Highlights
-
•
The burden of Hib pneumonia in Southeast Asia has been quantified in few studies.
-
•
We estimate the impact of routine vaccination against Hib in Nha Trang.
-
•
Population-based surveillance allowed statistical adjustment for confounding viral pneumonia.
-
•
Hib vaccination has substantially reduced the burden of childhood pneumonia.
Keywords: Hib, Immunisation, Southeast Asia
Abstract
Introduction
Despite the global success of Hib vaccination in reducing disease and mortality, uncertainty about the disease burden and the potential impact of Hib vaccination in Southeast Asia has delayed the introduction of vaccination in some countries in the region. Hib vaccination was introduced throughout Vietnam in July 2010 without catch-up. In an observational, population based surveillance study we estimated the impact of routine Hib vaccination on all cause radiologically confirmed childhood pneumonia in Nha Trang, Vietnam.
Materials and methods
In 2007 active hospital based surveillance was established in Khanh Hoa General Hospital, the only hospital in Nha Trang, Khanh Hoa province. Nasopharyngeal samples and chest radiographs are taken routinely from all children diagnosed with acute respiratory illness on admission. For admissions between 02/2007 and 03/2012 chest radiographs were interpreted for the presence of WHO primary endpoint pneumonia and nasopharyngeal swabs were analysed by PCR for the presence of Influenza A or B, RSV and rhinovirus. We employed Poisson regression to estimate the impact of Hib vaccination on radiologically confirmed pneumonia (RCP) while statistically accounting for potential differences in viral circulation in the post vaccination era which could have biased the estimate.
Results
Of 3151 cases admitted during the study period, 166 had RCP and major viruses were detected in 1601. The adjusted annual incidence of RCP in children younger than 5 years declined by 39% (12–58%) after introduction of Hib vaccination. This decline was most pronounced in children less than 2 years old, adjusted IRR: 0.52 (0.33–0.81), and no significant impact was observed in the 2–4 years old who were not eligible for vaccination, adjusted IRR: 0.96 (0.52–1.72).
Discussion
We present early evidence that the burden of Hib associated RCP in Nha Trang before vaccination was substantial and that shortly after introduction to the routine childhood immunisation scheme vaccination has substantially reduced that burden.
1. Introduction
Haemophilus influenzae type b (Hib) is a leading cause for childhood mortality and morbidity worldwide. Hib was estimated to cause more than 8 million serious cases of illness and 350,000 deaths in children under 5 years of age in 2000 [1]. Common disease manifestations include pneumonia, septicemia and meningitis. In 2006 the World Health Organisation estimated that about 20% of all cause pneumonia and a substantial proportion of meningitis (varying geographically) could potentially be prevented by Hib vaccination and hence recommended the inclusion of Hib in all routine childhood immunisation programmes [2].
The conjugate vaccine against Hib has proven safe and effective in reducing the burden of Hib disease in various settings [3], [4], [5], [6], [7], [8]. In contrast to most other regions the burden of vaccine preventable disease associated with Hib in Southeast Asia has been perceived to be low [9], [10], [11]. As a result, Asian countries have been slow to include Hib vaccination into their routine schedules, and China and Thailand have still not introduced the vaccine [12]. An increasing body of evidence has been emerging that Hib substantially contributes to the burden of disease in Asia [13], [14], [15], [16], [17] and that the incidence and mortality associated with Hib in Southeast Asia are in fact similar to levels observed in many other parts of the world, in particular in the Americas [1]. This includes recent findings from Vietnam [18], [19].
Non-bacteraemic episodes of Hib pneumonia are thought to substantially contribute to the overall burden of Hib associated disease. Unlike bacteraemic infection, where isolation of a pathogen from blood provides well-established evidence of disease causality, the causal pathogen for non-bacteraemic pneumonia is typically unknown and no gold standard diagnostic techniques is available [20], [21], [22]. Widespread pre-hospitalisation use of antibiotics without prescription in Asian countries further limits proper aetiological identification. The lack of gold standard diagnostic techniques to identify the pathogen associated with an episode of non-bacteraemic pneumonia obscures assessment of the vaccine preventable burden of Hib pneumonia in common study designs [23]. Furthermore, vaccine-unrelated changes in the epidemiology of other pathogens contributing to total number of pneumonia cases can distort population impact estimates. Streptococcus pneumoniae and Haemophilus influeanzae are the leading causes of non-bacteraemic pneumonia supplemented by a sizeable contribution of respiratory viruses, including Rhinovirus, Influenza and respiratory syncytial virus (RSV) [24], [25]. While most of the serotypes causing pneumococcal disease show little variation over time in the absence of vaccination, serotypes 1 and 5 vary substantially in prevalence over time for yet unknown reasons [26] and are likely to cause a substantial amount of pneumonia despite low carriage rates [27]. Similarly the outbreak size of winter viruses can differ between seasons and can confound impact assessments [28].
Case control studies in various settings, including Pakistan and Bangladesh reported the Hib vaccine effectiveness against all cause radiologically confirmed pneumonia (RCP) in children to be between 20 and 60% [14], [29], [30], [31], [32], [33], although methodological problems may have led to inflated effectiveness estimates. A randomised controlled trial amongst Indonesian children could not detect any protective effect against this endpoint [17], despite reporting a substantial impact on meningitis. No estimates of rate reductions in RCP in the community with routine use of Hib vaccination have been reported amongst Asian countries to date.
With the support of the GAVI Alliance, Vietnam has included Hib as part of a DTwP-Hib-Hep (Pentavalent; Quinvaxem, Berna Biotech Korea, Seoul, South Korea) into the childhood immunisation programme since July 2010 and achieved a 3 dose coverage of 88% among children eligible for 3 doses by the end of 2010 [34]. Employing data from active hospital based surveillance in Nha Trang, central Vietnam, we here present early evidence that Hib vaccination in Vietnam is successfully reducing rates of all cause pneumonia in the targeted population.
