Abstract
Azidation (TMSN3, SnCl4) of a 9:1 mixture of trans- and cis-5-acetoxy-2-methylisoxazolidin-3-yl-3-phosphonates at the anomeric carbon atom led to the formation of the equimolar mixture of cis- and trans-5-azido-2-methylisoxazolidin-3-yl-3-phosphonates, which were efficiently separated. The 1,3-dipolar cycloaddition of pure trans- and cis-5-azidoisoxazolidin-3-yl-3-phosphonates with selected alkynes gave the respective nucleoside mimetics containing a 1,2,3-triazole linker. The (1,2,3-triazolyl)isoxazolidine phosphonates obtained herein were evaluated in vitro for activity against a variety of DNA and RNA viruses. None of the compounds were endowed with antiviral activity at subtoxic concentrations. Compounds 15f–j and 16f–j were cytostatic in the higher micromolar range.
Keywords: Phosphonates, Isoxazolidines, Triazoles, Synthesis, Antiviral, Cytostatic
Graphical abstract
Inhibitory activity of cell proliferation for HEL cells as well as L1210, CEM and HeLa cells of several (1,2,3-triazolyl)isoxazolidine phosphonates was evaluated. The unsubstituted and fluoro-substituted phenyl derivatives appeared cytostatic (CC50 40–250 μM).
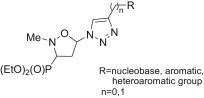
Highlights
► An isoxazolidine-3-yl-3-phosphonate skeleton and nucleobases linked by 1,2,3-triazole. ► Efficient azidation of 5-acetoxy-2-methylisoxazolidin-3-yl-3-phosphonates. ► Nucleoside mimetics from 5-azido-2-methylisoxazolidin-3-yl-3-phosphonates. ► Cytostatic substituted (1,2,3-triazolyl)isoxazolidine phosphonates.
1. Introduction
An intensive search for drugs effective in chemotherapy of viral infections and/or various cancers has been under way for decades. The idea of structural modifications of nucleosides and nucleotides gained interest fairly early and appeared fruitful supplying a wide variety of marketed compounds such as ribavirin, zidovudine, adefovir and many others (Fig. 1 ). In general, the modifications included replacements of the phosphate P(O)–O–C bond for the non-hydrolysable phosphonate P(O)–C linkage, a furanose ring for other heterocyclic or even acyclic fragments or natural nucleobases for unnatural, substituted heteroaromatic or even homoaromatic systems. Several functionalities have been tried as linkers and aliphatic chains as well as 3- to 6-membered aliphatic and aromatic homo- and heterocyclic systems exemplify a long list of examples [1], [2], [3], [4].
Fig. 1.
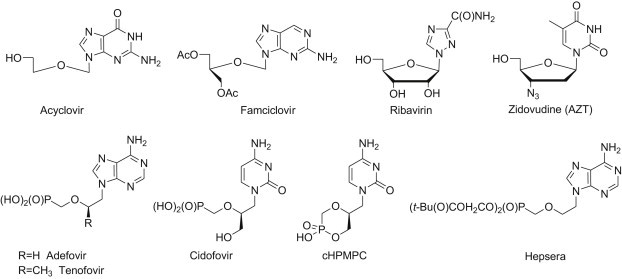
Examples of marketed nucleoside and nucleotide analogues.
Among commonly applied linkers a 1,2,3-triazole ring is of special interest since it is extremely easily to prepare by a 1,3-dipolar cycloaddition and biological activity of many 1,2,3-triazole derivatives has been recognised (Fig. 2 ). The idea of retaining the natural nucleobases with simultaneous introduction of the functionalised 1,2,3-triazole unit into a furanose ring led to compounds 1 and 2 which showed an antiviral activity [5], [6]. On the other hand 2′,3′-diethanethio-2′,3′,5′-trideoxy-5′-triazolonucleosides 3 displayed antitumour activity [7]. Recently, Kim et al. designed novel 1,2,3-triazole-appended C5-modified nucleosides 4 and their anticancer activity was demonstrated [8], [9]. Various acyclonucleosides [10], [11] and acyclonucleoside phosphonates [12] containing a 1,2,3-triazole unit were obtained from N-propargyl nucleobases and their antiviral activity was evaluated. Among them, pyrimidine- and purine-containing derivatives 5 and 6 appeared to be the most potent so far and exhibited anti-HCV activity (IC50 values of 16 μM) without any cytotoxicity at a concentration up to 100 μM. On the basis of a similar idea, novel 1,2,3-triazole nucleosides 7 linked to DNA nucleobases as a recognition element have been synthesised and their antiviral activity was evaluated [13].
Fig. 2.
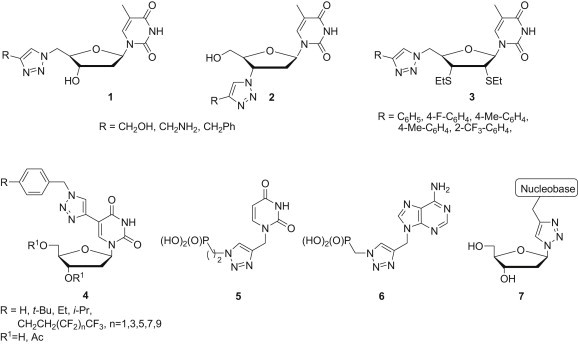
Biologically active nucleoside mimetics having functionalised 1,2,3-triazole unit.
Recently, we succeeded in the synthesis of N-substituted C-phosphorylated nitrone 8 [14] and its application in the 1,3-dipolar cycloaddition with vinyl acetate was described [15]. Furthermore, the usefulness of C5-acetoxyisoxazolidines 9 as precursors in the synthesis of isoxazolidine nucleoside analogues 10 has recently been demonstrated (Scheme 1 ) [15], [16].
Scheme 1.
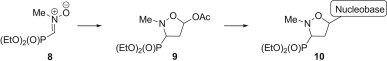
Transformation of the C-phosphorylated nitrone 8 into isoxazolidine nucleoside analogues 10.
In continuation of these efforts, a novel class of 1,2,3-triazole-containing isoxazolidine nucleosides 11 (Scheme 2 ) was designed. Although glycosyl as well as furanosyl azides have commonly been used in a direct synthesis of substituted 1,2,3-triazoles [13], [17], [18], [19], [20], to the best of our knowledge isoxazolidine analogues of 1-azidofuranose remain unknown. Herein, we wish to report the efficient synthesis of 5-azidoisoxazolidines 12 from 5-acetoxyisoxazolidines 9 and further transformation of the azides 12 into the respective nucleoside mimetics 11. The aim of this study was the preparation of a small library of nucleotide analogues 11 which was based on the idea of replacing of a furanose ring by an isoxazolidine system and introduction of a 1,2,3-triazole linker between isoxazolidine and the respective purine or pyrimidine nucleobase or other aromatic function as a nucleobase replacer (Scheme 2).
Scheme 2.
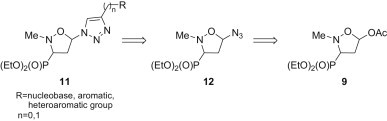
Retrosynthesis of nucleoside mimetics 11.
2. Results and discussion
2.1. Chemistry
A 9:1 mixture of trans- and cis-5-acetoxyisoxazolidines 9a and 9b was obtained from N-methyl-C-phosphorylated nitrone 8 and vinyl acetate as previously described [21]. This mixture was immediately treated with TMSN3 in the presence of SnCl4 as a catalyst to give 5-azidoisoxazolidines 12 and 13 in a ∼1:1 ratio (Scheme 3 ) [22]. They were cleanly separated on a silica gel column into the less polar diastereoisomer 13 (50%) and more polar 12 (46%).
Scheme 3.
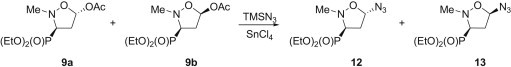
Synthesis of 5-azidoisoxazolidines 12 and 13.
Since almost an equimolar mixture of both anomeric azides 12 and 13 was detected in a crude product, the replacement of the acetoxy group at C5 with an azide function occurred via the formation of the respective oxonium intermediate. Under these circumstances the nucleophile can attack both faces of the isoxazolidinium ion to form the azides 12 and 13 as a ca. 1:1 anomeric mixture.
The relative configurations in the diastereoisomeric isoxazolidines 12 and 13 were established based on 1H and 13C NMR spectroscopic data [23], [24]. Analysis of vicinal coupling constants extracted from the spectra of the isomer 12 [J(H3–H4α) = 6.8 Hz, J(H3–H4β) = 10.8 Hz, J(H4α–P) = 4.0 Hz, J(H4β–P) = 17.0 Hz, J(H4α–H5) = 0.8 Hz, J(H4β–H5) = 5.3 Hz and J(CCCP) = 10.3 Hz] shows that the isoxazolidine ring exists in the single 4 E conformation. In this conformation the P(O)(OEt)2 and azido groups occupy the pseudoequatorial and pseudoaxial positions, respectively (Fig. 3 ) and thus reveal the trans relationship of H3 and H5. On the other hand, from the NMR spectra of the isoxazolidine 13 the following couplings were calculated: J(H3–H4α) = 9.3 Hz, J(H3–H4β) = 9.1 Hz, J(H4α–P) = 4.8 Hz, J(H4β–P) = 17.0 Hz, J(H4α–H5) = 7.5 Hz, J(H4β–H5) = 2.4 Hz and J(CCCP) = 10.3 Hz. On the basis of these values, a preferred E 3 conformation having the diethoxyphosphoryl and azido groups in equatorial and pseudoequatorial positions was established (Fig. 3). At the same time, the cis relationship of the substituents at C3 and C5 in 13 was unambiguously proved.
Fig. 3.
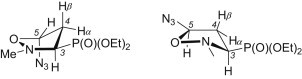
The preferred conformations of 5-azidoisoxazolidines 12 (left) and 13 (right).
Next the 1,3-dipolar cycloadditions of the 5-azidoisoxazolidines 12 and 13 with propargylated nucleobases and selected ethynyl aryls were examined (Scheme 4 , Table 1 ). Because we were interested in the formation of single regioisomers of C4′-substituted 1,2,3-triazoles, all reactions were carried out with equimolar amounts of the respective dipolarophiles 14 using Cu(I) as a catalyst, according to the procedure described by Sharpless [25], [26]. Indeed, 5-azidoisoxazolidin-3-yl-3-phosphonate trans-12 was cleanly transformed into C4′-substituted 1,2,3-triazoles trans-15 in good to excellent yields, whereas from cis-13 and the respective alkynes 14, isoxazolidines cis-16 were produced exclusively. In all cycloadditions no epimerization at C5 in the isoxazolidine ring was observed.
