Abstract
Although, to our knowledge, there has been no exhaustive or credible review of the evidence of the disease burden of COPD in China, COPD has become an increasing public health concern to the Chinese medical community. The purpose of this article is to review the evidence and evaluate and clarify the disease burden of COPD in China with the aim of improving effective management. We reviewed previous studies of COPD in China, which included data on prevalence, mortality, disease burden, risk factors, diagnosis, and management by searching related Web sites, including PubMed, ProQuest, and Thomson Reuters' Web of Knowledge, as well as major Chinese databases and government Web sites. Reported COPD prevalence varied between 5% and 13% in different provinces/cities across China. In 2008, COPD ranked fourth as a leading cause of death in urban areas and third in rural areas. In addition, COPD accounted for 1.6% of all hospital admissions in China in that year. The high prevalence of smoking and biomass fuel use acted as major contributors to the high occurrence of COPD in China. Management of COPD in China should focus on adjusting the distribution of medical resources and on addressing public health policies to facilitate earlier diagnosis in rural areas, aim to reduce smoking prevalence, improve patients' self-management, and keep physicians' knowledge up to date and consistent with current guidelines. COPD is one of the most challenging medical issues facing China because of its influence on both personal and public health and its impact on the economy. Optimal management strategies should be adopted and strengthened immediately.
Abbreviations
- DALY
disability-adjusted life year
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
COPD is characterized by a progressive deterioration of lung function and mental and physical comorbidities such as depression, dystrophy, and heart failure.1 The data suggest that COPD imposes an enormous burden on patients, health-care professionals, and society. COPD contributes to morbidity and mortality and to a significant use of health-care resources and expense on a global scale; this is especially true in developing countries.2, 3, 4 The burden of COPD in China is currently greater than that found in developed countries. This is probably due to greater exposure to epidemic risk factors, an imbalance in economic development, and health-care disparities between urban and rural areas. The reported prevalence of COPD morbidities and genetic susceptibility varies widely among geographic regions in China, and there is still a great need to learn more about the disease burden of COPD. In this review, we analyzed available studies on COPD in China, including data on prevalence, mortality, disease burden, risk factors, diagnosis, and patient management, to improve awareness of COPD and highlight its importance to facilitate the implementation of public health policies.
Epidemiology
Prevalence
There is still a need for well-designed nationwide epidemiologic studies of COPD in China. A population-based, cross-sectional survey of COPD conducted between 2002 and 2004 showed that the overall prevalence of the disease in people aged > 40 years was 8.2%,5 which would result in a COPD patient population of > 43 million in that time period. It was reported that the prevalence of COPD varied widely among locations across the country, from 5% to 13% (Table 1 ).6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 Most locations had a higher prevalence of COPD than the World Health Organization model-estimated prevalence for China (6.7%)20 and the reported prevalence rates in Western countries (4%-10%).21 But the cross-sectional survey of COPD results were consistent with the pooled prevalence (meta-analysis) of the Western Pacific Region (9.0%).3 The crude prevalence of COPD was significantly higher in men than in women (8.3%-18.9% vs 3.8%-7.1%), in rural areas than in urban areas (4.4%-16.7% vs 6.7%-8.3%), in the elderly, and in nonsmoking rural women. These differences were associated with gender, smoking status, biomass fuel use, occupational dust exposure, socioeconomic status, and availability of health-care resources. It was reported that approximately two-thirds of the patients were underdiagnosed, and the prevalence of diagnosed COPD in rural areas was apparently lower than that in urban areas,21 suggesting an inferior diagnostic rate in rural areas.
Table 1.
COPD Prevalence in Various Provinces in China
| Prevalence, % |
||||||||
|---|---|---|---|---|---|---|---|---|
| Study/Year | City/Province | Age, y | N | Diagnostic Criteria | M/F | R/U | T | Risk Factors |
| Tang et al6/2001 | Anhui | 9.8/5.4 | 7.3/… | 7.3 | Gender; smoking; age of starting smoking; sites of inhaling smoke; time of heating; dust exposure; marital status; pepper consumption | |||
| Xu et al7/2005 | Nanjing/Jiangsu | ≥ 35 | 29,319 | Physician diagnosis | 7.2/4.7 | 4.4/6.7 | 5.9 | Gender; age; living conditiona; smoking; TACS |
| Liu et al8/2005 | Guangdong | ≥ 40 | 3,286 | Post-BD spirometry | 15.9/4.8 | 12.0/7.4 | 9.4 | Gender; age; smoking; biomass fuel use; ventilation in the kitchen; family history; respiratory infection during childhood |
| Shan and Chen9/2007 | Tianjin | ≥ 40 | 3,008 | Post-BD spirometry | … | 11.4/8.3 | 9.6 | Age; gender; smoking; family history; living conditiona |
| Ma et al10/2005 | Shanghai | ≥ 65 | 1,214 | Post-BD spirometry | 18.9/6.5 | 16.7/8.0 | 12.1 | Gender; living conditiona; educational level; ventilation in the kitchen and living room; smoking; TACS; occupational exposure |
| Cai et al11/2009 | Shilin/Yunnan | ≥ 45 | 6,006 | Ph | 8.3/5.1 | 6.7/… | 6.7 | Gender; age; biomass fuel exposure |
| Wang et al12/2005 | Shaoguan/Guangdong | ≥ 40 | 1,468 | Post-BD spirometry | 18.3/7.1 | 12.0/… | 12.0 | Smoking; age; frequent cough during childhood; biomass fuel exposure |
| Yao et al13/2005 | Yanqing/Beijing | ≥ 40 | 1,624 | Post-BD spirometry | 15.1/3.8 | 9.1/… | 9.1 | Gender; age; smoking; family history; frequent cough before 14 y; BMI |
| Jiang et al14/2007 | Hubei | ≥ 40 | 1,883 | Post-BD spirometry | 13.7/6.6 | 9.9/… | 9.9 | Gender; age; smoking; cooking time; family history; frequent cough before age 14 y |
| Chen et al15/2008 | Lianjiang/Guangdong | ≥ 40 | 1,368 | Post-BD spirometry | 11.1/4.3 | 7.0/… | 7.0 | Age; gender; smoking; BMI; biomass fuel exposure; ventilation in the kitchen; occupational exposure; respiratory infection during childhood; family history |
| Li et al16/2009 | Chongqing | ≥ 40 | 1,518 | Post-BD spirometry | 23.0/7.5 | …/12.8 | 12.8 | Smoking; biomass fuel exposure |
| Hong et al17/2009 | Changsha/Hunan | ≥ 15 | 8,243 | Spirometry | 7.6/2.6 | 5.3/4.8 | 5.1 | Smoking; biomass fuel exposure; gender; family history; educational level |
| Zhang et al18/2006 | Qingdao/Shandong | ≥ 40 | 410 | Spirometry | 7.9/5.9 | 6.9/… | 6.9 | Age; smoking; biomass fuel exposure; living area; family history; BMI |
| Hou et al19/2007 | Shenyang, Jinzhou/Liaoning | ≥ 40 | 1,100 | Post-BD spirometry | … | … | 5.9 | Age; family history; low BMI |
Data collected from literature covered by SCI or Chinese Core Journals. Postbronchodilator FEV1/FVC of 70% was defined as COPD according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) diagnostic criteria. BD = bronchodilator; F = female; M = male; Ph = physician diagnosis; R = rural; T = total; TACS = total amount of cigarettes smoked; U = urban.