2. Materials and methods
2.1. Design
We conducted an observational, population-based surveillance study of hospitalised respiratory illness in children under 5 years of age. Cases were assessed with chest radiography and viral testing of NP specimens. Cases with RCP were analysed in a ‘before-after’ approach (i.e. before and after Hib vaccine introduction) controlling for secular trends in common viral causes of respiratory illness.
2.2. Surveillance
From February 2007 onwards active surveillance monitoring hospitalised cases of acute respiratory infections (ARI) in children has been conducted in Nha Trang, the capital of Khanh Hoa province in central Vietnam. Details of the data collection and the study population are described in detail elsewhere [18], [35], [36].
Briefly: the catchment area of the surveillance study consists of 16 of the 27 communes in Nha Trang excluding those with an increased rate of foreign inhabitants. Children admitted to Khanh Hoa General Hospital (KHGH) from February 1st 2007 through March 31st 2012 with acute respiratory illness according to WHO definitions (any case with cough and/or difficulty breathing), aged between 1 month and 60 months on admission, residing in the catchment area were enrolled to the study. On admission, after obtaining informed consent from the parents or guardians, clinical and epidemiological information was collected by the respective paediatrician. Nasopharyngeal swabs and blood samples were taken by trained research nurses. Chest X-rays were taken within 24 h of admissions. In 2013 all chest X-rays were read independently by two designated radiologists according to WHO standards [37]. An expert panel reviewed discordant readings and a sample of positive readings. KHGH is the only hospital in Nha Trang, Khanh Hoa province and the only one accessible for residents of the catchment area. Hence for incidence calculations we assume that all children with RCP are enrolled into the study and use the population of the catchment area as denominator.
Viral nucleic acid was extracted from nasopharyngeal samples and four multiplex PCR assays were performed to detect 13 different respiratory viruses (influenza A and B, RSV, human metapneumovirus (hMPV), parainfluenza virus (PIV) 1–4, rhinovirus, coronaviruses OC43 and 229E, adenovirus and bocavirus) as described previously [36]. All of these viruses have been linked to pneumonia in children [38].
The study was approved by the Institutional Review Board at the National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, and the Institutional Review Board at the Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan.
2.3. Analysis
We present both the crude relative risk (RR) of pneumonia before and after the introduction of Hib vaccination and also an adjusted RR estimate. To calculate the adjusted RR we statistically adjust for differences in the circulation of viruses associated with pneumonia which can present a potential confounder for the estimation of Hib vaccination impact.
The crude RR (ψ) for all cause pneumonia was estimated through a segmented Poisson regression model. The model regresses the monthly number of cases of radiologically confirmed pneumonia (P t) with an intercept (α) to mark pre vaccination levels and a factor () to indicate the post vaccination period (T p); e t represents the residual errors:
Hence, the crude estimated impact of Hib vaccination on childhood pneumonia (1 − e ψ) is calculated as one minus the change in logged average monthly numbers of RCP before and after the introduction of routine Hib vaccination.
In a ‘before-after’ design, secular changes in the outcome (RCP) attributable to other pathogens can confound the association between the exposure of interest (Hib vaccine) and the outcome (RCP). To address this we have calculated an adjusted RR that adjusts for viral circulation using viral ARI episodes as a proxy for circulation. The adjusted RR for non-viral associated RCP (NVP) was similarly estimated through a segmented Poisson regression model. With the same naming conventions as before the model additionally attributes excess cases of RCP (deemed virus associated pneumonia – VP), which includes both viral pneumonia and secondary bacterial pneumonia infections, to changes in the monthly number of ARI due to influenza A, RSV, hMPV, parainfluenza 3, rhinovirus, adenovirus and bocavirus (N t,1–7). Less than one case per month on average of influenza B, parainfluenza virus 1, 2 and 4 and corona virus respectively was reported in the pre vaccination period; hence those were excluded from the analysis to limit the occurrence of statistical artefacts. Any potential negative association of viruses and pneumonia rates was omitted; i.e. presence of viruses was assumed not to prevent pneumonia (ϕ 1–7 ≥ 0).
In other words, we estimate the excess number of RCP due to influenza A, RSV, hPMV, parainfluenza 3, rhinovirus, adenovirus and bocavirus to obtain adjusted number of RCP (RCP excluding excess VP) during the study period. Based on the adjusted number of RCP, the NVP, we then calculate the impact of Hib vaccination on NVP (1 − e ψ) as one minus the change in logged average monthly numbers of NVP before and after the introduction of routine Hib vaccination. Because of multiplicity the impact on NVP in this model also corresponds to the impact of Hib vaccination on RCP adjusted for the circulation of viruses. Hence, in the following we refer to (1 − e ψ) in the adjusted analysis as the adjusted IRR for RCP.
S. pneumoniae is the dominant cause of pneumonia and hence presents a potential confounder for impact estimates in an observational “before-after” study. Not accounting for pneumococcal pneumonia presents a potential limitation to our study. However, no nasopharyngeal carriage of the major epidemic pneumococcal serotypes 1 or 5 has been detected in those presenting with ARI between 29/01/2007 and 15/04/2008 suggesting low contribution of serotypes 1 and 5 to the burden of pneumonia in Nha Trang [27], [35].
Cases with missing information on viral testing (4% of all cases) were attributed proportionally according to the distribution of ARI cases tested positive for influenza A, influenza B, RSV and rhinovirus or tested negative in the respective month. Similarly ARI episodes where no chest radiograph was taken (2% of all cases) were attributed proportionally according to the proportion of radiologically confirmed pneumonia in the respective month.
Two-tailed t-test and chi-square tests are used as appropriate to determine the significance of the change in the presence of clinical features from the pre vaccination era to the post vaccination era. Confidence intervals presented refer to a confidence level of 95%. Statistical analysis was performed in R 3.01 [39].
