salt stock solutions were prepared in doubly distilled water, and the concentration of Cu(II), Mg(II) and Al(III) was 1.0 mol L−1, respectively. Briton–Robinson buffer (pH 7.4) was used to control the pH of the working solution and NaCl was used to adjust the ionic strength of the solution. The solutions were adjusted with the Tris–HCl buffer solution (0.05 mol L−1, pH 7.4) when the binding of the flavonoid–metal complexes to DNA was studied. All other reagents were of analytical reagent grade, and doubly distilled water was used throughout. All working solutions were prepared by appropriate dilution at a room temperature before used.
2.2. Procedures
2.2.1. Spectral measurements
All absorption spectra were conducted using a GBC Cintra 10e UV–vis spectrophotometer (Australia) and a matched set of 1 cm path length quartz cuvettes. The slit width was 1.5 nm, and the scan rate was 442 nm min−1. The UV–vis absorption spectra of flavonoids and the flavonoid–metal complexes were measured. The drug–Cu(II), drug–Mg(II) and drug–Al(III) binary systems were formed by titrating the flavonoids with CuCl2, MgCl2 and AlCl3 stock solutions in microliter quantities, respectively.
All fluorescence measurements were performed on a Shimadzu RF-5301PC fluorescence spectrophotometer (Kyoto, Japan) using a quartz cuvette of 1 cm path length. Samples were excited at 543 nm, and emission spectra were recorded in the range from 550 to 700 nm. Both emission and excitation slits were set at 5 nm. The fluorescence spectra were measured by fixing the concentration of DNA–EB while varying the flavonoid concentration.
All solutions were shaken thoroughly and allowed to equilibrate for 20 min before spectral measurements were made at room temperature. The pH value of solution was measured with a Delta 320 pH meter (Mettler-Toledo Instruments (Shanghai) Co. Ltd., Shanghai, China). An electric thermostat water-bath (Tianjin Taisite Instrument Company, Tianjin, China) was used for controlling the temperature. The primary data were transferred to the ORIGIN graphic program for plotting and analysis.
2.2.2. DNA melting studies
DNA melting experiments were carried out by monitoring the fluorescence intensities of the sample at various temperatures in the absence and presence of the flavonoids. The temperature of the sample was continuously monitored with a thermocouple attached to the sample holder. The fluorescence intensities were then plotted as a function of temperature ranging from 20 to 100 °C. The melting temperature (T m) of DNA was determined as the transition midpoint.
2.2.3. Viscosity measurements
Viscosity measurements were performed by using a viscometer, which was immersed in a thermostat water-bath at 30 ± 0.1 °C. Typically, 10.0 mL buffer and 10.0 mL DNA were transferred into the viscometer separately. An appropriate amount of drugs were then added into the viscometer to give a certain r (r = [drug]/[DNA]) value while keeping the DNA concentration constant. After a thermal equilibrium was achieved (15 min), the flow times of samples were repeatedly measured with an accuracy of ±0.2 s by using a digital stopwatch. Flow times were above 200 s, and each point measured was the average of at least five readings. The data were presented as (η/η 0)1/3 versus r, where η and η 0 are the viscosity of DNA in the presence and the absence of drug, respectively.
2.2.4. Effect of ionic strength
A series of assay solutions containing various amount of NaCl and a fixed amount of flavonoid–DNA–EB were prepared to measure the fluorescence intensity. Furthermore, the quenching constants of the flavonoid–DNA–EB complex at three salt concentrations (0, 0.1 and 0.2 mmol L−1) were determined.
3. Results and discussion
3.1. UV–vis spectral studies
The binding of drugs to DNA has been characterized classically through absorption titrations [39]. Generally, red shift (or blue shift) and hypochromic (or hyperchromic) effect are observed in the absorption spectra of small molecules if they intercalate with DNA. The UV–vis spectra of the flavonoids are characterized by two transitions: a lowest energy absorption in the 304–350 nm region (bandI), and a highest energy band in the 250–280 nm region (bandII). The absorption spectra of flavonoids are given in Fig. 2 . Baicalein has three peaks, 217, 275 and 335 nm, wogonin has four peaks, 214, 240, 280 and 360 nm, respectively and baicalin has three peaks, 215, 278 and 315 nm. The absorption peaks of 275, 280 and 278 nm are attributed to the absorption of benzoly of ring A (bandII), and the absorption peaks of 335, 360 and 315 nm are attributed to the absorption of cinnamoyl of ring B (bandI). The spectral changes in the presence of DNA are clearly observed. Because there is no substituent group of ring B, the absorption at 335, 360 and 315 nm is without any change. A blue shift of 14, 6 and 6 nm are observed from the absorption at 275, 280 and 278 nm, and the absorption intensity increase by 97.9%, 42.9% and 45.8% when 0.11 mmol L−1 DNA is added, respectively. Because the A ring in the flavonoids is coplanar with the B ring and the A ring is stabilized by the intra-molecular hydrogen bonds (O2–H–O5), which would also facilitate its entry into the intercalation site. The electronic absorption spectra of the three kinds of flavonoids in the presence of DNA show strong hyperchromicity and blue shift in the absorption of benzoly of ring A, indicating the intercalation of the three kinds of flavonoids with DNA.
Fig. 2.
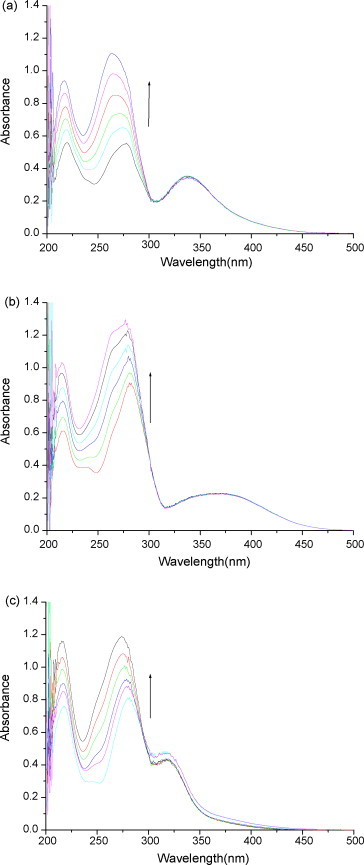
Absorption spectra of (a) baicalein, (b) wogonin and (c) baicalin in the presence of DNA at different concentrations. CDNA = 0.00, 2.21, 4.42, 6.62, 8.83 and 11.04 × 10−5 mol L−1 for curves 1–6. Arrow shows that the absorbance changes upon increasing DNA concentration.
