Abstract
Ethnopharmacological relevance
The radices of Glycyrrhiza uralensis Fisch. and herbal preparations containing Glycyrrhiza spp. have been used for thousands of years as an herbal medicine for the treatment of viral induced cough, viral hepatitis, and viral skin diseases like ulcers in China. Glycyrrhizic acid (GA) is considered the principal component in Glycyrrhiza spp. with a wide spectrum of antiviral activity.
Aim
The present study attempt to validate the medicinal use of Glycyrrhiza uralensis for hand, foot and mouth disease (HFMD) and further to verify whether GA is an active antiviral component in the water extract of Glycyrrhiza uralensis.
Materials and methods
Radices of Glycyrrhiza uralensis Fisch. were extracted with hot water. The chemical contents of the extract were profiled with HPLC analysis. The antiviral activity of the extract and the major components was evaluated against infection of enterovirus 71 (EV71) and coxsackievirus A16 (CVA16) on Vero cells. The cytopathic effect caused by the infection was measured with MTT assay. Infectious virion production was determined using secondary infection assays and viral protein expression by immunoblotting analysis.
Results
The extract at 1000 μg/ml suppressed EV71 replication by 1.0 log and CVA16 by 1.5 logs. The antiviral activity was associated with the content of GA in the extract since selective depletion of GA from the extract by acid precipitation resulted in loss of antiviral activity. In contrast, the acid precipitant retained antiviral activity. The precipitant at a concentration of 200 μg/ml inhibited EV71 and CVA16 replication by 1.7 and 2.2 logs, respectively. Furthermore, GA dose-dependently blocked viral replication of EV71 and CVA16. At 3 mM, GA reduced infectious CVA16 and EV71 production by 3.5 and 2.2 logs, respectively. At 5 mM, CVA16 production was reduced by 6.0 logs and EV71 by 4.0 logs. Both EV71 and CVA16 are members of Enterovirus genus, time-of-drug addition studies however showed that GA directly inactivated CVA16, while GA anti-EV71 effect was associated with an event(s) post virus cell entry.
Conclusions
This study validated the medicinal usefulness of radices Glycyrrhiza uralensis against the etiological agents of HFMD. In addition to the identification of GA as the antiviral component of Glycyrrhiza uralensis against EV71 and CVA16 infection, this study also reveals that GA inhibits EV71 and CVA16 with distinct mechanisms.
Keywords: Glycyrrhiza uralensis, Glycyrrhizic acid, Hand foot and mouth disease, Enterovirus 71, Coxsackievirus A16
1. Introduction
Hand, foot and mouth disease (HFMD) is a common viral illness that usually affects infants and young children of 5 years old or younger. The clinical features of HFMD typically begin with mild fever, sore throat, poor appetite, and diarrhea. A day or two later, blisters form in the mouth and a rash develops on the cheeks, gums, and the tongue, potentially with life-threatening neurological complications, such as encephalitis. The causative agents of HFMD have been identified as coxsackievirus A16 (CVA16) and enterovirus 71 (EV71) viruses, accounting for more than 70% of the cases during the 2008 outbreak in China (WHO, 2008). CVA16 and EV71 are single strand RNA viruses of the Enterovirus genus. The viruses can be spread through direct contact with blisters and other surfaces contaminated with virus-containing fluids or through fecal–oral route. HFMD occurs sporadically around the world since its first report in 1957. Large outbreaks of HFMD with higher morbidity and mortality have become common in the Asia Pacific region. An outbreak in 2008 affected nearly half million children with 126 deaths in eastern China (Yang et al., 2009). Data reported to the Ministry of Public Health of China showed that more than one million cases annually and hundreds of deaths associated with HFMD in China for the past 3 years (www.moh.gov.cn). There is no vaccine or specific antiviral drugs available for HFMD. Good hygiene, including hand washing and disinfection of surfaces in child care facilities, therefore remains as the most effective approach to reduce the transmission rate of HFMD (Solomon et al., 2010, Ma et al., 2011).
The radices of Glycyrrhiza spp., commonly known as “Gan Cao” in Chinese and licorice in English, have been used for thousands of years as an herbal medicine for the treatment of sore throat, cough, bronchitis, peptic ulcers (Das et al., 1989, Krausse et al., 2004), arthritis, and allergic diseases (Fiore et al., 2005, Asl and Hosseinzadeh, 2008, Kim et al., 2010). The plant extract has also been used as flavoring agents for food and beverages with a relative safe profile. During the outbreaks of HFMD in China, medicinal herbs or herbal preparations have demonstrated therapeutic efficacy by ameliorating the symptoms of the disease and/or shortening the course of the disease. Most of the herbs with reported therapeutic effectiveness have been used traditionally or folklorically for inflammatory and/or infective diseases (Xue et al., 2011, Cao et al., 2012).
Glycyrrhiza spp. have been reported with broad antiviral activity (Fiore et al., 2008) and glycyrrhizic acid (GA, also known as glycyrrhizin, glycyrrhizinic acid), the major bioactive component of Glycyrrhiza spp. has demonstrated activity against DNA and RNA viruses. GA at millimolar concentrations inhibits growth and cytopathology of several unrelated viruses, while not affecting cell activity and ability to replicate (Pompei et al., 1979). Subsequent studies have shown the antiviral activity of GA against several enveloped viruses, including HIV, and SARS related coronavirus (Cinatl et al., 2003, Hoever et al., 2005), Kaposi's Sarcoma-Associated herpesvirus (Curreli et al., 2005, Kang and Lieberman, 2011), hepatitis C virus (Ashfaq et al., 2011), respiratory syncytial virus, vaccinia virus, Epstein-Barr virus, and vesicular stomatitis virus (Fiore et al., 2005, Fiore et al., 2008, Pompei et al., 2009). The compound has not previously been reported to inhibit the infection by nonenveloped viruses.
Some of the plants like Glycyrrhiza uralensis and Houttuynia cordata have been demonstrated with strong antiviral activity against EV71 infection (Kuo et al., 2009, Lin et al., 2009). However, the antiviral activity against CVA16 infection and the active components in those herbs are still undetermined. In this study, we aimed to assess the anti-EV71 and CVA16 infection activity of the water extract of Glycyrrhiza uralensis, and further determine whether antiviral activity of Glycyrrhiza uralensis is attributable to GA.