3. Results
3.1. Characteristics of the enrolled cases
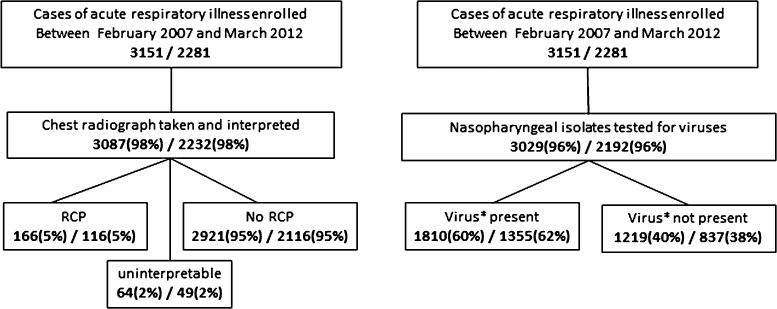
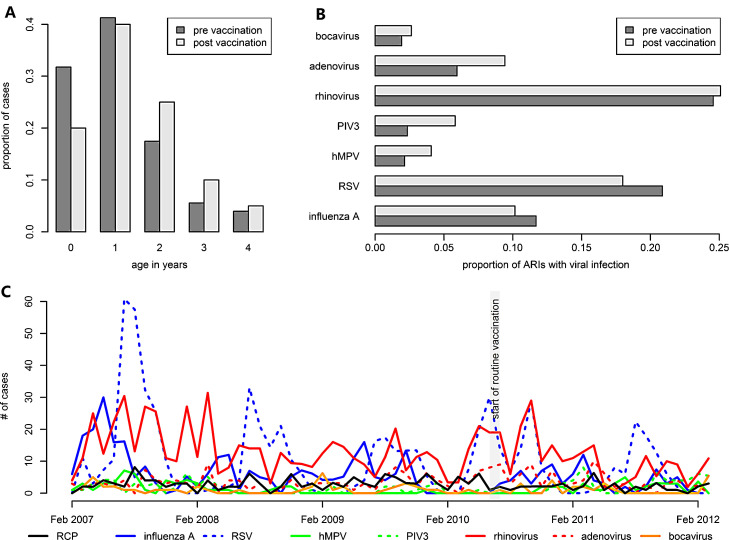
A total of 3151 children less than 5 years of age hospitalised for ARI were enrolled in the active ARI surveillance between February 2007 and March 2012 and a chest X-ray was taken and interpreted in 98% of the cases (Fig. 1 ). 166 (5%) of the radiographs were classified as cases of radiologically confirmed pneumonia (primary endpoint). 64 (2%) of the radiographs were found to be uninterpretable. The main burden of radiological pneumonia was amongst the 0–1 year olds but shifted slightly towards older ages in the post vaccination period (see Fig. 2A). Information on viral presence in the nasopharynx was available for 96% of all cases of ARI. At least one virus was detected in 1875 (61%) of the tested isolates (Table 1 ) with influenza A, RSV and rhinovirus accounting for 1545 (82%) of these isolates. Influenza A and RSV were more common among ARI in the period before vaccination (see Fig. 2B) while the less common viruses, including PIV3, hMPV and adenovirus, were more present in the post vaccination period. This presents a potential issue of confounding in a “before-after” analysis if not adjusted for.
Fig. 1.
Case counts of acute respiratory illnesses in children under five years old and under two years old that were included in the study and the stratification into data on RCP and viral circulation. The 166 samples with diagnosis of radiological confirmed pneumonia were used to estimate the impact of Hib vaccination in children younger than 5 years. *The 1810 samples of acute respiratory illness where either influenza A, RSV, human metapneumovirus, parainfluenza 3, rhinovirus, adenovirus or bocavirus was detected from the nasopharynx were employed as a proxy to represent monthly intensity of viral circulation in children less than 5 years of age.
Fig. 2.
Overview of data characteristics. (A) Age distribution of cases of radiological confirmed pneumonia. (B) Comparison of nasopharyngeal carriage of viruses amongst chidhood cases of ARI before and after introduction of Hib vaccination to the national childhood immunisation. (C) Timeline of the number of cases of ARI either classified as RCP or found to carry a viral infection.
Table 1.
Demographic and clinical features of enrolled cases. The number of positives is reported along with the proportion in all cases. Where appropriate the mean and the standard deviation is reported instead. Where information on clinical features in some reports the reduced sample size of complete reports is given.
| RCP |
Non-RCP |
|||||
|---|---|---|---|---|---|---|
| Pre-vaccine period | Post-vaccine period | P value* | Pre-vaccine period | Post-vaccine period | P value* | |
| N = 126 | N = 40 | N = 2136 | N = 785 | |||
| Age <60 month | ||||||
| Male | 67 (53%) | 19 (48%) | 0.53 | 1296 (61%) | 467 (60%) | 0.56 |
| Age <24 month | 92 (73%) | 24 (60%) | 0.12 | 1554 (73%) | 562 (72%) | 0.53 |
| Kindergarten attended | 60 (48%) | 24 (60%) | 0.17 | 895 (42%) | 321 (41%) | 0.62 |
| Clinical features | ||||||
| Increased respiratory rate | 39 (31%) | 5 (13%) | 0.02 | 379 (18%) | 70 (9%) | <0.001 |
| Chest indrawing | 13 (10%) | 1 (3%) | 0.19 | 123 (6%) | 38 (5%) | 0.34 |
| Stridor | 2 (2%) | 0 (0%) | 1 | 13 (1%) | 10 (1%) | 0.07 |
| Body temperature ≥38.0 °C | 94 (75%) | 18 (45%) | <0.001 | 1336 (63%) | 282 (36%) | <0.001 |
| Pre-hospital antibiotics | 38 (45%) | 14 (41%) | 0.73 | 617 (42%) | 342 (47%) | 0.03 |
| Days from onset to admission | 3.1 ± 2.6 | 3 ± 3.3 | 0.88 | 3.1 ± 2.9 | 2.3 ± 2.5 | <0.001 |
| WBC count, x10^3 | 16.8 ± 8.8 | 16 ± 8.6 | 0.59 | 12.8 ± 6.9 | 13.1 ± 7.3 | 0.31 |
| Any virus | 72 (61%) | 25 (64%) | 0.73 | 1287 (62%) | 491 (67%) | 0.01 |
| RCP |
Non-RCP |
|||||
|---|---|---|---|---|---|---|
| Pre-vaccine period | Post-vaccine period | P value* | Pre-vaccine period | Post-vaccine period | P value* | |
| N = 92 | N = 24 | N = 1554 | N = 561 | |||
| Age <24 month | ||||||
| Male | 51 (55%) | 14 (58%) | 0.8 | 985 (63%) | 351 (63%) | 0.73 |
| Kindergarten attended | 39 (42%) | 10 (42%) | 0.95 | 470 (30%) | 155 (28%) | 0.25 |
| Clinical features | ||||||
