Abstract
Background
Cardiac catheterization (CATH) is key in the diagnosis and management of coronary artery disease. Increasing demand coupled with limited resources in a publicly funded system (e.g. Ontario, the largest province in Canada) resulted in a waitlist for this procedure. Our province has recommended maximum wait times (RMWT) for patients referred to CATH. The purpose of this study is to describe our experience over the past decade in attempting to meet RMWTs for patients needing CATH at our centre, and to discuss issues concerning capacity planning in providing timely service.
Methods
We measured the proportion of patients undergoing a procedure within the RWMT, and calculated both the mean number of patients and mean length of time on the wait list for each year over a decade for those referred to CATH using prospectively collected registry data. We identified factors that increased referrals or improved capacity. Wait time was compared to community standard RMWTs in order to establish if and how RMWTs were achieved.
Results
Despite a number of systematic and capacity improvements, RMWTs were not achieved until after the addition of a 4th laboratory.
Interpretation
Improving access to CATH in our centre was reactive to the increasing need of the community rather than based on anticipation of need and continuity of service within RMWTs. Registry data can help monitor key indicators (e.g. RMWT). Prudent use of this information should help policy makers with future expansion in our region.
Keywords: Cardiac catheterization, Service delivery, Wait times, Capacity
1. Background
Coronary artery disease is a major cause of mortality and morbidity worldwide [1]. Cardiac catheterization (CATH) plays a key role in diagnosis and management of this disease [2]. Increasing demand coupled with limited resources in publicly funded systems (e.g. Ontario, the largest province in Canada) often results in a waitlist for this procedure [3], [4]. Previous reports have suggested an association between long wait times for CATH and increased mortality and morbidity [5]. In addition, long wait times are associated with anxiety in people needing a CATH, resulting in a decrease in quality of life [4].
In order to mitigate the effects of waiting for service, researchers and healthcare organizations have recommended maximum wait times (RMWT) based on urgency of need. Often these are local standards based on best evidence from scientific research and the needs of patients within a particular community. When a community standard has not been established, physicians will adopt an evidence-based standard. Early work in Ontario by Basinski et al. [6] used an expert panel process in establishing RMWTs for patients needing a CATH, which was later validated by Alter et al. [7]. Based on the research outlined above, the local monitoring organization (Cardiac Care Network of Ontario (CCN)) established benchmark RMWTs of 0–7, 8–28, and 28–84 days for urgent, semi-urgent, and elective patients undergoing CATH in Ontario, respectively [8]. Working outside the above-described ranges is thought to put undue risk to patients awaiting CATH.
Many factors affect wait time for service. Physical capacity improvements (e.g. additional laboratories, new centres, staff increases), and system design (e.g. advanced booking and queuing strategies) may allow for more cases to be completed, and thus decreasing wait for service [9]. Other factors, such as changes in practice, changes in population demographics, the emergence of new technologies (e.g. cardiac computed tomography), and public health crises (e.g. severe acute respiratory syndrome (SARS)) may direct patients towards or away from the cardiac catheterization laboratory; affecting the wait list in a less predictable manner. The understanding of how much these factors can influence delivery of cardiac services may be enhanced by the collection of data regarding the patient population in need (e.g. patient registries). Registry data has been used to develop models for improving wait times through eliminating inefficiencies in service delivery by optimizing patient throughput [9]. However, improved efficiency can only do so much, and additional resources may be necessary when squeezing the maximum capacity from existing resources is optimized.
Citing a lack of formal government programs in health technology assessment, health data collection, and service expansion planning, a local cardiac service task force published a report outlining recommendations to improve service in Ontario [10]. This document acknowledged the lack of adequate data/information at the time as a barrier to planning, and listed among their recommendations the expansion of patient monitoring systems. Because data was limited, many of the task force recommendations may not have been adequate to meet the needs of some cardiac patient populations in Ontario. Since the release of this report, some health care providers in Ontario have developed patient registries, thus improving on the previous lack of data. Registry data may provide an observational database in which to examine both factors that increase need for service and those which improve our ability to meet that demand. The purpose of this study is to demonstrate (with registry data) our experience over the past decade in attempting to meet RMWTs for patients needing CATH at our centre, and discuss issues concerning capacity planning (or lack of it) in providing timely service.