Rural areas or urban areas.
Mortality and Hospitalization
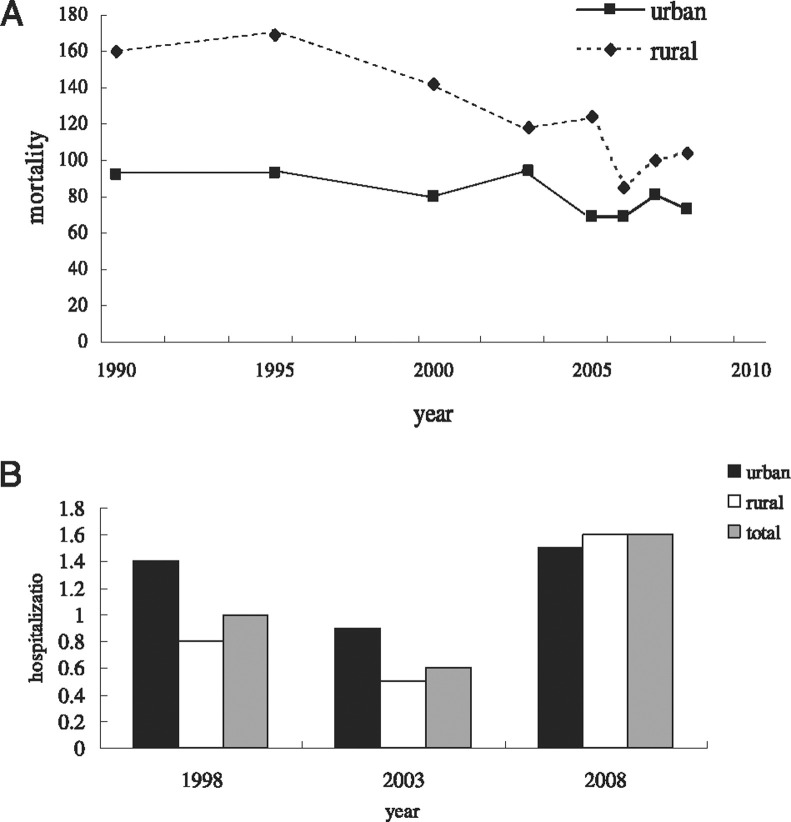
The Global Burden of Disease study conducted in 2004 showed that about 3 million people die of COPD each year, of which 1.8 million deaths occur in middle-income countries. COPD is expected to become the third leading cause of death globally by 2030, trailing only ischemic heart disease and cerebrovascular disease.4 According to data published by the Chinese Ministry of Health, COPD ranks as the fourth leading cause of death in urban areas and third leading in rural areas.22 Both crude and age-adjusted COPD mortality rates have fluctuated but have displayed a decreasing trend from 1990 (Fig 1A ),22 which is probably because of improved management of COPD, upgraded technologies, and awareness of the disease.
Figure 1.
A, COPD mortality trend in urban and rural areas from 1990 to 2008. Data were collected from the Chinese Ministry of Health.22 B, Hospitalization rate of COPD for 1998, 2003, and 2008 in China. The decline of hospitalization in 2003 could be attributable to the outbreak of severe acute respiratory syndrome.
Another nationwide, large-scale, long-term prospective cohort study between 1990 and 2000 demonstrated that COPD-related mortality was 27.3 for men and 21.3 for women per 100,000 persons > 40 years of age, making COPD the seventh leading cause of death in men and eighth leading in women.23 When cor pulmonale deaths with documented COPD history were included, COPD-related mortality increased to 179.9 for men and to 141.3 for women per 100,000 persons.24 Both rates were much higher than the corresponding estimated rates in the Asia-Pacific region (64-92 per 100,000 population in men and 21-35 per 100,000 population in women).25 It was reported that COPD-related mortality was higher in rural areas than in urban areas, mainly because of the unavailability of health-care resources and more exposure to indoor air pollution. Another finding showed that COPD-related mortality in northern China was higher than in other locations;24 the higher mortality rate in the north might be associated with a colder climate, more use of biomass fuel, heavier outdoor air pollution, and lower socioeconomic status. Although mortality rates fell, hospitalization rates continued to rise in the last decade. Hospitalization rates for COPD jumped from 1.0% in 1998 (urban vs rural was 1.4% vs 0.8%) to 1.6% in 2008 (urban vs rural was 1.5% vs 1.6%). Correspondingly, the gap between rural and urban areas narrowed (Fig 1B).22 This is partially a result of the increase in incidence but it also indicates that patients in rural areas are more aware of COPD, and that resources for rural residents have been increasing. The hospitalization rate trend is consistent with that of other Asian countries.26
Burden of COPD
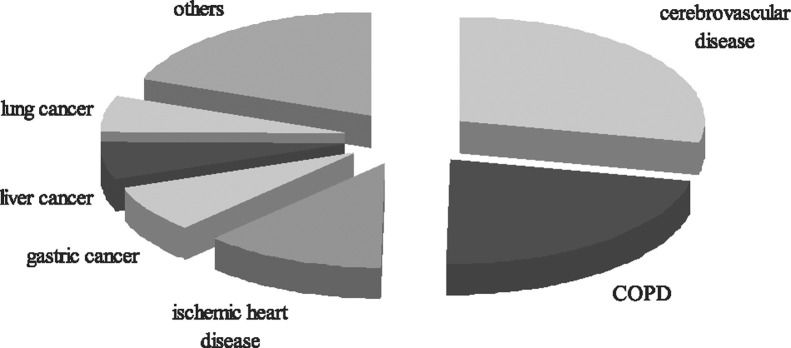
COPD treatments are extremely costly in China and impose an enormous economic burden on both families and society. Health-care expenses were investigated with person-to-person interviews in a cross-sectional survey conducted among 723 COPD outpatients in six large cities in China.27 It was found that the annual direct medical expense for urban patients in 2006 was $1732.24 per patient, whereas the estimated indirect expense spent on nutraceutics, transportation, and end-of-life care was $231.6 per patient. Total expense for one COPD patient ($1963.8) accounted for 40% of the average family's total income ($4849.8). Evidence showed that the cost of COPD was strongly correlated with the severity of disease and that hospitalization acted as a major contributor to total cost.26, 28 It was suggested that reducing hospitalization frequency through better management could result in a crucial decrease in health-care expenses. Moreover, the burden of COPD has been underestimated consistently because of premature mortality and impaired productivity of patients and their family members. The overall disease burden, measured by disability-adjusted life years (DALYs), was predicted to double over the next 2.5 decades worldwide, at which point COPD would move from the 13th-highest cause of DALYs worldwide to the seventh-highest by 2030. The burden of COPD in China was greater than that in developed Western countries, and it ranked second among chronic diseases as a leading cause of DALYs lost in 2001 (Fig 2 ).29
Figure 2.
