Abstract
Respiratory tract infections are a leading cause of morbidity and mortality worldwide. The human bocavirus (HBoV) is a newly recognized human parvovirus first reported in 2005. Since its discovery, this virus has been associated with upper and lower respiratory tract disease and gastroenteritis worldwide. This article is a comprehensive review of what is known about HBoV. It includes an evaluation of diagnostic modalities, symptoms occurring in affected patients, and a discussion as to whether HBoV is responsible for identified clinical manifestations. The article reviews the incidence and effect of coinfection and updates on related members (HBoV-2 and HBoV-3) recently reported. Understanding of respiratory viruses such as HBoV remains vitally important to the health of adult and pediatric patients.
Keywords: Human bocavirus, Human bocavirus 2, Human bocavirus 3, Viral infection, Viral gastroenteritis, Pneumonia
Respiratory tract infections are a major cause of morbidity and mortality. Lower respiratory tract disease accounts for approximately 4 million deaths annually worldwide. Of all hospital discharges in the United States, close to 10% are attributed to diseases of the respiratory system with an estimated mortality of 232,000 in 2005.1 The economic burden of these infections in the United States is projected to be more than $100 billion.2
Viruses, such as influenza, respiratory syncytial virus (RSV), and parainfluenza viruses, are known to be responsible for most respiratory tract infections. In a significant proportion of respiratory tract disease, however, no pathogen is identified. Over the last 10 years, the advancement of molecular detection and genomic amplification has led to the recognition of numerous potential respiratory pathogens, including the human metapneumovirus (HMPV),3 human coronaviruses NL63,4, 5, 6 HKU1,7 and SARS-CoV,8 Ki and WU polyomaviruses,9, 10 and the human bocavirus (HBoV).11 Although disease caused by respiratory viruses has been underestimated in the past, there is now a growing appreciation for the role these viruses play in community- and hospital-acquired pneumonia.
The HBoV is a novel parvovirus first isolated in 2005 from human respiratory secretions from patients who had pneumonia.11 Since that time, it has been associated with upper and lower respiratory tract disease and gastrointestinal illness in adult and pediatric patients throughout the world. Recently, two viruses closely related to the human bocavirus have been reported, provisionally named human bocavirus 2 (HboV-2) and human bocavirus 3 (HBoV-3).12, 13 For the sake of clarity, the remainder of this article refers to the original human bocavirus strain described by Allander and colleagues as human bocavirus 1 (HBoV-1).11 It remains unclear whether HBoV-1, HBoV-2, and HBoV-3 represent unique viral entities or distant genotypes of a single virus. Because HBoV-2 and HBoV-3 have only been recognized over the last several months, the epidemiology and clinical manifestations associated with these viruses are only now being realized. The incidence of human bocaviruses varies widely,14, 15 with various clinical presentations, and they are often found in association with other potential pathogens. This phenomenon has led to debate over the role of human bocaviruses as true pathogens. This article reviews the published studies and discusses the virologic, clinical, and diagnostic aspects of these newly recognized human viruses.
Discovery of human bocaviruses
Human Bocavirus 1
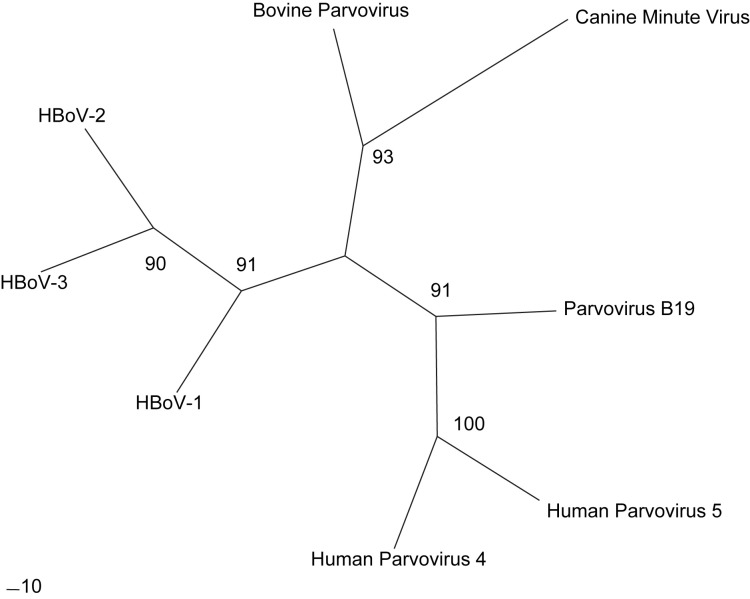
HBoV-1 was first described by Allander and colleagues11 in 2005. Pooled, cell-free supernatant from respiratory samples was examined using a random polymerase chain reaction (PCR) technique. Amplified PCR products were separated, ligated to a vector, and introduced into Escherichia coli. Clones were sequenced and evaluated through an automated protocol and compared against known sequences. The majority of the clones were determined to be human or recognized bacterial and viral pathogens. Of the remaining sequences, a parvoviruslike sequence was identified with no known correlation to recognized human parvoviruses. Phylogenetic analysis showed this to be a novel parvovirus most closely related to bovine parvovirus and canine minute virus (Fig. 1 ). It is now named the human bocavirus.
Fig. 1.
Phylogenetic analysis of Animal and Human Parvovirus Species. Unrooted radial tree of representative animal and human parvovirus strains (HBoV-1 [GenBank accession no. NC_007455.1], HBoV-2 [NC_012042.1]; HBoV-3 [NC_012564.1]; Bovine Parvovirus [DQ335247.1], Canine Minute Virus [NC_004442.1], Parvovirus B19 [AY386330.1]; Human Parvovirus 4 [EU874248.1], and Human Parvovirus 5 [DQ873391.1]) is displayed. Maximum-likelihood phylogenetic trees were constructed using the PHYLIP program DNAML, with the default transition to transversion ratio of 2.0 and 1 jumble. One hundred bootstrap data sets were created using the PHYLIP program SEQBOOT. Bootstrap values are displayed at major branch points.
Human Bocavirus 2
In January 2009, Kapoor and colleagues12 reported the identification of a related parvovirus, termed HBoV-2, from pediatric stool samples by random PCR analysis of nuclease-resistant virus particles. The resulting amplicons were subcloned into a plasmid vector, sequenced, and compared with genome entries on GenBank. Phylogenetic analysis of HBoV-2 shows that it is most closely related to HBoV-1. The amino acid identity of HBoV-2 to HBoV-1 is 78%, 67%, and 80% for the NS1, NP1, and VP1/VP2 proteins, respectively. There is significant divergence of the genomic targets used for detection of HBoV-1, which explains why HBoV-2 has remained unidentified to this point.
Human Bocavirus 3
A third bocavirus species was described in April 2009 from Australia, and has been provisionally named human bocavirus 3 (HBoV-3). Similar to the methods used in discovery of HBoV-1 and HBoV-2, Arthur and colleagues13 digested filtered fecal samples with nucleases, then pelleted virions and evaluated using degenerate oligonucleotide primed–PCR. During these investigations, the group simultaneously discovered HBoV-2 and a third species of bocavirus, HBoV-3. Analysis of the genome shows close homology to HBoV-1 in the nonstructural protein encoding regions (NS1, NP1), but HBoV-3 is more similar to HBoV-2 in the structural protein encoding regions (VP1/VP2). This finding suggests that HBoV3 may be the product of recombination between HBoV1 and HBoV2. Arthur and colleagues identified a recombination site that supports this hypothesis. As with HBov-2, the HBoV-3 genome is divergent enough from HBoV-1 to explain lack of detection using currently published primer sets (Table 1 ). Clinical presentation, seasonal distribution, and prevalence of HBoV3 have yet to be characterized in full. Further analysis of the phylogenetic relationships will be required to determine the correct taxonomy of this virus family.
Table 1.