2. Methods
2.1. Heart investigation unit profile
The Hamilton Health Sciences Heart Investigation Unit (HIU) is a tertiary regional cardiac care centre servicing a population of approximately 1.5 million people in Southern Ontario (area of approximately 7000 km2). The HIU expanded from two CATH laboratories with the addition of a third in April 1998, and a fourth in May 2005. Expansion in May 2005 also resulted in the addition of a dedicated 32-bed reception/recovery area for registration and post-procedural management. Referral to the HIU is highly selected. Only cardiologists, surgeons, and special internists can make a direct referral. In addition, elective and semi-urgent patients are required to undergo a triage process with a specially trained nurse coordinator. If deemed appropriate by the triage nurse, the patient is required to attend a pre-catheterization clinic with a designated cardiac nurse clinician. In consultation with the cardiologist scheduled to perform the procedure, the triage nurse can remove referred patients deemed inappropriate from the waitlist. Patients are referred either from home or one of 17 regional community hospitals by a cardiologist or internist on an emergent, urgent, semi-urgent, or elective basis for purposes of cardiac diagnosis (angiogram) and/or percutaneous coronary intervention (PCI).
Most angiograms are performed in order to assess presence and/or extent of coronary artery disease and prescribed one of three management strategies: medical therapy alone, PCI (performed within the catheterization laboratory) or cardiac surgery (on-site services available). Cases are scheduled from Monday to Friday, from 8 a.m. to 8 p.m. (each lab operating 10 h/day with start times staggered). Laboratory times are scheduled in this manner as per CCN consensus panel recommendations [8], [11]. An on-call team handles emergent cases (e.g. patients with ‘ST segment’ elevated myocardial infarction (STEMI)) presenting outside scheduled laboratory hours at the discretion of the cardiologist on call.
Patients referred from home on a semi-urgent or elective basis for diagnostic procedures are managed as surgical day-care referrals and sent home 4–6 h post-procedure. Inpatient (urgent) referrals requiring diagnostic procedures can either be repatriated back to the referring department or hospital, or may be discharged home 4–6 h post-procedure. Patients receiving PCI can be discharged home the morning after the procedure, or repatriated back to the referring centre. Emergent cases are often retained in the coronary care unit at our hospital post-procedure, though some may be repatriated to referring hospitals if critical care facilities are available.
2.2. HIU Registry
The HIU Registry was established in 1997 for use in tracking patient demographics for patients referred to, and clinical outcomes/procedural data for procedures performed in the Hamilton Health Sciences CATH laboratory. Data from referrals (completed by the referring specialist) are captured on a CATH laboratory referral form. Upon receiving the referral, the triage nurse reviews the referral for accuracy and completeness by cross-referencing with the physician notes accompanying the referral. Procedure related data are entered onto case forms by either a technologist or nurse during the procedure. Data are entered into the registry via the DATAFAX™ system, whereby optical recognition software captures data on referral and case forms that are sent to a registry facsimile line. An analyst then performs a manual check of the data entered in order to ensure the accuracy and integrity of the data. The registry can be queried using the DATAFAX software. Output is in the form of a spreadsheet (Microsoft Access or Excel).
2.3. Data collection
Registry data used in this study were wait time for each patient referred (defined as the time from point of referral to the point in which the procedure was performed), and patient demographics and clinical characteristics, including gender, diabetes, age, Canadian Cardiovascular Society (CCS) angina class, previous revascularization (PCI, CABG), and left ventricular function. Department operations data were used to observe both the total number of cases performed annually, and the number of patients awaiting CATH at the end of each month during the observation period. Staffing logs were used to estimate the average number of hours the laboratory was in operation each day, annually. The period of observation was fiscal year 1997–2007, inclusive (fiscal year: April 1–March 31; i.e. April 1997–March 2008). Registry data presented for 1997 were pilot data and were based on patients from a sample of 10 referring cardiologists. Inpatients (including emergent cases) are considered urgent cases, whereas outpatients comprise a combination of both semi-urgent and elective cases (a high majority semi-urgent, very few elective), with a minimal number of urgent cases amongst the group.