Scheme 4.
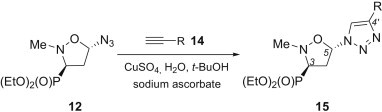
1,3-Dipolar cycloadditions of the 5-azidoisoxazolidines 12 with propargylated nucleobases and selected ethynyl aryls.
Table 1.
Isoxazolidines 15 and 16 produced via Scheme 4.
| Entry | Alkyne | Azide | Product; yield [%] |
|---|---|---|---|
| a | 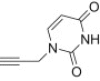 |
12 | 15a; 85 |
| 13 | 16a; 91 | ||
| b | 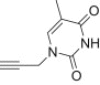 |
12 | 15b; 77 |
| 13 | 16b; 98 | ||
| c | 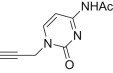 |
12 | 15c; 77 |
| 13 | 16c; 93 | ||
| d | 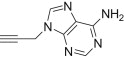 |
12 | 15d; 82 |
| 13 | 16d; 83 | ||
| e | 12 | 15e; 85 | |
| 13 | 16e; 98 | ||
| f | 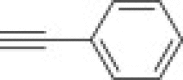 |
12 | 15f; 87 |
| 13 | 16f; 89 | ||
| g | 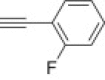 |
12 | 15g; 82 |
| 13 | 16g; 98 | ||
| h | 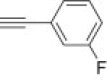 |
12 | 15h; 81 |
| 13 | 16h; 98 | ||
| i | 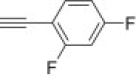 |
12 | 15i; 85 |
| 13 | 16i; 94 | ||
| j | 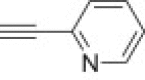 |
12 | 15j; 76 |
| 13 | 16j; 87 | ||
| k | 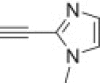 |
12 | 15k; 61 |
| 13 | 16k; 65 |
Additional evidence supporting our configurational assignments in the azidoisoxazolidines 12 and 13 comes from the comparison of the 1H and 13C NMR spectroscopic data of the respective (1,2,3-triazol-1-yl)isoxazolidines 15 and 16. Based on the vicinal couplings [J(H3–H4α) = 6.8–6.9 Hz, J(H3–H4β) = 10.5–11.1 Hz, J(H4α–P) = 4.5–6.8 Hz, J(H4β–P) = 16.8–71.7 Hz, J(H4α–H5) = 1.2–1.8 Hz, J(H4β–H5) = 6.6–6.9 Hz and J(CCCP) = 9.4–10.5 Hz] observed in the 1H and 13C NMR spectra of the isoxazolidines 15 it was concluded that they exist in the 4 E conformation, the same as observed for the azidoisoxazolidine 12 (Fig. 4 ). On the other hand, the preferred E 3 conformation for all (1,2,3-triazol-1-yl)isoxazolidines 16 was established based on the vicinal coupling constants derived from their NMR spectra [J(H3–H4α) = 9.0–9.9 Hz, J(H3–H4β) = 8.1–9.0 Hz, J(H4α–P) = 5.4–6.0 Hz, J(H4β–P) = 17.2–18.0 Hz, J(H4α–H5) = 7.8–7.9 Hz, J(H4β–H5) = 2.0–2.4 Hz and J(CCCP) = 8.6–9.7 Hz] (Fig. 4).
Fig. 4.
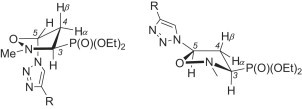
The preferred conformations of (1,2,3-triazol-1-yl)isoxazolidines 15 (left) and 16 (right).
2.2. Antiviral and cytostatic evaluation
All (1,2,3-triazol-1-yl)isoxazolidines trans-15 and cis-16 were evaluated for inhibitory activity against a wide variety of DNA and RNA viruses, using the following cell-based assays: (a) human embryonic lung (HEL) cells: herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), herpes simplex virus-1 (TK− KOS ACVr), vaccinia virus and vesicular stomatitis virus, cytomegalovirus (AD-169 strain and Davis strain) and varicella–zoster virus (TK+ VZV strain Oka, and TK− VZV strain 07/1); (b) Vero cell cultures: parainfluenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus; (c) HeLa cell cultures: vesicular stomatitis virus, Coxsackie virus B4 and respiratory syncytial virus; (d) MDCK cell cultures: influenza A virus (H1N1 and H3N2) and influenza B virus; (e) CrFK cell cultures: feline herpes virus (FHV) and feline corona virus (FIPV) and (f) CEM cell cultures: human immunodeficiency virus type 1 (HIV-1) and HIV-2. Ganciclovir, cidofovir, acyclovir, brivudin, (S)-9-(2,3-dihydroxypropyl)adenine [(S)-DHPA], oseltamivir and ribavirin were used as the reference compounds. The antiviral activity was expressed as the EC50: the compound concentration required to reduce virus plaque formation (VZV) by 50% or to reduce virus-induced cytopathogenicity by 50% (other viruses). Unfortunately, no inhibitory activity against any virus was detected for the evaluated compounds at 250 μM.
The cytotoxicity of the tested compounds towards the uninfected host cells was defined as the minimum compound concentration (MCC) that caused a microscopically detectable alteration of normal cell morphology. The 50% cytostatic concentration (CC50), causing a 50% decrease in cell proliferation was determined against murine leukaemia L1210, human lymphocyte CEM, human cervix carcinoma HeLa and human lung fibroblast HEL cells. None of the tested compounds affected cell morphology of HEL, HeLa, Vero, MDCK and CrFK cells at concentrations up to 100 μM. However, several compounds (trans-15f–j and cis-16f–j) were able to inhibit cell proliferation by 50% (CC50) at concentrations ranging from 40 to 78 μM for HEL cells, and from 120 to 250 μM for L1210, CEM and HeLa cells (Table 2 ).
Table 2.
Cytostatic activity of compounds 15a–k and 16a–k.
| Compound | CC50a (μM) |
|||
|---|---|---|---|---|
| L1210 | CEM | HeLa | HEL | |
| 15a | >250 | >250 | ≥250 | >100 |
| 15b | ≥250 | >250 | >250 | >100 |
| 15c | ≥250 | >250 | >250 | >100 |
| 15d | 140 | >250 | >250 | >100 |
| 15e | 191 | >250 | >250 | >100 |
| 15f | >250 | >250 | >250 | 40 |
| 15g | 120 | >250 | 202 | 73 |
| 15h | ≥250 | >250 | ≥250 | 40 |
| 15i | 131 | ≥250 | 162 | 59 |
| 15j | ≥250 | >250 | >250 | 62 |
| 15k | ≥250 | >250 | >250 | >100 |
| 16a | 235 | >250 | >250 | >100 |
| 16b | 280 | >250 | >250 | >100 |
| 16c | >250 | >250 | >250 | >100 |
| 16d | 147 | >250 | ≥250 | >100 |
| 16e | 166 | >250 | >250 | >100 |
| 16f | 172 | >250 | >250 | 54 |
| 16g | 122 | >250 | 247 | 41 |
| 16h | 104 | >250 | 212 | 43 |
| 16i | 124 | ≥250 | 135 | 42 |
| 16j | 206 | >250 | >250 | 78 |
| 16k | ≥250 | >250 | >250 | >100 |
50% Inhibitory concentration or compound concentration required to inhibit cell proliferation by 50%.
Active as cell proliferation inhibitors isomeric isoxazolidines 15/16f–j exhibit several common structural features such as a six-membered aromatic ring (benzene or pyridine) and substitution with fluorine. The aromatic fragments of these compounds fulfil the requirements to be considered as “nonpolar nucleoside isosteres” [27].
3. Conclusions
Azidation of a 9:1 mixture of trans- and cis-5-acetoxy-2-methylisoxazolidin-3-yl-3-phosphonates with trimethylsilyl azide in the presence of SnCl4 gave an easily separable 1:1 mixture of diethyl 5-azido-2-methylisoxazolidin-3-yl-3-phosphonates trans-12 and cis-13. A series of 1,2,3-triazole-containing isoxazolidin-3-yl-3-phosphonates was obtained in good to excellent yields from azides trans-12 and cis-13 via 1,3-dipolar cycloaddition with the respective propargylated nucleobases and ethynyl aryls.
The relative configuration in the 5-azidoisoxazolidines trans-12 and cis-13 as well as the 1,2,3-triazolyl cycloadducts trans-15 and cis-16 was established based on the conformational analysis using vicinal coupling constants extracted from 1H and 13C NMR spectra.
All synthesised trans- and cis-(1,2,3-triazolyl)isoxazolidinephosphonates trans-15 and cis-16 were evaluated against a broad-spectrum of viruses but found not active at 250 μM. The unsubstituted and fluoro-substituted phenyl derivatives proved slightly cytostatic (middle to higher micromolar range: 40–250 μM).
4. Experimental section
4.1. Chemistry
The 1H NMR spectra were taken in CDCl3 or C6D6 on the Varian Mercury-300 spectrometer with TMS as an internal standard. The 13C NMR spectra were recorded for CDCl3 solutions on a Varian Mercury-300 machine at 75.5 MHz. The 31P NMR spectra were taken in CDCl3 or C6D6 on Varian Mercury-300 at 121.5 MHz.
IR spectra were measured on an Infinity MI-60 FT-IR spectrometer. Melting points were determined on a Boetius apparatus and are uncorrected. Elemental analyses were performed by the Microanalytical Laboratory of this Faculty on Perkin–Elmer PE 2400 CHNS analyser.
The following adsorbents were used: column chromatography, Merck silica gel 60 (70–230 mesh); analytical TLC, Merck TLC plastic sheets silica gel 60 F254.
Starting Materials. All solvents were dried according to the literature methods. The nitrone 8 and isoxazolidines 9a and 9b were previously reported [21].
4.1.1. Synthesis of 5-azidoisoxazolidines 12 and 13
To a solution of a 9:1 mixture of 5-acetoxyisoxazolidines 10 and 11 (3.384 g, 12.03 mmol) in dry methylene chloride (40 mL) trimethylsilyl azide (3.97 mL, 30.08 mmol) was added at 0 °C under argon followed by SnCl4 (0.704 mL, 6.02 mmol). The reaction mixture was stirred at this temperature for 12 h and then saturated aqueous NaHCO3 (70 mL) was added at 0 °C. After stirring for 30 min at this temperature organic phase was separated, and an aqueous layer was extracted with methylene chloride (4 × 20 mL). The combined organic layers were washed with saturated aqueous NaHCO3 (2 × 20 mL), dried over MgSO4, filtered and solvent was removed under reduced pressure. Purification on a silica gel column with toluene:isopropanol (200:1 and then 100:1, v/v) gave azidoisoxazolidine 13 (1.587 g, 50%) and azidoisoxazolidine 12 (1.468 g, 46%), both as colourless oils.