3.2. Fluorescence spectroscopic studies
3.2.1. Binding characteristics of flavonoids with DNA in the presence of EB
The fluorescence method is very useful to estimate the biological activities of some drug molecules because their biological activities correlate well with DNA binding affinity. In terms of the mechanism, small molecules are bound to DNA double helix by three binding modes: electrostatic binding, groove binding and intercalative binding [40]. Fluorescence of EB is weak, but its fluorescence intensity in the presence of DNA can be greatly enhanced (see Fig. 3 ) because of its strong intercalation between the adjacent DNA base pairs. It was reported that the enhanced fluorescence can be quenched, at least partly by the addition of a second molecule [41], [42], [43]. So the binding of the flavonoids was evaluated using competitive binding studies involving EB.
Fig. 3.
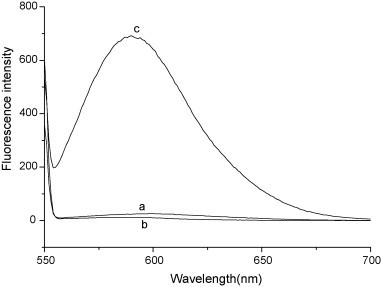
Fluorescence spectra of (a) EB, (b) DNA and (c) EB–DNA with excitation at 543 nm in a Britton–Robinson buffer. The concentrations of DNA and EB were 2.2 × 10−5 and 3.9 × 10−6 mol L−1, respectively.
The optimal ratio of DNA to EB in solution was determined by a fluorescence method. The concentration of EB (3.9 × 10−6 mol L−1) was fixed and the concentration of DNA was varied in assay solutions. The fluorescence signal increases by increasing the concentration of DNA (Fig. 4 ). When DNA concentration is higher than 6.6 × 10−5 mol L−1, the change of fluorescence intensity tends to be a constant. Based on this result, the DNA concentration in the experiment was selected as 1.1 × 10−5 mol L−1 and the ratio of DNA to EB was 2.82:1.
Fig. 4.
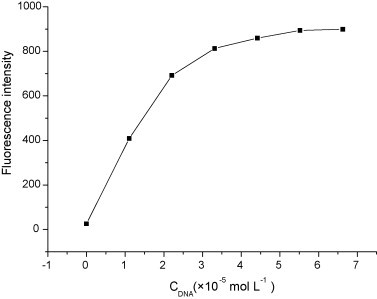
Variations in fluorescence intensity of EB with increasing the concentration of DNA. CEB = 3.9 × 10−6 mol L−1; CDNA = 0.00, 1.10, 2.21, 3.31, 4.42, 5.52 and 6.62 × 10−5 mol L−1.
The emission spectra of EB bound to DNA in the absence and the presence of flavonoids are given in Fig. 5 . There are a significant decrease in the fluorescence intensity (>50%) and a red shift in the emission wavelength from 593 to 600 nm. The shift is attributed to the overlap of the decreasing DNA–EB band at 593 nm and the progressively growing 600 nm band from the free EB in solution. Furthermore, the quenching is not saturated when the fluorescence intensity is reduced to nearly half the initial value. These results indicate that the DNA-bound EB fluorophore is partially replaced by flavonoids and the flavonoids intercalate into the DNA, which is consistent with the above results obtained by absorption measurement.
Fig. 5.
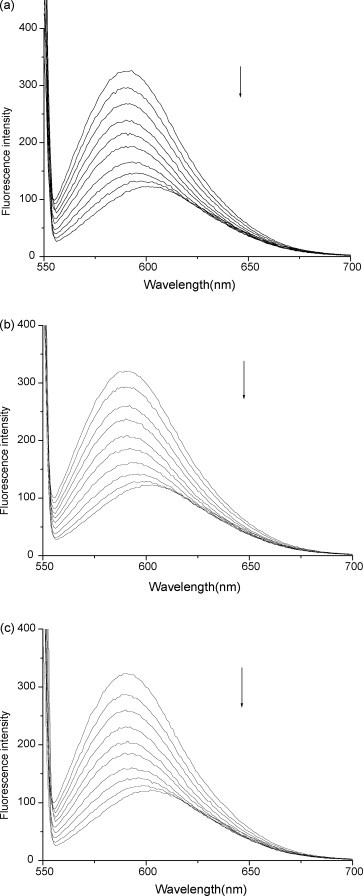
Fluorescence spectra of EB bound to DNA in the absence and presence of (a) baicalein, (b) wogonin and (c) baicalin. CEB = 3.9 × 10−6 mol L−1; CDNA = 1.1 × 10−5 mol L−1; Cbaicalein = 0.00, 1.11, 2.22, 3.34, 4.45, 5.56, 6.67, 7.78, 8.90 and 10.00 × 10−5 mol L−1; Cwogonin = 0.00, 2.02, 3.04, 4.05, 5.06, 6.07, 7.08, 8.10 and 9.11 × 10−5 mol L−1; Cbaicalin = 0.00, 1.26, 2.51, 3.77, 5.02, 6.28, 7.54, 8.79, 10.05 and 11.30 × 10−5 mol L−1. The arrow shows the intensity changes on increasing the flavonoids concentration.
However, at increasing concentrations of the fluorescent substrate, its increasing absorbance introduces the inner filter effect (IFE) that decreases the fluorescence emission and change. So the inner filter effect must be evaluated. The corrected fluorescence intensity is approximately given by [44], where F corr and F obs are the corrected and observed fluorescence intensities, and A ex and A em are the absorbance values of flavonoids at the excitation and emission wavelengths. The experimental results indicated that the sum of the absorbances at excitation and emission wavelengths was less than 0.001 and the value of was about 1.0 when the concentrations of flavonoids was less than 11.30 × 10−5 mol/L. So the observed fluorescence intensities were applied throughout the experiment.