The primary analysis focused on calculating the proportion of inpatients (i.e. urgent) that underwent a procedure within the recommended 7 days, and the proportion of outpatients (i.e. semi-urgent) within 28 days. Next, each of the variables collected (described above) were charted for each year so that annual trends could be determined. Both inpatient and outpatient mean wait times calculated for each year in the observation period were compared to the RMWT outlined by the local guidelines. As a subsequent focus we identified potential factors that increased the number of patient referrals to our centre (capacity stressors), and those improving our ability to deliver this service (capacity builders) over the observation period. Particular attention was paid to if and how RMWTs were achieved (especially after the implementation of a 4th laboratory at our centre). Fisher's exact and Chi-squared tests were used to determine the association between the onset of a capacity change (stressor or builder) and the effect on meeting RMWTs.
3. Results
The number of patients referred to CATH steadily increased during the observation period, rising from 3216 in 1998 to 5619 in 2007. In addition, patient demographics and clinical characteristics have changed considerably, including increases in the proportion of patients over 75 years old, those with diabetes, and those presenting with a previous myocardial infarction (MI). Both the number of scheduled hours of operation each day and the number of cases performed annually increased steadily, more than doubling in 2007 as compared to 1997. This data are presented in Table 1 .
Table 1.
Patient clinical information and demographics—all referrals.
| 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | 3571 | 3924 | 4746 | 5105 | 5172 | 6046 | 6512 | 7037 | 7482 | 8262 | 8056 |
| Lab hours/day | 18 | 24 | 26 | 26 | 26 | 26 | 30 | 36 | 40 | 40 | 40 |
| Patients | 604a | 3216 | 3543 | 3908 | 3526 | 4338 | 4071 | 4712 | 4942 | 5577 | 5619 |
| % | |||||||||||
| 21 ≤ age < 65 | 54.1 | 50.8 | 49.1 | 50.1 | 50.7 | 48.6 | 49.5 | 47.8 | 48.9 | 47.1 | 46.9 |
| 65 ≤ age < 75 | 30.1 | 33.9 | 32.9 | 30.5 | 29.9 | 30.8 | 28.8 | 28.7 | 27.0 | 26.9 | 27.3 |
| Age ≥ 75 | 14.9 | 15.0 | 17.9 | 19.1 | 19.2 | 20.2 | 21.3 | 22.9 | 23.0 | 25.2 | 25.4 |
| Female | 40.1 | 35.1 | 36.9 | 35.4 | 36.3 | 36.2 | 36.0 | 36.1 | 36.2 | 36.2 | 34.4 |
| CAD referral | 87.4 | 88.3 | 90.0 | 90.0 | 89.7 | 89.5 | 90.7 | 90.3 | 91.9 | 90.6 | 89.1 |
| Aortic stenosis | 7.6 | 5.8 | 6.5 | 6.4 | 5.9 | 6.0 | 5.0 | 5.1 | 4.4 | 5.8 | 3.6 |
| CCS: 3, 4 | 67.1 | 71.6 | 73.8 | 65.7 | 65.9 | 66.2 | 65.6 | 68.0 | 64.2 | 61.3 | 66.7 |
| NYHA: 3, 4 | 18.9 | 13.5 | 12.0 | 15.8 | 16.6 | 14.9 | 16.1 | 15.3 | 12.8 | 13.3 | 12.9 |
| MI | 28.1 | 28.9 | 27.5 | 41.4 | 44.6 | 44.9 | 45.7 | 44.9 | 40.9 | 39.9 | 44.6 |
| Diabetes | 21.5 | 19.2 | 20.4 | 22.4 | 24.7 | 24.3 | 25.7 | 28.3 | 25.9 | 26.7 | 27.7 |
| Previous CABG | 7.9 | 7.8 | 6.2 | 7.7 | 6.9 | 7.3 | 6.2 | 6.9 | 7.3 | 7.6 | 10.1 |
| Previous PTCA | 7.6 | 6.7 | 5.2 | 5.6 | 5.5 | 5.9 | 5.4 | 7.6 | 7.6 | 9.4 | 16.6 |
| LVEF: <35% | 14.0 | 12.4 | 13.1 | 13.7 | 12.6 | 12.8 | 12.8 | 11.3 | 11.0 | 17.7 | 10.1 |
Data presented in 1997 was based on a sample of referring cardiologists.