2. Materials and methods
2.1. Chemicals and antibodies
Antibodies to EV71 VP1 protein were purchased from Abnova (Taiwan), to human GAPDH from Bioworld Technology (Minneapolis, MN), and horse radish peroxidase (HRP) conjugated secondary antibodies from Sigma (St. Lois, MO). Chemicals including acyclovir (ACV), 18β-glycyrrhetinic acid (β–GA), ammonium glycyrrhizate, and methyl thiazolyl diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO). Liquiritin was from Chengdu Biopurify Pharmaceuticals (Chengdu). Chemiluminescent (ECL) reagent kit with enhanced detection sensitivity was purchased from Thermo Fisher (Pittsburgh, PA).
2.2. Cells and virus
African green monkey kidney Vero cells were purchased from Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen), supplemented with 10% fetal bovine serum (FBS, Invitrogen), 100 U/ml of penicillin and streptomycin, and 2 mM l-glutamine.
CVA16 was kindly provided by Dr. C. Zheng of Wuhan University. EV71 was a clinical isolate of the C4 subgenotype by VP1 sequence analysis (Zhang et al., 2010). The viruses were propagated in Vero cells and virus titers were determined on Vero cells by measuring 50% tissue culture infective dose (TCID50) for EV71 and by a plaque forming assay for CVA16.
2.3. Preparation of water extract of Glycyrrhiza uralensis
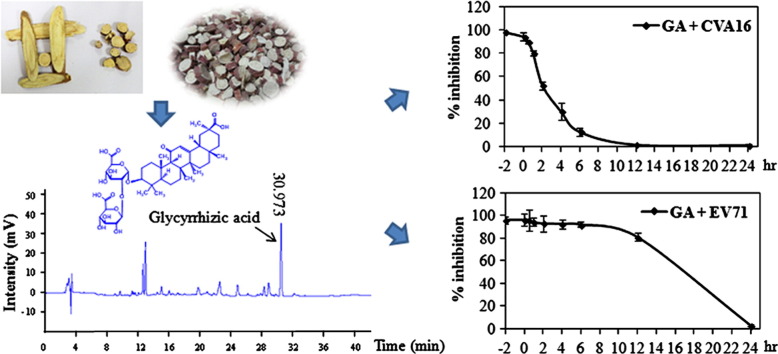
Slices of radices of Glycyrrhiza uralensis Fisch. were purchased from local drug stores in Nanjing and identified by Ms. Yunxia Xu (Research Assistant, Nanjing University) and with HPLC profiling following instructions in Chinese Pharmacopeia ( Fig. 1). Voucher specimens (2011-18-1 to -5) were stored at Laboratory of Microbial Science and Pharmacy, School of Medicine, Nanjing University. The materials were ground and extracted with boiling water as described in Kuo et al. (2009). After adjusting the pH of the liquid (∼6) with diluted NaOH, the liquid was filtered and tested for antiviral activities or lyophilized for future studies. The lyophilized powder was stored at −70 °C and reconstituted in de-ionized water (diH2O) prior to experiments.
Fig. 1.
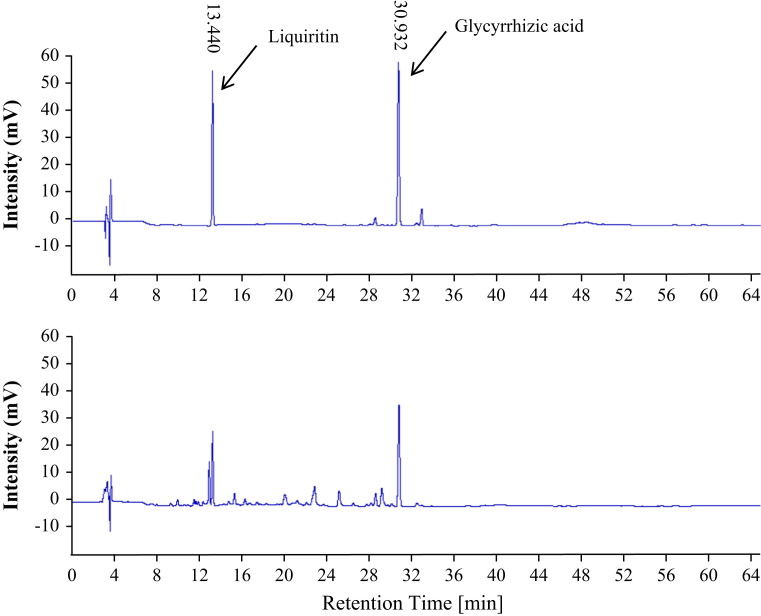
HPLC profiling of the water extract of Glycyrrhiza uralensis. The radices of Glycyrrhiza uralensis were ground and extracted with diH2O. The extract was profiled on a BosChroma ODS-AQ column (4.6×150 mm, 5 mm particle size), using acetonitrile and H2O containing 0.1% trifluoroacetic acid (32:68) as a mobile phase. Liquiritin and glycyrrhizic acid were detected at 13.440 and 30.932 min with a UV detector. Upper panel is HPLC chromatogram of standard compounds; lower panel is a sample of Glycyrrhiza uralensis water extract.
To deplete GA, the pH of extract was adjusted to pH 1.5 with 2 M H2SO4 to precipitate GA at 4 °C for overnight. The precipitant and the supernatant were collected by filtration. The precipitant was rinsed with cold water for two times and then lyophilized. Additionally, the supernatant was also lyophilized after adjusting pH with diluted NaOH. Both the precipitant and supernatant were reconstituted in diH2O prior to experiments. Acid precipitation to concentrate glycyrrhizic acid from the water extract is effective (Lu et al., 2006) and is a standard procedure recommended by the US Food and Drug Administration (CFR Title 21, Sec. 184.1408 Licorice and licorice derivatives).