3.2.2. Fluorescence quenching studies
Fluorescence quenching refers to any process in which the fluorescence intensity of a given fluorophore decreases upon adding quencher [45]. Stern–Volmer K sv is used to evaluate the fluorescence quenching efficiency. According to the classical Stern–Volmer equation [46]:
where F 0 and F are the fluorescence intensities in the absence and presence of flavonoids, respectively. K q is the DNA–EB quenching rate constant. τ 0 is the average lifetime of DNA–EB in the absence of the flavonoids and its value is 10−9 to 10−7 s [43], [47]. [D] is the flavonoids concentration. K sv is the quenching constant and equals K q multiplied by τ 0. Based on the quenching of EB fluorescence, an experimental strategy for determining the quenching constant of the ligand molecule interacting with DNA has become a standard method in nucleic acid chemistry [48]. Flavonoids with increasing concentration were added into the fixed amount of DNA–EB solution. The nature of the quenching process was inferred by comparison of the behavior of the Stern–Volmer plots at 25 and 37 °C. The quenching curves are shown in Fig. 6 , where each point in the plots is the average of five values measured at the maximum emission (λ max). For the concentration of flavonoids lower than 50 μmol L−1, which corresponds to the molar ratio drug/DNA of 5:1, the behaviors of the three plots are linear. However, the plots show a small upward concave curvature toward the y-axis at the flavonoid concentrations higher than 50 μmol L−1.
Fig. 6.
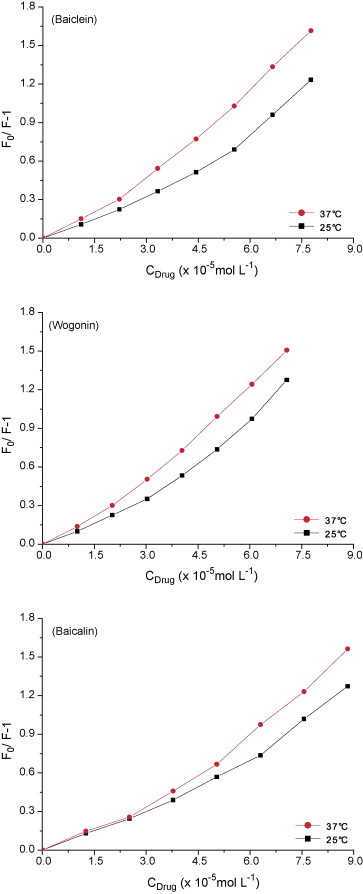
Stern–Volmer plots for the quenching of DNA–EB by flavonoids at 25 and 37 °C.
It is well known that there are two quenching processes: static and dynamic quenching [47]. Fluorescence quenching can be dynamic, resulting from the collisional encounters between the fluorophore and quencher, or static, resulting from the formation of a ground-state complex between the fluorophore and quencher [47]. The plots of the quenching of EB bound to DNA by the flavonoids progressively loose its linearity, which indicates that not only one type of quenching process occurred. First, based on the Stern–Volmer equation and curves in Fig. 6, the K sv can be obtained (Table 1 ). Based on K sv = K q τ 0 and τ 0 values [43], [47], the K q can be obtained (Table 1). For baicalein, wogonin and baicalin, the values of K q (Table 1) are much greater than 2.0 × 1010 L s−1 mol−1, the maximum diffusion collision quenching rate constant of various quenchers with biopolymers [49]. The experimental results demonstrate that the quenching process is static quenching. Secondly, dynamic quenching depends upon diffusion. Since increasing the temperature results in higher diffusion coefficients, the quenching constants are expected to increase [45], [50]. The deviation of the plots toward the y-axis and the upward concave curvature are significant when the temperature increases. The increasing of the angular coefficient caused by the higher temperature suggests the occurrence of dynamic quenching for DNA–EB quenching by flavonoids. On the other hand, a non-linear Stern–Volmer plot may be suggestive of the existence of more than one binding site with different accessibilities for flavonoids in the proximity of DNA, and such sites would facilitate flavonoids penetration into the hydrophobic region of DNA. It is also possible that the increasing number of binding sites can be the result of conformational changes in the DNA molecule, or it may indicate the occurrence of combined quenching.
Table 1.
The quenching constants and quenching rate constants for flavonoids and DNA at 25 and 37 °C
| Compound | T (°C) | Ksv (104 mol L−1) | Kq (1011–13 L s−1 mol−1) | r |
|---|---|---|---|---|
| Baicalein | 25 | 1.26 | 1.26 | 0.9960 |
| 37 | 1.87 | 1.87 | 0.9941 | |
| Wogonin | 25 | 1.45 | 1.45 | 0.9912 |
| 37 | 1.96 | 1.96 | 0.9935 | |
| Baicalin | 25 | 1.17 | 1.17 | 0.9854 |
| 37 | 1.50 | 1.50 | 0.9965 | |
r is regression coefficient
There are some differences of the three kinds of flavonoids in the structure, but the graphs (Fig. 6) indicate that quenching of EB–DNA by baicalein, wogonin and baicalin is similar.
3.2.3. Binding constants and binding sites
If the static binding reaction between the DNA and drug happens, and there are the similar and independent binding sites n in the DNA, namely, the binding capability of DNA at each binding site is equal. The binding constant K A and the number n of binding sites can be described by following equation [51]:
Based on the equation and curves in Fig. 5, the binding constant K A and the number n of binding sites are obtained and shown in Table 2 .
Table 2.
Binding constants and binding sites of flavonoids to DNA at 25 and 37 °C
| Compound | T (°C) | KA (105 mol L−1) | n | r |
|---|---|---|---|---|
| Baicalein | 25 | 0.53 | 1.15 | 0.9992 |
| 37 | 1.38 | 1.21 | 0.9978 | |
| Wogonin | 25 | 1.33 | 1.23 | 0.9985 |
| 37 | 1.76 | 1.22 | 0.9993 | |
| Baicalin | 25 | 0.24 | 1.08 | 0.9977 |
| 37 | 0.82 | 1.18 | 0.9923 | |
r is regression coefficient.
3.2.4. Thermodynamic parameters and the nature of the binding forces
To get a detailed view of the interaction, the approach of parsing the free energy into component terms is a powerful and insightful method. Intercalators are a very important class of DNA interactive ligand, while the number of detailed thermodynamic studies characterizing these interactions is relatively few. There are essentially four types of non-covalent interactions that can play a role in drugs binding to biomolecules such as hydrogen bonds, van der Waals forces, electrostatic and hydrophobic bonds interactions. Intercalation, a planar aromatic chromophore is inserted between two adjacent base pairs in a DNA helix, and minor groove binding, an isohelical drug molecule binds in the minor groove of DNA without inducing significant structural changes in the DNA [52]. The complex of intercalation is stabilized by hydrophobic interactions and van der Waals forces, however, the complex of minor groove binding is stabilized again mainly by hydrophobic interactions [52].
