Abstract
The present study investigated the long-term impact of antibiotic use policy on the rates of consumption (expressed as daily-defined doses/1000 patient-days) of various parenteral antibiotics and on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and the incidence of healthcare-associated MRSA (HA-MRSA) infection at a tertiary care hospital from 2001 to 2009. During this time, consumption of all antimicrobials for systemic use decreased by 33%. This change was driven by a 44% decrease in the consumption of unrestricted antibacterials, which was offset by a 42% increase in the consumption of restricted agents. The trends in MRSA prevalence (number of isolates/1000 patient-days) and HA-MRSA incidence (number of HA-MRSA-infected persons/1000 patient-days) correlated with the trend in overall consumption of antimicrobials. Significant positive correlations were observed between MRSA prevalence and the consumption of extended-spectrum and β-lactamase-resistant penicillins, first-generation cephalosporins, macrolides, lincosamides and streptogramins, aminoglycosides, and glycopeptides. Significant positive correlations were found between the incidence of HA-MRSA infection and the consumption of tetracyclines, extended-spectrum and β-lactamase-resistant penicillins, sulfonamides and trimethoprim, macrolides, lincosamides and streptogramins, and aminoglycosides. In conclusion, we have documented the ongoing successful reduction in total consumption of antimicrobials associated with a decrease in the incidence of HA-MRSA and the prevalence of MRSA over a 9-year period.
Keywords: Antibiotic consumption, Healthcare-associated infection, Methicillin-resistant, Staphylococcus aureus, Correlation, Taiwan
1. Introduction
Antimicrobial resistance has increased rapidly during the last 15 years and has become a global health issue [1], [2], [3], [4]. A relationship between the consumption of antibiotics and antimicrobial resistance has been widely documented and has been used to support the implementation of antimicrobial control policies [5], [6], [7], [8], [9], [10], [11], [12]. In Taiwan, healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA), which can cause life-threatening infections, has become endemic in hospitals [4]. A government policy restricting the use of antibiotics was established by the Bureau of National Health Insurance (BNHI) and was implemented in 2001 in Taiwan [4]. A few studies have evaluated the short-term relationship between consumption of antibiotics and antimicrobial resistance in Taiwan hospitals [9], [13], [14] before and after implementation of this policy.
In this report, we describe a long-term study on the impact of the policy on the rates of consumption of various parenteral antibiotics and the incidence of HA-MRSA infection and the prevalence of MRSA during a 9-year period at a medical centre in central Taiwan.
2. Materials and methods
2.1. Hospital setting and infection control measures
Chung Shan Medical University (CSMU) Hospital is a 1162-bed tertiary care university hospital in Taichung, central Taiwan. The infection control practices at CSMU during the study period of 2001–2009 have been standardised according to the guidelines of the Nosocomial Infection Control Society of Taiwan (NICST) and the Taiwan Center for Disease Control (CDC). The guidelines (http://www.cdc.gov.tw/np.asp?ctNode=1693&mp=1) can be accessed by any hospital in Taiwan. Briefly, the hospital distributed and implemented an infection control manual with specific protocols for prevention of infection by various routes (e.g. airborne and droplets); analysed incidences of infection with MRSA and other multidrug-resistant infections; performed active surveillance, reporting of notifiable infectious diseases and isolation of patients; performed surveillance and screening of healthcare workers (HCWs); trained HCWs in the appropriate use of personal protective equipment; provided continuing education programmes for HCWs; promoted hand hygiene; analysed the use of antibiotics and presented workshops on appropriate use of antibiotics; and adopted the measures for control, monitoring and review of antibiotic use described below.
Following the 2003 epidemic of severe acute respiratory syndrome (SARS), Taiwan CDC was aware of the importance of nosocomial infection control and implemented the Nosocomial Infection Control Regional Assistance Plan and Medical Service Quality Improvement Plan, which commissioned the NICST and the Taiwan Joint Commission on Hospital Accreditation (TJCHA). CSMU was audited for compliance with the infection control measures from 2007–2009 by the Taiwan CDC and TJCHA. The hospital secured accreditation at the level of medical centre.
2.2. Prescription of antibiotics
Before 2000 there was no well-established antibiotic control policy at CSMU. Although prescriptions for some restricted antibiotics required prior authorisation by infectious disease specialists (IDSs), most antibiotics were freely prescribed without documentation of indications or limitations on the duration of use. In 2000, the CSMU Infection Control Committee and Department of Pharmacy established a new antibiotic use policy with the following provisions. Restricted and unrestricted antimicrobials were clearly classified. The restricted antimicrobials included β-lactamase inhibitors and penicillin plus β-lactamase inhibitor combination (sulbactam sodium, ampicillin/sulbactam injection, amoxicillin/clavulanic acid injection and piperacillin/tazobactam), second-generation cephalosporins (cefuroxime and cefmetazole), third-generation cephalosporins (cefotaxime, ceftazidime, ceftriaxone, cefoperazone and flomoxef), fourth-generation cephalosporins (cefepime and cefpirome), carbapenems (ertapenem, meropenem and imipenem/cilastatin), aminoglycosides (amikacin and isepamicin), fluoroquinolones (ciprofloxacin, levofloxacin and moxifloxacin), glycopeptides (vancomycin and teicoplanin), linezolid, tigecycline and daptomycin. Furthermore, the indications for use of restricted antimicrobials became regulated by the BNHI in Taiwan and three important new regulations restricting antimicrobial use were implemented. Inappropriate prophylactic use of antibiotics for surgery was discouraged, for example by limiting the duration of antibiotic therapy to 3 days in some clean surgeries, e.g. total hip replacement, total knee replacement and coronary artery bypass graft. Antimicrobial agents used in the treatment of upper respiratory tract infections were restricted. The 10 guidelines for the use of antimicrobial agents in Taiwan can be freely downloaded from http://www.ejmii.com/guidelines.php to use as a reference.
At CSMU, the role of IDSs in making bedside consultations and providing authorisation was established. Furthermore, prescriptions for restricted antibiotics required authorisation from an IDS. Criteria that defined the need for consultation and authorisation by an IDS include use of restricted antibiotics, use of more than two different classes of antibiotics or use of antibiotics for longer than 7 days as well as the requested assessment of appropriate antibiotic use. The role of the IDSs in the antibiotic use programme is crucial. IDSs assess the appropriate use of antimicrobials and provide recommendations according to patient's clinical symptoms and signs, diagnosis, liver and renal function, co-morbidities, culture susceptibility tests and adherence to guidelines for the use of antimicrobial agents in Taiwan. Inappropriate use of antimicrobials is discontinued. IDSs permit appropriate antibiotic agents for 7 days after assessment and consent.