The number of patients awaiting CATH at the end of each month was averaged for each year during the observation period. Overall, there was a steady decline in the number of patients awaiting CATH, though there were occasional increases from one year to the next. These data are presented in Fig. 1 . The greatest magnitude of decline in the mean number of patients waiting was from 626 in 2003 to 378 in 2004 (difference: 248; 39.6% decrease). This was due to a combination of factors that will be discussed below. The greatest relative decrease in the mean number of patients awaiting CATH was from 317 to 178 (difference: 139; 43.8% decrease) in 2005 (the point in which the 4th laboratory was implemented).
Fig. 1.
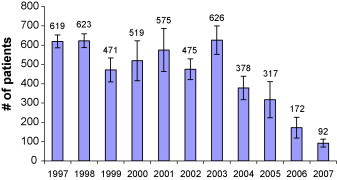
The number of patients awaiting cardiac catheterization at our centre at the end of each month, averaged for each year in the observation period. The error bars denote the standard deviation.
The proportion of inpatients (urgent) and outpatients (semi-urgent) that underwent a procedure within the RMWT each year is presented in Fig. 2, Fig. 3 . Compared to the previous year, the proportion of outpatients undergoing a procedure within the RMWT increased in 1999 after expansion to a third laboratory (15–26%, p < 0.0001). Similarly, improvements were seen after the increase in hours of operation from 30 to 36 h in 2004 (19.5–34.1%, p < 0.0001), and expansion to a 4th laboratory in 2005 (34.1–53.7%, p < 0.0001). Greater than 80% of outpatients underwent a procedure within the RMWT of 28 days once the realized capacity of the 4th laboratory developed. Increases in the proportion of inpatients undergoing a procedure within the RMWT coincided with increases in hours of operation in 1998 (39–62%, p < 0.0001) and 2004 (68–82%, p < 0.0001). Again, the addition of the third and 4th laboratories improved access for inpatients (62–76%, p < 0.0001, and 82–87%, p < 0.0009, respectively). The proportion of both outpatients and inpatients undergoing a procedure within the RMWTs declined between 1999 and 2000 (26–18%, p < 0.0001, and 76–47%, p < 0.0001, for outpatients and inpatients, respectively).
Fig. 2.
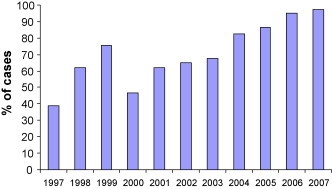
Proportion of inpatient (urgent) referrals who underwent a cardiac catheterization procedure within the RMWT (≤7 days).
Fig. 3.
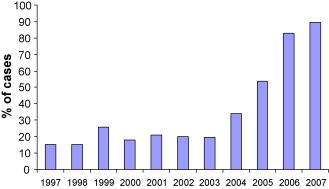
Proportion of outpatient (semi-urgent) referrals who underwent a cardiac catheterization procedure within the RMWT (≤28 days).
Mean wait time for all outpatients and inpatients (urgent and emergent) referred each year are presented in Fig. 4, Fig. 5 , respectively. Mean wait time for referred outpatients did not fall within the range of the RMWT for semi-urgent cases proposed until 2006; the year after the opening of the 4th laboratory (mean 20.6 days, SD 20.5; 75% within 24 days of referral). A mean wait time within the RMWT range for inpatients (urgent cases) was achieved with the expansion of laboratory hours from an average of 30–36 h/day in 2004, though the greatest improvement was seen after the 4th laboratory opened (decreasing mean wait time from 6.6 to 4 days).
Fig. 4.
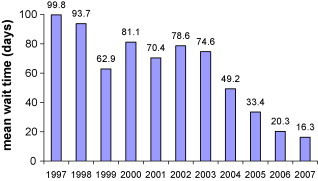
Average number of days waited for cardiac catheterization: outpatient referrals.
Fig. 5.
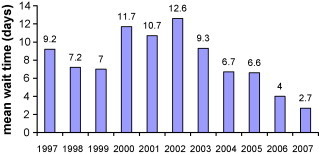
Average number of days waited for cardiac catheterization: inpatient referrals.