If the enthalpy change (ΔH) does not vary significantly over the temperature range studied, then the enthalpy change can be estimated indirectly by examining the temperature dependence of K A and using the van’t Hoff equation. That is to say, the enthalpy change is considered as 0 in little change of temperature.
The free energy change (ΔG) and the entropy change (ΔS) are estimated from the following relationship:
In this paper, based on the binding constant K A values obtained from the experimental results at 25 and 37 °C, ΔG, ΔH and ΔS obtained are listed in Table 3 . These results show that the binding of flavonoids to DNA is endothermic, and the acting force between flavonoids and DNA is mainly hydrophobic interaction forces. When the chromophore penetrates the DNA helix, the main interaction is hydrophobic contact, but the van der Waals forces and hydrogen bonds cannot be excluded. The stabilization of the flavonoid–DNA complexes is due to a hydrophobic interaction between the hydrophobic segment of the flavonoids and the intercalation site, which would permit this part of the chromophore to penetrate the DNA helix and to arrange its planar structure parallel to the adjacent planes of the nitrogenous bases. It is known that the hydrogen bond can be formed to stabilize the already intercalated molecules. A strong hydrogenous bond between a flavone (2,6-dimethoxyflavone) and orthophosphoric acid was found in an interesting study based on X-ray crystallographic data [53].
Table 3.
Thermodynamic parameters for the interactions of flavonoids and DNA at 25 °C
| Compound | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 k−1) |
|---|---|---|---|
| Baicalein | −26.97 | 60.77 | 294.44 |
| Wogonin | −29.23 | 17.93 | 158.26 |
| Baicalin | −28.02 | 77.80 | 449.11 |
3.3. Melting studies
The melting temperature (T m) of DNA characterizes the transition from double-stranded to single-stranded nucleic acid [54]. DNA melting experiments were carried out by controlling various temperatures in the absence and presence of flavonoids while monitoring the fluorescence intensity. Intercalation of small molecules into the double helix is known to cause stabilization of base stacking and increase the DNA melting temperature, at which the double helix denatures into single stranded DNA [55]. Intercalation binding can stabilize the double helix structure and T m increases by about 5–8 °C, but the non-intercalation binding causes no obvious increase in T m [56]. The DNA melting curves obtained in the absence and presence of flavonoids are shown in Fig. 7 , and the T m value decreases from 89 to 85 °C. Because the planar of flavonoids is different with the rigid planar of the typical intercalator, the decreased value is less than those obtained with classical intercacators. That is to say, the flavonoids provide lower accessibility for DNA binding than EB and the stability of the flavonoids–DNA complex is poorer than that of the EB–DNA complex.
Fig. 7.
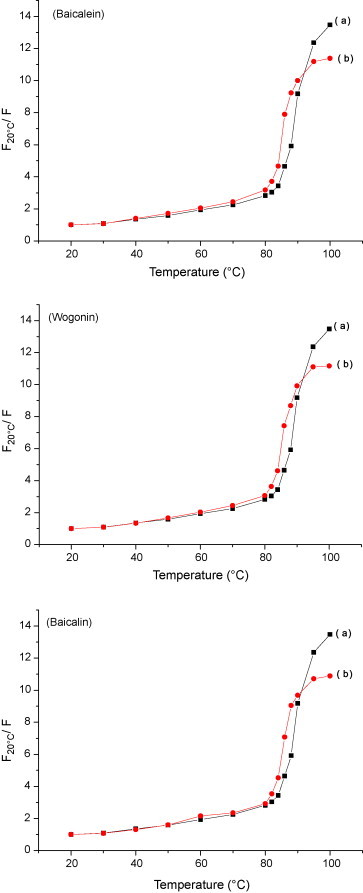
Melting curves of DNA–EB in the absence of flavonoids (a) and in the presence of flavonoids (b).
3.4. Viscosity studies
In addition to spectroscopic data we carried out viscosity experiment which is regarded as less ambiguous and the most critical test for a DNA binding model in solution, and provides stronger arguments for intercalation mode [56], [57]. A classical intercalation mode results in lengthening the DNA helix, as base pairs are separated to accommodate the binding ligand, leading to the increase of DNA viscosity [40]. However, a partial and/or nonclassical intercalation of ligand may bend (or kink) the DNA helix, resulting in the decrease of the effective length and the viscosity [57], [58], [59]. Fig. 8 shows the effect of increasing the flavonoid concentration on the relative viscosity of the DNA. The relative viscosity of DNA increases, which follows the order, baicalein > baicalin > wogonin. The increased relative viscosity of DNA indicates the intercalation of flavonoids into DNA, which can be also explained by the molecular structure, as the A ring in the flavonoids is coplanar with the B ring and the A ring is stabilized by the intra-molecular hydrogen bonds (O2–H–O5) that may lead to the intercalative mode.
Fig. 8.
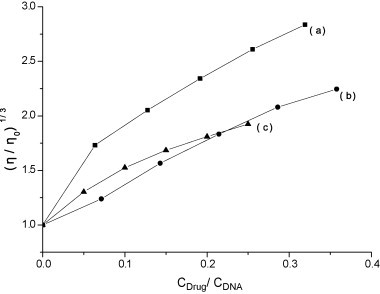
Effect on the relative viscosity of DNA–EB in the presence of (a) baicalein, (b) wogonin and (c) baicalin.
3.5. Effect of ionic strength on the fluorescence properties
The study of the ionic strength effect is also an efficient method to distinguish binding mode between molecules and DNA. It is apparent that intercalative binding and groove binding are related to the groove in the DNA double helix, but the electrostatic binding can take place out of the groove. When NaCl exists in the system, the electrostatic repulsion between the negatively charged phosphate skeletons on adjacent nucleotides is reduced with increasing the concentration of Na+. That is to say, electrostatic interactions are shielded by the presence of ions and the DNA chains will be tightened. However, for groove binding modes, a small molecule binds in the groove of DNA duplex and exposes much more to the solvent surrounding than it does for the intercalation [56], [60]. Thus, the groove bound molecules can be released from the helix by increasing the ionic strength, whereas it is difficult for the intercalating bound molecules to be released [56]. So the effect of NaCl on the fluorescence of flavonoid–DNA–EB and DNA–EB were studied. Classical intercalators are planar aromatic ring systems such as acridines, anthracyclines, EB, etc. [61]. In particular, EB molecule is a planar aromatic ring system positively charged. Thus, the interaction between EB and DNA has two binding modes: intercalative binding and electrostatic binding.