| Increased respiratory rate | 30 (33%) | 3 (13%) | 0.07 | 287 (18%) | 52 (9%) | <0.001 |
| Chest indrawing | 9 (10%) | 0 (0%) | 0.2 | 98 (6%) | 29 (5%) | 0.33 |
| Stridor | 2 (2%) | 0 (0%) | 1 | 10 (1%) | 5 (1%) | 0.55 |
| Body temperature ≥38.0 °C | 69 (75%) | 10 (42%) | 0.002 | 947 (61%) | 181 (32%) | <0.001 |
| Pre-hospital antibiotics | 27 (44%) | 9 (43%) | 0.91 | 420 (40%) | 237 (46%) | 0.016 |
| Days from onset to admission | 3.3 ± 2.8 | 2.6 ± 3.1 | 0.34 | 3.2 ± 3.0 | 2.4 ± 2.5 | <0.001 |
| WBC count, ×103 | 17.2 ± 8.9 | 15.3 ± 8.8 | 0.33 | 13.0 ± 6.6 | 13.5 ± 7.8 | 0.2 |
| Any virus | 60 (68%) | 18 (75%) | 0.52 | 957 (63%) | 367 (66%) | 0.21 |
| RCP |
Non-RCP |
|||||
|---|---|---|---|---|---|---|
| Pre-vaccine period | Post-vaccine period | P value* | Pre-vaccine period | Post-vaccine period | P value* | |
| N = 34 | N = 16 | N = 582 | N = 224 | |||
| Age 24–59 month | ||||||
| Male | 16 (47%) | 5 (31%) | 0.37 | 311 (53%) | 116 (52%) | 0.67 |
| Kindergarten attended | 21 (62%) | 14 (88%) | 0.1 | 425 (73%) | 166 (74%) | 0.76 |
| Clinical features | ||||||
| Increased respiratory rate | 9 (26%) | 2 (13%) | 0.47 | 92 (16%) | 18 (8%) | 0.004 |
| Chest indrawing | 4 (12%) | 1 (6%) | 1 | 25 (4%) | 9 (4%) | 0.86 |
| Stridor | 0 (0%) | 0 (0%) | 1 | 3 (1%) | 5 (2%) | 0.042 |
| Body temperature ≥38.0 °C | 25 (74%) | 8 (50%) | 0.1 | 389 (67%) | 101 (45%) | <0.001 |
| Pre-hospital antibiotics | 11 (46%) | 5 (38%) | 0.74 | 197 (50%) | 105 (51%) | 0.89 |
| Days from onset to admission | 2.5 ± 2.2 | 3.5 ± 3.5 | 0.27 | 2.8 ± 2.6 | 2.2 ± 2.3 | 0.002 |
| WBC count, ×103 | 15.8 ± 8.3 | 17.0 ± 8.4 | 0.61 | 12.2 ± 7.6 | 12.2 ± 6.0 | 1 |
| Any virus | 12 (40%) | 7 (44%) | 0.81 | 330 (58%) | 151 (68%) | 0.008 |
Chi-square tests for categorical variables and t-tests for numerical variables as appropriate.
Definitions: Chest indrawing: child's chest wall moves in or retracts during inhalation; increased respiratory rate for children younger than 2 month is defined as at least 60 breaths per minute, for children 2–12 month olds at least 50 breaths per minute, and for children older than 12 months at least 40 breaths per minute; antibiotic usage within 14 days prior to admission is recognised here as possibly affecting the susceptibility to bacterial pneumonia.
Of all study participants 56% presented with fever (Table 1). Other common symptoms included dyspnoea and increased respiratory rate. The rate of antibiotic usage prior to admission did not change significantly in the post vaccination period. A higher proportion of children who were found positive for RCP presented with increased respiratory rate, chest wall indrawing, stridor or fever than of those children who were found RCP negative. Changes in clinical features towards milder symptoms were observed in the period after introduction of Hib vaccination which were similar in both RCP positive and negative cases.
3.2. Presence of RCP before and after the introduction of Hib vaccination
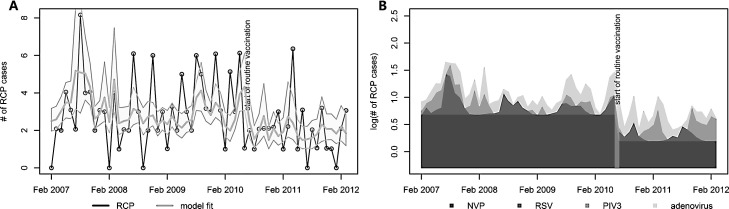
The average annual incidence of radiologically confirmed childhood pneumonia was 2.68 cases per 1000 population before vaccination and 1.71 cases per 1000 population after vaccination (Fig. 3A). The average annual incidence of radiologically confirmed pneumonia, adjusted for viral circulation (NVP), in children younger than 5 years declined from 1.7 cases per 1000 children before to 1.1 cases per 1000 children after introduction of Hib vaccination (Table 2 ). This corresponds to a reduction of 39% (12–58%) in NVT and also all cause RCP. The introduction of routine childhood vaccination of Hib coincided with a decrease in cases of acute respiratory illness where RSV was detected, however, more parainfluenza virus type 3 and more adenovirus was found (Fig. 2B and C). RSV was estimated to increase NVP incidence by 15% (2–29%) before and 9% (1–18%) after the start of Hib vaccination. Parainfluenza virus type 3 and adenovirus were found to increase NVP incidence by 10% (0–22%) and 19% (−2% to 43%) before and 20% (−1% to 44%) and 22% (−3% to 51%) after vaccination (see Fig. 3B). No correlation between the co-circulation of the remaining viruses and RCP was found in this age group.