4.1.1.1. Diethyl 5-azido-2-methylisoxazolidin-3-yl-3-phosphonate 12
IR (film, cm−1) ν max: 3473, 2962, 2114, 1443, 1260, 1240, 1100, 1054, 1026, 964, 800; 1H NMR (300 MHz, CDCl3) δ: 1.35 (t, 6H, J = 6.9 Hz, 2× POCH2CH 3), 2.47 (dddd, 1H, J = 12.6, 6.8, 4.0, 0.8 Hz, HbC4), 2.63 (dddd, 1H, J = 17.0, 12.6, 10.8, 5.3 Hz, HaC4), 3.04 (d, 3H, J = 1.0 Hz, CH 3–N), 3.27 (ddd, 1H, J = 10.8 Hz, 6.8, 2.4 Hz, HC3), 4.15–4.30 (m, 4H, 2× POCH 2CH3), 5.58 (dd, 1H, J = 5.3, 0.8 Hz, HC5); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 6.2 Hz), 40.2 (d, 2 J PCC = 1.9 Hz, C4), 49.5 (d, J = 6.9 Hz, CH3–N), 62.2 (d, 1 J PC = 175.2 Hz, C3), 62.8 (d, J = 7.0 Hz, C–O–P), 63.4 (d, J = 6.9 Hz, C–O–P), 89.8 (d, 3 J PCCC = 10.3 Hz, C5); 31P NMR (121.5 MHz, CDCl3) δ: 22.39. Anal. Calcd for C8H17N4O4P: C, 36.37; H, 6.49; N, 21.20. Found: C, 36.25; H, 6.59; N, 20.98.
4.1.1.2. Diethyl 5-azido-2-methylisoxazolidin-3-yl-3-phosphonate 13
IR (film, cm−1) ν max: 3580, 2981, 1442, 1257, 1053, 1027, 968; 1H NMR (300 MHz, CDCl3) δ: 1.36 (t, 3H, J = 7.1 Hz, POCH2CH 3), 1.38 (t, 3H, J = 7.1 Hz, POCH2CH 3), 2.40–2.60 (m, 1H, HbC4), 2.80–2.90 (m, 1H, HaC4), 2.85–2.98 (m, 1H, HC3), 2.96 (s, 3H, CH 3–N), 4.15–4.30 (m, 4H, 2× POCH 2CH3), 5.41 (very br d, 1H, HC5); 1H NMR (300 MHz, C6D6) δ: 0.99 (t, 3H, J = 7.1 Hz, POCH2CH 3), 1.11 (t, 3H, J = 7.1 Hz, POCH2CH 3), 2.16 (dddd, 1H, J = 12.8, 9.3, 7.5, 4.8 Hz, HbC4), 2.42 (dddd, 1H, J = 17.0, 12.8, 9.1, 2.4 Hz, HaC4), 2.55 (ddd, 1H, J = 9.3, 9.1, 2.0 Hz, HC3), 2.94 (d, 3H, J = 1.2 Hz, CH 3–N), 3.80–3.98 (m, 2H, POCH 2CH3), 4.00–4.23 (m, 2H, POCH 2CH3), 4.64 (dd, 1H, J = 7.5, 2.4 Hz, HC5); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 6.4 Hz), 16.7 (d, J = 6.0 Hz), 40.5 (d, 2 J PCC = 2.8 Hz, C4), 45.8 (d, J = 2.3 Hz, CH3–N), 62.6 (d, J = 6.5 Hz, C–O–P), 63.7 (d, J = 6.5 Hz, C–O–P), 63.9 (d, 1 J PC = 164.8 Hz, C3), 88.0 (d, 3 J PCCC = 10.3 Hz, C5); 31P NMR (121.5 MHz, C6D6) δ: 21.03; 31P NMR (121.5 MHz, CDCl3) δ: 21.38. Anal. Calcd for C8H17N4O4P: C, 36.37; H, 6.49; N, 21.20. Found: C, 36.45; H, 6.69; N, 21.43.
4.1.2. General procedure for the synthesis of (1,2,3-triazolyl)isoxazolidines 15 and 16
To a solution of the azidoisoxazolidine 12 or 13 (1.00 mmol) in tert-butanol (0.5 mL), CuSO4·4H2O (0.10 mmol) in water (1 mL) was added followed by sodium ascorbate (0.20 mmol) and the respective alkyne 14 (1.00 mmol). The reaction mixture was stirred at room temperature for 48 h, concentrated and co-evaporated with ethanol (3 × 10 mL). The residue was dissolved in chloroform (10 mL), dried over MgSO4, filtered through a pad of Celite and the solution was evaporated under reduced pressure. The crude product was purified on a silica gel column with chloroform:methanol (from 100:1 to 20:1, v/v) as eluent.
4.1.2.1. Diethyl 5-(4-((3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15a
From azidoisoxazolidine 12 (0.166 g, 0.628 mmol) and N 1-propargyluracil (0.094 g, 0.628 mmol), phosphonate 15a (0.222 g, 85%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3485, 3152, 2993, 1680, 1468, 1441, 1250, 1220, 1050, 1026, 971, 753; 1H NMR (300 MHz, CDCl3) δ: 1.35 (t, J = 7.1 Hz, 3H), 1.36 (t, J = 7.1 Hz, 3H), 2.88 (s, 3H), 3.00–3.25 (m, 1H), 3.40–3.55 (m, 1H), 3.58–3.65 (m, 1H), 4.15–4.30 (m, 4H), 4.95 (AB, 1H, J AB = 15.5 Hz), 5.03 (AB, 1H, J AB = 15.5 Hz), 5.70 (d, 1H, J = 7.9 Hz), 6.15 (d, 1H, J = 6.1 Hz), 7.51 (d, 1H, J = 7.9 Hz), 7.94 (s, 1H), 9.20 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.5 (d, J = 5.7 Hz), 16.6 (d, J = 5.7 Hz), 38.8, 43.1, 47.8 (d, J = 5.4 Hz), 62.5 (d, 1 J PC = 171.5 Hz, C3), 63.0 (d, J = 6.9 Hz, C–O–P), 63.4 (d, J = 6.6 Hz, C–O–P), 86.3 (d, 3 J PCCC = 9.7 Hz, C5), 102.6, 123.9, 142.1, 144.4, 151.0, 164.1; 31P NMR (121.5 MHz, CDCl3) δ: 21.33. Anal. Calcd for C15H23N6O6P: C, 43.48; H, 5.59; N, 20.28. Found: C, 43.56; H, 5.76; N, 20.38.
4.1.2.2. Diethyl 5-(4-((3,4-dihydro-2,4-dioxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16a
From azidoisoxazolidine 13 (0.164 g, 0.621 mmol) and N 1-propargyluracil (0.093 g, 0.621 mmol), phosphonate 16a (0.233 g, 91%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (20:1, v/v). IR (film, cm−1) ν max: 3464, 3155, 2996, 1706, 1690, 1457, 1390, 1235, 1110, 1044, 1020, 959, 755; 1H NMR (300 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.1 Hz), 1.34 (t, 3H, J = 7.1 Hz), 2.96 (d, 3H, J = 0,8 Hz), 3.00–3.20 (m, 2H), 3.20–3.40 (m, 1H), 4.00–4.25 (m, 4H), 5.00 (s, 2H), 5.70 (d, J = 7.9 Hz, 1H), 6.37 (d, J = 6.0 Hz, 1H), 7.53 (d, J = 7.9 Hz, 1H), 8.20 (s, 1H), 8.87 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 5.7 Hz), 40.2, 42.6, 45.6, 62.9 (d, J = 6.9 Hz, C–O–P), 63.4 (d, 1 J PC = 166.6 Hz, C3), 63.5 (d, J = 6.6 Hz, C–O–P), 85.4 (d, 3 J PCCC = 9.2 Hz, C5), 102.4, 122.3, 141.9, 144.4, 150.8, 164.2; 31P NMR (121.5 MHz, CDCl3) δ: 21.36. Anal. Calcd for C15H23N6O6P: C, 43.48; H, 5.59; N, 20.28. Found: C, 43.46; H, 5.53; N, 20.44.
4.1.2.3. Diethyl 5-(4-((3,4-dihydro-5-methyl-2,4-dioxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15b
From azidoisoxazolidine 12 (0.165 g, 0.628 mmol) and N 1-propargylthymine (0.103 g, 0.628 mmol), phosphonate 15b (0.206 g, 77%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3454, 3143, 2986, 1689, 1458, 1390, 1320, 1232, 1067, 1026, 973, 809, 783; 1H NMR (300 MHz, CDCl3) δ: 1.35 (t, 3H, J = 7.2 Hz), 1.36 (t, 3H, J = 7.2 Hz), 1.90 (s, 3H), 2.89 (s, 3H), 3.14 (dddd, 1H, J = 17.7, 12.9, 10.2, 6.6 Hz), 3.39–3.48 (m, 1H), 3.60 (ddd, 1H, J = 10.2, 7.2, 3.3 Hz), 4.10–4.35 (m, 4H), 4.91 (AB, 1H, J AB = 15.0 Hz), 5.02 (AB, 1H, J AB = 15.0 Hz), 6.15 (d, 1H, J = 6.7 Hz), 7.33 (s, 1H), 7.91 (s, 1H), 8.77 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 12.4, 16.4 (d, J = 5.7 Hz), 16.5 (d, J = 5.7 Hz), 38.8, 47.7 (d, J = 5.7 Hz), 50.2, 62.5 (d, 1 J PC = 171.5 Hz, C3), 63.0 (d, J = 6.9 Hz, C–O–P), 63.4 (d, J = 6.6 Hz, C–O–P), 86.3 (d, 3 J PCCC = 10.0 Hz, C5), 111.1, 123.9, 140.2, 142.4, 151.2, 164.5; 31P NMR (121.5 MHz, CDCl3) δ: 21.23. Anal. Calcd for C16H25N6O6P: C, 44.86; H, 5.88; N, 19.62. Found: C, 45.07; H, 5.70; N, 19.83.