2.4. HPLC profiling and quantitative determination of the chemical components in the extracts
The identity of the water extract was characterized by HPLC profiling for glycyrrhizic acid and liquiritin as specified in Chinese Pharmacopeia. A BosChroma ODS-AQ column (4.6×150 mm, 5 mm particle size) and Acetonitrile–H2O containing 0.1% trifluoroacetic acid (32:68) as a mobile phase were used. The components were monitored at 248 nm under a UV detector. Ammonium glycyrrhizate and liquiritin were used as standards for HPLC studies.
2.5. Infection assays
Vero cells in triplicate or duplicate were infected with EV71 or CVA16 virus at a multiplicity of infection (MOI) of 0.3. To test the inhibitory effect of a compound, a stock solution of a testing sample in PBS or in DMSO or solvent control (DMSO at 0.1%) was added to Vero cells for 2 h before infection. The compound was left in medium throughout the infection assays. The cytopathic effect caused by virus infection was quantitatively measured using MTT assay (Mosmann, 1983) at approximately 72 h post-infection (PI). An inhibition rate was calculated as a percentage of (ODtreated−ODinfected) over (ODuninfected−ODinfected). Alternatively, the cells were fixed with 3% paraformaldehyde and stained with 0.5% crystal violet prior to photograph with a digital camera.
For secondary infection assays, Vero cells were pretreated with or without an extract or GA for 2 h and then infected with CVA16 or EV71 at an MOI of 0.3. The cells and culture supernatants were collected at 48 h PI and freeze-thawed in liquid nitrogen to release infectious virus. Series-dilution of the supernatants was tested on Vero cells as described (Liu et al., 2011). The TCID50 numbers were calculated using the method of Reed and Muench (1938) and the plaque forming unit (PFU) numbers were calculated after enumeration of the samples with 10–100 plaques.
In time-of-drug addition experiment, Vero cells were infected with CVA16 or EV71 at 0.3 MOI. GA at 5 mM was added at 2 h prior to infection, during the infection or at varying times post infection. GA was left in the medium throughout the infection assay. The cytopathic effect was determined by measuring cell viability using MTT assay at 72 h PI.
Virucidal activity assay was carried out to determine whether GA directly inactivated the viruses, CVA16 or EV71 was resuspended in 100 μl culture medium, and treated with GA at 0, 3, and 5 mM in a 37 °C water bath for 60 min. Aliquots of 10 μl treated virus were used to infect Vero cells (MOI was at 0.3, but final concentrations of GA were at 0.03 and 0.05 mM). The samples were harvested at 48 h PI for titration of infectious viruses with a secondary infection assay.
2.6. Western blot assay
Vero cells were infected with EV71 at an MOI of 0.3 or 1.0 for 48 h in the presence or absence of GA. The cells were lysed by a lysis buffer containing 150 mM NaCl, 50 mM Tris–HCl (pH 7.4), 1% NP-40, and a cocktail of protease inhibitors (Roche). The cell lysates were then subjected to separation by SDS-PAGE. The proteins were transferred to PVDF membrane (Millipore). EV71 VP1 in the samples was detected by incubation with an antibody against VP1 protein, followed by HRP-conjugated secondary antibody and ECL reagent kit. GAPDH was used as a loading control. The images were captured using Alpha Innotech Flour Chem-FC2 imaging system (San Leandro, CA).
2.7. Determination of maximum non-cytotoxic concentrations of testing compounds
The maximum non-cytotoxic concentrations of testing compounds were determined on Vero cells by incubating Vero cells with a testing compound at varying concentrations for 72 h. The maximum non-cytotoxic concentration was defined as that at which no significant reduction of cell viability was determined by MTT assay.
3. Results
3.1. Evaluation of Glycyrrhiza uralensis water extract against CVA16 and EV71 infections
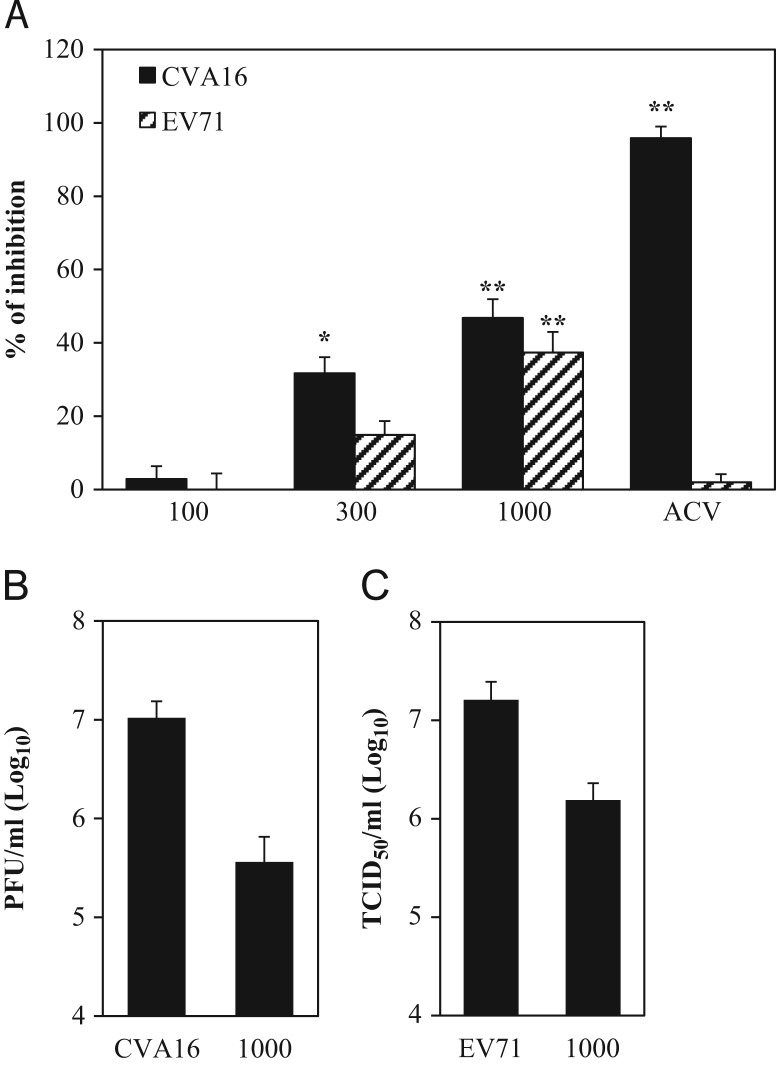
To investigate the antiviral effect of Glycyrrhiza uralensis, water extracts of Glycyrrhiza uralensis were prepared and tested for their antiviral activity against EV71 and CVA16 infections in cell culture system. The quality of the extracts was validated by HPLC analysis (Fig. 1). The content of GA and liquiritin in the extract used for antiviral studies was determined at 3.20% and 0.75%, exceeding the specified amounts in Chinese Pharmacopeia. When tested for its antiviral activity, we found the extract at 300 and 1000 μg/ml, but not 100 μg/ml, significantly blocked the cytopathic effect caused by CVA16 and EV71 infections. At 300 and 1000 μg/ml, the extract blocked CVA16 infection by 31.7%±4.4 and 46.8%±5.1, respectively ( Fig. 2A). The anti-EV71 effect of the extract was less potent but became significant at 1000 μg/ml. In addition, the antiviral activity was verified with secondary infection assays to titrate infectious virion production in samples treated with 1000 μg/ml of the extract. More than a log less of infectious virions in those samples were detected (Fig. 2B and C), indicating the water extract of Glycyrrhiza uralensis possessed antiviral activity.