The leading chronic diseases that caused disability-adjusted life years (DALYs) lost in China. Data were collected from the Chinese Center for Disease Control.29 The DALYs lost caused by chronic diseases accounted for 70% of the total DALYs lost. Cerebrovascular disease and COPD ranked as the top two leading causes of DALYs lost in China, responsible for 17.9% and 13.9%, respectively, followed by ischemic heart disease, gastric cancer, liver and lung cancer, and others.
Risk Factors
Tobacco Smoking
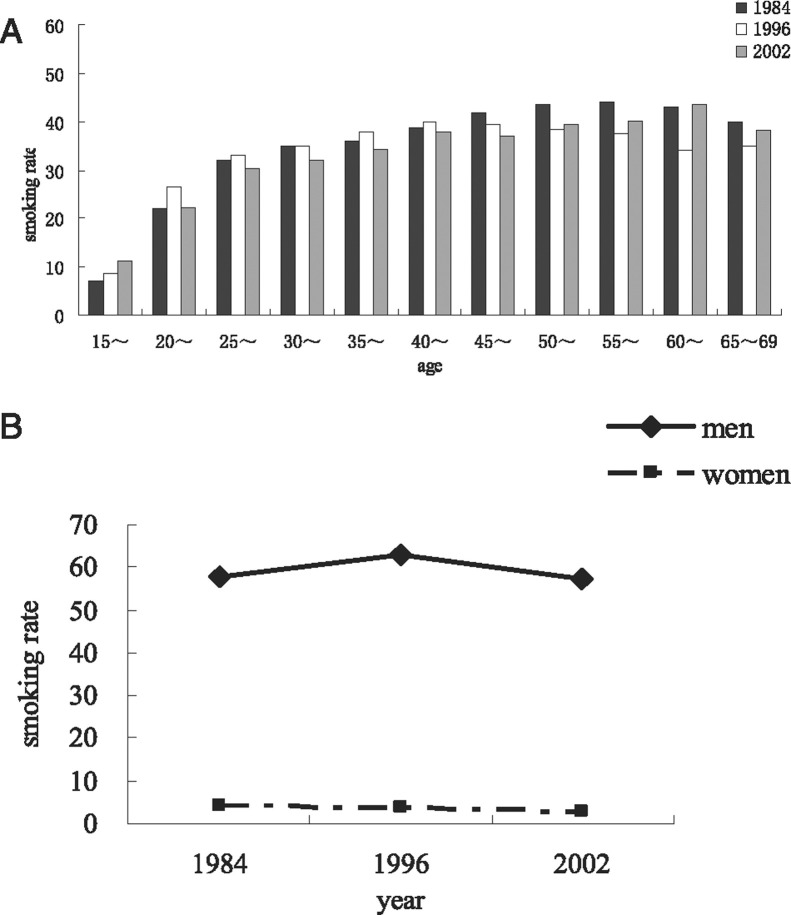
In 2002, about 30% of the world's cigarettes were manufactured and consumed in China,30 which had an estimated 350 million smokers2; furthermore, the prevalence of smoking in women has been increasing (Fig 3 ).30, 31, 32, 33 In addition, there has been a steady increase in the rate of household second-hand smoke exposure,34 and the rate of passive smoking among nonsmoking females has increased to 82.5%.35 Taking all smokers and passive smokers into consideration, 72% of Chinese aged > 15 years were tobacco exposed.36 Individuals who were less educated, poorer, and employed in stressful jobs were more likely to be heavy smokers and less likely to quit than were wealthier, better-educated individuals. Smoking the Chinese pipe was once prevalent in rural areas, but only a minority of people do it today. Smokers consumed cheaper cigarettes but spent a larger proportion of their personal income on smoking. (The cost of cigarettes per pack varied from $0.294 to $33.971 in China37). Smoking has been recognized as the most important causative factor in the pathogenesis of COPD.38 In 2005, a total of 673,000 deaths were attributed to smoking in China, whereas 268,000, 146,200, and 66,800 deaths were attributed to cancer, cardiovascular disease, and respiratory disease, respectively.39 In another case-control study, conducted from 2000 to 2001 in Nanjing, a significant dose-response relationship between COPD and total amount of cigarettes smoked was detected after adjusting for other risk factors. The study further showed that women smokers were more susceptible to COPD.21, 40
Figure 3.
Smoking prevalence in China. A, Overall prevalence of smoking for 1984, 1996, and 2002 by age group in China. B, Smoking rate trend for women and men from 1984 to 2002.
Because of a diversity of methodologies and exposure assessment criteria, there are conflicting results regarding an association between passive smoking and the risk of COPD. The aim of the Biobank Cohort Study was to demonstrate that the high prevalence of COPD among Chinese nonsmokers is the result of a positive dose-response relationship between passive smoking exposure and any respiratory symptom.41, 42 It was estimated that 1.9 million excessive deaths from COPD without smoking could be attributed to passive smoking.40 In contrast to these findings, no significant association between COPD and passive smoking was revealed in other localized surveys.7, 11, 40
In the survey conducted by Yang et al,43 about 74% of the smokers indicated that they did not want to quit, which may be partly because of ignorance about the harmful effects of smoking. About 20% of the smokers had intended to quit smoking at least once, of which about one-half reverted to smoking.43, 44 Smoking cessation has been the most cost-effective intervention in COPD management,1 and the importance of effective tobacco-control programs cannot be emphasized too strongly. Men and women who stopped smoking in 2003 were expected to lower their absolute risks of COPD by 56% and 63% after 5 years relative to those who continued smoking.45 Antismoking education should focus the public's attention on the risk of active and passive smoking, as well as the expense of smoking, especially among the poor.46
Use of Biomass Fuels
The use of biomass fuels has been considered one of the most important risk factors for COPD.47, 48, 49, 50, 51 Men and women who are exposed to biomass smoke have an odds ratio of 4.30 and 2.73, respectively, for developing COPD, relative to those not exposed to biomass smoke.51 It was reported that solid fuels, such as wood, crop residues, and coal, were used in > 70% of Chinese households, whereas in rural areas, the rate increased to 90%.40, 52 In a cluster disproportional random sampling survey performed in populations aged > 40 years in urban and rural areas in Guangdong, COPD prevalence among rural nonsmoking women was found to be significantly higher than that among urban nonsmoking women (7.2% vs 2.5%). Univariate analysis showed a significant association between COPD and exposure to biomass fuel for cooking.50 In a retrospective cohort study, installation of chimneys on household coal stoves led to a substantial reduction in COPD incidence.53 Other evidence showed that when biomass fuel was used in cooking and/or heating, the exposure time and ventilation status in the kitchen/living room were associated with COPD prevalence.5, 6, 9, 11, 12, 13, 14, 52 Biomass smoke may interact with cigarette smoking in the pathogenesis of COPD, with ORs of 4.39 and 2.55 for cigarette smokers and nonsmokers, respectively.51 A meta-analysis of many epidemiologic studies demonstrated that if the status of smoking and solid-fuel use persists, then 65 million deaths from COPD can be expected, and 82% of COPD deaths between now and 2033 will be attributed to the combined effect of smoking and solid-fuel use, whereas 26 million deaths from COPD will be avoided by reducing smoking and solid-fuel use with intermediate magnitude. Halving solid-fuel use would lower the annual number of COPD cases by 2.2 million in men and by 4.3 million in women.45, 54 Along with rapid development of the economy and urbanization in most rural areas, indoor air pollution and ventilation in the kitchen would be gradually improved and the negative influence of biomass fuel use on health would be expected to decrease.