3.1. Capacity stressors
During the observation period a number of factors were identified as having a stressing effect on the ability of the catheterization laboratory to deliver timely service to all eligible patients. Each of these factors resulted in increased referrals to the catheterization laboratory beyond contemporaneous capacity limits. The first was the widespread utilization of biomarkers (troponins, CK-MB) to assess cardiac injury [12], [13]. Troponin screening lowered the threshold for defining, and thus identifying, the occurrence of a MI. Biomarker utilization coupled with new guidelines for treating patients with acute coronary syndromes [14], [15] increased the number of patients eligible to benefit from referral for CATH. This scenario is reflected in our data with the proportion of patients who suffered a previous MI increasing dramatically from approximately 28% to over 40% each year after 1999. Such may also explain the increase in the number of patients awaiting CATH, the decrease in the proportion of inpatients and outpatients undergoing a catheterization laboratory procedure within the RMWTs, and the mean wait time for service in 2000 when compared to the previous year, as the increased referrals were not met with synchronous capacity improvements (the number of cases performed were similar in both years).
The second major stressor identified was the occurrence of a public health emergency. The onset of SARS in the beginning of 2003 restricted hospital services for non-urgent cases in the Toronto region (80 km from our centre) [16]. Consequently, many outpatient referrals for CATH were redirected to our centre, greatly increasing the number of patients on the waitlist (peaking at 740 in June 2003), and thus the length of wait for a procedure.
Other factors were identified as potential stressors, though it is not clear in the data presented how each affect service delivery. First, clinical characteristics of patients in the catchment area may change in a manner such that more patients are in need of cardiac services. Indeed, this is the case for data presented in this study, where both the proportion of patients over age 75, and the proportion of patients with diabetes (both risk factors for coronary disease) have increased over the observation period. Whether this observation constitutes more demand for cardiac service or is a change in referral practice is difficult to discern in this analysis.
Secondly, the availability of other screening diagnostics has also been identified as a potential reason for increased referrals. For instance, if catheterization lab capacity outpaces that for other important diagnostic tests, such as non-invasive cardiac stress and imaging tests, many patients may receive an angiogram before these other tests become available. Often, these diagnostic tests are used to screen patients whom would most benefit from referral to the catheterization laboratory. Without such tests the threshold for referral may decrease, resulting in increased referrals. Conversely, when catheterization laboratory resources are scarce, many patients may be required to undergo multiple non-invasive diagnostics as an alternative in order to better establish the likelihood of disease before referral for CATH. A decrease in the number of referrals may also result because of an increased threshold for evidence pertaining to disease burden prior to referral. Ultimately, this may cause a delay in referral to CATH because the patient is waiting for such tests (which may not be reflected in wait times if referral is after the point when test results are available).
Finally, it has been shown that regions with more laboratory capacity see a more intensive management style in practice, as has been shown for those presenting with acute MI, regardless of risk profile and presentation [17]. Overall, while the three factors listed above theoretically will increase the number of referrals to the catheterization laboratory, we have not been able to measure either the existence or the magnitude of effect on our wait list.
3.2. Capacity builders
Capacity improvements were achieved most notably by the addition of catheterization laboratories both within our department and in adjacent regions, and the increase in the hours of operation. Both decreases in wait time and increases in the number of cases performed, as compared to the previous year, coincided with the addition of a third and 4th lab in our facility in April 1998 and May 2005, respectively. This trend was also seen (to a lesser extent) with the opening of catheterization laboratories in two regional hospitals, one 50 km (April 1999) and the other 70 km (February 2001) away from our centre. In addition to lab openings, capacity in our centre was potentially improved by four other, primarily systematic, means. First we have observed a change in the same-sitting (ad hoc) rate of PCI, increasing from 59% in 1997 to 90.7% in 2007. By performing the procedure ad hoc we diminished the need to have the patient come back to the lab, effectively eliminating the number of potential future cases, along with the time it takes to prepare the patient and set-up the lab for an additional procedure. Second, by staggering lab start times we were able to effectively extend our hours of service (as of January 2004). This allowed more flexibility for providing care for late day inpatient referrals and emergent cases. Finally, in May 2005 we instituted two changes. One was the opening of a 32-bed recovery and reception area exclusive to the catheterization laboratory. The recovery area provided more flexibility for managing care post-procedure. That is, patients were registered and accepted directly to the HIU independent of availability in our hospital inpatient wards. The other change was mandatory Pre-Cath Clinic attendance for all elective outpatients booked for a procedure at our centre. The clinic took place 3–5 days before the procedure. Capacity could improve because the Pre-Cath Clinic staff addressed potential medication and other issues that might otherwise cause a cancellation on the day of the procedure. In addition, the clinic provided education that would otherwise take up CATH laboratory staff time. Although each will logically improve “realized” capacity, many of the above-mentioned changes coincided with laboratory openings, thus it is difficult to ascertain the precise impact on our ability to deliver service (with regards to wait list management and caseload improvements) [9].