Fig. 2.
Evaluation of antiviral activity of a water extract of Glycyrrhiza uralensis against coxsackievirus A16 and enterovirus 71. (A) Antiviral activity of Glycyrrhiza uralensis water extract. Vero cells in triplicate samples were pretreated with the water extract of Glycyrrhiza uralensis at 100, 300, and 1000 μg/ml for 2 h or mock treated, the cells were then infected with CVA16 or EV71 (both at MOI=0.3) for 72 h. Cytopathic effect due to virus infection was determined with an MTT assay. The inhibition rates, presented as a percentage, were calculated as described in Section 2. Acyclovir (ACV) at 10 μM was used as an antiviral positive control for CVA16. Statistical significance was determined by one-way ANOVA (* denotes P<0.05 and ** denotes P<0.01). (B, C) Glycyrrhiza uralensis water extract treatment reduces infectious virion production. Vero cells were treated with the extract at 1000 μg/ml or mock treated for 2 h. The cells were then infected with CVA16 (B) or EV71 (C). The infected cells and the culture supernatants were harvested at 48 h PI. Infectious virions in those samples were titrated with secondary infection assays. Data are presented as mean±standard errors of triplicate samples. The results are representative of three independent experiments.
3.2. The water extract is devoid of antiviral activity after depletion of GA by acid precipitation
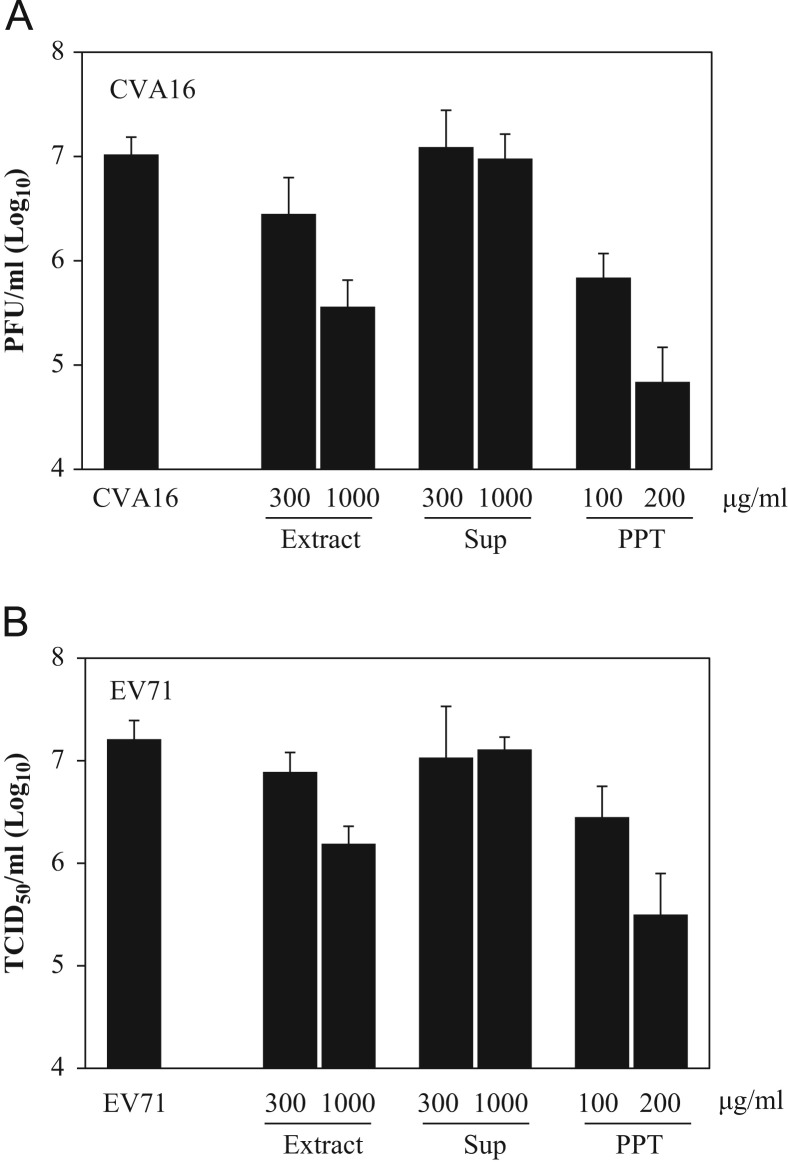
We therefore focused our attention on the identification of antiviral components in the water extract of Glycyrrhiza uralensis. The chemical components of Glycyrrhiza spp. have been well investigated and are relatively clear. Plants in the genus contain triterpenoid saponins such as glycyrrhizic acid, dihydroflavonoids like liquiritin, and polysaccharides (Asl and Hosseinzadeh, 2008), with GA as the principal component for antiviral activities. GA has three carboxylic acid groups and can be selectively precipitated by acidification of an aqueous solution. To preliminarily determine whether GA was responsible for Glycyrrhiza uralensis antiviral activity, we precipitate GA from water extract of Glycyrrhiza uralensis with H2SO4 and detected the antiviral activity of precipitant and supernatant. Consistent with GA as an antiviral component, depletion of GA by acid precipitation obviated the antiviral activity of the extract ( Fig. 3A and B). As expected, the acidic precipitant which has GA content significantly enriched showed strong antiviral activity against both CVA16 and EV71. At 200 μg/ml, the precipitant blocked infectious CVA16 and EV71 production by 2.2 and 1.7 logs, respectively, suggesting GA as a primary antiviral component of Glycyrrhiza uralensis.