Nucleic acid amplification methods and primer sets used for detection of human bocavirus 1
| Target Gene | Target Locationa | Predicted Size (bp) | Forward Primer | Reverse Primer | Probeb | % Detectedc (Mediand) | References | ||
|---|---|---|---|---|---|---|---|---|---|
| Polymerase chain reaction | Conventional | NP1 | 2351–2704 | 353 | GACCTCTGTAAGTCTATTAC | CTCTGTGTTGACTGAATACAG | 0.8–45.2 (5.4) | 11, 14, 20, 24, 28, 29, 34, 38, 39, 40, 47, 48, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 83, 102 | |
| NS1 | 2301–2700 | 399 | CCCAAGAAACGTCGTCTAAC | GTGTTGACTGAATACAGTGT | 2.8–2.9 (2.90) | 62, 63, 76, 103, 104, 105 | |||
| VP1/VP2 | 1545–1835 | 290 | TATGGCCAAGGCAATCGTCCAAG | GCCGCGTGAACATGAGAAACAGA | 0.8–11.3 (5.5) | 35, 40, 41, 42, 43, 49, 56, 59, 60, 77, 102 | |||
| VP1/VP2 | 4125–4966 | 841 | GTGACCACCAAGTACTTAGAACTGG | GCTCTCTCCTCCCAGTGACAT | 5.1–12.5 (8.8) | 24, 47, 66, 74 | |||
| VP1/VP2 | 4370–5189 | 819 | GGACCACAGTCATCAGAC | CCACTACCATCGGGCTG | 3.5–5.5% (4.50) | 42, 43 | |||
| VP1/VP2 | 3639–4286 | 647 | TTCAGAATGGTCACCTCTACA | CTGTGCTTCCGTTTTGTCTTA | 6.60 | 65 | |||
| VP1/VP2 | 4562–4966 | 404 | GCAAACCCATCACTCTCAATGC | GCTCTCTCCTCCCAGTAGACAT | 1.5–14.2 (7.6) | 14, 24, 47, 66, 74 | |||
| VP1/VP2 | 4268–4467 | 199 | AGACAAAACGGAAGCACAGC | TCAAAGCCAGATCCAAATCC | 3.10 | 84 | |||
| VP1/VP2 | 4269–4467 | 198 | CAGTGGTACCAGACACCAGAAG | GCCAGTTCTTTGTTGCGTATCT | 5.5–31.3 (14.0) | 25, 84 | |||
| VP1/VP2 | 4270–4467 | 197 | CCAAAAAAGACACTTTTACTTTGCTAACTCA | TGGACGCCAGTTCTTTGTTGCGTATCTTTC | 2.2–2.7 (2.40) | 48 | |||
| Nested | NP1 | 2259–3259 | 1000 | Outer: GAAGACACCGAGCCTGAGAC | Outer: GCTGATTGGGTTGTTCCTGAT | 7.10 | 106 | ||
| 2351–2565 | 214 | Inner: GAGCTCTGTAAGTACTATTAC | Inner: ATATGAGCCCGAGCCTCTCT | ||||||
| Paired Nestede | NP1 | 2318–2709 | 391 | Outer: TAACTGCTCCAGCAAGTCCTCCA | Outer: GGAAGCTCTGTGTTGACTGAAT | 7.4–11.1 (9.3) | 107, 108 | ||
| 2344–2709 | 365 | Inner: CTCACCTGCGAGCTCTGTAAGTA | Inner: GGAAGCTCTGTGTTGACTGAAT | ||||||
| VP1/VP2 | 4085–4988 | 903 | Outer: GCACTTCTGTATCAGATGCCTT | Outer: CGTGGTATGTAGGCCGTGTAG | |||||
| 4139–4988 | 849 | Inner: CTTAGAACTGGTGAGAGCACTG | Inner: CGTTGGTATGTAGGCGTGTAG | ||||||
| NS1 | 1579–1794 | 215 | Outer: TATGGGTGTGTTAATCATTTGAAYA | Outer: GTAGATATCGTGRTTRGTKGATAT | 5.80 | 50 | |||
| 1600–1703 | 103 | Inner: AACAAAGGATTTGTWTTYAATGAYTG | Inner: CCCAAGATACACTTTGCWKGTTCCACCC | ||||||
| NS1 | 2325–2723 | 398 | Outer: CCAGCAAGTCCTCCAAACTCACCTGC | Outer: GGAGCTTCAGGATTGGAAGCTCTGTG | |||||
| 2351–2704 | 353 | Inner: GAGCTCTGTAAGTACTATTAC | Inner: CTCTGTGTTGACTGAATACAG | ||||||
| Real-time | NP1 | 22548–2628, 2570 | AGAGGCTCGGGCTCATATCA | CACTTGGTCTTGAGGTCTTCGAA | AGGAACACCCAATCARCCACCTATCGTCT | 2.9–32.3 (9.1) | 21, 36, 85 | ||
| NP1 | 2509–2605, 2534 | AGGAGCAGGAGCCGCAGCC | CAGTGCAAGACGATAGGTGGC | ATGAGCCCGAGCCTCTCTCCCCACTGTGTC | g | 22 | |||
| NP1 | 2397–2592, 2533 | CCACGTGACGAAGATGAGCTC | TAGGTGGCTGATTGGGTGTTC | CCGAGCCTCTCTCCCCACTGTGTCG | 11.9 | 78 | |||
| NP1 | 2405–2586, 2506 | CGAAGAATGAGCTCAGGGAAT | GCTGATTGGGTGTTCCTGAT | CACAGGAGCAGGAGCCGCAG | 9.0–11.3 (10.7) | 17, 51, 67, 86 | |||
| NP1 | 2427–2580, 2504 | GAAAGACAAGCATCGCTCC | TGGGTGTTCCTGATGATATG | CGCGCTGGAGCAGGAGCCGCAGCCCGATAGCGCG | 2.9 | 87 | |||
| NP1 | 2351–2704, 2480 | GAGCTCTGTAAGTACTATTAC | CTCTGTGTTGACTGAATACAG | GGAAGAGACATGGCAGACAAC | 12.8 | 79 | |||
| NP1 | 2391–2466, 2411 | GCACAGCCACGTGGCAGACAA | TGGACTCCCTTTTCTTTTGTAGGA | TGAGCTCAGGGAATATGAAAGACAAGCATCGTMR | 4.6 | 44 | |||
| NP1 | 2480–2579, 2504 | GGAAGAGACACTGGCAGACAA | GGGTGTTCCTGATGATATGAGC | CTGCGGCTCCTGCTCCTGTGAT | 2.4–18.9 (5.3) | 15, 26, 30, 88 | |||
| NP1 | 2424–2636, 2559 | TATGAAAGACAAGCATCGCTCCTA | GTCTTCATCACTTGGTCTGAGGTCT | CTCATATCATCAGGAACAC | 8.2 | 37 | |||
| NP1 | 2554–2628, 2577 | TCGGGCTCATATCATCAGGAA | CACTTGGTCTGAGGTCTTCGAA | CCCAATCAGCCACCTATCGTCTTGC | 19.3 | 83 | |||
| NP1 | 2488–2566, 2512 | CACTGGCAGACAACTCATCACA | GATATGAGCCCGAGCCTCTCT | AGCAGGAGCCGCAGCCCGA | 4.5 | 89 | |||
| NP1 | 1617–1710, 1667 | TAATGACTGCAGACAACGCCTAG | TGTCCCGCCCAAGATACACT | TTCCACCCAATCCTGGT | g | 90 | |||
| NP1 | 1637–1711, 1668 | TAGTTGTTTGGTGGGARGA | CTGTCCCGCCCAAGATACA | CCAGGATTGGGTGGAACCTGCAAA | 9.1 | 54 | |||
| NP1 | 1624–1711, 1668 | TGCAGACAACGCYTAGTTGTTT | CTGTCCCGCCCAAGATACA | CCAGGATTGGGTGGAACCTGCAAA | 2.9–32.3 (3.5) | 36, 42, 85 | |||
| NASBAf | NP1 | 2567–2424, 2456 | AATTCTAATACGACTRCACTATAGGGGCTGATTGGGTGTTCCTGAT | AATTCTAATACGACTCACTATAGGGCGAAGATGAGCTCAGGGAAT | CGATCGAGTCCAGAAAGAGGGGAGAGCGATCG | 0 | 94 |
Estimated location based on GenBank isolate EU262978.1, probe location added when applicable.