4.1.2.4. Diethyl 5-(4-((3,4-dihydro-5-methyl-2,4-dioxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16b
From azidoisoxazolidine 13 (0.150 g, 0.568 mmol) and N 1-propargylthymine (0.093 g, 0.568 mmol), phosphonate 16b (0.243 g, 98%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3484, 3156, 3058, 2984, 1690, 1679, 1468, 1367, 1236, 1110, 1050, 1024, 972, 786, 734; 1H NMR (300 MHz, CDCl3) δ: 1.30 (t, 3H, J = 7.1 Hz), 1.34 (t, 3H, J = 7.1 Hz), 1.90 (s, 3H), 2.96 (s, 3H), 2.95–3.20 (m, 2H), 3.20–3.40 (m, 1H), 4.05–4.25 (m, 4H), 4.98 (s, 2H), 6.36 (d, 1H, J = 6.3 Hz), 7.36 (s, 1H), 8.19 (s, 1H), 8.42 (s, 1H); 13C NMR (CDCl3, 75.5 MHz) δ: 12.4, 16.5 (d, J = 5.7 Hz), 40.2, 42.4, 45.6, 62.9 (d, J = 6.9 Hz, C–O–P), 63.4 (d, 1 J PC = 166.3 Hz, C3), 63.5 (d, J = 6.6 Hz, C–O–P), 85.4 (d, 3 J PCCC = 9.2 Hz, C5), 110.9, 122.2, 140.2, 142.2, 150.9, 164.6; 31P NMR (121.5 MHz, CDCl3) δ: 21.32. Anal. Calcd for C16H25N6O6P: C, 44.86; H, 5.88; N, 19.62. Found: C, 44.77; H, 5.90; N, 19.60.
4.1.2.5. Diethyl 5-(4-((acetylamino)-2-oxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15c
From azidoisoxazolidine 12 (0.169 g, 0.640 mmol) and N-(1,2-dihydro-2-oxo-1-(prop-2-ynyl)pyrimidin-4-yl)acetamide (0.122 g, 0.640 mmol), phosphonate 15c (0.224 g, 77%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3446, 2928, 1654, 1559, 1490, 1374, 1307, 1221, 1024, 962, 792; 1H NMR (300 MHz, CDCl3) δ: 1.35 (t, J = 7.2 Hz, 3H), 1.36 (t, J = 7.2 Hz, 3H), 2.25 (s, 3H), 2.87 (s, 3H), 3.14 (dddd, 1H, J = 17.4, 13.3, 10.9, 6.7 Hz), 3.40 (dddd, 1H, J = 13.3, 6.9, 5.0, 1.8 Hz), 3.63 (ddd, 1H, J = 10.5, 6.9, 3.0 Hz), 4.15–4.27 (m, 4H), 5.10 (AB, 1H, J AB = 14.5 Hz), 5.20 (AB, 1H, J AB = 14.5 Hz), 6.20 (dd, 1H, J = 6.7, 1.8 Hz), 7.42 (d, 1H, J = 7.1 Hz), 7.96 (d, 1H, J = 7.1 Hz), 8.09 (s, 1H), 9.40 (br s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 3.7 Hz), 24.9, 38.9, 45.1, 47.8 (d, J = 6.0 Hz, CH3–N), 62.7 (d, 1 J PC = 171.5 Hz, C3), 63.1 (d, J = 6.9 Hz, C–O–P), 63.4 (d, J = 6.6 Hz, C–O–P), 86.4 (d, 3 J PCCC = 9.4 Hz, C5), 97.3, 124.3, 142.1, 148.9, 155.8, 163.0, 171.1; 31P NMR (CDCl3, 121.5 MHz) δ: 20.82. Anal. Calcd for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 44.93; H, 5.76; N, 21.67.
4.1.2.6. Diethyl 5-(4-((acetylamino)-2-oxopyrimidin-1(2H)-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16c
From azidoisoxazolidine 13 (0.203 g, 0.768 mmol) and N-(1,2-dihydro-2-oxo-1-(prop-2-ynyl)pyrimidin-4-yl)acetamide (0.147 g, 0.768 mmol), phosphonate 16c (0.325 g, 93%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3446, 2982, 2924, 1718, 1684, 1662, 1600, 1497, 1374, 1308, 1240, 1050, 1025, 952, 797; 1H NMR (300 MHz, CDCl3) δ: 1.31 (t, 3H, J = 7.1 Hz,), 1.34 (t, 3H, J = 7.1 Hz,), 2.24 (s, 3H), 2.96 (d, 3H, J = 1.0 Hz,), 3.00–3.10 (m, 2H), 3.10–3.20 (m, 1H), 4.00–4.20 (m, 4H), 5.17 (s, 2H), 6.35 (dd, 1H, J = 7.3, 2.2 Hz), 7.38 (d, 1H, J = 7.3 Hz), 7.96 (d, 1H, J = 7.3 Hz), 8.25 (s, 1H), 9.00 (br s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 5.7 Hz), 24.8, 40.0, 44.8, 45.5 (s, CH3–N), 62.9 (d, J = 6.9 Hz, C–O–P), 63.4 (d, 1 J PC = 166.3 Hz, C3), 63.5 (d, J = 6.6 Hz, C–O–P), 85.4 (d, 3 J PCCC = 9.2 Hz, C5), 97.0, 122.6, 141.8, 148.9, 155.6, 162.9, 171.2; 31P NMR (CDCl3, 121.5 MHz) δ: 20.53. Anal. Calcd for C17H26N7O6P: C, 44.84; H, 5.75; N, 21.53. Found: C, 44.79; H, 5.64; N, 21.60.
4.1.2.7. Diethyl 5-(4-((6-amino-9H-purin-9-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15d
From azidoisoxazolidine 12 (0.150 g, 0.568 mmol) and N 9-(propargyl)adenine (0.098 g, 0.568 mmol), phosphonate 15d (0.204 g, 82%) was obtained as a white amorphous solid after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). M.p.: 100–102 °C. IR (KBr, cm−1) ν max: 3445, 3301, 3137, 2984, 1665, 1600, 1474, 1418, 1322, 1249, 1050, 1023, 969, 776; 1H NMR (300 MHz, CDCl3) δ: 1.33 (t, 3H, J = 7.0 Hz), 1.34 (t, 3H, J = 7.0 Hz), 2.82 (s, 3H), 3.10 (dddd, 1H, J = 17.4, 12.6, 10.2, 6.6 Hz), 3.60–4.30 (m, 2H), 4.10–4.30 (m, 4H), 5.50 (s, 2H), 6.01 (s, 2H), 6.08 (d, 1H, J = 6.6 Hz, H-C5), 7.88, (s, 1H), 8.00 (s, 1H), 8.35 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.5 (d, J = 5.4 Hz), 16.6 (d, J = 5.7 Hz), 38.5, 38.7, 47.9 (d, J = 5.7 Hz), 62.5 (d, 1 J PC = 172.0 Hz, C3), 63.0 (d, J = 6.9 Hz, C–O–P), 63.4 (d, J = 6.6 Hz, C–O–P), 86.4 (d, 3 J PCCC = 9.7 Hz, C5), 119.0, 123.1, 140.1, 142.7, 149.2, 152.7, 155.7; 31P NMR (121.5 MHz, CDCl3) δ: 21.38. Anal. Calcd for C16H24N9O4P: C, 43.94; H, 5.53; N, 28.82. Found: C, 44.03; H, 5.30; N, 29.01.
4.1.2.8. Diethyl 5-(4-((6-amino-9H-purin-9-yl)methyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16d
From azidoisoxazolidine 13 (0.150 g, 0.568 mmol) and N 9-(propargyl)adenine (0.098 g, 0.568 mmol), phosphonate 16d (0.207 g, 83%) was obtained as a white amorphous solid after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). M.p.: 139–140 °C. IR (KBr, cm−1) ν max: 3292, 2983, 1665, 1600, 1475, 1304, 1243, 1115, 1050, 1019, 960, 777; 1H NMR (300 MHz, CDCl3) δ: 1.21 (t, 3H, J = 7.1 Hz), 1.29 (t, 3H, J = 7.1 Hz), 2.93 (s, 3H), 2.98–3.17 (m, 2H), 3.20–3.40 (m, 1H), 4.00–4.20 (m, 4H), 5.49 (s, 2H), 5.99 (br s, 2H), 6.35 (d, 1H, J = 6.1 Hz, H-C5), 8.01 (s, 1H), 8.18 (s, 1H), 8.35 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 5.4 Hz), 16.2 (d, J = 5.7 Hz), 38.4, 40.2, 45.6 (s, CH3–N), 62.8 (d, J = 6.9 Hz, C–O–P), 63.3 (d, J = 6.9 Hz, C–O–P), 63.3 (d, 1 J PC = 166.6 Hz, C3), 85.4 (d, 3 J PCCC = 9.2 Hz, C5), 119.1, 121.6, 140.2, 142.4, 149.4, 152.8, 155.8; 31P NMR (121.5 MHz, CDCl3): 21.44. Anal. Calcd for C16H24N9O4P: C, 43.94; H, 5.53; N, 28.82. Found: C, 43.76; H, 5.60; N, 28.71.