2.3. Antibiotic consumption
Data on the annual consumption of oral and intravenous antibiotics in CSMU from 2001–2009 were collected from the computer database of the Pharmacy Department. Antibiotic consumption was standardised by using the defined daily dose (DDD) as established by the World Health Organization's ATC/DDD index [15], [16] and calculated using the European Society of Clinical Microbiology and Infectious Diseases Study Group on Antibiotic Policies (ESGAP) ABC Calc v.3.1 [17].
2.4. Microbiological data
The prevalence of MRSA was obtained from the Clinical Microbiology Laboratory of CSMU. Healthcare-associated isolates (HAIs) were defined according to criteria established by the CDC [18], [19] and the data relevant to HAIs in CSMU were obtained from the hospital's Infection Control Committee. All MRSA isolates were non-duplicate samples; a duplicate isolate was defined as an isolate of the same species of bacteria with the same antimicrobial susceptibility pattern isolated from the same patient and site within 1 month. Susceptibility testing was performed by the disk diffusion method and was interpreted according to Clinical and Laboratory Standards Institute (CLSI) guidelines [20], [21].
2.5. Statistical analysis
Consumption of restricted and unrestricted antibiotics was summarised as the DDD/1000 patient-days (PD). The Spearman correlation coefficient (ρ) was determined for the correlation between MRSA prevalence and incidence of HA-MRSA infections versus antibiotic consumption. Statistical hypothesis tests were set with a significance level of 0.05. All statistical analyses were performed using SPSS v.15.0 (SPSS Inc., Chicago, IL).
3. Results
3.1. Trends in antibiotic consumption
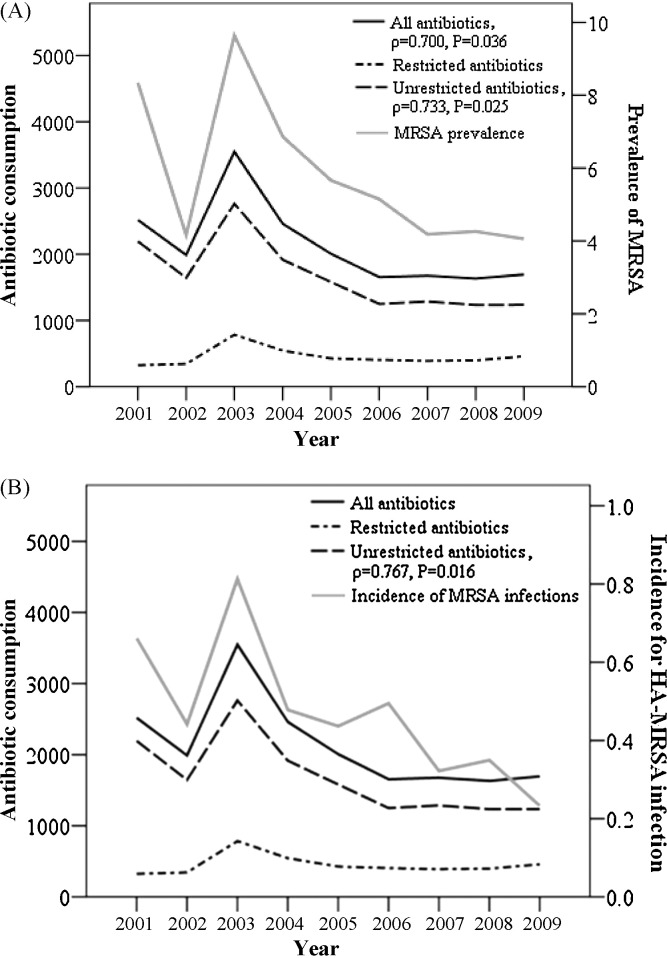
The total DDD of antibiotics consumed per 1000 PD decreased during the first 2 years following implementation of the new policies (2001 and 2002) and then steeply increased in 2003 (Fig. 1 ). Antibiotic consumption decreased continuously from 2004–2006 and remained stable from 2007–2009 (Table 1 ). The trend for consumption of unrestricted antibiotics was similar to that for overall consumption (Fig. 1; Table 2 ). Consumption of restricted antibiotics was stable during 2001–2002 but also increased in 2003 (Fig. 1; Table 3 ). The use of restricted antibiotics then decreased continuously until 2007, but began to increase slightly thereafter.
Fig. 1.
Relationship between annual consumption of antibiotics (DDD/1000 PD) (left-hand y-axis; derived from Table 1, Table 2, Table 3) and (A) the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) per 1000 PD (right-hand y-axis) and (B) the incidence of healthcare-associated MRSA (HA-MRSA) infections per 1000 PD (right-hand y-axis). The prevalence of MRSA and the incidence of HA-MRSA are derived from Table 4. DDD, defined daily doses; PD, patient-days.
Table 1.
Correlation between antibiotic consumption and the incidence of healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infection and the prevalence of MRSA at Chung Shan Medical University Hospital (Taichung, Taiwan), 2001–2009.