Fig. 3.
Model results. (A) Comparison of the reported number of radiological confirmed cases of pneumonia in under 5 year olds (line with data points) and the fit of the adjusted model including its 95% confidence intervals (grey shaded area). (B) The contribution of the estimated regression parameters to the modelled logged number of RCP cases. In both figures the vertical grey bar represents the introduction of Hib vaccination to Vietnam.
Table 2.
Annual incidence per 1000 population in the pre and post Hib vaccination era and the corresponding incidence risk ratio. Both results adjusted and not adjusted for viral circulation are shown.
| Incidence before vaccinationa | Incidence after vaccinationa | Incidence risk ratio | |
|---|---|---|---|
| <5y | |||
| Adjusted | 1.71 (1.21–2.39) | 1.05 (0.66–1.63) | 0.61 (0.42–0.88) |
| Crude | 2.68 (2.24–3.17) | 1.71 (1.24–2.28) | 0.64 (0.44–0.90) |
| <2y | |||
| Adjusted | 3.01 (1.98–4.46) | 1.57 (0.89–2.67) | 0.52 (0.33–0.81) |
| Crude | 5.84 (4.73–7.10) | 3.06 (2.01–4.41) | 0.52 (0.33–0.80) |
| 2-4y | |||
| Adjusted | 0.86 (0.49–1.45) | 0.82 (0.43–1.47) | 0.96 (0.52–1.72) |
| Crude | 1.09 (0.76–1.49) | 1.01 (0.60–1.59) | 0.93 (0.51–1.65) |
Note that the unadjusted incidence refers to all cause RCP while the adjusted incidence refers to non-virus associated RCP. Also the mean incidence presented here is assuming Poisson distributed count data and hence is lower than previously reported estimates which assumed normal distributed counts [18].
No children older than 2 years have been eligible for vaccination during the study period and hence were protected by direct vaccine effects. We find no significant reduction of RCP incidence amongst 2–4 year old children. However, most of the childhood pneumonia burden was in children under the age of 2 years with an annual RCP incidence of 5.8 cases per 1000 children before vaccination (Table 2). In this age group we estimated a substantial decline of pre vaccination RCP incidence to 3.1 cases per 1000 population after vaccination corresponding to a reduction of 48% (19–67%).
4. Discussion
We present here an early estimate of the impact of Hib vaccine on all cause pneumonia from an observational, population based surveillance study in children less than five years of age, accounting for differences in viral circulation in the pre and post vaccination era. We find early evidence that within 21 months after the introduction of Hib vaccination the burden of all cause pneumonia in children less than 2 years of age in Nha Trang has already been substantially reduced. However, only a small and not significant reduction in RCP in older children who have not been eligible for routine vaccination was observed.
Few studies have assessed the effect of Hib vaccination on RCP and even fewer in Asia. To date most studies that were conducted in Asia reported effectiveness estimates from case-control designs rather than population based incidence impact. Vaccine effectiveness was estimated to be 31% (−9% to 57%) in Brazil, 55% (7–78%) in children receiving three vaccine doses in Colombia, 21% (5–35%) in Gambia, 22% (−7% to 43%) in Chile, 45% (18–63%) in Ukraine and 62% (47–73%) in Pakistan [14], [29], [30], [31], [32]. All but Gambia and Chile were case control studies. The vaccine preventable proportion of RCP was 35% and 44% in a case control study in Bangladesh when using community and hospital controls respectively [33]. However, case control designs may overestimate vaccine effectiveness if vaccinated and unvaccinated communities differ significantly in their risk of pneumonia. The only study that did not detect any impact amongst children was a randomised controlled trial conducted in Indonesia, although a significant impact on meningitis and non-radiological pneumonia was shown in the same study [17]. Our findings suggest that routine childhood Hib vaccination is associated with a significant reduction in the burden of radiologically confirmed pneumonia in young children in Vietnam. This supports both the presence of a high burden of Hib pneumonia in Vietnam in the absence of vaccination and a high impact of routine vaccination with DTwP-Hib-Hep. The lack of a measurable impact of Hib vaccination in children between 2 and 4 years of age and too old to be vaccinated may suggest that herd protection has not been established yet and in subsequent years could further benefit the unvaccinated [40].
Only 5% of all children hospitalised with acute respiratory symptoms were classified as primary endpoint pneumonia according to WHO definitions in our study. This is substantially less than the respective fraction of RCP cases among hospitalised children in other studies [14], [31], [41]. This reflects a low threshold for admission for children with respiratory infections in this part of Vietnam. Free of charge health insurance for all children under the age of 6 years, good accessibility of the hospital within the study area and a 2 child only policy in Vietnam are likely to have contributed to the low threshold for admission, and therefore inclusion in our study and hence a low proportion of RCP cases among them.
While RCP incidence in Vietnam is low in comparison with other middle income settings [18] we estimate that annually 1.44 cases of RCP per 1000 population in children less than 2 years of age have already been prevented by the yet immature Hib vaccination programme. Few other estimates are available for the vaccine preventable burden of severe, non-bacteraemic pneumonia associated with Hib because of the lack of sensitivity and specificity of diagnostics. A randomised trial conducted in Chile has reported the burden of vaccine preventable RCP due to Hib at 1.1 per 1000 children of age 4–23 month [32]. A review on the global burden of Hib in 2000 estimated that in Southeast Asia the vaccine preventable burden of Hib meningitis and clinical pneumonia was 0.27 and 17.9 cases per 1000 children under 5 years of age respectively [1].