4.1.2.9. Diethyl 5-(4-(methoxycarbonyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15e
From azidoisoxazolidine 12 (0.150 g, 0.568 mmol) and methyl propiolate (0.051 mL, 0.568 mmol), phosphonate 15e (0.168 g, 85%) was obtained as colourless needles after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). M.p.: 78–80 °C. IR (KBr, cm−1) ν max: 3457, 2989, 2923, 1744, 1445, 1257, 1202, 1046, 1020, 804; 1H NMR (300 MHz, CDCl3) δ: 1.37 (t, 3H, J = 7.0 Hz, POCH2CH 3), 1.38 (t, 3H, J = 7.0 Hz, POCH2CH 3), 2.90 (s, 3H, CH 3–N), 3.10 (dddd, 1H, J = 17.4, 13.1, 10.8, 6.9 Hz, HbC4), 3.45–3.60 (m, 2H), 3.98 (s, 3H, COOCH 3), 4.17–4.30 (m, 4H, 2× POCH 2CH3), 6.20 (dd, 1H, J = 6.5, 1.0 Hz, HC5), 8.31 (s, 1H, HC5′); 1H NMR (300 MHz, C6D6,) δ: 1.00 (t, 3H, J = 6.9 Hz, POCH2CH 3), 1.54 (t, 3H, J = 6.9 Hz, POCH2CH 3), 2.69 (d, 3H, J = 0.4 Hz, CH 3–N), 2.84 (dddd, 1H, J = 17.4, 13.1, 10.8, 6.9 Hz, HbC4), 3.13 (dddd, 1H, J = 13.1, 6.8, 4.7, 1.6 Hz, HaC4), 3.47 (ddd, 1H, J = 10.8, 6.8, 2.8 Hz, HC3), 3.16 (s, 3H, COOCH 3), 3.86–3.99 (m, 2H, POCH 2CH3), 4.01–4.07 (m, 2H, POCH 2CH3), 5.24 (dd, 1H, J = 6.9, 1.6 Hz, HC5), 7.55 (s, 1H, HC5′); 13C NMR (75.5 MHz, CDCl3) δ: 16.70 (d, J = 5.8 Hz), 16.8 (d, J = 5.7 Hz), 39.3 (s, C4), 48.2 (s, CH3–N), 52.6 (s, COOCH3), 62.7 (d, 1 J PC = 171.8 Hz, C3), 63.2 (d, J = 6.9 Hz, C–O–P), 63.7 (d, J = 6.6 Hz, C–O–P), 87.0 (d, 3 J PCCC = 10.0 Hz, C5), 127.4 (s, C5′), 140.5 (s, C4′), 160.9 (s, C O); 31P NMR (121.5 MHz, CDCl3) δ: 21.03. 31P NMR (C6D6, 121.5 MHz): 21.33. Anal. Calcd for C12H21N4O6P: C, 41.38; H, 6.08; N, 16.09. Found: C, 41.42; H, 5.99; N, 16.20.
4.1.2.10. Diethyl 5-(4-(methoxycarbonyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16e
From azidoisoxazolidine 13 (0.150 g, 0.568 mmol) and methyl propiolate (0.051 mL, 0.568 mmol), phosphonate 16e (0.168 g, 85%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3480, 3156, 2983, 1741, 1545, 1440, 1368, 1241, 1200, 1163, 1098, 1050, 1026, 972, 809, 779; 1H NMR (300 MHz, C6D6) δ: 0.96 (t, 3H, J = 7.2 Hz, POCH2CH 3), 1.01 (t, 3H, J = 7.2 Hz, POCH2CH 3), 2.41 (dddd, 1H, J = 13.5, 9.9, 7.9, 5.4 Hz, HbC4), 2.53 (ddd, 1H, J = 9.9, 8.1, 1.8 Hz, HC3), 2.74 (dddd, 1H, J = 17.2, 13.5, 8.1, 2.0 Hz, HaC4), 2.76 (d, 3H, J = 1.0 Hz, CH 3–N), 3.48 (s, 3H, COOCH3), 3.93–3.75 (m, 4H, 2× POCH 2CH3), 5.87 (dd, 1H, J = 7.9, 2.0 Hz, HC5), 8.74 (s, 1H, HC5′); 13C NMR (75.5 MHz, CDCl3) δ: 16.70 (d, J = 5.5 Hz), 16.6 (d, J = 5.7 Hz), 40.8 (d, 2 J PCC = 2.0 Hz, C4), 45.7 (d, J = 1.7 Hz, CH3–N), 52.3 (s, COOCH3), 63.0 (d, J = 6.8 Hz, C–O–P), 63.4 (d, 1 J PC = 166.9 Hz, 140.0 (s, C4′), C3), 63.5 (d, J = 6.9 Hz, C–O–P), 85.7 (d, 3 J PCCC = 8.6 Hz, C5), 126.6 (s, C5′), 161.1 (s, C O); 31P NMR (121.5 MHz, CDCl3) δ: 21.12; 31P NMR (121.5 MHz, C6D6) δ:21.30. Anal. Calcd for C12H21N4O6P: C, 41.38; H, 6.08; N, 16.09. Found: C, 41.23; H, 6.18; N, 16.28.
4.1.2.11. Diethyl 2-methyl-5-(4-phenyl-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 15f
From azidoisoxazolidine 12 (0.130 g, 0.492 mmol) and phenylacetylene (0.054 mL, 0.492 mmol), phosphonate 15f (0.157 g, 87%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3469, 3129, 2983, 2910, 1484, 1441, 1250, 1235, 1160, 1060, 1027, 972, 768, 698; 1H NMR (300 MHz, C6D6) δ: 1.02 (t, 3H, J = 7.2 Hz, POCH2CH 3), 1.08 (t, 3H, J = 7.2 Hz, POCH2CH 3), 2.77 (s, 3H, CH 3–N), 2.93 (dddd, 1H, J = 17.1, 12.9, J = 10.8, 6.6 Hz, HbC4), 3.38 (dddd, 1H, J = 12.9, 6.9, 5.4, 1.5 Hz, HaC4), 3.66 (ddd, 1H, J = 10.8, 6.9, 2.4 Hz, HC3), 3.90–3.98 (m, 2H, POCH 2CH3), 4.01–4.12 (m, 2H, POCH 2CH3), 5.40 (dd, 1H, J = 6.6, 1.5 Hz, HC5), 7.08 (s, 1H), 7.09–7.42 (m, 3H), 7.90–7.93 (m, 2H); 13C NMR (75.5 MHz, CDCl3) δ: 16.8 (d, J = 5.7 Hz), 16.9 (d, J = 5.7 Hz), 38.9 (s, C4), 48.4 (d, J = 6.3 Hz, CH3–N), 63.0 (d, 1 J PC = 172.6 Hz, C3), 63.3 (d, J = 6.9 Hz, C–O–P), 63.7 (d, J = 6.9 Hz, C–O–P), 86.7 (d, 3 J PCCC = 9.7 Hz, C5), 119.7, 125.9, 129.0, 129.1, 130.2, 148.5; 31P NMR (121.5 MHz, CDCl3) δ: 21.63; 31P NMR (121.5 MHz, C6D6) δ: 21.68. Anal. Calcd for C16H23N4O4P: C, 52.46; H, 6.33; N, 15.29. Found: C, 52.45; H, 6.32; N, 15.25.
4.1.2.12. Diethyl 2-methyl-5-(4-phenyl-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 16f
From azidoisoxazolidine 13 (0.100 g, 0.378 mmol) and phenylacetylene (0.042 mL, 0.378 mmol), phosphonate 16f (0.124 g, 89%) was obtained as a white amorphous solid after purification on silica gel with chloroform–methanol (from 100:1 to 50:1, v/v). M.p.: 80–81 °C. IR (KBr, cm−1) ν max: 3522, 2455, 2988, 2891, 1609, 1430, 1250, 1053, 1027, 949; 1H NMR (300 MHz, C6D6) δ: 0.93 (t, 3H, J = 7.2 Hz, POCH2CH 3), 0.95 (t, J = 7.2 Hz, 3H, POCH2CH 3), 2.41 (dddd, 1H, J = 13.8, 9.9, 7.8, 6.0 Hz, HaC4), 2.82 (d, 3H, J = 1.0 Hz, CH 3–N), 2.56 (ddd, 1H, J = 9.9, 8.4, 2.4 Hz, HC3), 2.92 (dddd, 1H, J = 18.0, 13.8, 8.4, 2.4 Hz, HbC4), 3.75–3.92 (m, 4H, POCH 2CH3), 5.95 (dd, 1H, J = 7.8, 2.4 Hz, HC5), 7.00–7.20 (m, 3H), 8.02–8.06 (m, 2H), 8.46 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.8 (d, J = 5.7 Hz), 40.6 (s, C4), 45.9 (d, J = 1.7 Hz, CH3–N), 63.1 (d, J = 6.9 Hz, C–O–P), 63.5 (d, J = 6.9 Hz, C–O–P), 63.7 (d, 1 J PC = 166.9 Hz, C3), 85.6 (d, 3 J PCCC = 9.2 Hz, C5), 118.6, 125.9, 128.2, 128.9, 130.7, 148.1; 31P NMR (121.5 MHz, CDCl3) δ: 21.04; 31P NMR (121.5 MHz, C6D6) δ: 21.99. Anal. Calcd for C16H23N4O4P: C, 52.46; H, 6.33; N, 15.29. Found: C, 52.29; H, 6.52; N, 15.20.
4.1.2.13. Diethyl 5-(4-(2-fluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15g
From azidoisoxazolidine 12 (0.102 g, 0.386 mmol) and 1-ethynyl-2-fluorobenzene (0.043 mL, 0.386 mmol), phosphonate 15g (0.122 g, 82%) was obtained as a white amorphous solid after purification on silica gel with chloroform–methanol (from 100:1 to 50:1, v/v). M.p.: 102–105 °C. IR (KBr, cm−1) ν max: 3426, 2983, 2922, 1488, 1437, 1243, 1047, 1027, 948, 762; 1H NMR (300 MHz, C6D6) δ: 1.01 (t, 3H, J = 7.1 Hz, POCH2CH 3), 1.07 (t, 3H, J = 7.1 Hz, POCH2CH 3), 2.78 (s, 3H, CH 3–N), 2.90 (dddd, 1H, J = 16.9, 13.2, 11.1, 6.9, HbC4), 3.30 (dd, 1H, J = 13.2, 6.9, 4.5, 1.5 Hz, HaC4), 3.67 (ddd, 1H, J = 10.8, 6.9, 2.1 Hz, HC3), 3.87–4.10 (m, 4H, 2× POCH 2CH3), 5.34 (dd, 1H, J = 6.9, 1.5 Hz, HC5), 6.83–6.93 (m, 3H), 7.69 (d, J = 3.8 Hz, 1H), 8.62–8.68 (m, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.7 (d, J = 5.7 Hz), 16.8 (d, J = 5.4 Hz), 38.9 (d, J = 1.7 Hz, C4), 48.3 (d, J = 6.3 Hz, CH3–N), 62.9 (d, 1 J PC = 172.6 Hz, C3), 63.1 (d, J = 6.7 Hz, C–O–P), 63.6 (d, J = 6.7 Hz, C–O–P), 86.6 (d, 3 J PCCC = 9.7 Hz, C5), 115.7 (d, J = 21.5 Hz), 118.1 (d, J = 12.6 Hz), 122.6 (d, J = 12.9 Hz), 124.6 (d, J = 3.4 Hz), 127.7 (d, J = 3.4 Hz), 129.6 (d, J = 8.6 Hz), 141.6 (s, C4′), 159.2 (d, J = 247.9 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 21.47; 31P NMR (121.5 MHz, C6D6) δ: 21.79. Anal. Calcd for C16H22FN4O4P: C, 50.00; H, 5.77; N, 4.94. Found: C, 49.90; H, 5.84; N, 5.01.