| ATC code/drug name | Antibiotic consumption (DDD/1000 PD) |
Correlation coefficient |
|||||
|---|---|---|---|---|---|---|---|
| 2001 (%) | 2009 (%) | 2009–2001 (fold change) | Incidence of HA-MRSA |
Prevalence of MRSA |
|||
| ρ | P-value | ρ | P-value | ||||
| J01A–Tetracyclines | 509.28 (20.22) | 136.29 (8.06) | −372.99 (3.74) | 0.717 | 0.030* | 0.483 | 0.187 |
| J01C–β-Lactam antibacterials, penicillins | 476.65 (18.93) | 512.74 (30.34) | 36.09 (1.08) | 0.217 | 0.576 | 0.517 | 0.154 |
| J01CA–Penicillins with extended spectrum | 148.86 (5.91) | 110.25 (6.52) | −38.61 (1.35) | 0.667 | 0.050* | 0.800 | 0.010* |
| J01CE–β-Lactamase-sensitive penicillins | 11.00 (0.44) | 4.41 (0.26) | −6.59 (2.49) | 0.133 | 0.732 | 0.150 | 0.700 |
| J01CF–β-Lactamase-resistant penicillins | 172.49 (6.85) | 124.09 (7.34) | −48.40 (1.39) | 0.733 | 0.025* | 0.717 | 0.030* |
| J01CG–β-Lactamase inhibitors | 0.00 (0) | 4.20 (0.25) | 4.20 | −0.525 | 0.146 | −0.441 | 0.235 |
| J01CR–Combination of penicillins | 144.30 (5.73) | 274.00 (16.21) | 129.70 (1.90) | −0.017 | 0.966 | 0.367 | 0.332 |
| Penicillins without antipseudomonal activity + β-lactamase inhibitor (J01CR01 and J01CR02)a | 131.93 (5.24) | 243.42 (14.40) | 111.49 (1.85) | 0.233 | 0.546 | 0.483 | 0.187 |
| Penicillins with antipseudomonal activity + β-lactamase inhibitor (J01CR05) | 12.38 (0.49) | 30.58 (1.81) | 18.20 (2.47) | −0.300 | 0.433 | −0.067 | 0.865 |
| J01D–Other β-lactam antibacterials | 746.41 (29.64) | 607.08 (35.92) | −139.33 (1.23) | 0.450 | 0.224 | 0.633 | 0.067 |
| J01DB–First-generation cephalosporins | 641.43 (25.47) | 449.06 (26.57) | −192.37 (1.43) | 0.600 | 0.088 | 0.667 | 0.050* |
| J01DC–Second-generation cephalosporins | 60.51 (2.40) | 62.63 (3.71) | 2.12 (1.04) | 0.117 | 0.765 | 0.433 | 0.244 |
| J01DD–Third-generation cephalosporins | 26.45 (1.05) | 64.43 (3.81) | 37.98 (2.44) | −0.133 | 0.732 | 0.300 | 0.433 |
| J01DE–Fourth-generation cephalosporins | 3.48 (0.14) | 4.59 (0.27) | 1.11 (1.32) | 0.433 | 0.244 | 0.550 | 0.125 |
| J01DH–Carbapenems | 14.54 (0.58) | 26.36 (1.56) | 11.82 (1.81) | −0.033 | 0.932 | 0.117 | 0.765 |
| J01E–Sulfonamides and trimethoprim | 129.08 (5.13) | 38.77 (2.29) | −90.31 (3.33) | 0.750 | 0.020* | 0.650 | 0.058 |
| J01F–Macrolides, lincosamides and streptogramins | 305.32 (12.12) | 98.38 (5.82) | −206.94 (3.10) | 0.817 | 0.007* | 0.800 | 0.010* |
| J01FA–Macrolides | 192.04 (7.63) | 76.36 (4.52) | −115.68 (2.51) | 0.817 | 0.007* | 0.783 | 0.013* |
| J01FF–Lincosamides | 113.28 (4.50) | 22.02 (1.30) | −91.26 (5.14) | 0.867 | 0.002* | 0.650 | 0.058 |
| J01G–Aminoglycoside antibacterials | 144.44 (5.74) | 39.65 (2.35) | −104.79 (3.64) | 0.783 | 0.013* | 0.700 | 0.036* |
| J01M–Quinolone antibacterials | 177.11 (7.03) | 211.73 (12.53) | 34.62 (1.20) | 0.335 | 0.379 | 0.159 | 0.683 |
| J01X–Other antibacterials | 30.14 (1.20) | 45.35 (2.68) | 15.21 (1.50) | 0.183 | 0.637 | 0.350 | 0.356 |
| J01XA–Glycopeptide antibacterials | 17.77 (0.71) | 17.56 (1.04) | −0.21 (1.01) | 0.583 | 0.099 | 0.717 | 0.030* |
| J01XC–Steroid antibacterials | 7.16 (0.28) | 11.63 (0.69) | 4.47 (1.62) | 0.067 | 0.865 | 0.267 | 0.488 |
| J01XD–Imidazole derivatives | 5.21 (0.21) | 6.57 (0.39) | 1.36 (1.26) | 0.067 | 0.865 | 0.333 | 0.381 |
| J01XX–Other antibacterials | 0.00 (0) | 9.60 (0.57) | 9.60 | −0.092 | 0.814 | 0.042 | 0.915 |
| Total (J01–Antibacterials for systemic use) | 2518.43 (100) | 1689.99 (100) | −828.44 (1.49) | 0.633 | 0.067 | 0.700 | 0.036* |
ATC, Anatomical Therapeutic Chemical; DDD, defined daily doses; PD, patient-days.
Includes ampicillin and enzyme inhibitor (parenteral) (J01CR01) and amoxicillin and enzyme inhibitor (parenteral and oral) (J01CR02).
P < 0.05 indicates that the 95% confidence interval for the Spearman correlation coefficient (ρ) did not include zero.
Table 2.
Correlation between consumption of unrestricted antibiotic and the incidence of healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infection and the prevalence of MRSA at Chung Shan Medical University Hospital (Taichung, Taiwan), 2001–2009.