Genetic Susceptibility
COPD is a complex condition resulting from the interaction of environmental and genetic factors. Familial clustering was reported in the studies of COPD in China.6, 7, 9, 10, 11, 15, 51 Several gene polymorphisms have been investigated in the Chinese population, but there is a need for further clarification and confirmation. Individuals with α1-antiprotease deficiency had a markedly increased risk of developing COPD in Europe,55 whereas few patients with α1-antiprotease deficiency developed COPD in China.56 Another case-control study among the Han population in southwest China failed to obtain evidence of the contribution of SERPINE2 polymorphism to COPD susceptibility,57 although it has been accepted as a candidate gene in other populations.58 It was suggested that there might be other genetic factors involved in the development of COPD in the Chinese population.
Microsomal epoxide hydrolase is an enzyme that potentially reduces oxidative stress by detoxifying epoxide compounds such as those found in cigarette smoke. An updated meta-analysis of 16 studies on such hydrolase polymorphisms in COPD in the Asian population suggested that the slow activity phenotype of the enzyme is associated with an increased risk of COPD, and the fast activity phenotype is a protective factor in the development of COPD.59 Genetic polymorphism in Heme oxygenase-1, one of the antioxidant enzymes, was found to be associated with the severity of COPD in southwest China.60, 61 γ-Glutamylcysteine synthetase and 8-hydroxy-guanine glycosylase both play roles in the antioxidant defenses, and both were reported to be not associated with susceptibility to COPD in a southern Chinese population.62 Polymorphisms of IL-27 gene,63 matrix metalloproteinase-9 gene,64 tumor necrosis factor-α,65, 66, 67 and aquaporin 568, 69 were demonstrated to play roles in susceptibility to COPD. Clara cell 16 kDa secretory protein, which plays a potential role in the control of inflammatory response, was not found to be associated with the development of COPD.59 The study of gene polymorphisms might be helpful in defining patient subgroups, leading to the development of different approaches to the prevention and treatment of COPD.
Others
Studies have shown that smoking and the individual's socioeconomic status are correlated to the influence of occupational exposure on COPD prevalence.70, 71, 72, 73 Studies have investigated green tea as an antioxidant in the treatment of COPD and have found that its use might prevent the occurrence of the disease.74 There is a need to evaluate how tea drinking or other dietary habits may influence the development or treatment of COPD in China. Malnutrition may aggravate dyspnea and exercise intolerance in COPD patients by weakening respiratory muscle strength. Low BMI was reported to be closely connected with a high incidence of COPD,75, 76 as well as an increased hospitalization rate and poor prognosis.77, 78 Outdoor air pollution is also considered an important risk factor for COPD, evidenced by the fact that ambient concentrations of air pollutants have an adverse effect on hospital admissions for COPD.79 Another case-crossover analysis of air pollution and daily mortality in Shanghai showed an association between air pollution and mortality with COPD and cardiovascular causes.80 The results confirmed the deleterious role played by the current air pollution level in COPD, especially in large cities like Shanghai. In addition, low educational level, chronic cough or respiratory infection in childhood, low socioeconomic status, and reduced physical activities have all been reported to contribute to the increased incidence of COPD independently or jointly.
Management of COPD in China
A national strategy for the management of COPD, drafted by the Chinese Medical Association, was first published in 2002 and then updated annually.81 Doctors are also encouraged to adopt the GOLD (Global Initiative for Chronic Obstructive Lung Disease) guideline for the care of patients with COPD.1 Despite widely disseminated evidence-based clinical practice guidelines, current knowledge of primary care physicians and management of patients with COPD remain suboptimal.82, 83, 84 Barriers to the implementation of guidelines are multifarious, whether physician related or patient related.
Delayed Diagnosis and Intervention
A lack of awareness about biomarkers and risk factors for COPD among physicians had led to delayed diagnosis and interventions for patients. Early diagnosis may help patients quit smoking and lead to the adoption of effective therapies to prevent progression of COPD. However, more than one-third (35.3%) of COPD patients were asymptomatic, whereas only one-third of subjects with GOLD stage I COPD were diagnosed with respiratory disease.5 In a recent study, the diagnosis of COPD was made in 148 of the 1,624 subjects aged > 40 years in five villages of northern China according to the standards set in the GOLD guideline, whereas none of the patients had ever been diagnosed previously.85 Another retrospective study demonstrated that only 15.9% of the patients in southern China were diagnosed with mild-degree COPD, whereas most of the patients were at a severe or extremely severe stage when first diagnosized.86 This could be attributed to the fact that most Chinese patients, especially those in rural areas with major health-care disparities, are used to bottling up their discomfort until the development of significant symptoms and/or exacerbations.
Diagnosis Without Spirometry
Physicians continue to diagnose and manage patients with COPD without verifying the diagnosis and assessing the severity with spirometry. According to GOLD guidelines, spirometry is the gold standard in the measurement of airflow limitation1 because of its reliable, simple, safe and inexpensive grading, monitoring, and assessing of the disease. However, less than one-third of COPD diagnoses were made with the aid of spirometry87 and in some rural areas none of the patients diagnosed with COPD received a spirometry test.6, 7, 8 In 185 previously diagnosed COPD patients, 67 were confirmed by spirometry based on diagnostic criteria, 47 had normal lung function, and 63 had mixed ventilation disorder.88 Profound underuse of spirometry was considered to be a barrier to decent care of Chinese COPD patients.89 Barriers hindering use of spirometry in clinical practice include ignorance of the crucial role of spirometry, facility inaccessibility, and limited budgets, especially in most of the primary care settings.
Patient Self-Management of COPD
Patients are not knowledgeable about how to manage their COPD. Health education on self-management for patients should be taken into account to reduce the frequency and severity of exacerbations and to improve quality of life.90, 91 However, COPD patients were found to lack general knowledge about the disease. For example, the term “COPD” was recognized by < 30% of the patients who regularly visited hospitals, whereas an even lower number of rural patients recognized the term.85, 92 Patients also lacked awareness of COPD risk factors, the importance of lung function tests, inhalation therapy, and oxygen therapy.93, 94, 95 About 20% of patients were current smokers, of which 23% did not know the harmful effect of smoking on the disease.96 Unfortunately, no health education program was available in rural areas, although all patients were willing to read information about COPD.84, 85, 96 Patients took the initial advice for smoking cessation from primary care physicians, so there is a great need to implement evidence-based guidelines and demand that physicians take immediate action. Studies in some urban hospitals demonstrated that 64.5% of the COPD patients quit smoking after application of general interventions, whereas the cessation rate of the control group was only 28%.86 Underuse of home oxygen therapy, lung function training, and nutrition support among patients94, 95 also suggested the necessity of patient education to facilitate comprehensive rehabilitation.