Added when applicable.
Range of percent detected in cited studies.
Median of percent detected in cited studies when applicable.
In cited studies, a sample positive for HBoV-1 required amplification from both sets of nested PCR primers.
Nucleic acid sequence based amplification.
Case reports.
Biology and taxonomy
Human bocaviruses are parvoviruses belonging to the family Parvoviridae, subfamily Parvovirinae, and are most closely related to bovine parvovirus and canine minute virus, in the genus Betaparvovirus (see Fig. 1). Parvoviruses are single-stranded DNA viruses with a small genome size between 4 kilobases (kb) and 6 kb that have three open reading frames that encode two nonstructural proteins, NS1 and NP1, and the structural VP1 and VP2 proteins. The HBoV genome does not encode a polymerase and depends on host cell DNA polymerases for replication. Parvoviruses have unenveloped capsids with icosahedral symmetry.16 Based on electron micrographs, HBoV-1 appears structurally similar to other members of the Parvoviridae family and is approximately 25 nm in diameter.17
The VP2 gene is nested in the reading frame of VP1 and uses an alternate initiator codon.11 The sequence of VP1 and VP2 differ only in the N-terminal extension of VP1. This region, referred to as the VP1 unique region, plays a critical role in virus infectivity in some parvoviruses.18 In several parvoviruses, VP3 arises from posttranslational proteolytic cleavage of VP2 proteins. It remains unclear if VP3 exists in human bocavirus. The capsid of parvoviruses consists of about 60 copies of each viral capsid protein. The role of NS1 and NP1 proteins in human bocavirus species remain unknown. Zhi and colleagues19 created an infectious clone of the related parvovirus B19 to determine the functions of these genes. Their findings showed that NS and VP1 knockout mutants abolished the viral infectivity. The NS protein also seemed to be vital in genome transcription and to play a role in the regulation of capsid gene expression.
Parvoviruses have been shown to be transmitted by various routes, including respiratory, urine, and fecal–oral contact.16 The mode of bocavirus transmission of HBoV is unknown. Most studies have reported the presence of HBoV-1 DNA in respiratory secretions and fecal samples. HBoV-1 DNA has also been detected in addition to serum, urine, and lymph nodes.20, 21, 22 So far, HBoV-2 and HBoV-3 have only been identified in stool samples. Similar to other respiratory viruses, transmission may occur through several routes, including inhalation, contact with infected secretions (through direct contact or possibly contaminated surfaces), and fecal–oral from the standpoint of gastrointestinal illness.
Although some parvoviruses cannot replicate without helper viruses, the mechanisms of cell entry and replication have not been described for human bocavirus. This lack of information is largely because of the inability to grow human bocaviruses in standard cell lines.
Clinical presentation
Bocaviruses and Respiratory Illness
Following Allander's initial description of HBoV-1 in the human respiratory tract11, more than 60 studies have been published investigating the role of this virus in respiratory tract illness. Clinical findings reported from HBoV-1–positive patients from 9 representative studies are summarized in Table 2 . Most patients present with symptoms of upper respiratory tract illness, including cough, fever, and rhinorrhea. Pharyngitis and rash are less common (11%–13%). Additionally, patients tended to report earache.14, 23
Table 2.
Clinical findings of patients positive for human bocavirus 1
| Na | Respiratory Findings |
GI Findings |
References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cough | Fever | Rhinorrhea | O2 Therapy | Hypoxia | Tachypnea | Wheeze | Pharyngitis | Other Respiratory Symptomsb | Nausea or Vomiting | Diarrhea | Other GI Symptomsc | ||
| 32 | 26 | 18 | 22 | 10 | 10 | 11 | 10 | — | 1 | 8 | 5 | 0 | 38 |
| 68 | 58 | 42 | 46 | 30 | 28 | — | — | — | 59 | 21 | 14 | — | 65d |
| 14 | — | 10 | — | — | 7 | 11 | — | — | 14 | 0 | 0 | 0 | 11 |
| 30 | 23 | 13 | 27 | — | — | 6 | 6 | — | — | 6 | 7 | — | 73 |
| 9 | 9 | 9 | 0 | 5 | — | 2 | 2 | — | — | — | — | — | 70 |
| 95 | 81 | 75 | 72 | — | — | — | — | 11 | 24 | 28 | 7 | 11 | 26d |
| 18 | 12 | 12 | 6 | — | — | — | — | 7 | — | 2 | — | — | 14d |
| 32 | 28 | 21 | 28 | 12 | — | — | — | — | 26 | 4 | 5 | — | 49d |
| 33 | 13 | — | 9 | 12 | — | — | 10 | 1 | 23 | — | — | 3 | 42 |
| Totale | 250 | 200 | 210 | 69 | 45 | 30 | 28 | 19 | 147 | 69 | 38 | 14 | — |
| No. evaluatedf | 317 | 298 | 317 | 174 | 14 | 85 | 104 | 146 | 304 | 289 | 271 | 174 | — |
| Occurrence (%) | 78.9 | 67.1 | 66.2 | 39.7 | 39.5 | 35.3 | 26.9 | 13.0 | 48.4 | 23.9 | 14.0 | 8.0 | — |
Abbreviation: GI, gastrointestinal.
Number evaluated in each study.
Includes respiratory distress, increased work of breathing, cyanosis, apnea, rhonchi, rales, shortness of breath.
Includes abdominal pain, poor feeding, unspecified GI complaints.
Clinical analysis includes patients who have respiratory copathogens identified in addition to HBoV-1.
Cumulative patients reported with listed findings.
Cumulative patients evaluated for listed finding.
Several studies raise the possibility that HBoV-1 is associated with severe respiratory disease. Reports describe this virus in children hospitalized for bronchiolitis,24 asthma exacerbations,15, 25 and first-time episodes of wheezing.26 Wheezing is described in numerous studies. In one prospective study comparing 16 respiratory viruses in children admitted with respiratory infection, wheezing was seen in more than 50% of children in whom HBoV-1 was recognized as the sole agent. Surprisingly, children who had coinfections had less wheezing.27 Other common findings include tachypnea, fever, and hypoxia. One report from Jordan found as many of 18% of children hospitalized with pneumonia were positive for HBoV-1.28 In Thailand, Fry and colleagues found that patients who had HBoV-1 were more likely to be hospitalized with pneumonia (odds ratio of 3.56) compared with controls.
Most HBoV-1 reports are retrospective analyses based from hospital settings In these studies, up to 6.6% of patients required stays in intensive care units and up to 40% required oxygen therapy at some point during their hospitalization.20, 29 These studies likely overestimate the severity of disease associated with HBoV-1 because of selection bias. In studies based on community samples, disease is mild and only rarely requires admission. To date, there have been no reported deaths associated with HBoV-1.
Few studies list underlying comorbidities in HBoV-1–infected patients. When reported, more than half of patients had an underlying medical condition.20 The most common underlying conditions involve primary cardiac or pulmonary disease, including congenital heart lesions, heart failure, asthma, chronic obstructive pulmonary disease, and prematurity with chronic lung disease.11, 20, 29
HBoV-1 has been reported in immunocompromised patients who have respiratory and gastrointestinal illness. Schenk and colleagues22 reported a child coinfected with rhinovirus and HBoV-1 following hematopoietic stem cell transplant. This patient developed fever, dyspnea, wheezing, and infiltrates on chest radiograph. In this patient, HBoV-1 DNA was isolated from nasopharyngeal, stool, and blood samples. Koskenvuo and colleagues30 reported a series of children who had acute lymphoblastic leukemia in whom HBoV-1 was identified. Presenting symptoms were febrile upper respiratory infection with otitis media, fever with vomiting and diarrhea, and isolated fever. Garbino and colleagues31 reported a case of HBoV-1 in a hospitalized adult infected with HIV. All patients eventually recovered from their acute illness.