In several instances we chose assumptions in our estimates that may have led to an under estimation of the true impact of Hib vaccination on RCP (i.e. conservative assumptions). (A) In the absence of a catch up campaign a large proportion of individuals younger than 2 years of age have not been eligible for vaccination in the 21 months after vaccine introduction that we consider here. Hence our early estimate the impact of routine Hib vaccination is may be an underestimate of the full impact of Hib vaccination, although most Hib pneumonia is likely to occur in the first year. With an increasing duration of follow up a transition period can be included in the regression model to account for the initial period of low vaccination eligibility in the considered age groups and provide an estimate of the full impact of Hib vaccination. (B) We assume that a reduction of virus circulation among children presenting with acute respiratory symptoms is not associated with Hib vaccination. However, the observed decline could partly be due to a reduction in co-infections of viruses and Hib. In this case the crude estimate would be a more accurate estimate of the actual impact of Hib vaccination on RCP. (C) The decrease in severity of symptoms of children diagnosed with RCP at Khanh Hoa General Hospital in the post vaccination period could suggest an increase in health care seeking of children with milder symptoms. This could have led to an increase in the number of acute respiratory cases and RCPs reported over time, and in particular to an overrepresentation of ARI in the post vaccination era. On the other hand, it could reflect the rapid disappearance of severe childhood pneumonia associated with improved care seeking and overall socioeconomic development. When tested in a linear regression model we found no evidence for a significant decrease in RCP incidence during the pre vaccination era. (D) We do not account for possible changes in pneumococcal pneumonia, in particular due to volatile serotypes 1 and 5. In a subset of the pre vaccination period no serotype 1 or 5 carriage in children presenting with ARI was detected suggesting a low contribution of those types to pneumonia during this period [35] so any bias would likely underestimate the impact of Hib vaccination. There is no evidence available that allowed assessment of the circulation of pneumococcal serotypes and hence potential biases during the study period. However, a previous study did not find evidence of serotype 1 and 5 causing invasive pneumococcal diseases in Vietnam during the decade ending in 2002 [42] so their impact may be generally low in this setting and hence unlikely to greatly impact on our estimates. However in settings where those serotypes are more dominant, e.g. in Africa, this adjustment can be essential.
Among the non-RCP ARI cases antibiotic usage during the 2 weeks prior to hospital admission has increased by about 12% in the post vaccination era and hence potentially reduced the likelihood of developing RCP. Also the likelihood for the development of RCP on admission has potentially been reduced in the post vaccination era as the time from illness onset to hospitalisation decreased. Both, changes in antibiotic usage and timeliness of health care seeking could have led to an overestimation of the impact of Hib vaccination in our analysis. However, antibiotic usage and timeliness of health care seeking was similar between RCP cases and ARI cases without RCP suggesting that those factors had only modest impact on the development of RCP for a case admitted with acute respiratory symptoms in the study population. Furthermore, after the introduction of Hib vaccination we find little or no change in RCP incidence in 2–4 year old children, who were not eligible for vaccination in the study period tentatively suggesting that the observed impact among less than 2 year old children is vaccine specific and that potential changes in treatment and healthcare seeking behaviour in the population had little impact on RCP incidence.
The regression analysis includes several assumptions and limitations. Negative associations of viral circulation and RCP were not permitted. If virus associated illnesses were extensively treated with antibiotics, the circulation of such viruses could have a preventive rather than a supportive effect on bacterial pneumonia. Nevertheless, influenza, RSV and rhinovirus are generally found to increase the risk of pneumonia [43], [44], [45], [46], [47]. However, we could not find seasonally increased circulation of influenza A or B to be associated with a concurrent increase in radiologically confirmed pneumonia (Fig. 2C). Furthermore the limited number of cases only allowed for monthly resolution of the data. Hence, possible lags between viral infection and associated secondary bacterial pneumonia were not accounted for. Moreover Influenza A subtypes were not differentiated in the analysis. If these subtypes were associated with significantly different risk for pneumonia this could have masked the true contribution of the subtypes on VP. Also the adequacy of chest radiographs as gold standard for pneumonia diagnosis may be questioned [48], however, we use of multiple independent readers to minimise non-specificity.
We further tested the sensitivity of our results to the choice of model. Instead of limiting the viruses in the model to those with sufficiently high detection frequency we included all viruses in the model. The corresponding incidence risk ratio in children less than two years of age was estimated at 0.53 (0.33–0.82). Although no significant trend was found prior to vaccination we estimated the effects of Hib vaccination in children less than 2 years of age via the crude analysis but adjusting for a linear trend in both the pre and post vaccination era. The corresponding incidence risk ratio was estimated at 0.43 (0.22–0.85). Furthermore we excluded the period from February 2007 to February 2008 from the analysis to assess whether this period of increased RCP incidence could have confounded our analysis. When excluding this period the estimated incidence risk ratio in children less than two years old was 0.51 (0.32–0.81). Hence the substantial impact of Hib vaccination on all cause primary endpoint pneumonia in Nha Trang is relatively robust to model choice.
5. Conclusions
This study provides evidence that less than 2 years after introduction of routine vaccination against Hib in Vietnam a substantial impact on all cause RCP in children less than 5 years old has been observed. This is driven by children under 2 years of age, of whom most have been vaccinated. Although an underlying secular trend cannot be ruled out, this study adds to the body of evidence that the preventable burden of Hib pneumonia in Asian countries may be substantial. More post vaccination surveillance data is needed to provide more robust estimates and to detect evidence for herd protection.
Conflict of interest
WJE's partner works for GSK. EKM has served on advisory boards for GSK. All other authors declare that they have no competing interests.
Funding
This study was supported by the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID), MEXT, Japan, Japan Society for Promotion of Science, Japan and the GAVI Hib initiative Project, through the London School of Hygiene and Tropical Medicine. The study sponsors had no role in study design, the collection, analysis and interpretation of data, the writing of the report and the decision to submit the manuscript for publication.
Acknowledgements
We are grateful to the participants of this study and their parents as well as to the staff from Khanh Hoa Health Service and the medical staff from of Khanh Hoa General Hospital for their support. We also thank the staff from the Japan-Vietnam Friendship Laboratory at National Institute of Hygiene and Epidemiology, Hanoi, and Institute of Tropical Medicine, Nagasaki University. We also thank anonymous reviewers for thorough comments that helped improving this manuscript.