4. Discussion
Our region experienced substantial change with regards to need for cardiac catheterization services during the observation period. Planning for such changes by policy makers and service providers is limited in that it may be difficult to anticipate changes in practice (prompted by new evidence in the research literature; i.e. acute coronary syndrome (ACS) guidelines, CK-MB and troponin screening), or, even rarer, public health crisis (SARS) that put substantial stress on service capacity; capacity which in our experience was already stretched thin. Our experience suggests that while projecting change may be difficult, prospective collection of data may provide a measure of how service is delivered and specifically, when service is not delivered in a timely manner, and thus, provide quantifiable evidence for a need to improve. That is, by using registry data we were able to estimate the magnitude of improvements to our service delivery by measuring changes in key wait list indicators (in this case RMWT) that followed the implementation of each successive capacity building strategy. Likewise, we were able to estimate the detriment to the timeliness of service delivery each capacity stressor brought. Despite the awareness raised from this data that RMWTs were not being met during this time, the increase in capacity was slow. Reasons for the delay in expansion may have been exacerbated by competing interests for government funding, bureaucratic processes (that delay realized capacity even though funding has been allocated) [9], a lack of standardized methods for monitoring and reporting wait times and wait list lengths, and/or government opinion differing from service providers on the seriousness of wait list problems [18], [19].
The data presented in this study alludes to the absence of a consistent and explicit government policy regarding the criteria or benchmarks driving expansion of CATH laboratories in our region. With increasing community need, fixed or moderately increasing resources, and no immediate or transparent plan to expand the number of funded laboratories, the only recourse available to the HIU leadership for improving wait times was to stretch contemporaneous resources for maximal benefit, either through improved efficiencies and system redesign, or less formally, through increasing effective employment (overtime, staggered lab start times). While it can be argued that improving efficiency should be preferred to building a new lab as a method for increasing capacity, our experience shows that the effect of capacity improvements through systematic means (i.e. improved efficiencies and resource management) in absence of additional physical capacity was not enough to allow for a delivery of service within the RMWT. In other words, you can only get so much out of available resources before additional resources must be made available.
As part of a publicly funded health care system our mandate is to deliver service in a timely manner. In Ontario, CATH providers are required to deliver care within the CCN outlined RMWTs. Despite our best efforts, it is evident that we were unable deliver care within the RMWTs for a high majority of outpatients and a significant minority of inpatients referred to our centre until we opened the 4th laboratory (and a self-contained recovery unit registering and admitting overnight patients independent of the hospital ward). The fact that the addition of the 4th laboratory at our centre (with concurrent systematic improvements) resulted in an improvement to greater than 80% of outpatients and almost 100% of inpatients receiving care within RMWT benchmarks indicates that additional physical capacity was needed in our community (compared to <40% of outpatients and <80% of inpatients before expansion). In addition it is evident that, from a service delivery perspective, significant improvements in timely access for outpatients were only achieved by the addition of physical resources (i.e. additional laboratories). It seems that many of the systematic changes, such as increasing hours of operation (most often through the use of overtime), were aimed at improving timely access for inpatients.