Fig. 3.
The water extract is devoid of antiviral activity after depletion of glycyrrhizic acid by acid precipitation; whereas the precipitant has improved antiviral activity. The pH of the water extract of Glycyrrhiza uralensis was lowered with dilute H2SO4 to precipitate glycyrrhizic acid using a standard protocol. The precipitant was collected by filtration. After neutralizing the pH of the supernatant devoid of glycyrrhizic acid, the antiviral activity against CVA16 (A) and EV71 (B) of this supernatant (Sup), the precipitant (PPT), and the water extract of Glycyrrhiza uralensis (Extract) was assayed on Vero cells. Acid precipitation removes glycyrrhizic acid from the water extract of Glycyrrhiza uralensis, resulting in loss of antiviral activity of the supernatant. As expected, the antiviral activity was enriched in the precipitant. Data are presented as mean±standard errors of triplicate samples from three independent experiments.
3.3. Inhibition of virus production and viral protein expression by glycyrrhizic acid
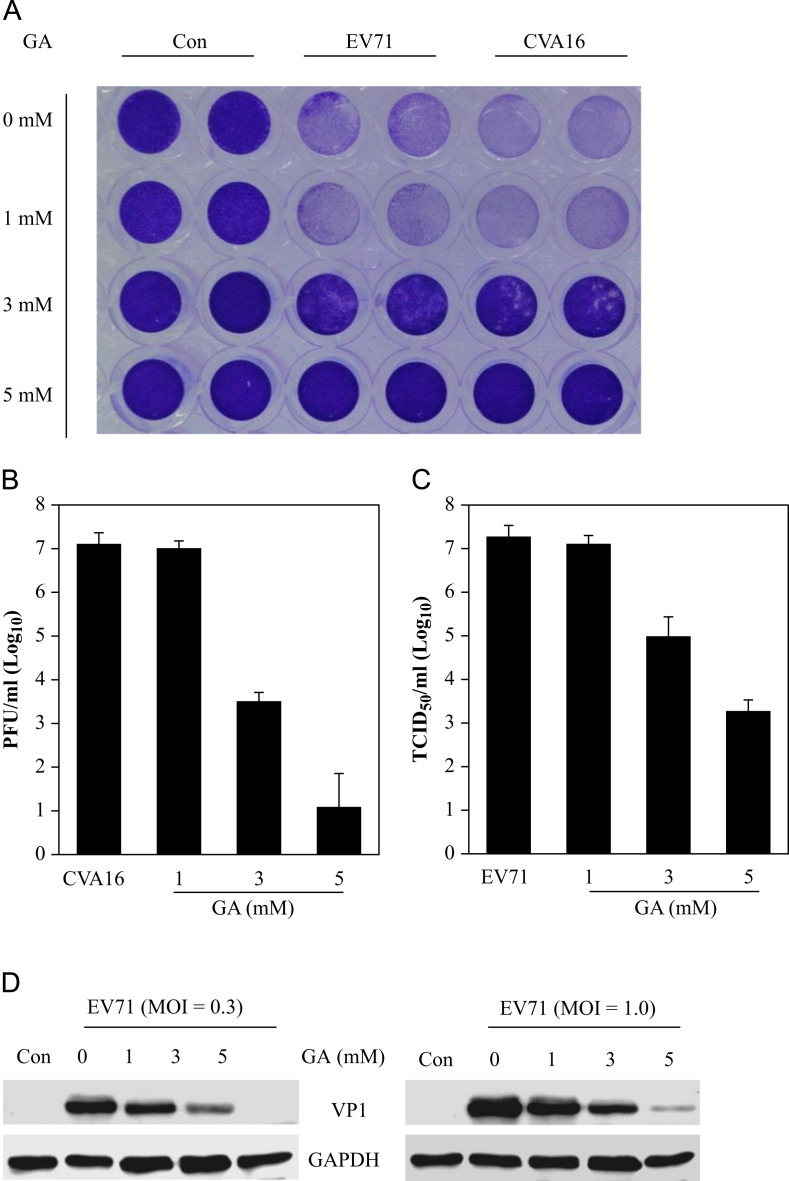
To confirm whether GA was the active component of Glycyrrhiza uralensis against CVA16 and EV71 infections, we first have determined the maximum nontoxic concentration of GA in Vero cells was 7 mM. The antiviral studies showed that treatment with GA at 3 and 5 mM concentrations noticeably suppressed the cytopathic effect associated with CVA16 and EV71 infections at 72 h ( Fig. 4A).
Fig. 4.
Inhibition of coxsackievirus A16 and enterovirus 71 infection by glycyrrhizic acid. (A) Glycyrrhizic acid treatment blocks the cytopathic effect by virus infection. Vero cells in duplicate were untreated or treated with glycyrrhizic acid at concentrations as indicated for 2 h. The cells were then infected with CVA16 or EV71 (MOI=0.3). The cells were fixed with 3% paraformaldehyde at 72 h PI and stained with 0.5% crystal violet. Inhibition of CVA16 or EV71 infection prevents cell death and hence increased staining compared infected but untreated controls. The results are representative from two independent experiments. (B, C) Glycyrrhizic acid treatment reduces infectious virion production. To assay for inhibition of virion production, Vero cells were treated with glycyrrhizic acid at 1, 3, and 5 mM or remained untreated. The cells were then infected with CVA16 (B) or with EV71 (C) at MOI=0.3. The samples were harvested at 48 h PI and infectious virions in those samples were assayed with secondary infection assays. Data are presented as mean±standard errors of triplicate samples from three independent experiments. (D) Glycyrrhizic acid treatment inhibits VP1 protein expression of EV71. Vero cells were infected with EV71 in the presence or absence of glycyrrhizic acid at concentrations as indicated. The cells were harvested at 48 h PI and VP1 expression in the samples was detected by immunoblotting analysis. GAPDH was used as a loading control. The results are representative from two independent experiments.
