Graphical abstract
Keywords: Adamantane, Amantadine, NMDA receptor antagonist, M2 channel, Ritter reaction
Abstract
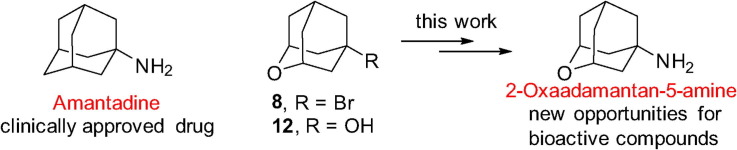
Two alternative syntheses of 2-oxaadamantan-5-amine, a novel analog of the clinically approved drug amantadine, are reported. The compound has been tested as an anti-influenza A virus agent and as an NMDA receptor antagonist. While the compound was not antivirally active, it displayed moderate activity as an NMDA receptor antagonist.
Introduction
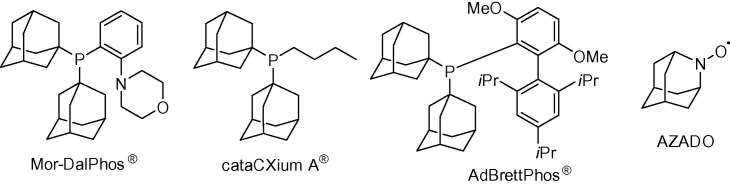
The highly symmetrical structure of adamantane is a very common building block in organic chemistry. Among the several applications of adamantane derivatives, two are of the highest interest. First, the adamantane scaffold is used as a sterically demanding group in the synthesis of ligands for transition metal catalysts. Currently there are several adamantane-derived phosphines, such as Me-DalPhos®, Mor-DalPhos®, AdBrettPhos®, cataCXium® A, that are commercially available (Fig. 1 ).1 Of note, the use of heteroadamantanes in catalysis has been less studied. The nitroxyl radical AZADO, a highly active catalyst for alcohol oxidation with superior catalytic proficiency to the well-known TEMPO, is a remarkable exception (Fig. 1).2
Figure 1.
Structures of selected adamantyl- and heteroadamantyl-based compounds of interest in catalysis.
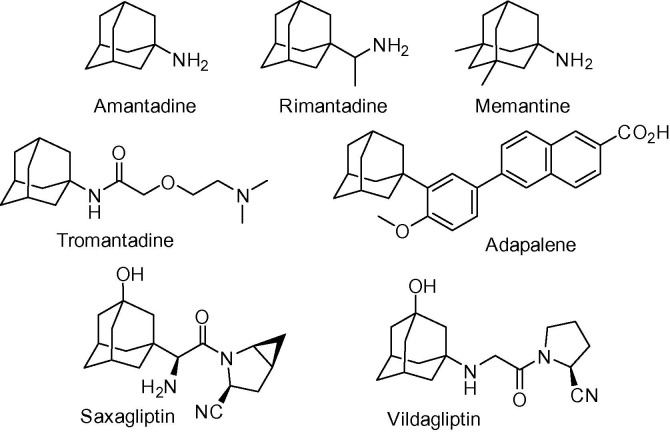
On the other hand, adamantane is of great interest in medicinal chemistry. The first clinically approved adamantane derivative, amantadine, was already introduced in the clinic in 1966.3 Since then, thousands of adamantane derivatives have been pharmacologically evaluated and, so far, seven of them have been approved for clinical use (Fig. 2 ). Many more are in development as potential therapeutics against a plethora of targets.4
Figure 2.
Structures of adamantyl-based compounds in clinical use.
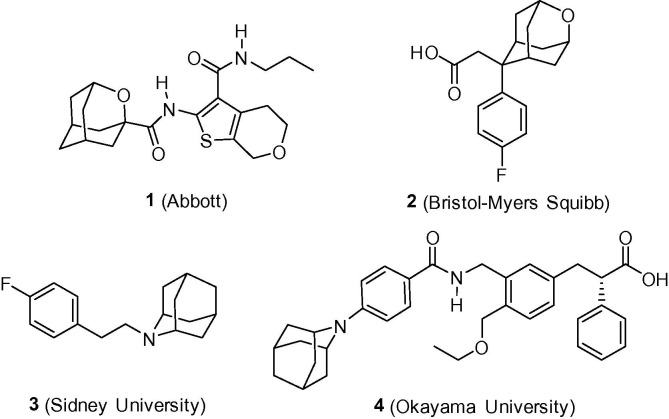
Although there are thousands of adamantyl derivatives that have been tested for biological activity, the number of heteroadamantanes that have been used in medicinal chemistry is only very small. Indeed, several oxaadamantanes, and azaadamantanes have been synthesized and pharmacologically tested, but no derivative has reached clinical trials so far. Some examples from either academic laboratories or the pharmaceutical industry are presented in Figure 3 .5
Figure 3.
Selected heteroadamantyl-based compounds. 1 is a cannabinoid receptor 2 agonist; 2 an inhibitor of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1); 3 a low nanomolar inhibitor of σ receptors; and 4 a nanomolar agonist of the human peroxisome proliferator activated receptor-γ.5
Some time ago, we reported the synthesis and pharmacological evaluation of several 2-oxaadamantan-1-amines as analogs of amantadine (Fig. 4 ). Taking into account that amantadine shows NMDA receptor antagonist and anti-influenza A virus activities, we evaluated the 2-oxaanalogs for these two activities. We found that although all the compounds were devoid of antiviral activity, several of them displayed NMDA receptor antagonism, with some having lower IC50 values than amantadine.6
Figure 4.