Although few studies have systematically examined immunocompromised patients for HBoV-1, the incidence seems to be low. One study by Muller and colleagues32 examined bronchoalveolar lavage specimens from immunocompromised patients suspected of having Pneumocystis jiroveci pneumonia. Only 1 specimen of 128 (0.8%) was found to have HBoV-1. Miyakis and colleagues33 prospectively examined bronchoalveolar lavage specimens from 53 adult lung transplant recipients and 67 symptomatic nontransplant patients and failed to find HBoV-1 in any sample. These studies argue against HBoV-1 as a pathogen of lower respiratory tract disease in these vulnerable populations.
Because many of these reports observe high coinfection rates, it is difficult to draw conclusions as to whether HBoV-1 plays a role in pathogenicity of infected patients. To answer this several studies have compared the presence of HBoV-1 in asymptomatic control groups to symptomatic individuals.34, 35, 36, 37 All but one found HBoV-1 more often in symptomatic children than in healthy controls. In the remaining study, asymptomatic carriage of HBoV-1 was as frequent in asymptomatic infants as those who had acute respiratory tract disease. In a recent report by Allander and colleagues15 HBoV-1 DNA is also present in the serum of acutely ill patients suggesting that dissemination of this virus is possible. These findings provide support for HBoV-1 as a cause for respiratory illness. When in vitro culture systems and animal models are available, a better understanding of the role of HBoV-1 in human respiratory disease can be established.
Bocaviruses and Gastrointestinal Symptoms
Gastrointestinal symptoms are reported in up to 25% of patients who have HBoV-1 respiratory infection (see Table 2) suggesting HBoV-1 may not be limited to the respiratory tract alone. The closely related canine and bovine parvoviruses are well described as both respiratory and enteric pathogens.16 Subsequent investigations have identified HBoV-1 DNA in stool samples from patients with gastrointestinal illness. The most common gastrointestinal symptoms include nausea, vomiting, and diarrhea. Both watery and bloody diarrhea have been reported in conjunction with HBoV- 1.38, 39, 40, 41, 42, 43, 44, 45
HBoV-1 has been identified in 0.8% to 9.1% of stool samples from patients presenting with gastrointestinal illness.38, 39, 40, 41, 42, 43 HBoV-1 viral load in stool is significantly less than viral loads found in respiratory tract specimens of symptomatic patients.44 Similar to studies of respiratory illness, HBoV-1 is commonly codetected with recognized enteric pathogens, with coinfection rates ranging from 21% to 77.6%.41, 43 Identified copathogens include norovirus, rotavirus, human calicivirus, astrovirus, adenovirus, Campylobacter, Salmonella, and Clostridium difficile.38, 39, 40, 42, 43, 44 In a recent case-control study on acute gastroenteritis, Arthur and colleagues13 examined stool specimens for potential pathogens, including all three species of human bocavirus. DNA from human bocaviruses 1, 2, or 3 was isolated from 54 (27.4%) stool samples, making these human bocaviruses the second most common viral agents identified after rotavirus (37.1%). Clinical illness associated with HBoV-1 and rotavirus coinfection did not seem to be significantly different than that of rotavirus illness alone, suggesting the presence of HBoV-1 did not worsen disease.42
HBoV-1 is infrequently reported in stools originating from immunocompromised patients. In a 4-year-old patient who had diarrhea, bocavirus was recognized on day 21 and again on day 75 following stem cell transplant. This patient went on to develop disseminated disease.22 One patient who had a T cell deficiency was reported to have hepatitis associated with HBoV-1.46 HBoV-1 was also associated with gastroenteritis in one patient who had B cell immunodeficiency.39 Unlike HBoV-1–associated respiratory disease, large investigations for HBoV-1–associated gastrointestinal illness have not been performed for the immunocompromised population.
As with respiratory disease, the role of HBoV-1 as a cause for gastrointestinal disease is unclear. Several reports have identified HBoV-1 in stool samples from asymptomatic patients.42, 44 Arthur and colleagues compared stool samples positive for HBoV-1, HBoV-2, and HBoV-3 from symptomatic patients and age-matched asymptomatic controls. The presence of HBoV-1 failed to achieve statistical significance, supporting the hypothesis that HBoV-1 may not be a cause of acute gastroenteritis.13 The authors note that the lack of HBoV-1 in symptomatic individuals may be because symptoms may appear before viral particles are shed in stool. Similar findings were made for astrovirus and adenovirus, which are widely accepted causes of gastroenteritis.13 Also, HBoV-2 was significantly found more often in patients with gastroenteritis. Because of the low incidence in this study, the clinical significance of HBoV-3 remains unclear. Further data on higher viral load in stool samples corresponding with active disease would support causality.
Human bocavirus 1 coinfections
Most studies identify coinfecting viral and bacterial pathogens in a substantial percentage of HBoV-1–positive samples. Although all studies did not examine samples for all pathogens, commonly reported pathogens include enterovirus, rhinovirus, RSV, parainfluenza, influenza, adenovirus, and HMPV (Table 3 ).
Table 3.
Human bocavirus 1 detection and coinfection in relation to common respiratory viruses
| RSV (%) | Influenzad(%) | PIVe(%) | HMPV (%) | Rhinovirus (%) | Enterovirus (%) | Coronavirusesf (%) | |
|---|---|---|---|---|---|---|---|
| Frequency of HBoV-1a | 1.6–31.3 (7.1) | 1.6–31.2 (9.8) | 1.6–31.3 (8.0) | 1.7–19.3 (7.7) | 1.6–19.3 (7.7) | 2.4–19.3 (12.5) | 1.7–19.3 (7.8) |
| Bocavirus 1 samples with copathogenb | 15.0 | 9.0 | 3.8 | 5.6 | 8.0 | 13.0% | 0.0 |
| Listed virus-positive samples coinfected with HBoV-1c | 6.5 | 10.3 | 6.5 | 5.9 | 9.5 | 13.45 | 0.0 |
Abbreviations: HMPV, human metapneumovirus; HBoV-1, human bocavirus 1; RSV, respiratory syncytial virus; PIV, parainfluenza virus.
Frequency (median) of human bocavirus 1 in studies that also screened for listed virus.
Median number of human bocavirus 1–positive samples found to be coinfected with listed pathogen.
Median number of samples positive for listed virus found to be coinfected with human bocavirus 1.
Includes pooled results for Influenza A, B, and C. Not all studies included all members.
Includes types 1–4. Not all studies included all members.
Includes 229E, OC43, HKU1, and NL 63. Not all studies included all members.
HBoV-1 coinfection was a common occurrence in all studies with a median rate of 42.5%. HBoV-1 coinfection is described with numerous pathogens; however, those that screen positive for rhinovirus, enteroviruses, and influenza have the highest incidence of HBoV-1 coinfection. Similarly, in samples found positive for HBoV-1, a high rate of enterovirus, influenza, and RSV is seen. Although this likely reflects the high frequency of these viruses in the population at large, one may speculate that these particular coinfecting viruses may provide a biologic benefit to HBoV.
Epidemiology of human bocaviruses
HBoV-1 has been recognized worldwide in association with upper and lower respiratory disease. Most HBoV-1–positive samples originate from children predominantly younger than 2 years of age.11, 20, 27, 29, 34, 35, 36, 47, 48, 49, 50, 51, 52, 53 Within this age group, children less than 6 months of age seem to be less frequently affected, perhaps because of passive maternal immunity. Several studies have detected HBoV-1 in adults.20, 54, 55 In one study screening 1539 children and 273 adults, HBoV-1 occurred at a similar frequency in both groups.20
Seasonal Distribution
Most studies report circulation of HBoV-1 predominantly during the winter season.20, 47 Seasonal peaks vary but most often are described in the early winter. Most of these studies are retrospective, using archived respiratory samples submitted for analysis of common respiratory pathogens. This approach may lead to misrepresentation of when this virus circulates. Several reports show increased frequency of HBoV-1 infections during the spring. Choi and colleagues56 screened samples over a 5-year period from patients who had lower respiratory tract illness (LRTI) in Korea and found a higher frequency of HBoV-1 between May and July. Bastien and colleagues14 studied samples originating from patients in Canada and reported no apparent seasonal prevalence. Only a few prospective studies of HBoV-1 have been performed. In a 1-year prospective investigation in children less than 5 years of age hospitalized with respiratory tract disease, HBoV-1 was detected in every month except August.51 In this study HBoV-1 peaked in the month of December, with 80% of HBoV-1 isolates occurring between November and March.