Physician Recommendations
The recommendations made by physicians often do not meet the standard of the GOLD guidelines. According to the GOLD guidelines, bronchodilators, including β2-agonists, anticholinergics, and methylxanthines, should be the basic agents for all patients, whereas inhaled corticosteroids are recommended only for patients with moderate/severe airflow obstruction and/or frequent exacerbations. An investigation of the application of pharmacologic therapy conducted in six cities, using face-to-face interviews among 723 patients with stable COPD and 258 pulmonary physicians, showed that expectorants were the most often prescribed medications, followed by β2-agonists and anticholinergics.97 Instead of long-acting bronchodilators, more than one-half of the patients were prescribed short- or medium-acting bronchodilators, which was inconsistent with the guidelines.81 Most of the physicians underused oxygen therapy, noninvasive intermittent positive pressure ventilation, and antiinflammation therapy.82 Only 20% of the primary care physicians were fully knowledgeable about pharmacologic therapies for COPD.98
Prospects
COPD represents a challenging issue because of its impact on both personal and public health and its economic consequences. There is an urgent need to understand the present and future burden of COPD in China from well-designed epidemiologic studies on its prevalence and morbidity. Because of ethnic differences, studies on genetic susceptibility to COPD among the Chinese population should be carried out to improve prevention and lead to early diagnosis. Because China is the world's largest cigarette manufacturer and because it is the world's largest consumer of cigarettes, there is a great challenge to restrict and regulate smoking in China with an intent to reduce the burden of COPD. During the last decade, smoking prevention and cessation programs have been implemented widely and preliminary results have been observed (Tables 2 , 3 ).29, 99, 100 However, there are many factors affecting the execution of smoking laws and regulations by the government. Rural workers, teenagers, and women with high or rising rates of smoking need to be targeted specifically for tobacco-control measures. The existing public health policies should be strengthened and new strategies, including raising taxes and the price of cigarettes, should be put into effect. On the other hand, physicians should take the leading role in the fight against smoking. It has been found that about 40% of male physicians are current smokers,101 which adds to the difficulty of smoking control.
Table 2.
Laws Related to Smoking Control in China
| Laws Related to Smoking Control | Year | Content |
|---|---|---|
| Regulations on management of public places | 1987 | Prohibit smoking in public places |
| Law of the People's Republic of China on the Prevention of Minors to Commit Crime | 1999 | Parents and teachers should educate minors not to smoke; Business units should not sell cigarettes to minorsa |
| Law of the People's Republic of China on Tobacco Sales | 1991 | Forbid or restrict cigarette smoking on public conveyances or in public places; dissuade adolescents from smoking and forbid primary and middle school students from smoking |
| Law of the People's Republic of China on Advertisement | 1994 | Smoking-related advertisements are prohibited through media or in any public places; all smoking-related advertisements should include the following label: “smoking is harmful to health” |
| Law of the People's Republic of China on the Protection of Minors, article11, 37,67 | 2006 | The sale of cigarettes to minors is banned; no smoking in schools, kindergartens or any other places where minors are present |
| Framework Convention on Tobacco Control | 2006 | Raise taxes on cigarettes; prohibit smoking in public places; warning notices or pictures of fearful consequences of smoking are printed on the cigarette packets |
“Minors” as used in this law refers to citizens under the age of 18 y.
Table 3.
Smoking Control Mechanisms in China
| Smoking control is required for the criterion of “national clean city” |
| Smoking is prohibited in public places |
| Knowledge that “smoking is harmful to health” is spread through media |
| Attend international smoking cessation contest, encourage the building of a no smoking community and no smoking unit |
| Smoking by teenagers is reduced through laws or local regulations and school-based prevention programs |
| Doctors are encouraged to give up smoking to raise society's awareness of its harmful effects |
| Smoking cessation clinic has been set up |
Another challenge is delayed diagnosis and underestimation of frequent exacerbations in the management of COPD. Screening by spirometry is impractical in most primary care settings and rural areas, indicating the urgent need for disease-specific biomarkers to facilitate early diagnosis and identify high-risk populations.102, 103 Management techniques of COPD should take into consideration cultural variations and mental and physical comorbidities, such as psychologic depression. Comorbidities could influence patients' quality of life, increase hospitalization, and reduce survival. The role played by finances should also be taken into account to improve the poor management of COPD. For example, the cost for drug therapy ($443-$738 per year) in the maintenance phase is a huge economic burden for families and individuals, especially for rural residents. Less than 40% of patients with chronic hypoxemia use oxygen therapy because the cost ($517 per year) is too high. It is necessary to implement a community-based comprehensive intervention, which requires support from national polices, to ensure the availability of medical resources, such as essential drugs, equipment, and human resources, and to improve the affordability of COPD therapy for patients. Continuous educational programs on quality-improving skills and management strategies should be more effective and comprehensive in China to improve health-care quality and physicians' clinical practice skills and to reduce health-care disparities among regions and economic groups.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We express our special appreciation to Mr Mark Danderson for his valuable comments and editorial assistance.
Footnotes
Funding/Support: This work was supported by Shanghai Leading Academic Discipline Project [Grant B115].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Rabe KF, Hurd S, Anzueto A, Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization The global burden of disease: 2004 update, The World Health Organization Web site. 2004. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html Accessed December 2006.