| ATC code/drug name | Antibiotic consumption (DDD/1000 PD) |
Spearman correlation coefficients |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Incidence of HA-MRSA |
Prevalence of MRSA |
||||
| ρ | P-value | ρ | P-value | |||||||||||
| J01AA02 | Doxycycline | 403.83 | 242.28 | 308.49 | 181.70 | 112.45 | 119.19 | 102.69 | 109.28 | 130.00 | 0.686 | 0.041* | 0.483 | 0.187 |
| J01AA07 | Tetracycline | 4.20 | 4.96 | 0.38 | 2.42 | 2.72 | 1.51 | 1.80 | 1.00 | 0.94 | 0.084 | 0.831 | −0.017 | 0.966 |
| J01AA08 | Minocycline | 101.25 | 64.32 | 56.02 | 18.38 | 2.09 | 1.00 | 1.38 | 0.96 | 0.81 | 0.678 | 0.045* | 0.567 | 0.112 |
| J01CA01 | Ampicillin | 7.02 | 2.19 | 2.21 | 0.23 | 1.16 | 0.67 | 4.04 | 0.14 | 2.25 | 0.008 | 0.983 | −0.017 | 0.966 |
| J01CA04 | Amoxicillin | 136.49 | 116.93 | 207.19 | 144.17 | 125.00 | 122.82 | 142.34 | 116.08 | 107.59 | 0.619 | 0.075 | 0.767 | 0.016* |
| J01CA12 | Piperacillin | 5.35 | 3.47 | 0.02 | 0.00 | 0.00 | 0.00 | 0.01 | 1.53 | 0.42 | −0.034 | 0.931 | −0.170 | 0.663 |
| J01CE01 | Penicillin G benzathine | 1.25 | 0.91 | 1.58 | 1.20 | 1.01 | 0.83 | 0.88 | 0.81 | 1.09 | 0.527 | 0.145 | 0.600 | 0.088 |
| J01CE08 | Penicillin G | 9.75 | 5.39 | 7.66 | 4.08 | 2.71 | 2.54 | 11.91 | 3.05 | 3.32 | 0.100 | 0.797 | 0.150 | 0.700 |
| J01CF01 | Dicloxacillin | 118.72 | 110.12 | 190.56 | 101.52 | 57.64 | 57.97 | 58.41 | 58.79 | 64.16 | 0.485 | 0.185 | 0.367 | 0.332 |
| J01CF04 | Oxacillin | 53.77 | 51.00 | 109.39 | 82.08 | 73.72 | 73.31 | 36.59 | 55.91 | 56.03 | 0.494 | 0.177 | 0.600 | 0.088 |
| J01CF05 | Flucloxacillin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 36.76 | 11.68 | 3.90 | −0.785 | 0.012* | −0.584 | 0.099 |
| J01CR02 | Amoxicillin/clavulanic acid | 92.20 | 70.38 | 212.82 | 233.87 | 159.98 | 131.93 | 127.28 | 132.98 | 131.09 | 0.268 | 0.486 | 0.533 | 0.139 |
| J01CR04 | Sultamicillin tosylate | 3.23 | 13.76 | 41.18 | 28.30 | 19.73 | 26.88 | 25.49 | 30.59 | 31.26 | −0.092 | 0.814 | 0.017 | 0.966 |
| J01DB01 | Cefalexin monohydrate | 419.89 | 350.26 | 630.12 | 451.57 | 354.39 | 314.04 | 315.64 | 323.46 | 328.17 | 0.561 | 0.116 | 0.683 | 0.042* |
| J01DB04 | Cefazolin | 221.54 | 182.85 | 300.96 | 191.03 | 124.00 | 114.61 | 118.21 | 95.74 | 67.00 | 0.795 | 0.010* | 0.783 | 0.013* |
| J01DB09 | Cefradine | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 36.41 | 53.90 | −0.642 | 0.062 | −0.548 | 0.127 |
| J01DC04 | Cefaclor | 42.03 | 37.26 | 74.77 | 57.55 | 276.97 | 31.72 | 42.57 | 30.92 | 38.63 | 0.268 | 0.486 | 0.533 | 0.139 |
| J01DD14 | Ceftibuten | 0.00 | 2.64 | 12.90 | 22.28 | 14.64 | 16.95 | 21.99 | 25.85 | 27.85 | −0.686 | 0.041* | −0.483 | 0.187 |
| J01EC01 | Sulfamethoxazole/trimethoprim | 129.08 | 101.29 | 159.92 | 89.62 | 67.91 | 62.31 | 67.46 | 32.30 | 38.77 | 0.736 | 0.024* | 0.650 | 0.058 |
| J01FA01 | Erythromycin | 93.43 | 32.27 | 45.06 | 8.93 | 1.95 | 0.21 | 0.08 | 0.00 | 0.03 | 0.803 | 0.009* | 0.667 | 0.050* |
| J01FA09 | Clarithromycin | 71.26 | 68.98 | 96.34 | 59.35 | 32.79 | 45.34 | 50.22 | 62.23 | 56.39 | 0.469 | 0.203 | 0.367 | 0.332 |
| J01FA10 | Azithromycin | 27.35 | 35.07 | 80.82 | 100.09 | 56.68 | 33.65 | 23.59 | 22.89 | 19.93 | 0.661 | 0.053 | 0.650 | 0.058 |
| J01FF01 | Clindamycin | 113.28 | 54.23 | 67.91 | 33.83 | 24.15 | 27.65 | 25.93 | 22.79 | 22.02 | 0.837 | 0.005* | 0.650 | 0.058 |
| J01GB03 | Gentamicin sulphate | 127.23 | 83.75 | 127.91 | 79.49 | 53.12 | 45.40 | 49.29 | 40.99 | 31.60 | 0.770 | 0.015* | 0.700 | 0.036* |
| J01XC01 | Fusidic acid | 7.16 | 5.23 | 16.70 | 14.99 | 7.87 | 10.72 | 10.80 | 7.64 | 11.63 | 0.092 | 0.814 | 0.267 | 0.488 |
| J01XD01 | Metronidazole | 5.21 | 4.39 | 11.38 | 9.23 | 8.14 | 8.37 | 10.65 | 9.59 | 6.57 | 0.092 | 0.814 | 0.333 | 0.381 |
| Total | 2194.53 | 1643.95 | 2762.28 | 1915.93 | 1580.83 | 1249.62 | 1286.02 | 1233.62 | 1235.32 | 0.767 | 0.016* | 0.733 | 0.025* | |
ATC, Anatomical Therapeutic Chemical; DDD, defined daily doses; PD, patient-days.
P < 0.05 indicates that the 95% confidence interval for the Spearman correlation coefficient (ρ) did not include zero.
Table 3.
Correlation between consumption of restricted antibiotics and the incidence of healthcare-associated methicillin-resistant Staphylococcus aureus (HA-MRSA) infection and the prevalence of MRSA at Chung Shan Medical University Hospital (Taichung, Taiwan), 2001–2009.