A policy for expansion based on meeting RMWT goals would have meant that the addition of the 4th laboratory would have been desirable many years before it was implemented [5]. The lesson learned from above analysis is that effective service expansion requires a plan based on benchmarks for care (in this case RMWT). Prospective registries can be an effective way of monitoring these benchmarks. It is essential that planning accommodate expanding or contracting need in a timely manner, such that indicators do not fall too far outside benchmarks for best care. Although improvements were made throughout the period of observation, the desire to improve was driven by a strategy of “putting out fires” rather than by a transparent planning policy based on quantifiable benchmarks. This is evidenced during the observation period, where a capacity builder immediately followed each capacity stressor; where with the exception of the concerted effort in May 2005 bringing simultaneous systematic changes and a 4th laboratory, no attempt was successful in decreasing the wait time to meet RMWTs. CCN currently monitors and reports data available to the public regarding wait times for Ontario CATH laboratories and other cardiac procedures. Prudent use of this data by policy makers when planning capacity should improve access for patients in future.
The other lesson we have learned is that registry data are useful for monitoring improvements at the local level. For example, the 32-bed unit design helped in decreasing the number of patients on the waitlist. This strategy was exceptionally helpful in situations where laboratory capacity existed, but inpatients were not brought to the laboratory because there was nowhere to manage the patients before and after the procedure. This emphasizes the importance of planning capacity based on patient risk and management rather than procedural capacity alone. The implementation of local registries may assist centres in developing efficiency models based on community needs. Such registries may also be useful in monitoring the appropriateness of referrals. Increases in referrals of patients who would derive little or no benefit by undergoing a diagnostic procedure may result in longer wait times for others on the waitlist. Although our study did not specifically address this issue, strategies to ensure appropriate referral were undertaken at our centre. These included prioritizing patients based on an urgency score (which was developed with data from the registry). As each community may have its own standards, the registry may allow for the ability to improve on its delivery of service by using information specific to the patients it services.
This study demonstrates the use of a registry in tracking the changes in the proportion of patients undergoing a procedure within RMWTs coinciding with both capacity improvements and increases in referrals to the catheterization laboratories in our region. Thus, a registry may be a useful tool for investigating the results of interventions designed to improve access. In order to fully appreciate any observed association between changes in practice/capacity improvements and the degree of access, comparisons with centres in our region that did not implement similar changes (i.e. additional laboratories, biomarker screening, etc.) would have been desirable. Unfortunately, we are not aware of similar registries in our region. The implementation of such registries may assist both investigators and policy makers in understanding the effects of capacity improvement decisions. This systematic approach may help align the views of policy maker and healthcare providers.
5. Conclusion
Planning for service delivery in healthcare is a complex issue due to rapid changes in technology, evidence and available resources. Service delivery is most effective when service providers can anticipate what the community will need before access becomes restricted due to capacity limits. In retrospect, improving access to CATH services in our centre was reactive to the increasing need of the community rather than based on anticipation of need and continuity of service within RMWTs. Still, it may be difficult to know whether emerging technologies, such as cardiac computed tomography, will divert diagnostic cases away from the catheterization laboratory [20], or if the use of this or other modalities will result in more referrals to the catheterization laboratory for purposes of clarification or intervention. In addition, further investigation may be needed to ascertain the impact clinical studies (e.g. COURAGE [21]) on disease management. In light of our experience over the last decade, it is recommended that governments work with researchers, cardiac service providers, and biotechnology/pharmaceutical industry representatives in order to plan for maintaining and improving access to CATH where needed rather than appropriating resources where service is failing to meet demand. It is also recommended that centres develop registries to provide a means of both monitoring changes in access and evaluating the effectiveness of strategies designed to improve timely access.
Disclosures: None.