- 5.Zhong N, Wang C, Yao W. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi: 10.1164/rccm.200612-1749OC. [DOI] [PubMed] [Google Scholar]
- 6.Tang G, Yuan F, Pang Y. Survey on prevalence and risk factors of COPD in rural areas in Anhui province [in Chinese] Chin J Tuberc Respir Dis. 2001;24(4):245. [Google Scholar]
- 7.Xu F, Yin XY, Zhang M, Shen H, Lu L, Xu Y. Prevalence of physician-diagnosed COPD and its association with smoking among urban and rural residents in regional mainland China. Chest. 2005;128(4):2818–2823. doi: 10.1378/chest.128.4.2818. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Wang X, Wang D. Epidemiologic analysis of COPD in Guangdong province [in Chinese] Zhonghua Yi Xue Za Zhi. 2005;85(11):747–752. [PubMed] [Google Scholar]
- 9.Shan S, Chen B. Epidemiology Investigation of chronic obstructive pulmonary disease in city and country of Tianjin [in Chinese] Med J Tianjin Univ. 2007;35(7):488–490. [Google Scholar]
- 10.Ma R, Cheng Q, Yao D. Epidemiological survey of chronic obstructive pulmonary disease in the elders in Shanghai. Acad J Shanghai Second Med Univ. 2005;25(5):521–524. [Google Scholar]
- 11.Cai L, Zhao K, Tang P. Analysis on burden of chronic obstructive pulmonary disease in rural Kunming [in Chinese] Chin J Prev Control Chron Dis. 2009;17(1):80–81. [Google Scholar]
- 12.Wang X, Zhou Y, Zeng X. Study on the prevalence rate of chronic obstructive pulmonary disease in northern part of Guangdong province [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(3):211–213. [PubMed] [Google Scholar]
- 13.Yao W, Zhu H, Shen N. Epidemiological data of chronic obstructive pulmonary disease in Yanqing County in Beijing [in Chinese] Beijing Da Xue Xue Bao. 2005;37(2):121–125. [PubMed] [Google Scholar]
- 14.Jiang R, Luo D, Huang C. Study on the prevalence rate and risk factors of chronic obstructive pulmonary disease in rural community population in Hubei province [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2007;28(10):976–979. [PubMed] [Google Scholar]
- 15.Chen H, Su W, Chen X. Epidemiologic survey and risk factors analysis of COPD in People aged 40ys or older in rural areas of Lianjiang, Guangdong [in Chinese] J Guangdong Med. 2008;26(5):560–564. [Google Scholar]
- 16.Li Q, Liao X, Zhang Q. Epidemiological sampling survey on chronic obstructive pulmonary disease in urban area of Chongqing. Chin J Respir Crit Care Med. 2009;8(1):12–15. [Google Scholar]
- 17.Hong XQ, Xiao SY, Dai AG. Epidemic Situation and Risk Factors Analysis of COPD in Partial Areas of Hunan Province. Central-South University; Hunan Province, China: 2009. [Google Scholar]
- 18.Zhang YM, Li YC. Epidemiologic Investigation of Risk Factors of COPD in Peripheral Rural of Qingdao. Qingdao University; Shandong Province, China: 2006. [Google Scholar]
- 19.Hou G, Li M, Feng X. Epidemiologic analysis of COPD in rural females in Liaoning province. J Chin Med Univ. 2007;36(6):671–691. [Google Scholar]
- 20.Regional COPD Working Group COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology. 2003;8(2):192–198. doi: 10.1046/j.1440-1843.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 21.Halbert RJ, Isonaka S, George D, Iqbal A. Interpreting COPD prevalence estimates: what is the true burden of disease? Chest. 2003;123(5):1684–1692. doi: 10.1378/chest.123.5.1684. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Ministry Of Health The top-ten disease-specific rates and causes of death among urban and rural residents. The Ministry of Health of the People's Republic of China Web site. 2008. http://www.moh.gov.cn/publicfiles/business/htmlfiles/zwgkzt/ptjnj/200908/42635.htm Accessed October 2009.
- 23.He J, Gu DF, Wu XG. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- 24.Reilly KH, Gu DF, Duan XF. Risk factors for chronic obstructive pulmonary disease mortality in Chinese adults. Am J Epidemiol. 2008;167(8):998–1004. doi: 10.1093/aje/kwm393. [DOI] [PubMed] [Google Scholar]
- 25.Tan WC, Seale P, Ip M. Trends in COPD mortality and hospitalizations in countries and regions of Asia-Pacific. Respirology. 2009;14(1):90–97. doi: 10.1111/j.1440-1843.2008.01415.x. [DOI] [PubMed] [Google Scholar]
- 26.Tan WC, Ng TP. COPD in Asia: where East meets West. Chest. 2008;133(2):517–527. doi: 10.1378/chest.07-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang CH. Cost analysis of chronic obstructive pulmonary disease in a tertiary care setting in Taiwan. Respirology. 2008;13(5):689–694. doi: 10.1111/j.1440-1843.2008.01308.x. [DOI] [PubMed] [Google Scholar]
- 28.He QY, Zhou X, Xie CM. Impact of chronic obstructive pulmonary disease on quality of life and economic burden in Chinese urban areas [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2009;32(4):253–257. [PubMed] [Google Scholar]
- 29.Chinese Center for Disease Control and Prevention Report on Chronic Disease in China, 2006. http://wenku.baidu.com/view/9113c5aedd3383c4bb4cd274.html Accessed December 2006.
- 30.Wipfli H, Samet JM. Global economic and health benefits of tobacco control: part 1. Clin Pharmacol Ther. 2009;86(3):263–271. doi: 10.1038/clpt.2009.93. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Ma J, Chen AP, Brown S, Taylor CE, Samet JM. Smoking among adolescents in China: 1998 survey findings. Int J Epidemiol. 2004;33(5):1103–1110. doi: 10.1093/ije/dyh225. [DOI] [PubMed] [Google Scholar]
- 32.Anderson Johnson C, Palmer PH, Chou CP. Tobacco use among youth and adults in Mainland China: the China Seven Cities Study. Public Health. 2006;120(12):1156–1169. doi: 10.1016/j.puhe.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 33.Mao R, Li XM, Stanton B. Psychosocial correlates of cigarette smoking among college students in China. Health Educ Res. 2009;24(1):105–118. doi: 10.1093/her/cyn002. [DOI] [PubMed] [Google Scholar]
- 34.Wang CP, Ma SJ, Xu XF, Wang JF, Mei CZ, Yang GH. The prevalence of household second-hand smoke exposure and its correlated factors in six counties of China. Tob Control. 2009;18(2):121–126. doi: 10.1136/tc.2008.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han JX, Ma L, Zhang HW. A cross sectional study of passive smoking of non-smoking women and analysis of influence factors on women passive smoking [in Chinese] Wei Sheng Yan Jiu. 2006;35(5):609–611. [PubMed] [Google Scholar]
- 36.Zhang H, Cai BQ. The impact of tobacco on lung health in China. Respirology. 2003;8(1):17–21. doi: 10.1046/j.1440-1843.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 37.Hesketh T, Lu L, Jun YX, Mei WH. Smoking, cessation and expenditure in low income Chinese: cross sectional survey. BMC Public Health. 2007;7(29):29. doi: 10.1186/1471-2458-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin P, Glasgow H, Patterson J. Chronic obstructive pulmonary disease (COPD): smoking remains the most important cause [abstract] N Z Med J. 2005;118(1213):U1409. [PubMed] [Google Scholar]