| ATC code/drug name | Antibiotic consumption (DDD/1000 PD) |
Spearman correlation coefficient |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | Incidence of HA-MRSA |
Prevalence of MRSA |
||||
| ρ | P-value | ρ | P-value | |||||||||||
| J01AA12 | Tigecycline | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.25 | 3.48 | 4.54 | −0.825 | 0.006* | −0.663 | 0.051 |
| J01CG01 | Sulbactam sodium | 0.00 | 0.00 | 0.00 | 1.01 | 2.27 | 7.83 | 4.67 | 4.54 | 4.20 | −0.502 | 0.168 | −0.441 | 0.235 |
| J01CR01 | Ampicillin/sulbactam injection | 0.00 | 0.00 | 19.18 | 51.23 | 34.75 | 34.68 | 40.77 | 28.76 | 30.71 | −0.315 | 0.409 | −0.050 | 0.898 |
| J01CR02 | Amoxicillin/clavulanic acid injection | 36.49 | 27.42 | 41.32 | 41.20 | 50.09 | 52.43 | 49.70 | 44.99 | 50.36 | −0.351 | 0.354 | −0.283 | 0.460 |
| J01CR05 | Piperacillin/tazobactam | 12.38 | 13.01 | 37.99 | 22.85 | 17.28 | 17.10 | 24.73 | 27.54 | 30.58 | −0.285 | 0.458 | −0.067 | 0.865 |
| J01DC02 | Cefuroxime | 12.49 | 7.01 | 13.41 | 7.68 | 8.59 | 8.24 | 6.67 | 7.67 | 11.90 | 0.552 | 0.123 | 0.583 | 0.099 |
| J01DC09 | Cefmetazole | 5.99 | 7.14 | 20.08 | 13.45 | 14.62 | 12.27 | 12.64 | 12.74 | 12.11 | 0.167 | 0.667 | 0.450 | 0.224 |
| J01DD01 | Cefotaxime | 1.64 | 2.96 | 4.25 | 2.13 | 22.30 | 0.92 | 1.02 | 0.66 | 1.40 | 0.368 | 0.330 | 0.400 | 0.286 |
| J01DD02 | Ceftazidime | 8.32 | 2.54 | 13.57 | 10.49 | 7.25 | 5.06 | 6.15 | 5.11 | 5.39 | 0.477 | 0.194 | 0.783 | 0.013* |
| J01DD04 | Ceftriaxone | 13.47 | 10.84 | 28.02 | 24.19 | 21.34 | 16.03 | 14.00 | 10.43 | 12.90 | 0.577 | 0.104 | 0.700 | 0.036* |
| J01DD12 | Cefoperazone | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 1.26 | 0.76 | 0.61 | 1.71 | −0.642 | 0.062 | −0.627 | 0.071 |
| Flomoxefa | 3.01 | 2.96 | 8.45 | 8.64 | 5.38 | 5.91 | 8.89 | 9.40 | 15.18 | −0.586 | 0.097 | −0.333 | 0.381 | |
| J01DE01 | Cefepime | 3.48 | 2.37 | 12.27 | 12.21 | 9.41 | 5.70 | 2.01 | 2.70 | 2.49 | 0.728 | 0.026* | 0.833 | 0.005* |
| J01DE02 | Cefpirome | 0.00 | 1.61 | 3.61 | 2.38 | 0.57 | 0.31 | 0.68 | 2.46 | 2.09 | −0.100 | 0.797 | 0.017 | 0.966 |
| J01DH02 | Meropenem | 11.27 | 9.69 | 19.74 | 19.85 | 11.22 | 9.31 | 9.22 | 6.27 | 5.45 | 0.795 | 0.010* | 0.833 | 0.005* |
| J01DH03 | Ertapenem | 0.00 | 0.00 | 0.00 | 0.00 | 3.20 | 9.38 | 5.29 | 7.55 | 8.42 | −0.520 | 0.151 | −0.540 | 0.134 |
| J01DH51 | Imipenem/cilastatin | 3.27 | 1.29 | 5.31 | 7.27 | 4.60 | 4.23 | 4.83 | 7.73 | 12.50 | −0.444 | 0.232 | −0.150 | 0.700 |
| J01GB06 | Amikacin | 17.20 | 11.50 | 21.10 | 10.56 | 6.08 | 3.08 | 2.27 | 2.35 | 1.71 | 0.870 | 0.002* | 0.750 | 0.020* |
| J01GB11 | Isepamicin | 0.00 | 0.34 | 12.52 | 4.96 | 4.45 | 3.70 | 6.67 | 8.00 | 6.35 | −0.234 | 0.544 | 0.017 | 0.966 |
| J01MA02 | Ciprofloxacin | 39.76 | 36.88 | 62.43 | 24.44 | 10.22 | 14.34 | 17.42 | 21.52 | 25.97 | 0.402 | 0.284 | 0.267 | 0.488 |
| J01MA12 | Levofloxacin | 137.35 | 187.11 | 422.25 | 241.31 | 156.98 | 162.02 | 139.57 | 151.27 | 172.20 | 0.259 | 0.500 | 0.117 | 0.765 |
| J01MA14 | Moxifloxacin | 0.00 | 3.23 | 8.53 | 9.33 | 14.74 | 13.49 | 14.04 | 17.06 | 13.56 | −0.653 | 0.057 | −0.367 | 0.332 |
| J01XA01 | Vancomycin | 14.03 | 9.97 | 18.11 | 15.35 | 12.02 | 11.80 | 12.06 | 11.63 | 14.09 | 0.310 | 0.417 | 0.517 | 0.154 |
| J01XA02 | Teicoplanin | 3.74 | 6.15 | 9.18 | 10.72 | 7.10 | 4.53 | 2.43 | 2.32 | 3.46 | 0.611 | 0.081 | 0.567 | 0.112 |
| J01XX01 | Fosfomycin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.54 | 6.49 | −0.642 | 0.062 | −0.548 | 0.127 |
| J01XX08 | Linezolid | 0.00 | 0.59 | 4.08 | 3.21 | 1.73 | 1.73 | 0.36 | 0.79 | 0.75 | 0.391 | 0.298 | 0.460 | 0.213 |
| J01XX09 | Daptomycin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.50 | 0.12 | 2.36 | −0.845 | 0.004* | −0.683 | 0.042* |
| Total | 323.90 | 344.61 | 785.41 | 544.49 | 426.37 | 405.35 | 389.58 | 398.25 | 458.86 | 0.100 | 0.798 | 0.283 | 0.460 | |
ATC, Anatomical Therapeutic Chemical; DDD, defined daily doses; PD, patient-days.
Flomoxef is a third-generation cephalosporin; there is no ATC code or World Health Organization definition or DDD at present. The package was 1 g per vial and the normal adult dosage was 1 g every 6 h in patients with normal renal function, hence the daily dosage was 4 g. We pre-emptively defined the DDD of flomoxef as 4 g.
P < 0.05 indicates that the 95% confidence interval for the Spearman correlation coefficient (ρ) did not include zero.
3.2. Trends in the prevalence of MRSA and the incidence of healthcare-associated MRSA infections
The trend in the prevalence of MRSA (defined as the number of isolates/1000 PD) was similar to the trend in overall consumption of antibiotics (Fig. 1A; Table 4 ). The prevalence of MRSA decreased from 2001–2002, steeply increased in 2003 and then continuously decreased from 2003–2007. After 2007, the prevalence of MRSA remained stable. Similarly, the incidence of HA-MRSA infection (defined as the number of MRSA-infected persons/1000 PD) also decreased in 2002 and increased in 2003. After 2003, the incidence of HA-MRSA fluctuated somewhat but generally declined through to 2009.
Table 4.