The inhibition of GA on CVA16 and EV71 infections was validated using secondary infection assays. As shown in Fig. 4B and C, as compared with untreated controls, treatment with GA at 3 and 5 mM resulted in reduced CVA16 production by 3.5 and 6.0 logs, respectively (Fig. 4B). At the same concentrations, EV71 production was reduced by approximately 2.2 and 4.0 logs, respectively (Fig. 4C). In contrast, 18β-glycyrrhetinic acid (β–GA) and liquiritin, components of Glycyrrhiza uralensis, at their maximum nontoxic concentrations (100 μM for β–GA and 300 μM for liquiritin, respectively) showed no antiviral effect (data not shown).
The inhibition of EV71 infection by GA was also confirmed by immunoblotting assay for EV71 VP1 protein production. As shown in Fig. 4D, GA at 5 mM blocked VP1 protein expression by more than 90%. The results together demonstrated that GA functions as an active component for Glycyrrhiza uralensis antiviral activity against CVA16 and EV71 infections.
3.4. GA directly inactivates CVA16, while inhibition of EV71 infection by GA is associated with events post virus cell entry
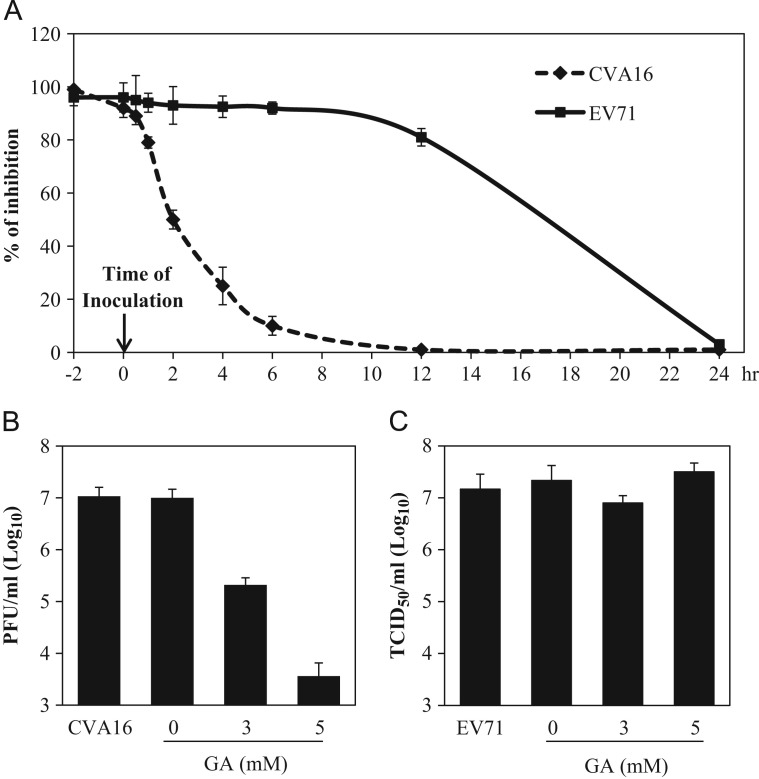
To investigate the mechanism(s) of GA on inhibition of CVA16 and EV71 infections, we performed time-of-drug addition experiments to determine at what stages GA inhibited CVA16 and EV71 infections. As shown in Fig. 5A, pre-treatment or addition of GA within the first 60 min, but not at later stages of the infection, significantly reduced CVA16 caused cytopathic effect of Vero cells, indicating that GA potentially deactivated the virus or inhibited CVA16 infection by targeting an early event(s) of CVA16 infection. In contrast, pretreatment of the host cells or treatment within the first 12 h PI blocked EV71 infection to comparable levels, suggesting that GA inhibited EV71 infection by targeting an event(s) post cell entry by EV71.
Fig. 5.
Glycyrrhizic acid inhibition of coxsackievirus A16 infection by direct inactivation, while the inhibition of enterovirus 71 infection is linked to a post cell entry event. (A) Time-of-drug addition study. Glycyrrhizic acid at 5 mM was added at 2 h prior to (−2 h), during (0 h), or post virus inoculation at time points as indicated (0.5 h, 1 h, 2 h, 4 h, 6 h, 12 h and 24 h PI). Virus infection was determined at 72 h PI using MTT assay. An inhibition rate was calculated as described in Section 2 and was plotted against time-of-drug addition. Data are presented as mean±standard errors of triplicate samples. The results are representative of two independent experiments. (B, C) Glycyrrhizic acid directly deactivates coxsackievirus A16, but not EV71. Duplicate samples of CVA16 (B) or EV71 (C) in 100 μl culture medium were incubated with glycyrrhizic acid at 0, 3, and 5 mM in a 37 °C water bath for 60 min. Ten microliters of the pre-treated virus or equal amount of untreated virus (final MOI=0.3) were then added to Vero cells (final concentrations of GA applied to Vero cells were 0.03 and 0.05 mM for GA-treated samples). Preincubation of CVA16 with 5 mM GA resulted in a loss of infectious virion production by more than 3 logs, whereas the same treatment did not significantly affect EV71 infectivity. Data are presented as mean±standard errors of duplicate samples. The results are representative of two independent experiments.
To demonstrate if GA inactivated CVA16 directly, we performed virucidal activity assay. As shown in Fig. 5B and C, co-incubation of CVA16 with GA significantly reduced CVA16 infectivity, while the same treatment showed no effect on EV71 infection, demonstrating GA blocked CVA16 infection likely through direct inactivation of the virus.