4.1.2.14. Diethyl 5-(4-(2-fluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16g
From azidoisoxazolidine 13 (0.153 g, 0.579 mmol) and 1-ethynyl-2-fluorobenzene (0.066 mL, 0.579 mmol), phosphonate 16g (0.122 g, 98%) was obtained as a white amorphous solid after purification on silica gel with chloroform–methanol (from 100:1 to 50:1, v/v). M.p.: 97–98 °C. IR (KBr, cm−1) ν max: 3482, 3092, 2983, 1620, 1590, 1479, 1454, 1360, 1240, 1129, 1068, 1034, 971, 864, 790; 1H NMR (300 MHz, C6D6) δ: 0.98 (t, 3H, J = 7.2 Hz, POCH2CH 3), 1.05 (t, 3H, J = 7.2 Hz, POCH2CH 3), 2.43 (dddd, 1H, J = 13.5, 9.0, 7.8, 5.4 Hz, HbC4), 2.58 (dd, 1H, J = 9.0, 9.0, 2.4 Hz, HC3), 2.83 (d, 3H, J = 1.0 Hz, CH 3–N), 2.93 (dddd, 1H, J = 17.2, 13.5, 9.0, 2.1 Hz, HaC4), 3.80–3.90 (m, 2H, POCH 2CH3), 3.93–4.00 (m, 2H, POCH 2CH3), 5.95 (dd, 1H, J = 7.8, 2.1 Hz, HC5), 6.80–6.92 (m, 3H), 8.61 (d, J = 3.9 Hz, 1H), 8.70–8.65 (m, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 6.3 Hz), 16.7 (d, J = 5.7 Hz), 40.7 (d, J = 2.6 Hz, C4), 45.8 (s, CH3–N), 62.9 (d, J = 6.7 Hz, C–O–P), 63.7 (d, J = 6.8 Hz, C–O–P), 63.8 (d, 1 J PC = 166.9 Hz, C3), 85.6 (d, 3 J PCCC = 9.7 Hz, C5), 115.7 (d, J = 21.7 Hz), 118.7 (d, J = 13.0 Hz), 121.4 (d, J = 13.2 Hz), 124.6 (d, J = 3.6 Hz), 127.9 (d, J = 3.9 Hz), 129.4 (d, J = 8.5 Hz), 141.6 (s, C4′), 159.2 (d, J = 247.6 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 21.41; 31P NMR (121.5 MHz, C6D6) δ: 21.37. Anal. Calcd for C16H22FN4O4P: C, 50.00; H, 5.77; N, 4.94. Found: C, 50.13; H, 6.01; N, 5.12.
4.1.2.15. Diethyl 5-(4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15h
From azidoisoxazolidine 12 (0.103 g, 0.390 mmol) and 1-ethynyl-3-fluorobenzene (0.045 mL, 0.390 mmol), phosphonate 15h (0.122 g, 81%) was obtained as colourless plates after purification on silica gel with chloroform–methanol (from 100:1 to 50:1, v/v). M.p.: 93–95 °C. IR (KBr, cm−1) ν max: 3123, 2988, 1618, 1590, 1486, 1448, 1343, 1242, 1052, 1023, 976, 945, 862; 1H NMR (300 MHz, C6D6) δ: 1.02 (t, 3H, J = 6.9 Hz, POCH2CH 3); 1.09 (t, 3H, J = 6.9 Hz, POCH2CH 3), 2.78 (s, 3H, CH 3–N), 2.94 (dddd, 1H, J = 17.7, 13.2, 10.8, 6.9 Hz, HbC4), 3.36 (dddd, 1H, J = 13.2, 6.9, 5.7, 1.5 Hz, HaC4), 3.65 (ddd, 1H, J = 10.8, 6.9, 2.7 Hz, HC3), 3.89–4.00 (m, 2H, POCH 2CH3), 4.01–4.11 (m, 2H, POCH 2CH3), 5.39 (dd, 1H, J = 6.9, 1.5 Hz, HC5), 6.72–6.80 (m, 1H), 6.95 (s, 1H), 6.93–7.00 (m, 1H), 7.58 (ddd, 1H, J = 9.9, 2.6, 1.6 Hz), 7.67 (dt, 1H, J = 7.9, 1.2 Hz); 13C NMR (75.5 MHz, CDCl3) δ: 16.7 (d, J = 6.4 Hz), 16.8 (d, J = 6.4 Hz), 38.8 (s, C4), 48.3 (d, J = 5.4 Hz, CH3–N), 62.9 (d, 1 J PC = 172.4 Hz, C3), 63.1 (d, J = 6.9 Hz, C–O–P), 63.6 (d, J = 6.9 Hz, C–O–P), 86.7 (d, 3 J PCCC = 10.4 Hz, C5), 112.8 (d, J = 22.9 Hz), 115.3 (d, J = 21.7 Hz), 119.9, 121.4 (d, J = 3.6 Hz), 130.5 (d, J = 8.5 Hz), 132.3 (d, J = 8.7 Hz), 147.3, 163.1 (d, J = 245.4 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 21.56; 31P NMR (121.5 MHz, C6D6) δ: 21.75. Anal. Calcd for C16H22FN4O4P: C, 50.00; H, 5.77; N, 4.94. Found: C, 50.03; H, 5.60; N, 4.91.
4.1.2.16. Diethyl 5-(4-(3-fluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16h
From azidoisoxazolidine 13 (0.148 g, 0.560 mmol) and 1-ethynyl-3-fluorobenzene (0.065 mL, 0.560 mmol), phosphonate 16h (0.213 g, 98%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 100:1 to 50:1, v/v). IR (film, cm−1) ν max: 3470, 2980, 1619, 1589, 1478, 1454, 1440, 1359, 1240, 1044, 1020, 972, 954, 863; 1H NMR (300 MHz, C6D6) δ: 0.92 (t, 3H, J = 6.9 Hz, POCH2CH 3), 0.94 (t, 3H, J = 6.9 Hz, POCH2CH 3), 2.39 (dddd, 1H, J = 13.5, 9.6, 7.8, 6.0 Hz, HbC4), 2.55 (ddd, 1H, J = 9.6, 8.4, 1.8 Hz, HC3), 2.81 (s, 3H, CH 3–N), 2.88 (dddd, 1H, J = 17.7, 13.5, 8.4, 2.1 Hz, HaC4), 3.77–3.87 (m, 4H, 2× POCH 2CH3), 5.91 (dd, 1H, J = 7.8, 2.1 Hz, HC5), 6.68–6.75 (m, 1H), 6.88–6.95 (m, 1H), 7.73–7.77 (m, 2H), 8.41 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 6.3 Hz), 16.7 (d, J = 6.3 Hz), 40.5 (d, J = 2.4 Hz, C4), 45.8 (d, J = 2.5 Hz, CH3–N), 63.1 (d, J = 6.9 Hz, C–O–P), 63.4 (d, J = 6.9 Hz, C–O–P), 63.5 (d, 1 J PC = 166.7 Hz, C3), 85.6 (d, 3 J PCCC = 9.2 Hz, C5), 112.7 (d, J = 22.7 Hz), 115.0 (d, J = 21.1 Hz), 119.0, 121.4 (d, J = 3.4 Hz), 130.4 (d, J = 8.6 Hz), 132.8 (d, J = 8.0 Hz), 146.9 (d, J = 3.4 Hz), 163.1 (d, J = 245.2 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 21.93; 31P NMR (121.5 MHz, C6D6) δ: 22.00. Anal. Calcd for C16H22FN4O4P: C, 50.00; H, 5.77; N, 4.94. Found: C, 49.87; H, 5.60; N, 4.93.
4.1.2.17. Diethyl 5-(4-(2,4-difluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 15i
From azidoisoxazolidine 12 (0.105 g, 0.397 mmol) and 1-ethynyl-2,4-difluorobenzene (0.055 g, 0.397 mmol), phosphonate 15i (0.136 g, 85%) was obtained as colourless needles after purification on silica gel with chloroform–methanol (20:1, v/v). M.p.: 112–113 °C. IR (KBr, cm−1) ν max: 3435, 3140, 2984, 2924, 1621, 1561, 1495, 1444, 1390, 1336, 1243, 1142, 1065, 1029, 951; 1H NMR (300 MHz, C6D6) δ: 1.01 (t, 3H, J = 6.9 Hz, POCH2CH 3), 1.07 (t, 3H, J = 6.9 Hz, POCH2CH 3), 2.80 (s, 3H, CH 3–N), 2.92 (dddd, 1H, J = 17.7, 12.9, 10.8, 6.9 Hz, HbC4), 3.30 (dddd, 1H, J = 12.9, 6.9, 4.5, 1.5 Hz, HaC4), 3.66 (ddd, 1H, J = 10.8, 6.9, 2.7 Hz, HC3), 3.90–4.00 (m, 2H, POCH 2CH3), 4.03–4.10 (m, 2H, POCH 2CH3), 5.35 (dd, 1H, J = 6.9, 1.5 Hz, HC5), 6.49–6.89 (m, 2H), 7.55 (dt, 1H, J = 3.8 Hz), 8.36–8.44 (m, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.7 (d, J = 5.7 Hz), 16.8 (d, J = 5.7 Hz), 38.9 (s, C4), 48.3 (d, J = 5.7 Hz, CH3–N), 63.0 (d, 1 J PC = 172.4 Hz, C3), 63.1 (d, J = 6.9 Hz, C–O–P), 63.6 (d, J = 6.6 Hz, C–O–P), 86.7 (d, 3 J PCCC = 9.7 Hz, C5), 104.3 (t, J = 25.4 Hz), 112.2 (dd, J = 21.7, 3.9 Hz), 114.7 (dd, J = 13.6, 3.8 Hz), 122.1 (d, J = 13.3 Hz), 128.8 (dd, J = 9.7, 5.1 Hz), 141.2 (d, J = 4.0 Hz), 159.3 (dd, J = 254.4, 12.0 Hz, CF), 162.9 (dd, J = 254.4, 12.5 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 21.45; 31P NMR (121.5 MHz, C6D6) δ: 21.68. Anal. Calcd for C16H21F2N4O4P: C, 47.76; H, 5.26; N, 13.93. Found: C, 48.00; H, 5.37; N, 13.85.