The experimental results show that fluorescence intensities of DNA–EB and flavonoid–DNA–EB decrease linearly with increasing NaCl concentration at the same solution conditions. Furthermore, we studied the quenching of the flavonoid–DNA–EB at three salt concentrations (0, 0.1 and 0.2 mmol L−1). The quenching constants are obtained and shown in Table 4 . It can be seen from Table 4 that the quenching constants increase with the increase of the ionic strength. The reason of the increase for quenching constants is that the EB molecule by electrostatic binding is released from the helix due to the electrostatic attraction between phosphate group and Na+. However, the EB molecule by intercalative binding cannot be influenced by the ionic strength. On the other hand, the existence of Na+ ions makes the double helix structure of DNA gather together longitudinally, and flavonoids are likely to intercalate the helix through the addition of salt. These results indicate that ionic strength has an influence on the binding behavior of EB with DNA, however, the effect of ionic strength on the flavonoids–DNA system is very limited. So the interaction between flavonoids and DNA is intercalative binding.
Table 4.
Effect of ionic strength on the quenching constants of flavonoids–DNA
| CNaCl (mol L−1) |
Ksv (104 mol L−1) |
||
|---|---|---|---|
| Baicalein | Wogonin | Baicalin | |
| 0 | 1.07(0.9868) | 0.90(0.9949) | 0.96(0.9963) |
| 0.1 | 1.73(0.9906) | 1.40(0.9869) | 1.43(0.9862) |
| 0.2 | 2.26(0.9973) | 1.80(0.9940) | 1.84(0.9897) |
The data in the parentheses is the regression coefficient.
3.6. Effect of metal ions
Some metal ions, such as Cu(II), Mg(II) and Al(III), especially transition metals, not only play vital roles in a vast number of widely biological processes, but also may be potentially toxic in their free state. The studies of interactions between flavonoid–metal complexes and DNA are important to further the understanding of pharmacology of flavonoids. Some literatures dealt with the complex formation of quercetin with metals, such as Al(III), Fe(II), Fe(III) and Cu(II), etc. However, data on the composition, structure and complex formation features are sometimes incomplete and contradictory [62]. Although the structures of the three flavonoids are similar, some differences are observed in their spectra.
3.6.1. Binding characteristics of flavonoids with Cu(II), Mg(II) and Al(III)
Titrating flavonoids solution with Cu(II), Mg(II) and Al(III) in increasing concentration in the Tris–HCl buffer (pH 7.4) separately. In the UV–vis spectra, baicalein absorption peaks at 270 (bandII) and 344 nm (bandI) are observed (Fig. 9 ). The peak of 270 nm with addition of Cu(II) or Mg(II) first shows a decrease in the absorbance, and then an increase with blue shift (13 nm). Red shift occurs at peak of 344 nm (14 nm). But the absorption spectra of baicalein–Al(III) complex only shows a decrease of the absorption intensity. The titration of baicalein–metal complexes is characterized by forming two isosbestic points, and the absorption spectra appear a new peak at the wavelength of 426 nm and increase progressively in absorbance, which indicate that baicalein can form stable complexes with metal ions.
Fig. 9.
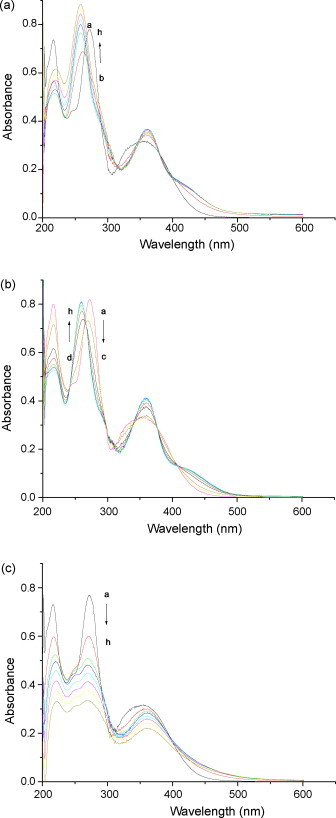
Absorption spectra of baicalein in the presence of (a) Cu2+, (b) Mg2+ and (c) Al3+. Cbaicalein = 2.66 × 10−5 mol L−1; [Cu2+] = 0.00, 1.00, 2.00, 3.00, 4.00, 5.00, 6.00 and 7.00 × 10−5 mol L−1; [Mg2+] = 0.00, 0.25, 0.50, 0.75, 1.00, 1.25, 1.50 and 1.75 × 10−2 mol L−1; [Al3+] = 0.00, 0.40, 0.80, 1.20, 1.60, 2.00, 2.40 and 2.80 × 10−4 mol L−1.
The UV–vis absorption spectra of wogonin and baicalin in the presence of Cu(II), Mg(II) or Al(III) were examined (data not displayed). There are two possible groups on flavonoids that can interact with metal ions: the 5-hydroxyls and the 4-carbonyl of ring C. These results indicate that flavonoids can bind to Cu(II), Mg(II) or Al(III) and the bound chromophore is converted to a new species, and the binding modes is similar.
3.6.2. The influence of metal ions on the fluorescence quenching of DNA with flavonoids
In order to make the results comparable, the same procedure was applied to estimate the quenching of the flavonoid–metal complexes binding to DNA in the Tris–HCl buffer (pH 7.4). Fluorescence quenching experiments were conducted by adding flavonoids into the samples containing the fixed of DNA–EB and metal ions concentration. When the molar ratio of the flavonoid to DNA increased, the extent of the fluorescence quenching increased gradually at the same concentration of the metal ions. The quenching curves are shown in Fig. 10 . It can also be found from Fig. 10 that the fluorescence of DNA–EB is quenched efficiently by the baicalein–Al(III) complex. When baicalein concentration increases to 2.66 × 10−5 mol L−1, the change of fluorescence intensity tends to be constant. However, the action of baicalin–Al(III) to quench the DNA–EB is weakened in the system. The planarity of baicalein–Al(III) complex efficiently intercalates into DNA base pairs, but the branch chain of baicalin is not helpful to intercalate. The branch chain of baicalin is likely to bind DNA by out-side self-stacking along the DNA helix, or bind DNA in the groove. This can be explained that baicalein–Al(III) has a higher binding affinity with DNA compared with wogonin–Al(III) and baicalin–Al(III).
Fig. 10.