Summary of the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and the incidence of healthcare-associated MRSA (HA-MRSA) infection at Chung Shan Medical University Hospital (Taichung, Taiwan), 2001–2009.
| 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | |
|---|---|---|---|---|---|---|---|---|---|
| Prevalence of MRSA | |||||||||
| No. of resistant isolates | 1577 | 1140 | 1530 | 1637 | 1623 | 1394 | 1169 | 1157 | 993 |
| No. of susceptible isolates | 916 | 755 | 1063 | 1185 | 1083 | 1018 | 894 | 905 | 842 |
| Total isolates | 2493 | 1895 | 2593 | 2822 | 2706 | 2412 | 2063 | 2062 | 1835 |
| % Resistant | 63.3 | 60.2 | 59.0 | 58.0 | 60.0 | 57.8 | 56.7 | 56.1 | 54.1 |
| Prevalence of MRSA/1000 PDa | 8.35 | 4.16 | 9.64 | 6.87 | 5.67 | 5.15 | 4.18 | 4.26 | 4.06 |
| Incidence of HA-MRSA | |||||||||
| No. of MRSA-infected persons | 125 | 121 | 129 | 114 | 125 | 134 | 90 | 95 | 57 |
| No. of susceptible strain-infected persons | 75 | 79 | 19 | 50 | 46 | 38 | 58 | 50 | 38 |
| Total patient no. | 200 | 200 | 148 | 164 | 171 | 172 | 148 | 145 | 95 |
| % Resistant | 62.5 | 60.5 | 87.2 | 69.5 | 73.1 | 77.9 | 60.8 | 65.5 | 60.0 |
| Incidence of HA-MRSA/1000 PDb | 0.662 | 0.442 | 0.813 | 0.478 | 0.436 | 0.495 | 0.322 | 0.350 | 0.233 |
| No. of infected persons | 776 | 1038 | 519 | 739 | 914 | 940 | 869 | 832 | 665 |
| Incidence of HAIc | 4.11 | 3.79 | 3.27 | 3.1 | 3.19 | 3.47 | 3.11 | 3.06 | 2.72 |
| Total PD | 188 903 | 273 968 | 158 683 | 238 393 | 286 457 | 270 756 | 279 443 | 271 697 | 244 670 |
| DDD/1000 PD | 2518.43 | 1988.56 | 3547.69 | 2459.42 | 2004.93 | 1647.14 | 1670.93 | 1627.34 | 1689.99 |
PD, patient-days; HAI, healthcare-associated infection; DDD, defined daily doses.
Prevalence of MRSA/1000 PD = [no. of MRSA isolates/total number of PD] × 1000.
Incidence of HA-MRSA/1000 PD = [no. of HA-MRSA-infected persons/total no. of PD] × 1000.
Incidence of HAI = [no. of infected persons/total no. of PD] × 1000.
3.3. Correlations between antibiotic consumption and the incidence of healthcare-associated MRSA
Although there was no significant correlation between total consumption of antibiotics (category J01–Antibacterials for systemic use) and the incidence of HA-MRSA (Table 1), significant positive correlations were found between the incidence of HA-MRSA infection and consumption of the following individual categories of antibiotics: tetracyclines (ρ = 0.717, P = 0.030); penicillins with extended spectrum (ρ = 0.667, P = 0.050); β-lactamase-resistant penicillins (ρ = 0.733, P = 0.025); sulfonamides and trimethoprim (ρ = 0.750, P = 0.020); macrolides, lincosamides and streptogramins (ρ = 0.817, P = 0.007); macrolides (ρ = 0.817, P = 0.007); lincosamides (ρ = 0.867, P = 0.002); and aminoglycosides (ρ = 0.783, P = 0.013).
Consumption of all unrestricted antibiotics was significantly positively correlated with the incidence of HA-MRSA infection (ρ = 0.767, P = 0.016) (Table 2). In addition, significant positive correlations were found between the incidence of HA-MRSA infection and consumption of following individual unrestricted antibiotics: doxycycline (ρ = 0.686, P = 0.041); minocycline (ρ = 0.678, P = 0.045); cefazolin (ρ = 0.795, P = 0.010); sulfamethoxazole/trimethoprim (ρ = 0.736, P = 0.024); erythromycin (ρ = 0.803, P = 0.009); clindamycin (ρ = 0.837, P = 0.005); and gentamicin sulphate (ρ = 0.770, P = 0.015). In contrast, consumption of flucloxacillin and ceftibuten were significantly negatively correlated with the incidence of HA-MRSA infection (ρ = −0.785, P = 0.012 and ρ = −0.686, P = 0.041, respectively).
Amongst the restricted antibiotics summarised in Table 3, significant positive correlations were observed between the incidence of HA-MRSA infection and consumption of the following antibiotics: cefepime (ρ = 0.728, P = 0.026); meropenem (ρ = 0.795, P = 0.010); and amikacin (ρ = 0.870, P = 0.002). In addition, consumption of tigecycline and daptomycin had a significant negative correlation with the incidence of HA-MRSA infection (ρ = −0.825, P = 0.006 and ρ = −0.845, P = 0.004, respectively).
3.4. Correlations between consumption of antibiotics and MRSA prevalence
There was a significant positive correlation between the consumption of all antibiotics and MRSA prevalence (ρ = 0.700, P = 0.036) (Table 1). In addition, there were significant positive correlations between MRSA prevalence and consumption of the following individual classes of antibiotics: penicillins with extended spectrum (ρ = 0.800, P = 0.010); β-lactamase-resistant penicillins (ρ = 0.717, P = 0.030); first-generation cephalosporins (ρ = 0.667, P = 0.050); macrolides, lincosamides and streptogramins (ρ = 0.800, P = 0.010); macrolides (ρ = 0.783, P = 0.013); aminoglycosides (ρ = 0.700, P = 0.036); and glycopeptides (ρ = 0.717, P = 0.030).
Consumption of the following restricted antibiotics was significantly positively correlated with MRSA prevalence: ceftazidime (ρ = 0.783, P = 0.013); ceftriaxone (ρ = 0.700, P = 0.036); cefepime (ρ = 0.833, P = 0.005); meropenem (ρ = 0.833, P = 0.005); and amikacin (ρ = 0.750, P = 0.020) (Table 3). In contrast, consumption of daptomycin had a significant negative correlation with HA-MRSA prevalence (ρ = −0.683, P = 0.042).
Consumption of all unrestricted antibiotics displayed a significantly positive correlation with MRSA prevalence (ρ = 0.733, P = 0.025) (Table 2), as did consumption of the following individual unrestricted antibiotics: amoxicillin (ρ = 0.767, P = 0.016); cefalexin monohydrate (ρ = 0.683, P = 0.042); cefazolin (ρ = 0.783, P = 0.013); erythromycin (ρ = 0.667, P = 0.050); and gentamicin sulphate (ρ = 0.700, P = 0.036).