4.1.2.18. Diethyl 5-(4-(2,4-difluorophenyl)-1H-1,2,3-triazol-1-yl)-2-methylisoxazolidin-3-yl-3-phosphonate 16i
From azidoisoxazolidine 13 (0.152 g, 0.575 mmol) and 1-ethynyl-2,4-difluorobenzene (0.079 g, 0.575 mmol), phosphonate 16i (0.218 g, 94%) was obtained as colourless plates after purification on silica gel with chloroform–methanol (20:1, v/v). M.p.: 72–74 °C. IR (KBr, cm−1) ν max: 3445, 3121, 3100, 2983, 2905, 1625, 1600, 1559, 1492, 1447, 1355, 1257, 1140, 1046, 1025, 967, 946; 1H NMR (300 MHz, CDCl3) δ: 1.32 (t, 3H, J = 6.9 Hz), 1.36 (t, 3H, J = 6.9 Hz), 3.00 (s, 3H), 3.00–3.21 (m, 2H), 3.25–3.40 (m, 1H), 4.12–4.30 (m, 4H), 6.46 (dd, 1H, J = 7.8, 2.4 Hz), 6.85–6.95 (m, 1H), 6.96–7.03 (m, 1H), 8.28 (dt, 1H, J = 8.7, 6.3 Hz), 8.44 (d, 1H, J = 3.6 Hz); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 5.5 Hz), 40.3, 45.6, 62.7 (d, J = 6.6 Hz, C–O–P), 63.4 (d, J = 6.6 Hz, C–O–P), 63.5 (d, 1 J PC = 166.0 Hz, C3), 85.4 (d, 3 J PCCC = 9.1 Hz, C5), 104.0 (t, J = 25.8 Hz), 111.7 (dd, J = 21.2, 3.4 Hz), 115.0 (dd, J = 13.5, 4.0 Hz), 120.8 (d, J = 12.0 Hz), 128.6 (dd, J = 11.5, 5.2 Hz), 140.6, 158.9 (dd, J = 238.5, 13.1 Hz, CF), 162.2 (dd, J = 242.2, 12.7 Hz, CF); 31P NMR (121.5 MHz, CDCl3) δ: 20.75. Anal. Calcd for C16H21F2N4O4P: C, 47.76; H, 5.26; N, 13.93. Found: C, 47.67; H, 5.06; N, 13.99.
4.1.2.19. Diethyl 2-methyl-5-(4-(pyridin-2-yl)-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 15j
From azidoisoxazolidine 12 (0.152 g, 0.575 mmol) and 2-ethynylpyridine (0.058 mL, 0.575 mmol), phosphonate 15j (0.160 g, 76%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (100:1, v/v). IR (film, cm−1) ν max: 3466, 3138, 2983, 2930, 1603, 1572, 1474, 1424, 1343, 1254, 12,110, 1160, 1050, 1025, 972, 788; 1H NMR (300 MHz, C6D6) δ: 0.99 (t, 3H, J = 6.9 Hz, POCH2CH 3), 1.06 (t, 3H, J = 6.9 Hz, POCH2CH 3), 2.76 (s, 3H, CH 3–N), 2.85 (dddd, 1H, J = 17.7, 12.9, 11.1, 6.9 Hz, HbC4), 3.22 (dddd, 1H, J = 12.9, 6.9, 4.5, 1.2 Hz, HaC4), 3.58 (ddd, 1H, J = 10.5, 6.6, 1.8 Hz, HC3), 3.86–3.99 (m, 2H, POCH 2CH3), 4.00–4.08 (m, 2H, POCH 2CH3), 5.39 (d, 1H, J = 6.9, 1.2 Hz, HC5), 6.59–6.64 (m, 1H), 7.09–7.16 (m, 1H), 8.18 (s, 1H), 8.36 (d, 1H, J = 7.9 Hz), 8.44–8.47 (m, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.5 (d, J = 6.2 Hz), 16.6 (d, J = 5.8 Hz), 38.9 (d, J = 2.4 Hz, C4), 48.0 (d, J = 6.0 Hz, CH3–N), 62.6 (d, 1 J PC = 172.4 Hz, C3), 62.9 (d, J = 7.2 Hz, C–O–P), 63.4 (d, J = 6.9 Hz, C–O–P), 86.5 (d, 3 J PCCC = 10.5 Hz, C5), 120.2, 121.7, 123.0, 136.9, 148.7, 149.2, 149.5; 31P NMR (121.5 MHz, CDCl3) δ: 21.44; 31P NMR (121.5 MHz, C6D6) δ: 21.62. Anal. Calcd for C15H22N5O4P: C, 49.04; H, 6.04; N, 19.07. Found: C, 48.97; H, 5.95; N, 19.19.
4.1.2.20. Diethyl 2-methyl-5-(4-(pyridin-2-yl)-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 16j
From azidoisoxazolidine 13 (0.156 g, 0.590 mmol) and 1-ethynylpyridine (0.060 g, 0.590 mmol), phosphonate 16j (0.188 g, 87%) was obtained as white amorphous solid after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). M.p.: 108–109 °C. IR (KBr, cm−1) ν max: 3447, 3113, 2983, 2906, 1595, 1446, 1358, 1252, 1167, 1041, 969, 804; 1H NMR (300 MHz, CDCl3) δ: 1.31 (t, 3H, J = 7.2 Hz), 1.33 (t, 3H, J = 7.2 Hz), 2.99 (s, 3H), 3.00–3.22 (m, 2H), 3.24–3.40 (m, 1H), 4.10–4.25 (m, 4H), 6.43 (dd, J = 6.9, 1.8 Hz, 1H), 7.20–7.25 (m, 1H), 7.75–7.82 (m, 1H), 8.17 (d, J = 8.1 Hz, 1H), 8.57–8.60 (m, 1H), 8.70 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 6.3 Hz), 16.5 (d, J = 6.0 Hz), 40.6 (d, J = 2.3 Hz, C4), 45.6 (s, CH3–N), 62.7 (d, J = 6.9 Hz, C–O–P), 63.6 (d, J = 6.3 Hz, C–O–P), 63.6 (d, 1 J PC = 166.0 Hz, C3), 85.4 (d, 3 J PCCC = 9.2 Hz, C5), 120.1, 120.9, 122.8, 136.7, 148.4, 149.3, 150.1; 31P NMR (121.5 MHz, CDCl3) δ: 21.19. Anal. Calcd for C15H22N5O4P: C, 49.04; H, 6.04; N, 19.07. Found: C, 49.05; H, 5.90; N, 19.01.
4.1.2.21. Diethyl 2-methyl-5-(4-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 15k
From azidoisoxazolidine 12 (0.151 g, 0.571 mmol) and 5-ethynyl-1-methyl-1H-imidazole (0.058 mL, 0.571 mmol), phosphonate 15k (0.128 g, 61%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3113, 2980, 1692, 1613, 1555, 1444, 1239, 1160, 1037, 1020, 967; 1H NMR (300 MHz, C6D6) δ: 1.02 (t, 3H, J = 7.1 Hz, POCH2CH 3), 1.08 (t, 3H, J = 7.1 Hz, POCH2CH 3), 2.80 (s, 3H, CH 3–N), 2.94 (dddd, 1H, J = 17.4, 13.2, 10.8, 6.9 Hz, HbC4), 3.28 (dddd, 1H, J = 13.2, 6.9, 5.1, 1.8 Hz, HaC4), 3.29 (s, 3H, CH 3–N), 3.66 (ddd, 1H, J = 10.8, 6.9, 2.7 Hz, HC3), 3.91–3.99 (m, 2H, POCH 2CH3), 3.99–4.10 (m, 2H, POCH 2CH3), 5.47 (dd, 1H, J = 6.9, 1.8 Hz, HC5), 6.97 (s, 1H), 7.08 (s, 1H), 7.44 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.6 (d, J = 6.4 Hz), 16.7 (d, J = 6.3 Hz), 33.8, 38.8 (s, C4), 48.1 (d, J = 5.4 Hz, CH3–N), 62.8 (d, 1 J PC = 172.1 Hz, C3), 63.0 (d, J = 6.8 Hz, C–O–P), 63.5 (d, J = 6.6 Hz, C–O–P), 86.5 (d, 3 J PCCC = 10.0 Hz, C5), 120.9, 122.9, 128.5, 138.9, 139.5; 31P NMR (121.5 MHz, CDCl3) δ: 21.41; 31P NMR (121.5 MHz, C6D6) δ: 21.68. Anal. Calcd for C14H23N6O4P: C, 45.40; H, 6.26; N, 22.69. Found: C, 45.45; H, 6.09; N, 22.50.
4.1.2.22. Diethyl 2-methyl-5-(4-(1-methyl-1H-imidazol-5-yl)-1H-1,2,3-triazol-1-yl)isoxazolidin-3-yl-3-phosphonate 16k
From azidoisoxazolidine 13 (0.135 g, 0.511 mmol) and 5-ethynyl-1-methyl-1H-imidazole (0.052 mL, 0.511 mmol), phosphonate 16k (0.122 g, 65%) was obtained as a colourless oil after purification on silica gel with chloroform–methanol (from 50:1 to 20:1, v/v). IR (film, cm−1) ν max: 3110, 2982, 1612, 1502, 1442, 1240, 1161, 1114, 1045, 1020, 975; 1H NMR (300 MHz, CDCl3) δ: 1.32 (t, 3H, J = 7.1 Hz), 1.36 (t, 3H, J = 7.1 Hz), 2.99 (d, 3H, J = 1.0 Hz), 3.00–3.20 (m, 2H), 3.20–3.40 (m, 1H), 3.93 (s, 3H), 4.10–4.25 (m, 2H), 6.45 (dd, J = 7.8, 2.1 Hz, 1H), 7.54 (s, 1H), 8.30 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ: 16.4 (d, J = 6.2 Hz), 33.5, 40.4 (d, J = 2.2 Hz, C4), 45.6 (d, J = 2.0 Hz, CH3–N), 62.8 (d, J = 7.0 Hz, C–O–P), 63.2 (d, J = 6.9 Hz, C–O–P), 63.5 (d, 1 J PC = 166.8 Hz, C3), 85.5 (d, 3 J PCCC = 9.5 Hz, C5), 119.8, 123.5, 128.7, 138.8, 139.4; 31P NMR (121.5 MHz, CDCl3) δ: 20.97. Anal. Calcd for C14H23N6O4P: C, 45.40; H, 6.26; N, 22.69. Found: C, 45.30; H, 6.21; N, 22.80.