- 39.Gu DF, Kelly TN, Wu XG. Mortality attributable to smoking in China. N Engl J Med. 2009;360(2):150–159. doi: 10.1056/NEJMsa0802902. [DOI] [PubMed] [Google Scholar]
- 40.Xu F, Yin XM, Shen HB, Xu Y, Ware RS, Owen N. Better understanding the influence of cigarette smoking and indoor air pollution on chronic obstructive pulmonary disease: a case-control study in Mainland China. Respirology. 2007;12(6):891–897. doi: 10.1111/j.1440-1843.2007.01178.x. [DOI] [PubMed] [Google Scholar]
- 41.Yin P, Jiang CQ, Cheng KK. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet. 2007;370(9589):751–757. doi: 10.1016/S0140-6736(07)61378-6. [DOI] [PubMed] [Google Scholar]
- 42.Menezes AM, Hallal PC. Role of passive smoking on COPD risk in non-smokers. Lancet. 2007;370(9589):716–717. doi: 10.1016/S0140-6736(07)61353-1. [DOI] [PubMed] [Google Scholar]
- 43.Yang G, Ma J, Liu N, Zhou LN. Smoking and passive smoking in Chinese, 2002 [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(2):77–83. [PubMed] [Google Scholar]
- 44.Cheng Y, Jin Y, Gu H, Zhao C. Prevalence survey of smoking pattern among peasants in China [in Chinese] Wei Sheng Yan Jiu. 2003;32(4):366–368. 406. [PubMed] [Google Scholar]
- 45.Lin HH, Murray M, Cohen T, Colijn C, Ezzati M. Effects of smoking and solid-fuel use on COPD, lung cancer, and tuberculosis in China: a time-based, multiple risk factor, modelling study. Lancet. 2008;372(9648):1473–1483. doi: 10.1016/S0140-6736(08)61345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang GH, Ma JM, Chen AP. Smoking cessation in China: findings from the 1996 national prevalence survey. Tob Control. 2001;10(2):170–174. doi: 10.1136/tc.10.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ekici A, Ekici M, Kurtipek E. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93–98. doi: 10.1016/j.envres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 48.Pérez-Padilla R, Regalado J, Vedal S. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. Am J Respir Crit Care Med. 1996;154(3 Pt 1):701–706. doi: 10.1164/ajrccm.154.3.8810608. [DOI] [PubMed] [Google Scholar]
- 49.Kiraz K, Kart L, Demir R. Chronic pulmonary disease in rural women exposed to biomass fumes. Clin Invest Med. 2003;26(5):243–248. [PubMed] [Google Scholar]
- 50.Liu SM, Zhou YM, Wang XP. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62(10):889–897. doi: 10.1136/thx.2006.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu GP, Zhou YM, Tian J. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi: 10.1378/chest.08-2114. [DOI] [PubMed] [Google Scholar]
- 52.Ran PX, Wang C, Yao WZ. The risk factors for chronic obstructive pulmonary disease in females in Chinese rural areas [in Chinese] Zhonghua Nei Ke Za Zhi. 2006;45(12):974–979. [PubMed] [Google Scholar]
- 53.Chapman RS, He XZ, Blair AE, Lan Q. Improvement in household stoves and risk of chronic obstructive pulmonary disease in Xuanwei, China: retrospective cohort study. BMJ. 2005;331(7524):1050–1052. doi: 10.1136/bmj.38628.676088.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang GH, Zhong NS. Effect on health from smoking and use of solid fuel in China. Lancet. 2008;372(9648):1445–1446. doi: 10.1016/S0140-6736(08)61346-X. [DOI] [PubMed] [Google Scholar]
- 55.Lieberman J, Winter B, Sastre A. Alpha 1-antitrypsin Pi-types in 965 COPD patients. Chest. 1986;89(3):370–373. doi: 10.1378/chest.89.3.370. [DOI] [PubMed] [Google Scholar]
- 56.Zhu YJ. Epidemiological survey of chronic obstructive pulmonary disease and alpha-1-deficiency in China. Respirology. 2001;6(Suppl):S13–S15. doi: 10.1046/j.1440-1843.2001.00306.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhong L, Fu WP, Sun C, Dai LM, Zhang YP. Absence of association between SERPINE2 genetic polymorphisms and chronic obstructive pulmonary disease in Han Chinese: a case-control cohort study. BMC Med Genet. 2009;10:66. doi: 10.1186/1471-2350-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu G, Warren L, Aponte J, International COPD Genetics Network (ICGN) Investigators The SERPINE2 gene is associated with chronic obstructive pulmonary disease in two large populations. Am J Respir Crit Care Med. 2007;176(2):167–173. doi: 10.1164/rccm.200611-1723OC. [DOI] [PubMed] [Google Scholar]
- 59.Hu GP, Shi Z, Hu JX, Zou G, Peng G, Ran P. Association between polymorphisms of microsomal epoxide hydrolase and COPD: results from meta-analyses. Respirology. 2008;13(6):837–850. doi: 10.1111/j.1440-1843.2008.01356.x. [DOI] [PubMed] [Google Scholar]
- 60.Fu WP, Zhao ZH, Fang LZ. Heme oxygenase-1 polymorphism associated with severity of chronic obstructive pulmonary disease. Chin Med J (Engl) 2007;120(1):12–16. [PubMed] [Google Scholar]
- 61.Fu WP, Sun C, Dai LM, Yang LF, Zhang YP. Relationship between COPD and polymorphisms of HOX-1 and mEPH in a Chinese population. Oncol Rep. 2007;17(2):483–488. [PubMed] [Google Scholar]
- 62.Liu SM, Li B, Zhou YM, Zhong N, Ran P. Genetic analysis of CC16, OGG1 and GCLC polymorphisms and susceptibility to COPD. Respirology. 2007;12(1):29–33. doi: 10.1111/j.1440-1843.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 63.Huang N, Liu L, Wang XZ, Liu D, Yin SY, Yang XD. Association of interleukin (IL)-12 and IL-27 gene polymorphisms with chronic obstructive pulmonary disease in a Chinese population. DNA Cell Biol. 2008;27(9):527–531. doi: 10.1089/dna.2007.0715. [DOI] [PubMed] [Google Scholar]
- 64.Yuan YM, Liu XJ, Li JW. The association of matrix metalloproteinase-9 gene polymorphism with chronic obstructive pulmonary disease susceptibility in Han nationality of southwestern China. Chest. 2006;130(4):277S. [Google Scholar]
- 65.Hu GP, Peng GY, Hu JX, Ran PX. Association of tumor necrosis factor alpha 308 G/A gene promoter polymorphism with the presence of chronic obstructive pulmonary disease: a meta-analysis [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(8):588–594. [PubMed] [Google Scholar]
- 66.Sakao S, Tatsumi K, Igari H, Shino Y, Shirasawa H, Kuriyama T. Association of tumor necrosis factor alpha gene promoter polymorphism with the presence of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(2):420–422. doi: 10.1164/ajrccm.163.2.2006031. [DOI] [PubMed] [Google Scholar]
- 67.Jiang L, He B, Zhao MW, Ning LD, Li XY, Yao WZ. Association of gene polymorphisms of tumour necrosis factor-alpha and interleukin-13 with chronic obstructive pulmonary disease in Han nationality in Beijing. Chin Med J (Engl) 2005;118(7):541–547. [PubMed] [Google Scholar]
- 68.Ning YY, Ying BW, Han SX, Wang B, Wang X, Wen F. Polymorphisms of aquaporin5 gene in chronic obstructive pulmonary disease in a Chinese population. Swiss Med Wkly. 2008;138(39–40):573–578. doi: 10.4414/smw.2008.12240. [DOI] [PubMed] [Google Scholar]