4. Discussion
The restrictive policies for antibiotic use adopted by CSMU in 2001 had several ongoing effects on antibiotic consumption. Consumption of all antibacterials for systemic use decreased by 33% between 2001 and 2009. This was driven primarily by the 44% decrease in consumption of unrestricted antibacterials, which was offset by a 42% increase in the consumption of restricted agents. The patterns of antibacterial use also changed. In 2001 the most frequently used classes of antibacterials were other β-lactam antibacterials (predominantly cephalosporins), tetracyclines and β-lactam antibiotics (penicillins). By 2009, the other β-lactam antibacterials were still used most often, but use of penicillins had greatly outpaced that of the tetracyclines owing to a large decrease in consumption of the tetracyclines and an increase in the use of combinations of penicillins. Other classes with relatively large changes in use included the lincosamides (5.1-fold decrease), macrolides (2.5-fold decrease), aminoglycosides (3.6-fold decrease) and sulfonamides and trimethoprim (3.3-fold decrease). Simultaneous changes were observed in the incidence of HA-MRSA and the prevalence of MRSA. The proportion of patients infected with S. aureus in whom the strains were methicillin resistant (HA-MRSA incidence), the proportion of isolates that were resistant (MRSA prevalence) and the number of patients infected with HA-MRSA per total number of PD (infection density) all declined between 2001 and 2009. The prevalence of MRSA was significantly positively correlated with the decrease in total antibiotic use. These results are in general agreement with those from numerous other investigations that have shown a correlation between the prevalence of antibiotic-resistant bacteria and antibiotic use [7].
MacKenzie et al. [22] observed a strong statistical relationship between macrolide use and MRSA prevalence as well as a significant association with the use of cephalosporins and all antimicrobial agents except glycopeptides in a study of over 100 European hospitals. In Taiwan, one university hospital reported a significant correlation between the prevalence of MRSA and vancomycin-resistant enterococci with increased consumption of glycopeptides, β-lactam–β-lactamase inhibitor combinations, extended-spectrum cephalosporins, carbapenems and fluoroquinolones [13]. We found that the specific antibiotics for which consumption was positively correlated with MRSA prevalence included penicillins with extended spectrum, β-lactamase-resistant penicillins, first-generation cephalosporins, macrolides, lincosamides and streptogramins, aminoglycosides and glycopeptides. In a recent investigation of the association between the use of antibiotics and the incidence of MRSA in a German hospital, Kaier et al. [23] found a significant correlation with the use of second- and third-generation cephalosporins, fluoroquinolones and lincosamides.
In general, the trends in incidence of HA-MRSA were similar to those observed in the prevalence of MRSA, although the observed trend towards a decrease in the incidence of HA-MRSA infection that corresponded to the decrease in total antibacterial use did not reach statistical significance. However, some specific differences were apparent. The incidence of HA-MRSA infection (but not the prevalence) was positively correlated with the use of tetracyclines and ampicillin, for example. The correlation between incidence of HA-MRSA infection and the use of all first-generation cephalosporins did not reach significance, although the correlation with prevalence was significant.
Policies restricting the use of antibiotics in hospitals have been widely adopted over the past decade. Although the extent of reduction varies and the specific provisions differ, these policies have been reported to be generally successful in reducing the consumption of antibiotics. A hospital in Italy reported an overall decrease of 8.5% in antibiotic use in the first year following adoption of an antibiotic control programme [24]. A hospital in Taiwan that adopted a policy similar to ours reported a decline of 13.2% in DDD/100 PD for parenteral antimicrobials immediately before and after implementation [25]. A novel feature of our study is that it spans a 9-year period following the implementation of an antimicrobial control policy, allowing an evaluation of the extent to which the provisions have been followed over time. This is particularly important in light of the findings of a study investigating the relationship between antimicrobial control policies and antimicrobial resistance rates in 33 hospitals in the USA in 2007 [26]. The authors of this report found that only 10 of the hospitals had an antibiotic use policy and that there was no correlation between the existence of an antibiotic use policy and resistance rates. Because they had no evidence that the policies were actually practiced in the hospitals that had adopted them, it was impossible to conclude whether the policies were effective.
In this study, data for antibiotic consumption and MRSA prevalence and incidence of HA-MRSA infection during 2003 stand out as obvious anomalies. A key event occurred during that year, which may explain these anomalous data. An epidemic of SARS began in mid March 2003 in Taiwan and lasted for almost 4 months. During that time, significant reductions (35.2%) in the utilisation of inpatient care were observed [27], probably because individuals were fearful of becoming exposed to the virus in hospitals and clinics. We speculate that this fear may have led to a bias towards more seriously ill individuals seeking health care during this period, which may have distorted the baseline characteristics of the inpatient population in CSMU. This cohort of more seriously ill patients might have driven up the consumption of antibiotics. It is noteworthy that the incidence HA-MRSA infection and the prevalence of MRSA continued to mirror the use of antibiotics during this time.
This retrospective study had three noteworthy limitations. First, this study was conducted in only a single centre and, as such, its findings might not reflect the overall situation in Taiwan. Second, reduction in HA-MRSA infection rates in CSMU might be due to other factors rather than the policy of antibiotic restriction alone. Studies on the molecular epidemiology of MRSA isolates obtained from the study period are required to delineate the possibility of decreased clonal spread of isolates caused by improved infection control measures. Finally, we did not evaluate antibiotic usage as a time series and therefore cannot comment on the lag period and the significance of the immediate and sustained effects of the interventions.
In conclusion, we have provided data documenting the ongoing successful reduction in total antibiotic consumption over a 9-year period in a hospital in Taiwan. The overall reduction was correlated with the prevalence of MRSA, and reductions in individual antibacterials were significantly positively correlated with decreases in the incidence of HA-MRSA infections.
Funding: This study was supported by Chung Shan Medical University Hospital, Taichung, Taiwan (grant no. CSH-9614).
Competing interests: None declared.
Ethical approval: Not required.
Contributor Information
Meng-Chih Lee, Email: leey521@yahoo.com.tw.
Po-Ren Hsueh, Email: hsporen@ntu.edu.tw.