References
- 1.Rosamond W., Flegal K., Friday G., Furie K., Go A., Greenlund K. Heart disease and Stroke statistics—2007 update. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan M.K., Gafni A., Yusuf S. Determining optimal population rates of cardiac catheterization: a phantom alternative? Canadian Medical Association Journal. 2005;173(1):49–52. doi: 10.1503/cmaj.050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naylor C.D., Baigrie R.S., Goldman B.S., Basinski A. Assessment of priority for coronary revascularization procedures. Lancet. 1990;335:1070–1073. doi: 10.1016/0140-6736(90)92640-4. [DOI] [PubMed] [Google Scholar]
- 4.Harkness K., Morrow L., Smith K., Kiczula M., Arthur H.M. The effect of early education on patient anxiety while waiting for elective cardiac catheterization. European Journal of Cardiovascular Nursing. 2003;2:113–121. doi: 10.1016/S1474-5151(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan M.K., Mehta S.R., Holder D.H., Goodhart D.R., Gafni A., Shilton D. The risks of waiting for cardiac catheterization: a prospective study. Canadian Medical Association Journal. 2002;167(11):1233–1240. [PMC free article] [PubMed] [Google Scholar]
- 6.Basinski A.S.H., Almond D.G., James R.G.G., Naylor C.D. Rating the urgency of coronary angiography: results of an expert panel process. The Canadian Journal of Cardiology. 1993;9(4):313–321. [PubMed] [Google Scholar]
- 7.Alter D.A., Newman A.M., Cohen E.A., Sykora K., Tu J.V. The evaluation of a formalized queue management system for coronary angiography waiting lists. The Canadian Journal of Cardiology. 2005;21(13):1203–1209. [PubMed] [Google Scholar]
- 8.Cardiac Care Network of Ontario. 2006/07 Annual Report, www.ccn.on.ca; 2008.
- 9.Gupta D., Natarajan M.K., Gafni A., Wang L., Shilton D., Holder D. Capacity planning for cardiac catheterization: a case study. Health Policy. 2007;82(1):1–11. doi: 10.1016/j.healthpol.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Lawson JD, Glynn P, Goodman S, Graham W, Hutchinson W, et al. CCN Cardiac Services Task Force. Planning for the future of cardiac services in Ontario: Final Report and Recommendations. July 1996.
- 11.Cardiac Care Network of Ontario. Cardiac Care Network of Ontario Consensus Panel on Cardiac Catheterization Services. Final Report and Recommendations; September 1997, www.ccn.on.ca; 2008.
- 12.Antman E., Bassand J.-P., Klein W., Ohman M., Sendon J.L.L., Ryden L. Myocardial infarction redefined: A consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Journal of the American College of Cardiology. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 13.Christenson R.H., Azzazy H.M.E. CK-MB and troponins: a clinically focused update. Journal of Clinical Ligand Assay. 2002;25(2):132–149. [Google Scholar]
- 14.Braunwald E., Antman E.M., Beasley J.W., Califf R.M., Cheitlin M.D., Hochman J.S. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) Journal of the American College of Cardiology. 2000;36:970–1062. doi: 10.1016/s0735-1097(00)00889-5. [DOI] [PubMed] [Google Scholar]
- 15.Bertrand M.E., Simoons M.L., Fox K.A.A., Wallentin L.C., Hamm C.W., McFadden E. Management of acute coronary syndromes in patients without persistent ST-segment elevation. European Heart Journal. 2002;23:1809–1840. doi: 10.1053/euhj.2002.3385. [DOI] [PubMed] [Google Scholar]
- 16.Schull M.J., Stukel T.A., Vermeulen M.J., Zwarenstein M., Alter D.A., Manuel D.G. Effect of widespread restrictions on the use of hospital services during an outbreak of severe acute respiratory syndrome. Canadian Medical Association Journal. 2007;176(13):1827–1832. doi: 10.1503/cmaj.061174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stukel T.A., Lucas F.L., Wennberg D.E. Long-term outcomes of regional variations in intensity of invasive vs medical management of medicare patients with acute myocardial infarction. The Journal of the American Medical Association. 2005;293:1329–1337. doi: 10.1001/jama.293.11.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanmartin C., Shortt S.E.D., Barer M.L., Sheps S., Lewis S., McDonald P.W. Waiting for medical services in Canada: lots of heat, but little light. Canadian Medical Association Journal. 2000;162(9):1305–1310. [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis S., Barer M.L., Sanmartin C., Sheps S., Shortt S.E.D., McDonald P.W. Ending waiting-list mismanagement: principles and practice. Canadian Medical Association Journal. 2000;162(9):1297–1300. [PMC free article] [PubMed] [Google Scholar]
- 20.Achenbach S. Computed tomography coronary angiography. Journal of the American College of Cardiology. 2006;48(10):1919–1928. doi: 10.1016/j.jacc.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Boden W.E., O’Rourke R.A., Teo K.K., Hartigan P.M., Maron D.J., Kostuk W.J. Optimal medical therapy with or without PCI for stable coronary disease. New England Journal of Medicine. 2007;356(15):1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]