- 69.Wang K, Feng YL, Wen FQ. Decreased expression of human aquaporin-5 correlated with mucus overproduction in airways of chronic obstructive pulmonary disease. Acta Pharmacol Sin. 2007;28(8):1166–1174. doi: 10.1111/j.1745-7254.2007.00608.x. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, Wang C, Yao WZ. Occupational exposure to dust/gases/fumes is contributed to chronic obstructive pulmonary disease and respiratory symptoms. Chin J Respir Crit Care Med. 2009;8(1):6–11. [Google Scholar]
- 71.Hu YP, Pan JS, Wang XJ. An epidemiologic survey of chronic obstructive pulmonary disease among coke oven workers. J Environ Occup Med. 2003;20(3):191–194. [Google Scholar]
- 72.Hu Y, Chen B, Yin Z, Jia L, Zhou Y, Jin T. Increased risk of chronic obstructive pulmonary diseases in coke oven workers: interaction between occupational exposure and smoking. Thorax. 2006;61(4):290–295. doi: 10.1136/thx.2005.051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang XR, Yano E, Nonaka K, Wang M, Wang Z. Respiratory impairments due to dust exposure: a comparative study among workers exposed to silica, asbestos, and coalmine dust. Am J Ind Med. 1997;31(5):495–502. doi: 10.1002/(sici)1097-0274(199705)31:5<495::aid-ajim2>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 74.Rahman I, Kilty I. Antioxidant therapeutic targets in COPD. Curr Drug Targets. 2006;7(6):707–720. doi: 10.2174/138945006777435254. [DOI] [PubMed] [Google Scholar]
- 75.Ran PX, Wang C, Yao WZ. A study of correlation of body mass index with COPD and quality of life [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2007;30(1):18–22. [PubMed] [Google Scholar]
- 76.Huang Z, Luo Q, Ling M. The correlation between body mass index and pulmonary function in patients with chronic obstructive pulmonary disease. J Clin Pulm Med. 2009;14(1):14–16. [Google Scholar]
- 77.Xue B, Yang G, Li X. The impact of body mass index on the prognosis of COPD. J Clin Pulm Med. 2008;13(3):281–282. [Google Scholar]
- 78.Du YJ. Luo Y, Huo Z. The relationship between body mass index and rehospitalization in patients with COPD. J Med Theor Pract. 2007;20(2):183–184. [Google Scholar]
- 79.Viegi G, Maio S, Pistelli F, Baldacci S, Carrozzi L. Epidemiology of chronic obstructive pulmonary disease: health effects of air pollution. Respirology. 2006;11(5):523–532. doi: 10.1111/j.1440-1843.2006.00886.x. [DOI] [PubMed] [Google Scholar]
- 80.Kan HD, Chen BH. A case-crossover analysis of air pollution and daily mortality in Shanghai. J Occup Health. 2003;45(2):119–124. doi: 10.1539/joh.45.119. [DOI] [PubMed] [Google Scholar]
- 81.Respiratory Diseases Branch of Chinese Medical Association A national guideline for the diagnosis, management, and prevention of chronic obstructive pulmonary disease in China. Chin J Tuberc Respir Dis. 2007;30(1):8–17. [Google Scholar]
- 82.Zhang HQ, Li YQ, Qin H. Survey on diagnosis and treatment of chronic obstructive pulmonary disease in physicians working in local hospitals in Shanghai. Shanghai Med J. 2008;31(11):806–811. [Google Scholar]
- 83.Liu Z, Gu YT, Cai Y. Investigating physician master the significance of pulmonary function in the diagnosis of COPD. Chin Clin Med J. 2004;11(1):39–41. [Google Scholar]
- 84.Lou PA, An XH, Zhang L. Analyzing the difference of the knowledge between men and women COPD patients for COPD in rural area. Chin J Health Care Med. 2009;11(3):198–201. [Google Scholar]
- 85.Shen N, Yao WHSC, Zhu H. Patient's perspective of chronic obstructive pulmonary disease in Yanqing County of Beijing. Chin J Tuberc Respir Dis. 2008;31(3):206–208. [PubMed] [Google Scholar]
- 86.Li ZP, Huang JQ, Tang KJ. Retrospective studies on 713 cases chronic obstructive pulmonary disease [in Chinese] Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24(8):722–724. [PubMed] [Google Scholar]
- 87.He Q, Zhao Q. Spirometry utilization for COPD in several provinces in China. Chin J Tuberc Respir Dis. 2003;26(1):39–40. [Google Scholar]
- 88.Li XF, Zhang FY, Hang JQ. Pulmonary function tests in diagnosis of chronic obstructive pulmonary disease. Clin Focus. 2009;24(5):387–389. [Google Scholar]
- 89.Sin DD, Tan WC. Breaking down the “Great Wall” of COPD care in China. Am J Respir Crit Care Med. 2007;176(8):732–733. doi: 10.1164/rccm.200706-927ED. [DOI] [PubMed] [Google Scholar]
- 90.Chen L, Zhang G, Lin S. The effect of health education on lung function and quality of life among stabilized patients with chronic obstructive pulmonary disease. Chin J Epidemiol. 2005;26(10):808–810. [PubMed] [Google Scholar]
- 91.Xie GQ, Cheng XS, Xu XS. Effects of comprehensive interventions in community on smoking, chronic bronchitis, and asthma in rural areas of Beijing [in Chinese] Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2005;27(1):92–98. [PubMed] [Google Scholar]
- 92.Chen K. Survey on Awareness Degree of COPD Patients. China Medical University; Liaoning Province: 2009. [Google Scholar]
- 93.Yan LH, Leng BZ, Xu B. The cognition and requirement for health education of COPD. Zhong Guo Chu Ji Wei Sheng Bao Jian. 2009;23(2):62–63. [Google Scholar]
- 94.Zhang X, Xu L, Zhou XY. Survey on oxygen therapy among elderly stabilized patients with COPD. Morden Prev Med. 2008;35(11):2196–2200. [Google Scholar]
- 95.Chen Y, Wu S, Xiang X. Current status of long-term domiciliary oxygen therapy in patients with chronic obstructive pulmonary disease. Chin J Modem Med. 2007;17(21):2658–2660. [Google Scholar]
- 96.Lou PA, Yu JX, An XH. Disease perception and awareness in patients with chronic obstrutive pulmonary disease in rural Xuzhou. Chin J General Practitioners. 2009;8(3):157–159. [Google Scholar]
- 97.He Q, Zhou X, Xie C. The investigation of the treatment conditions in stable COPD patients in partial cities in China. Chin J Practic Intern Med. 2009;29(4):354–357. [Google Scholar]
- 98.Zhang RB, He QY. Awareness of knowledge of COPD by doctors in district and community hospitals. Chin J Prev Control Chronic Dis. 2009;17(1):61–63. [Google Scholar]
- 99.Zhu WH, Yang L, Jiang CQ. Characteristics of smokers and predictors of quitting in a smoking cessation clinic in Guangzhou, China. J Public Health (Oxf) 2010;32(2):267–276. doi: 10.1093/pubmed/fdp107. [DOI] [PubMed] [Google Scholar]
- 100.The laws in China, The fz-china Web site. http://www.fz-china.com.cn/fz-china/law.php?m=lf Accessed June 2009.
- 101.Jiang H, Li X, Wu X. Smoking behavior of Chinese physician. Chin J Prev Control Chron Dis. 2009;17(3):224–227. [Google Scholar]
- 102.Price DB, Tinkelman DG, Nordyke RJ, Isonaka S, Halbert RJ, COPD Questionnaire Study Group Scoring system and clinical application of COPD diagnostic questionnaires. Chest. 2006;129(6):1531–1539. doi: 10.1378/chest.129.6.1531. [DOI] [PubMed] [Google Scholar]
- 103.Price DB, Tinkelman DG, Halbert RJ. Symptom-based questionnaire for identifying COPD in smokers. Respiration. 2006;73(3):285–295. doi: 10.1159/000090142. [DOI] [PubMed] [Google Scholar]