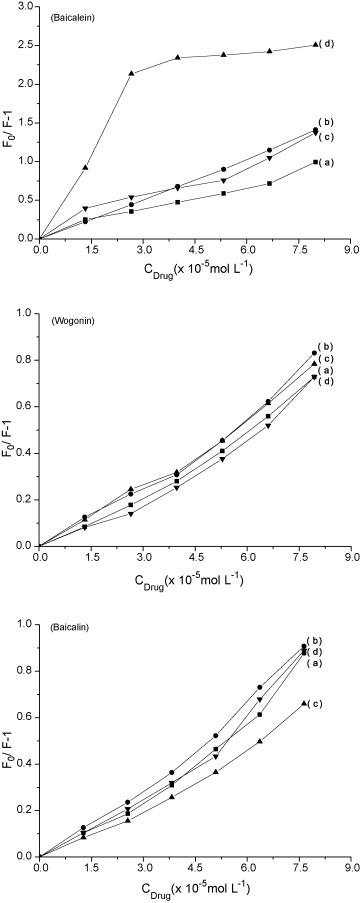
Fluorescence quenching curve of EB bound to DNA at the same concentration of the metal ions (16 μmol L−1) by flavonoids: (a) DNA–EB–flavonoid, (b) DNA–EB–flavonoid–Cu2+, (c) DNA–EB–flavonoid–Mg2+ and (d) DNA–EB–flavonoid–Al3+.
All of the above facts indicate that the 5-hydroxy-4-carbonyl group of flavonoids presents the strongest chelating power with metal ions, and extends the planarity of flavonoids so that these complexes can intercalate into the double helix of DNA with varying binding affinities.
4. Conclusions
The binding of flavonoids to DNA results in hyperchromic effect and blue shift in UV–absorption spectra and fluorescence quenching in fluorescence emission spectra. There are a significant decrease in the fluorescence intensity (>50%) and a red shift in the emission wavelength. These spectral characteristics strongly support intercalation of flavonoids into DNA, and the intercalated EB is displaced from the DNA–EB complex by flavonoids. This conclusion is further reinforced by melting experiments, viscosity measurements and salt effects. From a variety of experiments, it is concluded that baicalein, wogonin and baicalin intercalate into the double-stranded DNA molecules, which accounts for the high binding constant. In addition to the binding mode, the complete thermodynamic profiles for the binding of flavonoids to DNA are also constructed, and results show that the binding of flavonoids to DNA is endothermic. The different behavior of baicalein, wogonin and baicalin binding to DNA duplex is due to the different side chain of the benzoly of ring A, and the branch chain of baicalein provides greater accessibility for DNA binding than those of wogonin and baicalin. This is also confirmed by the spectral and viscosity measurements, so that the benzoly of ring A of the planarity of flavonoids is important for binding.
The effects of flavonoid–metal on the binding to the DNA by applying UV–absorbtion and fluorescence spectrometry were studied. The results clearly show that these metal ions form complexes with baicalein, wogonin and baicalin, and these complexes influence the fluorescence quenching in the system.
Biographies
Yantao Sun is a lecturer at College of Chemistry, Jilin Normal University. She received her master degree in Chemistry, Department of Yanbian University in 2002. She is currently a PhD student in College of Chemistry, Jilin University. Her interest is spectral analysis.
Shuyun Bi is an associate professor at College of Chemistry, Changchun Normal University. She received her doctor's degree from Jilin University in 2006.
Daqian Song gained his doctor's degree from College of Chemistry, Jilin University in 2003 and he is an associate professor in that school.
Chunyu Qiao is a PhD student of College of Chemistry, Jilin University.
Dan Mu is a Master student of College of Chemistry, Jilin University.
Hanqi Zhang is a professor of College of Chemistry, Jilin University. He graduated from Jilin University in 1977. His research area is spectrochemical analysis.
References
- 1.Hartshorn R.M., Barton J.K. Molecular “Light Switch” for DNA: Ru(bpy)2(dppz)2+ J. Am. Chem. Soc. 1990;112:4960–4962. [Google Scholar]
- 2.Thornton N.B., Schanze K.S. A chromophore–quencher-based luminescence probe for DNA. Inorg. Chem. 1993;32:4994–4995. [Google Scholar]
- 3.Yarmoluk S.M., Kovalska V.B., Kryvorotenko D.V., Balanda A.O., Ogul’chansky T.Yu. Interaction of cyanine dyes with nucleic acids. XXV. Influence of affinity-modifying groups in the structure of benzothiazol-4-[2,6-dimethylpyridinium] dyes on the spectral properties of the dyes in the presence of nucleic acids. Spectrochim. Acta A. 2001;57:1533–1540. doi: 10.1016/s1386-1425(01)00435-8. [DOI] [PubMed] [Google Scholar]
- 4.Keek M.V., Lippard S.J. Unwinding of supercoiled DNA by platinum–ethidium and related complexes. J. Am. Chem. Soc. 1992;114:3386–3390. [Google Scholar]
- 5.Dardlier P.J., Holmlin R.E., Barton J.K. Science. 1997;275:1465–1468. doi: 10.1126/science.275.5305.1465. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y., He X.W. Studies of interaction between safranine T and double helix DNA by spectral methods. Spectrochim. Acta A. 1998;54:883–892. doi: 10.1016/s1386-1425(97)00277-1. [DOI] [PubMed] [Google Scholar]
- 7.Krishna A.G., Kumar D.V., Khan B.M., Rawal S.K., Ganesh K.N. Taxol–DNA interactions: fluorescence and CD studies of DNA groove binding properties of taxol. Biochim. Biophys. Acta. 1998;1381:104–112. doi: 10.1016/s0304-4165(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 8.Radi A., Ries M.A.E., Kandil S. Electrochemical study of the interaction of levofloxacin with DNA. Anal. Chim. Acta. 2003;495:61–67. [Google Scholar]
- 9.Rauf S., Gooding J.J., Akhtar K., Ghauri M.A., Rahman M., Anwar M.A., Khalid A.M. Electrochemical approach of anticancer drugs–DNA interaction. J. Pharm. Biomed. Anal. 2005;37:205–217. doi: 10.1016/j.jpba.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 10.Udenfriend S., Zaltzman P. Fluorescence characteristics of purines, pyrimidines, and their derivatives: measurement of guanine in nucleic acid hydrolyzates. Anal. Biochem. 1962;3:49–59. doi: 10.1016/0003-2697(62)90043-x. [DOI] [PubMed] [Google Scholar]