Seroepidemiology
Several seroepidemiology studies have been performed to assess the prevalence of HBoV-1 infection during childhood. Kahn and colleagues reported anti–HBoV-1 antibodies are common in children.57 Screening serum samples using a viruslike particle–based ELISA, Kahn and colleagues found evidence of prior HBoV-1 infection in 195 of 270 (72.2%) serum samples.57 Children between 4 and 8 months of age had the lowest prevalence of antibodies against HBoV-1, whereas children younger than 2 months of age and those older than 5 years had the highest.57, 58 The high proportion of seropositive children younger than 2 months of age suggests vertical transfer of antibodies. The subsequent decline in the percentage of seropositive children over the first 4 to 6 months of life likely represents waning maternal immunity. The low percentage of anti-HBoV antibodies in children younger than 12 months of age correlates with population-based studies that demonstrate children younger than 1 year of age have a higher occurrence of infection.20, 29, 34 By 5 years of age, most people have circulating antibodies against HBoV-1, similar to other respiratory viruses, such as RSV,59 rhinovirus,60 and HMPV.61
Because of the difficulties of growing HBoV-1 in vitro, it has yet to be determined which antibodies offer protection. Also it is unclear if antibodies against HBoV-1 will confer protection against HBoV-2 or HBoV-3 and vice versa. Most protective antibodies are speculated to target the two viral capsid proteins VP1 and VP2. HBoV-2 and HBoV-3 both have approximately 80% AA identity with HBoV-1 in this region with most sequence diversion occurring in the N terminal portion. The high similarity of viral capsid proteins suggest cross-reactive antibodies between bocavirus species may occur. Studies on HBoV-2 and HBoV-3 seroepidemiology have not been performed thus far and are warranted.
Sequence Analysis of Bocavirus Species
As more bocavirus species are recognized, our understanding of this family of viruses grows. Based on the available sequence data of HBoV-1, there seem to be two closely related genotypes. The two prototype strains (ST1, DQ000495.1; ST2, NC_007455.1) have 99.5% identity at the nucleotide and protein level. Because the amino acid identity between both genotypes differs by so little, it may be assumed they represent one serotype. Analysis of 24 HBoV-1 unique isolates (12 originating from respiratory samples and 12 from fecal samples) demonstrated little sequence variation from the prototypic strains.39 There was also no difference in the proportion of each HBoV-1 genotype identified in nasopharyngeal and fecal isolates, suggesting that each lineage infects both respiratory and enteric systems.
The recent recognition of HBoV-2 and HBoV-3 demonstrates that the bocavirus genus has substantial diversity. HBoV-2 and HBoV-3 have similar genomic organization of the putative open reading frames (ORFs) to HBoV-1. Phylogenetic analysis of HBoV-2 ORFs show that it is more closely related to HBoV-1 (pairwise distance 22.2%–26.2%) than to the animal bocaviruses (pairwise distance 46.2%–55.8%).12 HBoV-3 nonstructural proteins NS1 and NP1 are closely related to HBoV-1, whereas the structural viral capsid proteins are more similar to HBoV-2 (see Fig. 1). Arthur and colleagues13 hypothesize that HBoV-3 may be a result of a distant recombination event between HBoV-1 and HBoV-2. Because only several strains of HBoV-2 and HBoV-3 have been recognized from a handful of regions worldwide, however, the true diversity is unclear.
Detection of human bocaviruses
Polymerase Chain Reaction
Following the discovery of human bocavirus, numerous studies have used conventional PCR to detect HBoV-1.14, 20, 24, 28, 29, 34, 35, 38, 39, 40, 41, 43, 48, 49, 56, 58, 60, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82 Primers, target genes, and predicted amplicon sizes are listed in Table 1. Most primer sets used for HBoV-1 screening target the NP1 gene. Although there is a wide variation in detection of HBoV-1 between study sites with detection rates as high as 45.2% reported in one study,72 primers targeting the VP1/VP2 region have a slightly higher detection rate (median 7.5%, see Table 1). This difference is not surprising because the VP1/VP2 is more conserved than NS or NP. In addition, nested and real-time PCR have been used to detect HBoV-1. Real-time PCR yields a slightly higher median frequency of detection of HBoV-1.15, 17, 21, 26, 30, 36, 37, 44, 51, 52, 54, 67, 68, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93 The variation in detection of HBoV-1 in these studies is likely attributable to multiple factors, including sensitivity of primer sets, laboratory technique, true variation in incidence of HBoV-1, and methods used in sample collection.
Ziegler and colleagues94 reported using nucleic acid sequence based amplification (NASBA) to screen for HBoV-1 in stool samples. NASBA is an isothermic nucleic acid amplification technique used as an alternative to PCR for the detection of viruses.95, 96 In the study by Ziegler and coworkers,94 no HBoV-1 was detected using NASBA. It is unclear if this represents a failure of the method, lack of HBoV-1 in the samples, reaction inhibitors to stool samples, or other variables.
An increasing number of studies are screening stool samples for the presence of HBoV-1.38, 39, 41, 42, 43, 44 Based on fecal and respiratory HBoV sequence similarities reported by Lau and colleagues genomic targets used for respiratory samples should be as successful in stool samples.39 The current literature finds that HBoV-1 is found in stool samples less often than in respiratory samples (median 2% versus 7.5%). It is unclear whether this lower rate of detection is due to a truly lower incidence of HBoV-1 in stool, decreased viral loads, or presence of reaction inhibitors commonly found in feces.
Cell Culture and Animal Models
To date, HBoV-1 has not been successfully propagated in standard cell lines or animal models. This failure has been one of the major impediments to our understanding of this virus. Parvoviruses have a large host range and tissue tropism, even among closely related strains. The viral capsid proteins seem to be the major determinant of host specificity.16 The related human parvovirus B19 causes viremia and replicates in the bone marrow of children and adults and in the liver of the fetus.16 Two studies identified HBoV-1 DNA in serum of affected patients. Fry and colleagues36 demonstrated HBoV-1 in acute serum from HBoV-1–positive children who had respiratory disease. Allander and colleagues15 detected HBoV-1 DNA in more than half of acute-phase serum samples and in 19% of the convalescent-phase serum specimens. Detection of HBoV-1 in serum was associated with a high viral load in the nasopharynx. Even with the detection of HBoV-1 DNA in the blood of affected patients, however, it remains unclear if HBoV-1 viremia truly occurs.
Therapy
Among healthy children, viral respiratory disease is often a self-limited and uncomplicated disease. Therapy against most viral respiratory pathogens has not been shown to be effective. Treatment of viral pathogens, such as influenza, may shorten the duration of illness in low-risk patients but has not been shown to reduce the duration of illness in the high-risk population.97 Treatment with ribavirin or intravenous immune globulin (IVIG) remain controversial in the setting of viral respiratory disease and are only used in select populations with severe RSV and human parainfluenza virus (HPIV) disease.98, 99
The relative ineffectiveness of antiviral compounds is, in part, because host inflammatory reaction leads to a substantial amount of pathology. In addition, many patients present for medical attention at a time past peak viral replication. For these reasons, it is difficult to demonstrate effectiveness of most antiviral compounds.
In several case reports, patients have received antiviral treatment either as prophylaxis or as treatment of confirmed or presumed viral infections. Schenck and colleagues22 reported a child who had undergone hematopoietic stem cell transplant who was receiving acyclovir prophylaxis; however, he subsequently developed symptomatic HBoV-1 disease with HBoV-1 DNA identified in respiratory, blood, and stool samples. During this patient's complicated hospital course, he was placed on ganciclovir and eventually foscarnet for cytomegalovirus (CMV) viremia. Both the CMV viral load and the HBoV-1 serum viral load became undetectable after initiating foscarnet. This patient continued to have HBoV-1 DNA detectable in stool samples, however. Kupfer and colleagues100 reported a patient undergoing treatment of B-cell lymphoma who received ganciclovir for CMV viremia who was also found to have HBoV-1 in respiratory secretions. There was no clinical improvement in symptoms after initiating ganciclovir.