References
- 1.Cohen M.L. Epidemiology of drug resistance: implications for a post-antimicrobial era. Science. 1992;257:1050–1055. doi: 10.1126/science.257.5073.1050. [DOI] [PubMed] [Google Scholar]
- 2.Webb G.F., D’Agata E.M.C., Magal P., Ruan S. A model of antibiotic-resistant bacterial epidemics in hospitals. Proc Natl Acad Sci U S A. 2005;102:13343–13348. doi: 10.1073/pnas.0504053102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsueh P.R., Liu C.Y., Luh K.T. Current status of antimicrobial resistance in Taiwan. Emerg Infect Dis. 2002;8:132–137. doi: 10.3201/eid0802.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsueh P.R., Shyr J.M., Wu J.J. Changes in macrolide resistance among respiratory pathogens after decreased erythromycin consumption in Taiwan. Clin Microbiol Infect. 2006;12:296–298. doi: 10.1111/j.1469-0691.2005.01348.x. [DOI] [PubMed] [Google Scholar]
- 5.Mouton R.P., Hermans J., Simoons-Smit A.M., Hoogkamp-Korstanje J.A., Degener J.E., van Klingeren B. Correlations between consumption of antibiotics and methicillin resistance in coagulase negative staphylococci. J Antimicrob Chemother. 1990;26:573–583. doi: 10.1093/jac/26.4.573. [DOI] [PubMed] [Google Scholar]
- 6.Seppälä H., Klaukka T., Vuopio-Varkila J., Muotiala A., Helenius H., Lager K. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 7.Monnet D.L. Methicillin-resistant Staphylococcus aureus and its relationship to antimicrobial use: possible implications for control. Infect Control Hosp Epidemiol. 1998;19:552–559. doi: 10.1086/647872. [DOI] [PubMed] [Google Scholar]
- 8.Crowcroft N.S., Ronveaux O., Monnet D.L., Mertens R. Methicillin-resistant Staphylococcus aureus and antimicrobial use in Belgian hospitals. Infect Control Hosp Epidemiol. 1999;20:31–36. doi: 10.1086/501555. [DOI] [PubMed] [Google Scholar]
- 9.McDonald L.C., Yu H.T., Yin H.C., Hsiung C.A., Hung C.C., Ho M. Correlates of antibiotic use in Taiwan hospitals. Infect Control Hosp Epidemiol. 2001;22:565–571. doi: 10.1086/501953. [DOI] [PubMed] [Google Scholar]
- 10.Weber S.G., Gold H.S., Hooper D.C., Karchmer A.W., Carmeli Y. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9:1415–1422. doi: 10.3201/eid0911.030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philmon C., Smith T., Williamson S., Goodman E. Controlling use of antimicrobials in a community teaching hospital. Infect Control Hosp Epidemiol. 2006;27:239–244. doi: 10.1086/500419. [DOI] [PubMed] [Google Scholar]
- 12.Kolar M., Urbanek K., Vagnerova I., Koukalova D. The influence of antibiotic use on the occurrence of vancomycin-resistant enterococci. J Clin Pharm Ther. 2006;31:67–72. doi: 10.1111/j.1365-2710.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 13.Hsueh P.R., Chen W.H., Teng L.J., Luh K.T. Nosocomial infections due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci at a university hospital in Taiwan from 1991 to 2003: resistance trends, antibiotic usage and in vitro activities of newer antimicrobial agents. Int J Antimicrob Agents. 2005;26:43–49. doi: 10.1016/j.ijantimicag.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh P.R., Chen W.H., Luh K.T. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int J Antimicrob Agents. 2005;26:463–472. doi: 10.1016/j.ijantimicag.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Collaborating Centre for Drug Statistics and Methodology. ATC/DDD index 2006. http://www.whocc.no/atc_ddd_index/ [accessed 3 March 2010].
- 16.Monnet D.L. Measuring antimicrobial use: the way forward. Clin Infect Dis. 2007;44:671–673. doi: 10.1086/511649. [DOI] [PubMed] [Google Scholar]
- 17.Monnet DL. ABC Calc—antibiotic consumption calculator [Microsoft Excel application], version 3.1. Copenhagen, Denmark: Statens Serum Institut; 2006. http://www.escmid.org/research_projects/study_groups/esgap/abc_calc [accessed 3 March 2010].
- 18.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 19.Horan T.C., Gaynes R.P. Surveillance of nosocomial infections. In: Mayhall C.G., editor. Hospital epidemiology and infection control. 2nd ed. Williams & Wilkins; Baltimore, MD: 1996. pp. 1017–1031. [App-A-1-14] [Google Scholar]
- 20.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility tests: approved standard. 9th ed. Document M2-A9. Wayne, PA: CLSI; 2006.
- 21.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. Document M100-S19. Wayne, PA: CLSI; 2009.
- 22.MacKenzie F.M., Bruce J., Struelens M.J., Goossens H., Mollison J., Gould I.M. Antimicrobial drug use and infection control practices associated with the prevalence of methicillin-resistant Staphylococcus aureus in European hospitals. Clin Microbiol Infect. 2007;13:269–276. doi: 10.1111/j.1469-0691.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaier K., Hagist C., Frank U., Conrad A., Meyer E. Two time-series analyses of the impact of antibiotic consumption and alcohol-based hand disinfection on the incidences of nosocomial methicillin-resistant Staphylococcus aureus infection and Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30:346–353. doi: 10.1086/596605. [DOI] [PubMed] [Google Scholar]
- 24.Basssetti M., Di Biagio A., Rebesco B., Cenderello G., Amalfitano M.E., Bassetti D. Impact of an antimicrobial formulary and restriction policy in the largest hospital in Italy. Int J Antimicrob Agents. 2000;16:295–299. doi: 10.1016/s0924-8579(00)00249-1. [DOI] [PubMed] [Google Scholar]
- 25.Chang M.T., Wu T.H., Wang C.Y., Jang T.N., Huang C.Y. The impact of an intensive antimicrobial control program in a Taiwanese medical center. Pharm World Sci. 2006;28:257–264. doi: 10.1007/s11096-006-9035-5. [DOI] [PubMed] [Google Scholar]
- 26.Larson E.L., Quiros D., Giblin T., Lin S. Relationship of antimicrobial control policies and hospital and infection control characteristics to antimicrobial resistance rates. Am J Crit Care. 2007;16:110–120. [PMC free article] [PubMed] [Google Scholar]
- 27.Chang H.J., Nuang N., Lee C.H., Hsu Y.J., Hsieh C.J., Chou Y.J. The impact of the SARS epidemic on the utilization of medical services: SARS and the fear of SARS. Am J Public Health. 2004;94:562–564. doi: 10.2105/ajph.94.4.562. [DOI] [PMC free article] [PubMed] [Google Scholar]