- 11.Pastre D., Pietrement O., Zozime A., Le Cam E. Study of the DNA/ethidium bromide interactions on mica surface by atomic force microscope: influence of the surface friction. Biopolymers. 2005;77:53–62. doi: 10.1002/bip.20185. [DOI] [PubMed] [Google Scholar]
- 12.Burns V.W.F. Fluorescence decay time characteristics of the complex between ethidium bromide and nucleic acids. Biochem. Biophys. 1969;133:420–424. doi: 10.1016/0003-9861(69)90471-8. [DOI] [PubMed] [Google Scholar]
- 13.Wahl P.J., Paoletti C., Le Pecq J.B. Decay of fluorescence emission anisotropy of the ethidium bromide-DNA complex evidence for an internal motion in DNA. Proc. Natl. Acad. Sci. U.S.A. 1970;65:417–421. doi: 10.1073/pnas.65.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresloff J.L., Crothers D.M. Equilibrium studies of ethidium–polynucleotide interactions. Biochemistry. 1981;20:3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- 15.Nafisi S., Saboury A.A., Keramat N., Neault J.-F., Tajmir-Riahi H.-A. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J. Mol. Struct. 2007;827:35–43. [Google Scholar]
- 16.Youdim K.A., Spencer J.P.E., Schroeter H., Rice-Evans C. Dietary flavonoids as potential neuroprotectants. Biol. Chem. 2002;383:503–519. doi: 10.1515/BC.2002.052. [DOI] [PubMed] [Google Scholar]
- 17.Commenges D., Scotet V., Renaud S., Jacqmin-Gadda H., Barberger-Gateau P., Dartigues J.-F. Eur. Intake of flavonoids and risk of dementia. J. Epidemiol. 2000;16:357–363. doi: 10.1023/a:1007614613771. [DOI] [PubMed] [Google Scholar]
- 18.2000 ed. China Chemical Industry Press; Beijing: 1999. China Pharmacopoeia Committee Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 19.Ye F., Wang H., Jiang S., Wu J., Shao J., Cheng X., Tu Y., Zhang D.Y. Quality evaluation of commercial extracts of Scutellaria baicalensis. Nutr. Cancer. 2004;49:217–222. doi: 10.1207/s15327914nc4902_14. [DOI] [PubMed] [Google Scholar]
- 20.Koda A., Watanabe S., Yanagihara Y., Nagai H., Sakamoto K. A comparative study of the anti allergic effects of disodium baicalein 6 phosphate (BPS) and disodium cromoglycate (DSCG) Jpn. J. Pharmacol. 1977;27:31–38. doi: 10.1254/jjp.27.31. [DOI] [PubMed] [Google Scholar]
- 21.Gao Z., Huang K., Yang X., Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim. Biophys. Acta. 1999;1472:643–650. doi: 10.1016/s0304-4165(99)00152-x. [DOI] [PubMed] [Google Scholar]
- 22.Shieh D.E., Liu L.T., Lin C.C. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Cancer Res. 2000;20:2861–2866. [PubMed] [Google Scholar]
- 23.Li B.Q., Fu T., Yan Y.D., Baylor N.W., Ruscetti F.W., Kung H.F. Inhibition of HIV infection by baicalin-A flavonoid compound purified from Chinese herbal medicine. Cell Mol. Biol. Res. 1993;39:119–124. [PubMed] [Google Scholar]
- 24.Wu J.A., Attele A.S., Zhang L., Yuan C.S. Anti-HIV activity of medicinal herbs: usage and potential development. Am. J. Chin. Med. 2001;29:69–81. doi: 10.1142/S0192415X01000083. [DOI] [PubMed] [Google Scholar]
- 25.Li B.Q., Fu T., Gong W.H., Dunlop N., Kung H.F., Yan Y.D., Kang J., Wang J.M. The flavonoid baicalin exhibits anti-inflammatory activity by binding to chemokines. Immunpharmacology. 2000;49:295–306. doi: 10.1016/s0162-3109(00)00244-7. [DOI] [PubMed] [Google Scholar]
- 26.Konoshima T., Kokumai M., Kozuka M., Iinuma M., Mizuno M., Tanaka T., Tokuda H., Nishino H., Iwashima A. Studies on inhibitors of skin tumor promotion. XI. Inhibitory effects of flavonoids from Scutellaria baicalensis on Epstein-Barr virus activation and their anti-tumor-promoting activities. Chem. Pharm. Bull. 1992;40:531–533. doi: 10.1248/cpb.40.531. [DOI] [PubMed] [Google Scholar]
- 27.Chan F.L., Choi H.L., Chen Z.Y., Chan P.S.F., Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000;160:219–228. doi: 10.1016/s0304-3835(00)00591-7. [DOI] [PubMed] [Google Scholar]
- 28.Ikemoto S., Sugimura K., Yoshida N., Yasumoto R., Wada S., Yamamoto K., Kishimoto T. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55:951–955. doi: 10.1016/s0090-4295(00)00467-2. [DOI] [PubMed] [Google Scholar]
- 29.Hui K., Huen M., Wang H., Zheng H., Sigel E., Baur R., Ren H., Li Z.W., Wong J.T.F., Xue H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002;64:1415–1424. doi: 10.1016/s0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- 30.Huang R.L., Chen C.C., Huang H.L., Chang C.G., Chen C.F., Chang C.M., Hsieh M.T. Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med. 2000;66:694–698. doi: 10.1055/s-2000-9775. [DOI] [PubMed] [Google Scholar]
- 31.Lee B.H., Lee S.J., Kang T.H., Kim D.H., Sohn D.H., Ko G.I., Kim Y.C. First-order system least-squares for elliptic problems with Robin boundary conditions. Planta Med. 2000;66:70–104. [Google Scholar]
- 32.Chen F., Chan K.H., Jiang Y., Kao R.Y.T., Lu H.T., Fan K.W., Cheng V.C.C., Tsui W.H.W., Lee T.S.W., Guan Y., Peiris J.S.M., Yuen K.Y. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solimani R., Bayon F., Domini I., Pifferi P.G., Todesco P.E., Marconi G., Samorì B. Flavonoid–DNA interaction studied with flow linear dichroism technique. J. Agric. Food Chem. 1995;43:876–882. [Google Scholar]