Supportive care, even in the intensive care setting, plays a large role in the management of patients who have severe viral illness. Supplemental oxygen22 and mechanical ventilation101 have been used to support patients who have HBoV-1–associated disease. In one study, up to 28% of HBoV-1–positive children required intensive care, although not all required respiratory assistance.20 One patient, coinfected with RSV and HBoV-1, required support through extracorporeal membrane oxygenation until underlying cardiac physiology could be repaired.
There have been no comparative studies using antiviral agents for the treatment of human bocaviruses. The large percentage of coinfections associated with human bocavirus infections suggests that evaluation for further pathogens should be undertaken for any patient diagnosed with HBoV-1. Treatment of copathogens (ie, influenza) may lead to patient improvement. Future therapeutic strategies may include vaccines, HBoV-neutralizing antibodies, and small interfering RNA.
Discussion
Three human bocavirus species are now recognized and are reported in association with respiratory and gastrointestinal diseases. Because of wide variation in clinical presentations, severity of disease, and a high rate of coinfection, the role of the bocaviruses in causing disease has been debated. One consideration is that bocaviruses are passenger viruses with little or no clinical effect. Another possibility is that bocaviruses require the presence of a copathogen for infection. Several studies suggest that this is not the case, however. Bocavirus is identified in patients who have disease despite the absence of copathogens. Case-control studies have shown an increased risk for pneumonia in patients who have HBoV-1–positive respiratory secretions and increased risk for gastroenteritis in patients who have HBoV-2–positive fecal samples. Two studies found no increase in HBoV-1 frequency in symptomatic versus asymptomatic stool samples. One report finds HBoV-1 did not worsen gastrointestinal disease when coinfected with known pathogens such as rotavirus.13 The question of whether HBoV-2 or HBoV-3 is associated with respiratory infection has not been answered.
Little is known about the recently described bocaviruses HBoV-2 and HBoV-3. Clinical epidemiology and seroepidemiology have yet to be described in detail. Although antibodies to HBoV-1 are common in human sera, cross-reactivity with HBoV-2 and HBoV-3 has not been investigated. Furthermore, although HBoV-2 and HBoV-3 have been described in stool, their presence in the respiratory tract should be investigated.
Areas for further research on HBoV-1 include examining objective measures of disease severity in patients who have coinfections and examining HBoV-1 for evidence of disease distant to the respiratory and gastrointestinal tracts. Presence of HBoV-1 has been described in serum,22 lymphatic tissue,21 and in conjunction with elevated hepatic enzymes,46 the clinical significance of which has yet to be determined.
In summary, although the evidence for pathogenicity of human bocaviruses is increasing, more research is needed to determine severity of HBoV-associated disease and clinical outcomes.
References
- 1.Merrill C., Elixhauser A. Agency for Healthcare Research and Quality; Rockville (MD): 2005. Hospitalization in the United States, 2002: HCUP fact book no. 6. [Google Scholar]
- 2.Birnbaum H.G., Morley M., Greenberg P.E. Economic burden of respiratory infections in an employed population. Chest. 2002;122(2):603–611. doi: 10.1378/chest.122.2.603. [DOI] [PubMed] [Google Scholar]
- 3.van den Hoogen B.G., de Jong J.C., Groen J. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Hoek L., Pyrc K., Jebbink M.F. Identification of a new human coronavirus. Nat Med. 2004;10(4):368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fouchier R.A., Hartwig N.G., Bestebroer T.M. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci U S A. 2004;101(16):6212–6216. doi: 10.1073/pnas.0400762101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esper F., Weibel C., Ferguson D. Evidence of a novel human coronavirus that is associated with respiratory tract disease in infants and young children. J Infect Dis. 2005;191(4):492–498. doi: 10.1086/428138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woo P.C., Lau S.K., Chu C.M. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. 2005;79(2):884–895. doi: 10.1128/JVI.79.2.884-895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drosten C., Gunther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348(20):1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 9.Allander T., Andreasson K., Gupta S. Identification of a third human polyomavirus. J Virol. 2007;81(8):4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaynor A.M., Nissen M.D., Whiley D.M. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;3(5):e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allander T., Tammi M.T., Eriksson M. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A. 2005;102(36):12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapoor A., Slikas E., Simmonds P. A newly identified bocavirus species in human stool. J Infect Dis. 2009;199(2):196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arthur J.L., Higgins G.D., Davidson G.P. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5(4):e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastien N., Brandt K., Dust K. Human bocavirus infection, Canada. Emerg Infect Dis. 2006;12(5):848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allander T., Jartti T., Gupta S. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44(7):904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns K., Parrish C.R. Parvoviridae. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields' virology. 5th edition. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2437–2466. [Google Scholar]
- 17.Brieu N., Gay B., Segondy M. Electron microscopy observation of human bocavirus (HBoV) in nasopharyngeal samples from HBoV-infected children. J Clin Microbiol. 2007;45(10):3419–3420. doi: 10.1128/JCM.00545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindner J., Modrow S. Human bocavirus—a novel parvovirus to infect humans. Intervirology. 2008;51(2):116–122. doi: 10.1159/000137411. [DOI] [PubMed] [Google Scholar]
- 19.Zhi N., Mills I.P., Lu J. Molecular and functional analyses of a human parvovirus B19 infectious clone demonstrates essential roles for NS1, VP1, and the 11-kilodalton protein in virus replication and infectivity. J Virol. 2006;80(12):5941–5950. doi: 10.1128/JVI.02430-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chow B.D., Huang Y.T., Esper F.P. Evidence of human bocavirus circulating in children and adults, Cleveland, Ohio. J Clin Virol. 2008;43(3):302–306. doi: 10.1016/j.jcv.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X., Gooding L.R., Erdman D.D. Human bocavirus in tonsillar lymphocytes. Emerg Infect Dis. 2008;14(8):1332–1334. doi: 10.3201/eid1408.080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schenk T., Strahm B., Kontny U. Disseminated bocavirus infection after stem cell transplant. Emerg Infect Dis. 2007;13(9):1425–1427. doi: 10.3201/eid1309.070318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monteny M., Niesters H.G., Moll H.A. Human bocavirus in febrile children, The Netherlands. Emerg Infect Dis. 2007;13(1):180–182. doi: 10.3201/eid1301.060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacques J., Moret H., Renois F. Human bocavirus quantitative DNA detection in French children hospitalized for acute bronchiolitis. J Clin Virol. 2008;43(2):142–147. doi: 10.1016/j.jcv.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gendrel D., Guedj R., Pons-Catalano C. Human bocavirus in children with acute asthma. Clin Infect Dis. 2007;45(3):404–405. doi: 10.1086/519505. [DOI] [PubMed] [Google Scholar]
- 26.Bosis S., Esposito S., Niesters H.G. Role of respiratory pathogens in infants hospitalized for a first episode of wheezing and their impact on recurrences. Clin Microbiol Infect. 2008;14(7):677–684. doi: 10.1111/j.1469-0691.2008.02016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia M.L., Calvo C., Pozo F. Detection of human bocavirus in ill and healthy Spanish children: a 2-year study. Arch Dis Child. 2009;94(3):249. [Google Scholar]