4.2. Antiviral activity assays
The antiviral assays [except anti-human immunodeficiency virus (HIV) assays] were based on inhibition of virus-induced cytopathicity in HEL [herpes simplex virus type 1 (HSV-1), HSV-2 (G), vaccinia virus and vesicular stomatitis virus], Vero (parainfluenza-3, reovirus-1, Sindbis, Coxsackie B4, and Punta Toro virus), HeLa (vesicular stomatitis virus, Coxsackie virus B4, and respiratory syncytial virus), MDCK (influenza A (H1N1 and H3N1) and influenza B virus) or CrFK (feline herpes virus; feline corona virus (FIPV)) cell cultures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 cell culture inhibitory dose-50 (CCID50) of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) in the presence of varying concentrations (250, 50, 10, … μM) of the test compounds. Viral cytopathicity was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. The methodology of the anti-HIV assays was as follows: human CEM (∼3 × 105 cells/cm3) cells were infected with 100 CCID50 of HIV-1(IIIB) or HIV-2(ROD)/mL and seeded in 200 μL wells of a microtiter plate containing appropriate dilutions of the test compounds. After 4 days of incubation at 37 °C, HIV-induced CEM giant cell formation was examined microscopically.
4.3. Cytostatic activity assays
Murine leukaemia L1210, human T-lymphocyte CEM, human cervix carcinoma (HeLa) and human lung fibroblast (HEL) cells were suspended at 300,000–500,000 cells/mL of culture medium, and 100 μL of a cell suspension was added to 100 μL of an appropriate dilution of the test compounds in wells of 96-well microtiter plates. After incubation at 37 °C for two (L1210) or three (CEM, HeLa, HEL) days, the cell number was determined using a Coulter counter. The IC50 was defined as the compound concentration required to inhibit cell proliferation by 50%.
Acknowledgements
The preliminary experiments in this project were conducted by Miss Zuzanna Pawłowska, M.Sc. The synthetic part of this work was supported by Medical University of Łódź internal found (503-3014-1). The virological part of this work was supported by the K. U. Leuven (GOA no. 10/14). We thank Mrs. Leentje Persoons, Mrs. Frieda De Meyer, Mrs. Lies Van den Heurck, Mrs. Anita Camps, Mr. Steven Carmans, Mrs. Leen Ingels and Mrs. Lizette van Berckelaer for excellent technical assistance.
References
- 1.Calderone V., Fiamingo F.L., Amato G., Giorgi I., Livi O., Martelli A., Martinotti E. 1,2,3-Triazol-carboxanilides and 1,2,3-triazol-(N-benzyl)-carboxamides as BK-potassium channel activators. XII. Eur. J. Med. Chem. 2008;43:2618–2626. doi: 10.1016/j.ejmech.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Kim E.-M., Joung M.-H., Lee C.-M., Jeong H.-J., Lim S.T., Sohn M.-H., Kim D.W. Synthesis of Tc-99m labeled 1,2,3-triazole-4-yl c-met binding peptide as a potential c-met receptor kinase positive tumor imaging agent. Bioorg. Med. Chem. Lett. 2010;20:4240–4243. doi: 10.1016/j.bmcl.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Giffin M.J., Heaslet H., Brik A., Lin Y.-C., Cauvi G., Wong C.-H., McRee D.E., Elder J.H., Stout C.D., Torbett B.E. A copper(I)-catalyzed 1,2,3-triazole azide-alkyne click compound is a potent inhibitor of a multidrug-resistant HIV-1 protease variant. J. Med. Chem. 2008;51:6263–6270. doi: 10.1021/jm800149m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q., Chittaboina S., Barnhill H.N. Highlights in organic chemistry advances in 1,3-dipolar cycloaddition reaction of azides and alkynes – a prototype of “click” chemistry. Lett. Org. Chem. 2005;2:293–301. [Google Scholar]
- 5.Zhou L., Amer A., Korn M., Burda R., Balzarini J., De Clercq E., Kern E.R., Torrence P.F. Synthesis and antiviral activities of 1,2,3-triazole functionalized thymidines: 1,3-dipolar cycloaddition for efficient regioselective diversity generation. Antivir. Chem. Chemother. 2005;16:375–383. doi: 10.1177/095632020501600604. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhary P.M., Chavan S.R., Shirazi F., Razdan M., Nimkar P., Maybhate S.P., Likhite A.P., Gonnade R., Hazara B.G., Deshpande M.V., Deshpande S.R. Exploration of click reaction for the synthesis of modified nucleosides as chitin synthase inhibitors. Bioorg. Med. Chem. 2009;17:2433–2440. doi: 10.1016/j.bmc.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Yu J.-L., Wu Q.-W., Zhang Q.-S., Xi X.-D., N-Liu N., Li Y.-Z., Liu Y.-H., Yin H.-Q. Synthesis and antitumor activity of novel 2′,3′-diethanethio-2′,3′,5′-trideoxy-5′-triazolonucleoside analogues. Eur. J. Med. Chem. 2010;45:3219–3222. doi: 10.1016/j.ejmech.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Park S.M., Yang H., Park S.-K., Kim H.M., Kim B.H. Design, synthesis, and anticancer activities of novel perfluoroalkyltriazole-appended 2′-deoxyuridines. Bioorg. Med. Chem. Lett. 2010;20:5831–5834. doi: 10.1016/j.bmcl.2010.07.126. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.-S., Park S.M., Kim H.M., Park S.-K., Lee K., Lee C.W., Kim B.H. C5-Modified nucleosides exhibiting anticancer activity. Bioorg. Med. Chem. Lett. 2009;19:4688–4691. doi: 10.1016/j.bmcl.2009.06.072. [DOI] [PubMed] [Google Scholar]
- 10.Lindsell W.E., Murray C., Preston P.N., Woodman T.A.J. Synthesis of 1,3-diynes in the purine, pyrimidine, 1,3,5-triazine and acridine series. Tetrahedron. 2000;56:1233–1245. [Google Scholar]
- 11.Lazrek H.B., Taourirte M., Oulih T., Barascut J.L., Imbach J.L., Pannecouque C., Witvrouw M., De Clercq E. Synthesis and anti-HIV activity of new modified 1,2,3-triazole acyclonucleosides. Nucleosides Nucleotides Nucleic Acids. 2001;20:1949–1960. doi: 10.1081/NCN-100108325. [DOI] [PubMed] [Google Scholar]
- 12.Elayadi H., Smietana M., Pannecouque C., Leyssen P., Neyts J., Vasseur J.-J., Lazrek H.B. Straightforward synthesis of triazoloacyclonucleotide phosphonates as potential HCV inhibitors. Bioorg. Med. Chem. Lett. 2010;20:7365–7368. doi: 10.1016/j.bmcl.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Chittepu P., Sirivolu V.R., Seela F. Nucleosides and oligonucleotides containing 1,2,3-triazole residues with nucleobase tethers: synthesis via the azide-alkyne ‘click’ reaction. Bioorg. Med. Chem. 2008;16:8427–8439. doi: 10.1016/j.bmc.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Piotrowska D.G. N-Substituted C-diethoxyphosphorylated nitrones as useful synthons for the synthesis of α-aminophosphonates. Tetrahedron Lett. 2006;47:5363–5366. [Google Scholar]
- 15.Piotrowska D.G. Stereochemistry of cycloaddition of (S)-N-(1-phenylethyl)-C-diethoxyphosphorylated nitrone with vinyl acetate. Studies on mutarotation of 3-(O,O-diethylphosphoryl)-5-hydroxyisoxazolidines. Tetrahedron: Asymmetry. 2008;19:279–287. [Google Scholar]
- 16.Piperno A., Giofrè S.V., Iannazzo D., Romeo R., Romeo G., Chiacchio U., Rescifina A., Piotrowska D.G. Synthesis of C-4′truncated phosphonated carbocyclic 2′-oxa-3′-azanucleosides as antiviral agents. J. Org. Chem. 2010;75:2798–2805. doi: 10.1021/jo902485m. [DOI] [PubMed] [Google Scholar]
- 17.Slámová K., Marhol P., Bezouška K., Lindkvist L., Hansen S.G., Křen V., Jensen H.H. Synthesis and biological activity of glycosyl-1H-1,2,3-triazoles. Bioorg. Med. Chem. Lett. 2010;20:4263–4265. doi: 10.1016/j.bmcl.2010.04.151. [DOI] [PubMed] [Google Scholar]
- 18.Dedola S., Hughes D.L., Nepogodiev S.A., Rejzek M., Field R.A. Synthesis of α- and β-d-glucopyranosyl triazoles by CuAAC ‘click chemistry’: reactant tolerance, reaction rate, product structure and glucosidase inhibitory properties. Carbohydr. Res. 2010;345:1123–1134. doi: 10.1016/j.carres.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 19.El Akri K., Bougrin K., Balzarini J., Farajd A., Benhida R. Efficient synthesis and in vitro cytostatic activity of 4-substituted triazolyl-nucleosides. Bioorg. Med. Chem. Lett. 2007;17:6656–6659. doi: 10.1016/j.bmcl.2007.08.077. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R., Tiwari P., Maulik P.R., Misra A.K. A generalized procedure for the one-pot preparation of glycosyl azides and thioglycosides directly from unprotected reducing sugars under phase-transfer reaction conditions. Eur. J. Org. Chem. 2006:74–79. [Google Scholar]
- 21.Piotrowska D.G. Stereochemistry of substituted isoxazolidines derived from N-methyl C-diethoxyphosphorylated nitrone. Tetrahedron. 2006;62:12306–12317. [Google Scholar]
- 22.Loukou C., Tosin M., Müller-Bunz H., Murphy P.V. Synthesis of sugar-lactams from azides of glucuronic acid. Carbohydr. Res. 2007;342:1953–1959. doi: 10.1016/j.carres.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Neeser J.-R., Tronchet J.M.J., Charollais E.J. Structural analysis of 3-C-phosphonates, -phosphinates, and -phosphine oxides of branched-chain sugars. Can. J. Chem. 1983;61:2112–2120. [Google Scholar]
- 24.Adiwidjaja G., Meyer B., Thiem J. Darstellung und Kristallstruktur von endo-2-Dimethylphosphono-exo-2-hydroxy-(–)-camphan zur Bestimmung von 3J(CCCP)-Vicinalkopplungen. Z. Naturforsch. 1979;34b:1547–1551. [Google Scholar]
- 25.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.Kolb H.C., Finn M.G., Sharpless K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Kool E.T., Sintim H.O. The difluorotoluene debate – a decade later. Chem. Commun. 2006:3665–3675. doi: 10.1039/b605414e. [DOI] [PubMed] [Google Scholar]