4. Discussion
Kuo et al. (2009) have recently reported that a water extract of radices of Glycyrrhiza uralensis Fisch. possessed strong antiviral activity against EV71 infection. The IC50 value was determined at 0.056 μg/ml. However, the components with such potent antiviral activity remain uncharacterized. In this study, we only detected moderate antiviral activity of the extract against EV71 and CVA16 infection. And we further demonstrated that GA contributed to the antiviral activity in Glycyrrhiza uralensis. In addition, GA is a signature component of Glycyrrhiza spp. and Chinese Pharmacopeia specifies its minimal content as 2.0%. It has been reported that GA contents in Glycyrrhiza uralensis Fisch. from different cultivated areas varied significantly (Gu et al., 2002). Consistent with this report, we found the contents of GA of five samples purchased from local market varied dramatically (data not shown). Therefore, we speculated that different GA contents and the cell lines may contribute to the discrepancy of reported activities of Glycyrrhiza spp. compared with our studies.
The use of Glycyrrhiza species for symptoms of viral respiratory tract infections can be traced back from ancient Chinese, Indian, and Greek manuscripts (Fiore et al., 2005, Fiore et al., 2008). Recent studies have linked glycyrrhizic acid to the antiviral activity of Glycyrrhiza uralensis against several DNA and RNA viruses, including HIV-1, SARS coronavirus, influenza virus, Epstein-Barr virus, Kaposi's sarcoma herpesvirus, and respiratory syncytial virus. Both CVA16 and EV71, along with poliovirus, are members of the Enterovirus genus of Picornaviridae family and are nonenveloped RNA viruses. In the earliest report of GA antiviral studies, no activity of GA against poliovirus-1 infection was detected (Pompei et al., 1979). This report represents the first case of GA antiviral activity against nonenveloped viruses.
A time-of-drug addition study revealed that GA inhibits CVA16 and EV71 infections through different mechanisms. The compound directly inactivates CVA16, while it inhibits EV71 infection apparently through an event post cell entry of the virus. Glycyrrhizic acid exhibits antiviral activity with multiple mechanisms, including direct deactivation of HSV-1 (Pompei et al., 1979), reduction of host cell membrane fluidity (Harada, 2005), inhibition of fusion of enveloped viruses (Wolkerstorfer et al., 2009), impairment of hepatitis B virus surface antigen transport (Sato et al., 1996), or disruption of RNA polymerase II pausing, resulting in loss of proper mRNA production and defects in sister chromatid cohesion (Kang and Lieberman, 2011). Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus infection and virus replication through its antioxidative effects in H5N1-infected cells (Michaelis et al., 2010, Michaelis et al., 2011). The mechanism of GA inhibition of EV71 infection remains unknown.
It is worth noting that similar to reported concentrations for GA to inhibit virus infection, we found the concentrations required for GA to inhibit CVA16 and EV71 infections are also in the millimolar ranges. Animal and clinical studies seem to suggest that a therapeutic effect of GA can be accomplished at lower concentrations. In a double-blind, randomized, placebo-controlled phase I/II trial of chronic hepatitis C, intravenous delivery of glycyrrhizin at 80, 160, and 240 mg, thrice weekly, lowers serum alanine aminotransferase (ALT) during the treatment, but no effect on HCV-RNA levels was detected (van Rossum et al., 1999). Whether drug metabolism process or other factors play a role in GA activity is unknown, but physiological proteins (Lampis et al., 2001) or biological lipids (Lampis et al., 1997) are known to enhance GA activities.
Both CVA16 and EV71 are transmitted through close contact with blisters and other surface contaminated by nose and throat discharge of infected person, or via fecal–oral route. Glycyrrhizic acid as a food additive is considered safe consumed at high doses. A recent assessment on the safety of GA and its salts by The Cosmetic Ingredient Review Expert Panel stated that those compounds are expected to be poorly absorbed through the skin (Andersen, 2007). The ingredients are not considered to be irritants, sensitizers, phototoxic agents, or photosensitizers at the current maximum concentration of use. Therefore, the identification of GA with antiviral activity against HFMD pathogens may lead to the development of hygiene products.
Acknowledgment
The work was supported by grants from NSFC (81121062 and 81172948), from Jiangsu Province University Program for Physicians (11KJB320002), and from Basic Research Foundations of Central Universities (1114021404). J. Wang and X. Chen are recipients of Jiangsu Innovation Program for Predoctoral Students.
References
- Andersen F.A. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. International Journal of Toxicology. 2007;26:79–112. doi: 10.1080/10915810701351228. [DOI] [PubMed] [Google Scholar]
- Ashfaq U.A., Masoud M.S., Nawaz Z. and Riazuddin S., Glycyrrhizin as antiviral agent against Hepatitis C Virus, Journal of Translational Medicine 2011, 112 [DOI] [PMC free article] [PubMed]
- Asl M.N., Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytotherapy Research. 2008;22:709–724. doi: 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H.J., Liu Z.L., Steinmann P., Mu Y.J., Luo H., Liu J.P. Chinese herbal medicines for treatment of hand, foot and mouth disease: a systematic review of randomized clinical trials. European Journal of Integrative Medicine. 2012;4:e85–e111. [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curreli F., Friedman-Kien A.E., Flore O. Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus latency, triggering p53-mediated apoptosis in transformed B lymphocytes. Journal of Clinical Investigation. 2005;115:642–652. doi: 10.1172/JCI23334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S.K., Das V., Gulati A.K., Singh V.P. Deglycyrrhizinated liquorice in aphthous ulcers. Journal of the Association of Physicians of India. 1989;37:647. [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Krausse R., Ragazzi E., Pellati D., Armanini D., Bielenberg J. Antiviral effects of Glycyrrhiza species. Phytotherapy Research. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Ragazzi E., Zanchin G., Armanini D. A history of the therapeutic use of liquorice in Europe. Journal of Ethnopharmacology. 2005;99:317–324. doi: 10.1016/j.jep.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H.Y., Gong L.D., Yu J.H. Measurement and comparison of glycyrrhizic acid contents in root of licorice (Glycyrrhiza uralensis Fisch.) from different cultivating areas. Journal of Forestry Research. 2002;13:141–143. [Google Scholar]
- Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochemical Journal. 2005;392:191–199. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., Kondratenko R., Tolstikov G.A., Doerr H.W., Cinatl J., Jr. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. Journal of Medicinal Chemistry. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Kang H., Lieberman P.M. Mechanism of glycyrrhizic acid inhibition of Kaposi's sarcoma-associated herpesvirus: disruption of CTCF-cohesin-mediated RNA polymerase II pausing and sister chromatid cohesion. Journal of Virology. 2011;85:11159–11169. doi: 10.1128/JVI.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.R., Jeong C.K., Park K.K., Choi J.H., Park J.H., Lim S.S., Chung W.Y. Anti-inflammatory effects of licorice and roasted licorice extracts on TPA-induced acute inflammation and collagen-induced arthritis in mice. Journal of Biomedicine & Biotechnology. 2010;2010:709378. doi: 10.1155/2010/709378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausse R., Bielenberg J., Blaschek W., Ullmann U. In vitro anti-Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. Journal of Antimicrobial Chemotherapy. 2004;54:243–246. doi: 10.1093/jac/dkh287. [DOI] [PubMed] [Google Scholar]
- Kuo K.K., Chang J.S., Wang K.C., Chiang L.C. Water extract of Glycyrrhiza uralensis inhibited enterovirus 71 in a human foreskin fibroblast cell line. The American Journal of Chinese Medicine. 2009;37:383–394. doi: 10.1142/S0192415X09006904. [DOI] [PubMed] [Google Scholar]
- Lampis G., Deidda D., Pinza M., Pompei R. Enhancement of anti-herpetic activity of glycyrrhizic acid by physiological proteins. Antiviral Chemistry & Chemotherapy. 2001;12:125–131. doi: 10.1177/095632020101200206. [DOI] [PubMed] [Google Scholar]
- Lampis G., Ingianni A., Pompei R. Synergistic effects of triterpenic compounds with prostaglandin A1 on vaccinia virus infected L929 cells. Antiviral Research. 1997;36:191–195. doi: 10.1016/s0166-3542(97)00051-x. [DOI] [PubMed] [Google Scholar]
- Lin T.Y., Liu Y.C., Jheng J.R., Tsai H.P., Jan J.T., Wong W.R., Horng J.T. Anti-enterovirus 71 activity screening of chinese herbs with anti-infection and inflammation activities. The American Journal of Chinese Medicine. 2009;37:143–158. doi: 10.1142/S0192415X09006734. [DOI] [PubMed] [Google Scholar]
- Liu C.C., Chou A.H., Lien S.P., Lin H.Y., Liu S.J., Chang J.Y., Guo M.S., Chow Y.H., Yang W.S., Chang K.H., Sia C., Chong P. Identification and characterization of a cross-neutralization epitope of Enterovirus 71. Vaccine. 2011;29:4362–4372. doi: 10.1016/j.vaccine.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Lu S.P., Sun Q., Wang J.H., Sun B.Q. Survey of study on the extraction, purification and determination methods of glycyrrhizic acid in Licorice. Zhongguo Zhong Yao Za Zhi. 2006;31:357–360. [PubMed] [Google Scholar]
- Ma E., Fung C., Yip S.H., Wong C., Chuang S.K., Tsang T. Estimation of the basic reproduction number of enterovirus 71 and coxsackievirus A16 in hand, foot, and mouth disease outbreaks. The Pediatric Infectious Disease Journal. 2011;30:675–679. doi: 10.1097/INF.0b013e3182116e95. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Doerr H.W., Cinatl J.J. Investigation of the influence of EPs(R) 7630, a herbal drug preparation from Pelargonium sidoides, on replication of a broad panel of respiratory viruses. Phytomedicine. 2011;18:384–386. doi: 10.1016/j.phymed.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Ogbomo H., Altenbrandt B., Leutz A., Doerr H.W., Cinatl J., Jr. Glycyrrhizin inhibits highly pathogenic H5N1 influenza A virus-induced pro-inflammatory cytokine and chemokine expression in human macrophages. Medical Microbiology and Immunology. 2010;199:291–297. doi: 10.1007/s00430-010-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Pompei R., Flore O., Marccialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- Pompei R., Laconi S., Ingianni A. Antiviral properties of glycyrrhizic acid and its semisynthetic derivatives. Mini-Reviews in Medicinal Chemistry. 2009;9:996–1001. doi: 10.2174/138955709788681636. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. American Journal of Hygiene. 1938;27:493–497. [Google Scholar]
- Sato H., Goto W., Yamamura J., Kurokawa M., Kageyama S., Takahara T., Watanabe A., Shiraki K. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antiviral Research. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. The Lancet Infectious Diseases. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- van Rossum T.G., Vulto A.G., Hop W.C., Brouwer J.T., Niesters H.G., Schalm S.W. Intravenous glycyrrhizin for the treatment of chronic hepatitis C: a double-blind, randomized, placebo-controlled phase I/II trial. Journal of Gastroenterology and Hepatology. 1999;14:1093–1099. doi: 10.1046/j.1440-1746.1999.02008.x. [DOI] [PubMed] [Google Scholar]
- WHO, 2008. Report on the Hand, Foot and Mouth Disease Outbreak in Fuyang City, Anhui Province and the Prevention and Control in China, 〈http://www2.wpro.who.int/NR/rdonlyres/591D6A7B-FB15-4E94-A1E9-1D3381847D60/0/HFMDCCDC20080515ENG.pdf〉.
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antiviral Research. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue B., Yao Z., Yu R. Studies on anti-EV71 virus activity of traditional Chinese medicine and its clinical application in treatment of HFMD. Zhongguo Zhong Yao Za Zhi. 2011;36:3366–3370. [PubMed] [Google Scholar]
- Yang F., Ren L., Xiong Z., Li J., Xiao Y., Zhao R., He Y., Bu G., Zhou S., Wang J., Qi J. Enterovirus 71 outbreak in the People's Republic of China in 2008. Journal of Clinical Microbiology. 2009;47:2351–2352. doi: 10.1128/JCM.00563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhu Z., Yang W., Ren J., Tan X., Wang Y., Mao N., Xu S., Zhu S., Cui A., Yan D., Li Q., Dong X., Zhang J., Zhao Y., Wan J., Feng Z., Sun J., Wang S., Li D., Xu W. An emerging recombinant human enterovirus 71 responsible for the 2008 outbreak of hand foot and mouth disease in Fuyang city of China. Virology Journal. 2010;7:94. doi: 10.1186/1743-422X-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]