- 28.Kaplan N.M., Dove W., Abu-Zeid A.F. Human bocavirus infection among children, Jordan. Emerg Infect Dis. 2006;12(9):1418–1420. doi: 10.3201/eid1209.060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold J.C., Singh K.K., Spector S.A. Human bocavirus: prevalence and clinical spectrum at a children's hospital. Clin Infect Dis. 2006;43(3):283–288. doi: 10.1086/505399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koskenvuo M., Mottonen M., Waris M. Human bocavirus in children with acute lymphoblastic leukemia. Eur J Pediatr. 2008;167(9):1011–1015. doi: 10.1007/s00431-007-0631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garbino J., Inoubli S., Mossdorf E. Respiratory viruses in HIV-infected patients with suspected respiratory opportunistic infection. AIDS. 2008;22(6):701–705. doi: 10.1097/QAD.0b013e3282f470ac. [DOI] [PubMed] [Google Scholar]
- 32.Muller A., Klinkenberg D., Vehreschild J. Low prevalence of human metapneumovirus and human bocavirus in adult immunocompromised high risk patients suspected to suffer from Pneumocystis pneumonia. J Infect. 2009;58(3):227–231. doi: 10.1016/j.jinf.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyakis S., van Hal S.J., Barratt J. Absence of human Bocavirus in bronchoalveolar lavage fluid of lung transplant patients. J Clin Virol. 2009;44(2):179–180. doi: 10.1016/j.jcv.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Kesebir D., Vazquez M., Weibel C. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J Infect Dis. 2006;194(9):1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maggi F., Andreoli E., Pifferi M. Human bocavirus in Italian patients with respiratory diseases. J Clin Virol. 2007;38(4):321–325. doi: 10.1016/j.jcv.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Fry A.M., Lu X., Chittaganpitch M. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195(7):1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.von Linstow M.L., Hogh M., Hogh B. Clinical and epidemiologic characteristics of human bocavirus in Danish infants: results from a prospective birth cohort study. Pediatr Infect Dis J. 2008;27(10):897–902. doi: 10.1097/INF.0b013e3181757b16. [DOI] [PubMed] [Google Scholar]
- 38.Chieochansin T., Thongmee C., Vimolket L. Human bocavirus infection in children with acute gastroenteritis and healthy controls. Jpn J Infect Dis. 2008;61(6):479–481. [PubMed] [Google Scholar]
- 39.Lau S.K., Yip C.C., Que T.L. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196(7):986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee J.I., Chung J.Y., Han T.H. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. J Infect Dis. 2007;196(7):994–997. doi: 10.1086/521366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albuquerque M.C., Rocha L.N., Benati F.J. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis. 2007;13(11):1756–1758. doi: 10.3201/eid1311.060671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng W.X., Jin Y., Duan Z.J. Human bocavirus in children hospitalized for acute gastroenteritis: a case-control study. Clin Infect Dis. 2008;47(2):161–167. doi: 10.1086/589244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J.M., Li D.D., Xu Z.Q. Human bocavirus infection in children hospitalized with acute gastroenteritis in China. J Clin Virol. 2008;42(3):280–285. doi: 10.1016/j.jcv.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Campe H., Hartberger C., Sing A. Role of Human Bocavirus infections in outbreaks of gastroenteritis. J Clin Virol. 2008;43(3):340–342. doi: 10.1016/j.jcv.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Schildgen O., Muller A., Simon A. Human bocavirus and gastroenteritis. Emerg Infect Dis. 2007;13(10):1620–1621. doi: 10.3201/eid1310.070436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kainulainen L., Waris M., Soderlund-Venermo M. Hepatitis and human bocavirus primary infection in a child with T-cell deficiency. J Clin Microbiol. 2008;46(12):4104–4105. doi: 10.1128/JCM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bastien N., Chui N., Robinson J.L. Detection of human bocavirus in Canadian children in a 1-year study. J Clin Microbiol. 2007;45(2):610–613. doi: 10.1128/JCM.01044-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Canducci F., Debiaggi M., Sampaolo M. Two-year prospective study of single infections and co-infections by respiratory syncytial virus and viruses identified recently in infants with acute respiratory disease. J Med Virol. 2008;80(4):716–723. doi: 10.1002/jmv.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung J.Y., Han T.H., Kim C.K. Bocavirus infection in hospitalized children, South Korea. Emerg Infect Dis. 2006;12(8):1254–1256. doi: 10.3201/eid1208.060261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manning A., Russell V., Eastick K. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194(9):1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brieu N., Guyon G., Rodiere M. Human bocavirus infection in children with respiratory tract disease. Pediatr Infect Dis J. 2008;27(11):969–973. doi: 10.1097/INF.0b013e31817acfaa. [DOI] [PubMed] [Google Scholar]
- 52.Catalano-Pons C., Bue M., Laude H. Human bocavirus infection in hospitalized children during winter. Pediatr Infect Dis J. 2007;26(10):959–960. doi: 10.1097/INF.0b013e3181256583. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Garcia M.L., Calvo C., Pozo F. Human bocavirus detection in nasopharyngeal aspirates of children without clinical symptoms of respiratory infection. Pediatr Infect Dis J. 2008;27(4):358–360. doi: 10.1097/INF.0b013e3181626d2a. [DOI] [PubMed] [Google Scholar]
- 54.Longtin J., Bastien M., Gilca R. Human bocavirus infections in hospitalized children and adults. Emerg Infect Dis. 2008;14(2):217–221. doi: 10.3201/eid1402.070851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zheng M.Q., Lin F., Zheng M.Y. [Clinical prospective study on maternal-fetal transmission of human bocavirus]Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2007;21(4):331–333. [PubMed] [Google Scholar]
- 56.Choi E.H., Lee H.J., Kim S.J. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43(5):585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kahn J.S., Kesebir D., Cotmore S.F. Seroepidemiology of human bocavirus defined using recombinant virus-like particles. J Infect Dis. 2008;198(1):41–50. doi: 10.1086/588674. [DOI] [PubMed] [Google Scholar]
- 58.Endo R., Ishiguro N., Kikuta H. Seroepidemiology of human bocavirus in Hokkaido prefecture, Japan. J Clin Microbiol. 2007;45(10):3218–3223. doi: 10.1128/JCM.02140-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilchrist S., Torok T.J., Gary H.E., Jr. National surveillance for respiratory syncytial virus, United States, 1985–1990. J Infect Dis. 1994;170(4):986–990. doi: 10.1093/infdis/170.4.986. [DOI] [PubMed] [Google Scholar]
- 60.Arden K.E., McErlean P., Nissen M.D. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol. 2006;78(9):1232–1240. doi: 10.1002/jmv.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Esper F., Martinello R.A., Boucher D., et al A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189(8):1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon A., Groneck P., Kupfer B. Detection of bocavirus DNA in nasopharyngeal aspirates of a child with bronchiolitis. J Infect. 2007;54(3):e125–e127. doi: 10.1016/j.jinf.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Volz S., Schildgen O., Klinkenberg D. Prospective study of Human Bocavirus (HBoV) infection in a pediatric university hospital in Germany 2005/2006. J Clin Virol. 2007;40(3):229–235. doi: 10.1016/j.jcv.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chieochansin T., Chutinimitkul S., Payungporn S. Complete coding sequences and phylogenetic analysis of Human Bocavirus (HBoV) Virus Res. 2007;129(1):54–57. doi: 10.1016/j.virusres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 65.Chieochansin T., Samransamruajkit R., Chutinimitkul S. Human bocavirus (HBoV) in Thailand: clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J Infect. 2008;56(2):137–142. doi: 10.1016/j.jinf.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cilla G., Onate E., Perez-Yarza E.G. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J Med Virol. 2008;80(10):1843–1849. doi: 10.1002/jmv.21271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Foulongne V., Olejnik Y., Perez V. Human bocavirus in French children. Emerg Infect Dis. 2006;12(8):1251–1253. doi: 10.3201/eid1208.060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foulongne V., Rodiere M., Segondy M. Human Bocavirus in children. Emerg Infect Dis. 2006;12(5):862–863. doi: 10.3201/eid1205.051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.IP M., Nelson E.A., Cheuk E.S. Pediatric hospitalization of acute respiratory tract infections with Human Bocavirus in Hong Kong. J Clin Virol. 2008;42(1):72–74. doi: 10.1016/j.jcv.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 70.Ma X., Endo R., Ishiguro N. Detection of human bocavirus in Japanese children with lower respiratory tract infections. J Clin Microbiol. 2006;44(3):1132–1134. doi: 10.1128/JCM.44.3.1132-1134.