References
- 1.Watt J.P., Wolfson L.J., O’Brien K.L., Henkle E., Deloria-Knoll M., McCall N. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organisation WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 2006;81:445–452. [PubMed] [Google Scholar]
- 3.Adams W.G., Deaver K.A., Cochi S.L., Plikaytis B.D., Zell E.R., Broome C.V. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269:221–226. [PubMed] [Google Scholar]
- 4.Adegbola R.A., Secka O., Lahai G., Lloyd-Evans N., Njie A., Usen S. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet. 2005;366:144–150. doi: 10.1016/S0140-6736(05)66788-8. [DOI] [PubMed] [Google Scholar]
- 5.Ramsay M.E., McVernon J., Andrews N.J., Heath P.T., Slack M.P. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J Infect Dis. 2003;188:481–485. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 6.Braikat M., Barkia A., El Mdaghri N., Rainey J.J., Cohen A.L., Teleb N. Vaccination with Haemophilus influenzae type b conjugate vaccine reduces bacterial meningitis in Morocco. Vaccine. 2012;30:2594–2599. doi: 10.1016/j.vaccine.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Pilishvili T., Chernyshova L., Bondarenko A., Lapiy F., Sychova I., Cohen A. Evaluation of the effectiveness of Haemophilus influenzae type b conjugate vaccine introduction against radiologically-confirmed hospitalized pneumonia in young children in Ukraine. J Pediatr. 2013;163:12–18. doi: 10.1016/j.jpeds.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Sigaúque B., Vubil D., Sozinho A., Quintó L., Morais L., Sacoor C. Haemophilus influenzae type b disease among children in rural Mozambique: impact of vaccine introduction. J Pediatr. 2013;163:19–24. doi: 10.1016/j.jpeds.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett J.V., Platonov A.E., Slack M.P., Mala P., Burton A., Robertson S.A. Haemophilus influenzae type b (Hib) meningitis in the pre-vaccine era: a global review of incidence, age distributions, and case-fatality rates. Vaccines Biol. 2002;1:92. [Google Scholar]
- 10.Morris S., Moss W., Halsey N. Haemophilus influenzae type b conjugate vaccine use and effectiveness. Lancet Infect Dis. 2008;8:435–443. doi: 10.1016/S1473-3099(08)70152-X. [DOI] [PubMed] [Google Scholar]
- 11.Knoll M.D., O’Brien K.L., Henkle E., Lee E., Watt J.P., McCall N. Global literature review of Haemophilus influenzae type b and Streptococcus pneumoniae invasive disease among children less than five years of age, 1980–2005. Immun Vaccines Biol. 2009:1–193. [Google Scholar]
- 12.World Health Organization . 2012. Global vaccination coverage of Haemophilus influenzae type b.http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/sentinel/Hib/en/index.html [accessed 04.12.13] [Google Scholar]
- 13.Shetty S., Cohen A.L., Edmond K., Ojo L., Loo J., O’Loughlin R. A systematic review and critical evaluation of invasive Haemophilus influenzae type B disease burden studies in Asia from the last decade: lessons learned for invasive bacterial disease surveillance. Pediatr Infect Dis J. 2010;29:653–661. doi: 10.1097/INF.0b013e3181d3ce19. [DOI] [PubMed] [Google Scholar]
- 14.Khowaja A.R., Mohiuddin S., Cohen A.L., Mirza W., Nadeem N., Zuberi T. Effectiveness of Haemophilus influenzae type b conjugate vaccine on radiologically-confirmed pneumonia in young children in Pakistan. J Pediatr. 2013;163:79–85. doi: 10.1016/j.jpeds.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sultana N.K., Saha S.K., Al-Emran H.M., Modak J.K., Sharker M.aY., El-Arifeen S. Impact of introduction of the Haemophilus influenzae type b conjugate vaccine into childhood immunization on meningitis in Bangladeshi infants. J Pediatr. 2013;163:73–78. doi: 10.1016/j.jpeds.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajjeh R., Mulholland K., Schuchat A., Santosham M. Progress towards demonstrating the impact of Haemophilus influenzae type b conjugate vaccines globally. J Pediatr. 2013;163:1–3. doi: 10.1016/j.jpeds.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gessner B.D., Sutanto A., Linehan M., Djelantik I.G.G., Fletcher T., Gerudug I.K. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet. 2005;365:43–52. doi: 10.1016/s0140-6736(04)17664-2. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida L.-M., Nguyen H.-A., Watanabe K., Le M.N., Nguyen A.T., Vu H.T. Incidence of radiologically-confirmed pneumonia and Haemophilus influenzae type b carriage before Haemophilus influenzae type b conjugate vaccine introduction in Central Vietnam. J Pediatr. 2013;163:38–43. doi: 10.1016/j.jpeds.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Nyambat B., Dang D.A., Nguyen H.A., Mai T.Q., Rani M., Slack M.P.E. Rapid assessment of Hib disease burden in Vietnam. BMC Public Health. 2011;11:260. doi: 10.1186/1471-2458-11-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammitt L.L., Kazungu S., Morpeth S.C., Gibson D.G., Mvera B., Brent A.J. A preliminary study of pneumonia etiology among hospitalized children in kenya. Clin Infect Dis. 2012;54(Suppl. 2):190–199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine O.S., O’Brien K.L., Deloria-Knoll M., Murdoch D.R., Feikin D.R., DeLuca A.N. The Pneumonia Etiology Research for Child Health Project: a 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54(Suppl. 2):93–101. doi: 10.1093/cid/cir1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murdoch D.R., O’Brien K.L., Driscoll A.J., Karron R.A., Bhat N. Laboratory methods for determining pneumonia etiology in children. Clin Infect Dis. 2012;54(Suppl. 2):146–152. doi: 10.1093/cid/cir1073. [DOI] [PubMed] [Google Scholar]