- 34.Solimani W.R. Quercetin and DNA in solution: analysis of the dynamics of their interaction with a linear dichroism study. Int. J. Biol. Macromol. 1996;18:287–295. doi: 10.1016/0141-8130(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 35.Riccardo S. The flavonols quercetin, rutin and morin in DNA solution: UV–vis dichroic (and mid-infrared) analysis explain the possible association between the biopolymer and a nucleophilic vegetable-dye. Biochim. Biophys. Acta. 1997;1336:281–294. doi: 10.1016/s0304-4165(97)00038-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J., Wang L.F., Wang J.Y., Tang N. Antioxidative and anti-tumour activities of solid quercetin metal (II) complexes. Transit. Metal Chem. 2001;26:57–63. [Google Scholar]
- 37.Zhou J., Wang L.F., Wang J.Y., Tang N. Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth(III) complexes. J. Inorg. Biochem. 2001;83:41–48. doi: 10.1016/s0162-0134(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 38.Reichmann M.E., Rice S.A., Thomas C.A., Doty P. A further examination of the molecular weight and size of desoxypentose nucleic acid. J. Am. Chem. Soc. 1954;76:3047–3053. [Google Scholar]
- 39.Pyle A.M., Rehmann J.P., Meshoyrer R., Kumar C.V., Turro N.J., Barton J.K. Mixed-ligand complexes of ruthenium(II): factors governing binding to DNA. J. Am. Chem. Soc. 1989;111:3051–3058. [Google Scholar]
- 40.Lerman L.S. Structural considerations in interaction of DNA and acridines. J. Mol. Biol. 1961;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- 41.Wolf A., Shimer G.H., Jr., Meehan T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry. 1987;26:6392–6397. doi: 10.1021/bi00394a013. [DOI] [PubMed] [Google Scholar]
- 42.Baguley B.C., Lebret M. Quenching of DNA–ethidium fluorescence by amsacrine and other antitumor agents: a possible electron-transfer effect. Biochemistry. 1984;23:937–943. doi: 10.1021/bi00300a022. [DOI] [PubMed] [Google Scholar]
- 43.Lakowicz J.R., Webber G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry. 1973;12:4161–4170. doi: 10.1021/bi00745a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steiner R.F., Weinryb L. Plenum Press; New York: 1971. Excited States of Protein and Nucleic Acid. pp.40. [Google Scholar]
- 45.Lakowitcz J.R. Plenum Press; New York: 1983. Principles of Fluorescence Spectroscopy. pp. 257–267. [Google Scholar]
- 46.Dewey T.G. Plenum; New York: 1991. Biophysical and Biochemical Aspects of Fluorescence Spectroscopy. pp. 1–41. [Google Scholar]
- 47.Lakowitcz J.R. Plenum Press; New York: 1999. Principles of Fluorescence Spectroscopy. pp. 237–259. [Google Scholar]
- 48.Ruckebusch C., Duponchel L., Huvenne J.P., Caudron A., Boilet L., Cornard J.P., Merlin J.C., de Juan A. Chemometric strategies for the study of the complexation of Al(III) ions with model molecule of humic substances from UV–vis data sets. Anal. Chim. Acta. 2005;544:337–344. [Google Scholar]
- 49.Ware W.R. Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process. J. Phys. Chem. 1962;66:455–458. [Google Scholar]
- 50.Silva D., Cortez C.M., Louro S.R.W. Chlorpromazine interactions to sera albumins: A study by the quenching of fluorescence. Spectrochim. Acta A. 2004;60:1215–1223. doi: 10.1016/j.saa.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Jiang M., Xie M.X., Zheng D., Liu Y., Li X.Y., Chen X. Spectroscopic studies on the interaction of cinnamic acid and its hydroxyl derivatives with human serum albumin. J. Mol. Struct. 2004;692:71–80. [Google Scholar]
- 52.Haq I. Part II: The thermodynamics of drug–bipolymer interaction thermodynamics of drug–DNA interactions. Arch. Biochem. Biophys. 2002;403:1–15. doi: 10.1016/S0003-9861(02)00202-3. [DOI] [PubMed] [Google Scholar]
- 53.Wallet J.C., Cody V., Wojtczac A., Blessing R.H. Structural and conformational studies on bio-active flavonoids. Crystal and molecular structure of a complex formed between 2′,6′-dimethoxyflavone and orthophosphoric acid: A model for flavone-nucleotide interactions. Anticancer Drug Des. 1993;8:325–332. [PubMed] [Google Scholar]
- 54.Kalouli E.T., Katsaros N. The interaction of [Ru(NH3)5Cl]2+ and [Ru(NH3)6]3+ ions with DNA. J. Inorg. Biochem. 1989;37:271–282. doi: 10.1016/0162-0134(89)85002-0. [DOI] [PubMed] [Google Scholar]
- 55.Patel D.J. Nuclear magnetic resonance studies of drug–nucleic acid interactions at the synthetic dna level in solution. Acc. Chem. Res. 1979;12:118–125. [Google Scholar]
- 56.Kumar C.V., Turner R.S., Asuncion E.H. Groove binding of a styrylcyanine dye to the DNA double helix: the salt effect. J. Photochem. Photobiol. A: Chem. 1993;74:231–238. [Google Scholar]
- 57.Satyanarayana S., Dabrowiak J.C., Chaires J.B. Neither DELTA-nor LAMBDA-tris(phenanthroline) ruthenium(II) binds to DNA by classical intercalation. Biochemistry. 1992;31:9319–9324. doi: 10.1021/bi00154a001. [DOI] [PubMed] [Google Scholar]
- 58.Kapicak L., Gabbay E.J. Effect of aromatic cations on the tertiary structures of deoxyribonucleic acid. J. Am. Chem. Soc. 1975;97:403–408. doi: 10.1021/ja00835a031. [DOI] [PubMed] [Google Scholar]
- 59.Song Y.F., Yang P. Mononuclear tetrapyrido [3,2-a:2′,3′-c:3‴, 2‴-h:2‴,3‴-j] phenazine (tpphz) cobalt complex. Polyhedron. 2001;20:501–506. [Google Scholar]
- 60.Wang A.H.J., Teng M.K., Bugg C.E., Ealick S.E., editors. Crystallographic and Modeling Methods in Molecular Design. Springer; New York: 1990. p. 123. [Google Scholar]
- 61.G.M. Blackburn, M.J. Gait (Eds.), IRL Press, 1990.
- 62.Torreggiani A., Tamba M., Trinchero A., Bonora S. Copper(II)–quercetin complexes in aqueous solutions spectroscopic and kinetic properties. J. Mol. Struc. 2005;744–747:759–766. [Google Scholar]