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naghipour M., Cuevas L.E., Bakhshinejad T. Human bocavirus in Iranian children with acute respiratory infections. J Med Virol. 2007;79(5):539–543. doi: 10.1002/jmv.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neske F., Blessing K., Tollmann F. Real-time PCR for diagnosis of human bocavirus infections and phylogenetic analysis. J Clin Microbiol. 2007;45(7):2116–2122. doi: 10.1128/JCM.00027-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redshaw N., Wood C., Rich F. Human bocavirus in infants, New Zealand. Emerg Infect Dis. 2007;13(11):1797–1799. doi: 10.3201/eid1311.070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vicente D., Cilla G., Montes M. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13(4):636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weissbrich B., Neske F., Schubert J. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;6:109. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Terrosi C., Fabbiani M., Cellesi C. Human bocavirus detection in an atopic child affected by pneumonia associated with wheezing. J Clin Virol. 2007;40(1):43–45. doi: 10.1016/j.jcv.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L.L., Tang L.Y., Xie Z.D. Human bocavirus in children suffering from acute lower respiratory tract infection in Beijing Children's Hospital. Chin Med J (Engl) 2008;121(17):1607–1610. [PubMed] [Google Scholar]
- 78.Christensen A., Nordbo S.A., Krokstad S. Human bocavirus commonly involved in multiple viral airway infections. J Clin Virol. 2008;41(1):34–37. doi: 10.1016/j.jcv.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleines M., Scheithauer S., Rackowitz A. High prevalence of human bocavirus detected in young children with severe acute lower respiratory tract disease by use of a standard PCR protocol and a novel real-time PCR protocol. J Clin Microbiol. 2007;45(3):1032–1034. doi: 10.1128/JCM.01884-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung J.Y., Han T.H., Kim S.W. Detection of viruses identified recently in children with acute wheezing. J Med Virol. 2007;79(8):1238–1243. doi: 10.1002/jmv.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sloots T.P., McErlean P., Speicher D.J. Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol. 2006;35(1):99–102. doi: 10.1016/j.jcv.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan B.H., Lim E.A., Seah S.G. The incidence of human bocavirus infection among children admitted to hospital in Singapore. J Med Virol. 2009;81(1):82–89. doi: 10.1002/jmv.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bonzel L., Tenenbaum T., Schroten H. Frequent detection of viral coinfection in children hospitalized with acute respiratory tract infection using a real-time polymerase chain reaction. Pediatr Infect Dis J. 2008;27(7):589–594. doi: 10.1097/INF.0b013e3181694fb9. [DOI] [PubMed] [Google Scholar]
- 84.Catalano-Pons C., Giraud C., Rozenberg F. Detection of human bocavirus in children with Kawasaki disease. Clin Microbiol Infect. 2007;13(12):1220–1222. doi: 10.1111/j.1469-0691.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 85.Lu X., Chittaganpitch M., Olsen S.J. Real-time PCR assays for detection of bocavirus in human specimens. J Clin Microbiol. 2006;44(9):3231–3235. doi: 10.1128/JCM.00889-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hindiyeh M.Y., Keller N., Mandelboim M. High rate of human bocavirus and adenovirus coinfection in hospitalized Israeli children. J Clin Microbiol. 2008;46(1):334–337. doi: 10.1128/JCM.01618-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hamano-Hasegawa K., Morozumi M., Nakayama E. Comprehensive detection of causative pathogens using real-time PCR to diagnose pediatric community-acquired pneumonia. J Infect Chemother. 2008;14(6):424–432. doi: 10.1007/s10156-008-0648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koskenvuo M., Mottonen M., Rahiala J. Respiratory viral infections in children with leukemia. Pediatr Infect Dis J. 2008;27(11):974–980. doi: 10.1097/INF.0b013e31817b0799. [DOI] [PubMed] [Google Scholar]
- 89.Regamey N., Frey U., Deffernez C. Isolation of human bocavirus from Swiss infants with respiratory infections. Pediatr Infect Dis J. 2007;26(2):177–179. doi: 10.1097/01.inf.0000250623.43107.bc. [DOI] [PubMed] [Google Scholar]
- 90.Qu X.W., Duan Z.J., Qi Z.Y. Human bocavirus infection, People's Republic of China. Emerg Infect Dis. 2007;13(1):165–168. doi: 10.3201/eid1301.060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schenk T., Huck B., Forster J. Human bocavirus DNA detected by quantitative real-time PCR in two children hospitalized for lower respiratory tract infection. Eur J Clin Microbiol Infect Dis. 2007;26(2):147–149. doi: 10.1007/s10096-006-0244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Regamey N., Kaiser L., Roiha H.L. Viral etiology of acute respiratory infections with cough in infancy: a community-based birth cohort study. Pediatr Infect Dis J. 2008;27(2):100–105. doi: 10.1097/INF.0b013e31815922c8. [DOI] [PubMed] [Google Scholar]
- 93.Esposito S., Bosis S., Niesters H.G. Impact of human bocavirus on children and their families. J Clin Microbiol. 2008;46(4):1337–1342. doi: 10.1128/JCM.02160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ziegler S., Tillmann R.L., Muller A. No gastroenteric Bocavirus in high risk patients stool samples. J Clin Virol. 2008;43(3):349–350. doi: 10.1016/j.jcv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Tillmann R.L., Simon A., Muller A. Sensitive commercial NASBA assay for the detection of respiratory syncytial virus in clinical specimen. PLoS ONE. 2007;2(12):e1357. doi: 10.1371/journal.pone.0001357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moore C., Corden S., Sinha J. Dry cotton or flocked respiratory swabs as a simple collection technique for the molecular detection of respiratory viruses using real-time NASBA. J Virol Methods. 2008;153(2):84–89. doi: 10.1016/j.jviromet.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 97.Williams J.M. 2009 update in prevention, evaluation, and outpatient treatment of influenza. Curr Med Res Opin. 2009;25(4):817–828. doi: 10.1185/03007990802708244. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez W.J., Gruber W.C., Welliver R.C. Respiratory syncytial virus (RSV) immune globulin intravenous therapy for RSV lower respiratory tract infection in infants and young children at high risk for severe RSV infections: Respiratory Syncytial Virus Immune Globulin Study Group. Pediatrics. 1997;99(3):454–461. doi: 10.1542/peds.99.3.454. [DOI] [PubMed] [Google Scholar]
- 99.Orange J.S., Hossny E.M., Weiler C.R. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(Suppl 4):S525–S553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 100.Kupfer B., Vehreschild J., Cornely O. Severe pneumonia and human bocavirus in adult. Emerg Infect Dis. 2006;12(10):1614–1616. doi: 10.3201/eid1210.060520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Calvo C., Garcia-Garcia M.L., Blanco C. Human bocavirus infection in a neonatal intensive care unit. J Infect. 2008;57(3):269–271. doi: 10.1016/j.jinf.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Arnold J.C., Singh K.K., Spector S.A. Undiagnosed respiratory viruses in children. Pediatrics. 2008;121(3):e631–e637. doi: 10.1542/peds.2006-3073. [DOI] [PubMed] [Google Scholar]
- 103.Fabbiani M., Terrosi C., Martorelli B. Epidemiological and clinical study of viral respiratory tract infections in children from Italy. J Med Virol. 2009;81(4):750–756. doi: 10.1002/jmv.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lin J.H., Chiu S.C., Lin Y.C. Clinical and genetic analysis of Human Bocavirus in children with lower respiratory tract infection in Taiwan. J Clin Virol. 2009;44(3):219–224. doi: 10.1016/j.jcv.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Norja P., Ubillos I., Templeton K. No evidence for an association between infections with WU and KI polyomaviruses and respiratory disease. J Clin Virol. 2007;40(4):307–311. doi: 10.1016/j.jcv.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Villa L., Melon S., Suarez S. Detection of human bocavirus in Asturias, Northern Spain. Eur J Clin Microbiol Infect Dis. 2008;27(3):237–239. doi: 10.1007/s10096-007-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smuts H., Hardie D. Human bocavirus in hospitalized children, South Africa. Emerg Infect Dis. 2006;12(9):1457–1458. doi: 10.3201/eid1209.051616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smuts H., Workman L., Zar H.J. Role of human metapneumovirus, human coronavirus NL63 and human bocavirus in infants and young children with acute wheezing. J Med Virol. 2008;80(5):906–912. doi: 10.1002/jmv.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]