- 23.Feikin D.R., Scott J.A.G., Gessner B.D. Use of vaccines as probes to define disease burden. Lancet. 2014;383:1762–1770. doi: 10.1016/S0140-6736(13)61682-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudan I., Boschi-Pinto C., Biloglav Z., Mulholland K., Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ. 2008;86:408–416. doi: 10.2471/BLT.07.048769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair H., Simões E.A., Rudan I., Gessner B.D., Azziz-Baumgartner E., Zhang J.S.F. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013;381:4–6. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black S. The volatile nature of pneumococcal serotype epidemiology: potential for misinterpretation. Pediatr Infect Dis J. 2010;29:301–303. doi: 10.1097/INF.0b013e3181c391fb. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg D., Givon-Lavi N., Newman N., Bar-Ziv J., Dagan R. Nasopharyngeal carriage of individual Streptococcus pneumoniae serotypes during pediatric pneumonia as a means to estimate serotype disease potential. Pediatr Infect Dis J. 2011;30:227–233. doi: 10.1097/INF.0b013e3181f87802. [DOI] [PubMed] [Google Scholar]
- 28.Baguelin M., Flasche S., Camacho A., Demiris N., Miller E., Edmunds W.J. Assessing optimal target populations for influenza vaccination programmes: an evidence synthesis and modelling study. PLoS Med. 2013;10:e1001527. doi: 10.1371/journal.pmed.1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Andrade A.L.S.S., de Andrade J.G., Martelli C.M.T., e Silva S.A., de Oliveira R.M., Costa M.S.N. Effectiveness of Haemophilus influenzae b conjugate vaccine on childhood pneumonia: a case–control study in Brazil. Int J Epidemiol. 2004;33:173–181. doi: 10.1093/ije/dyh025. [DOI] [PubMed] [Google Scholar]
- 30.De la Hoz F., Higuera A.B., Di Fabio J.L., Luna M., Naranjo A.G., de la Luz Valencia M. Effectiveness of Haemophilus influenzae type b vaccination against bacterial pneumonia in Colombia. Vaccine. 2004;23:36–42. doi: 10.1016/j.vaccine.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 31.Mulholland K., Hilton S., Adegbola R., Usen S., Oparaugo A., Omosigho C. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate for prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 32.Levine O.S., Lagos R., Muñoz A., Villaroel J., Alvarez A.M., Abrego P. Defining the burden of pneumonia in children preventable by vaccination against Haemophilus influenzae type b. Pediatr Infect Dis J. 1999;18:1060–1064. doi: 10.1097/00006454-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Baqui A.H., El Arifeen S., Saha S.K., Persson L., Zaman K., Gessner B.D. Effectiveness of Haemophilus influenzae type B conjugate vaccine on prevention of pneumonia and meningitis in Bangladeshi children: a case–control study. Pediatr Infect Dis J. 2007;26:565–571. doi: 10.1097/INF.0b013e31806166a0. [DOI] [PubMed] [Google Scholar]
- 34.The Government of Viet Nam . 2011. GAVI Alliance – Annual Progress Report 2010 for Viet Nam.http://www.gavialliance.org/country/vietnam/documents/#approvedproposal [accessed 11.07.12] [Google Scholar]
- 35.Vu H.T.T., Yoshida L.M., Suzuki M., Nguyen H.A.T., Nguyen C.D.L., Nguyen A.T.T. Association between nasopharyngeal load of Streptococcus pneumoniae, viral coinfection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr Infect Dis J. 2011;30:11–18. doi: 10.1097/INF.0b013e3181f111a2. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida L.M., Suzuki M., Yamamoto T., Nguyen H.A., Nguyen C.D., Nguyen A.T. Viral pathogens associated with acute respiratory infections in central Vietnamese children. Pediatr Infect Dis J. 2010;29:75–77. doi: 10.1097/INF.0b013e3181af61e9. [DOI] [PubMed] [Google Scholar]
- 37.Cherian T., Mulholland E.K., Carlin J.B., Ostensen H., Amin R., de Campo M. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 38.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.R Core Team . 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- 40.Chen W.-J., Moulton L.H., Saha S.K., Mahmud A. Al, Arifeen S. El, Baqui A.H. Estimation of the herd protection of Haemophilus influenzae type b conjugate vaccine against radiologically confirmed pneumonia in children under 2 years old in Dhaka, Bangladesh. Vaccine. 2014;32:944–948. doi: 10.1016/j.vaccine.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 41.Tregnaghi M.W., Sáez-Llorens X., López P., Abate H., Smith E., Pósleman A. Efficacy of pneumococcal nontypable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in young Latin American children: a double-blind randomized controlled trial. PLoS Med. 2014;11:e1001657. doi: 10.1371/journal.pmed.1001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parry C.M., Duong N.M., Zhou J., Mai N.T.H., Diep T.S., Thinh L.Q. Emergence in Vietnam of Streptococcus pneumoniae resistant to multiple antimicrobial agents as a result of dissemination of the multiresistant Spain23F-1 clone. Antimicrob Agents Chemother. 2002;46:3512–3517. doi: 10.1128/AAC.46.11.3512-3517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox N.J., Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 44.O’Brien K.L., Walters M.I., Sellman J., Quinlisk P., Regnery H., Schwartz B. Severe pneumococcal pneumonia in previously healthy children: the role of preceding influenza infection. Clin Infect Dis. 2000;30:784–789. doi: 10.1086/313772. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida L.-M., Suzuki M., Nguyen H.A., Le M.N., Vu T.D., Yoshino H. Respiratory syncytial virus: co-infection and paediatric lower respiratory tract infections. Eur Respir J. 2013;42:461–469. doi: 10.1183/09031936.00101812. [DOI] [PubMed] [Google Scholar]
- 46.Scott J.A.G., Brooks W.A., Peiris J.S.M., Holtzman D., Mulholland E.K. Pneumonia research to reduce childhood mortality in the developing world. J Clin Invest. 2008;118:1291–1300. doi: 10.1172/JCI33947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harada Y., Kinoshita F., Yoshida L.M., Minh L.N., Suzuki M., Morimoto K. Does respiratory virus coinfection increases the clinical severity of acute respiratory infection among children infected with respiratory syncytial virus? Pediatr Infect Dis J. 2013;32:441–445. doi: 10.1097/INF.0b013e31828ba08c. [DOI] [PubMed] [Google Scholar]
- 48.Izadnegahdar R., Cohen A.L., Klugman K.P., Qazi S.A. Childhood pneumonia in developing countries. Lancet Respir Med. 2013;1:574–584. doi: 10.1016/S2213-2600(13)70075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]