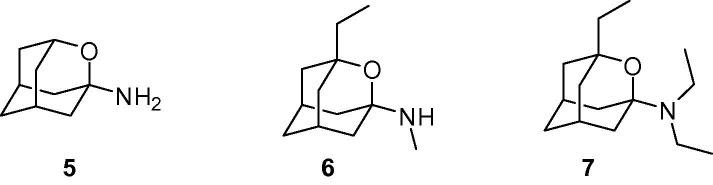
Selected 2-oxaadamantylamines previously reported by our group. NMDA receptor antagonist activities (IC50) are: >200 μM for 5; 32 ± 9 μM for 6, 14 ± 3 μM for 7, and 92 ± 29 μM for amantadine.
In order to further explore the biological interest of heteroadamantanes, in this Letter we report the synthesis of a novel scaffold, 2-oxaadamantan-5-amine, 11, of potential interest in medicinal chemistry. We have found that 11 is devoid of any inhibitory activity against the M2 channel of influenza A virus, but displays activity as an NMDA receptor antagonist, albeit being less active than amantadine.
Results and discussion
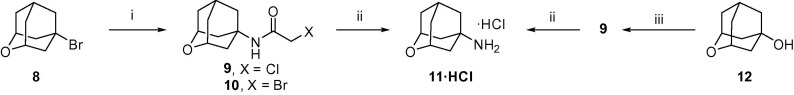
In order to synthesize the novel amantadine analog 11, we envisaged chloroacetamide 9 as a key intermediate. In turn 9, may be accessible from two already known 2-oxaadamantane derivatives, namely 8 and 12.7, 8 When we applied the Jirgensons’ modification of the classical Ritter reaction to 8,9 we recovered most of the starting material, along with an unseparable mixture of the expected chloroacetamide 9 and the corresponding bromoacetamide, 10, as evidenced by GC/MS analysis. As it is known that alcohols behave better in the Ritter reaction, we attempted to obtain 11 from the known alcohol 12.8 To our satisfaction, reaction of 12 with chloroacetonitrile in acidic medium proceeded uneventfully to furnish 9 in high yield. Finally, cleavage of the haloacetamide group by using thiourea, either from pure 9 or from a mixture of 9 and 10, furnished 2-oxaadamantane-5-amine, 11, in good yield. Amine 11 was fully characterized as its corresponding hydrochloride (Scheme 1 ).
Scheme 1.
Syntheses of 2-oxaadamantan-5-amine, 11, from known 2-oxaadamantane derivatives 8 and 12. (i) Chloroacetonitrile, acetic acid, concd H2SO4, 0–50 °C, 40 h, 27% yield. (ii) Thiourea, acetic acid, ethanol, reflux; then HCl/1,4-dioxane, 70% overall from the mixture of 9 and 10; 64% overall from pure 9. (iii) Chloroacetonitrile, acetic acid, concd H2SO4, 0 °C to room temperature, 70 h, 87% yield.
Amine 11 was tested for antiviral activity. However, it did not display activity against the influenza viruses A/H1N1, A/H3N2 or B. Also, it was found to be inactive against the enveloped DNA viruses herpes simplex virus (type 1 or type 2) or vaccinia virus; the enveloped RNA viruses HIV-1, HIV-2, feline coronavirus, parainfluenza-3 virus, respiratory syncytial virus, vesicular stomatitis virus, sindbis virus or Punta Toro virus; or the non-enveloped RNA viruses Coxsackievirus B4 and Reovirus-1. Of interest, 11 did not show cytotoxicity (CC50 >100 μM) in the human MT4 lymphoblast cells; human embryonic lung (HEL) fibroblast cells; HeLa cervix carcinoma cells; or African green monkey Vero cells.
Previously, we had found that although 5 did not show any anti-influenza virus activity,6 it was able to inhibit the wild-type (wt) M2 channel of influenza A virus, the target for the antiviral action of amantadine.10 In order to assess if 11 was an inhibitor of the M2 protein, its inhibitory activity was tested on A/M2 channels expressed in Xenopus oocytes using the two-electrode voltage clamp (TEV) technique. At 100 μM, amantadine was able to inhibit 91% of the activity of the wt A/M2 channel (IC50 = 16.0 ± 1.2 μM) and oxaamantadine 5 showed similar activity (IC50 = 29.2 ± 1.2 μM). On the other hand, the novel oxaamantadine 11, at 100 μM, produced only 18.9% inhibition of the activity of the wt M2 channel, and similar values were obtained when the compound was evaluated against the amantadine-resistant V27A (8.8% inhibition) and S31N (21.4% inhibition) M2 mutant channels.
Overall, taking into account the aforementioned pharmacological results, it seems that the introduction of the oxygen atom in the adamantane scaffold is much more deleterious for the anti-influenza A virus activity in 11 than in 5.
Finally, we measured the effect of 11 on the increase in intracellular calcium evoked by NMDA (at a concentration of 100 μM and in the presence of 10 μM of glycine) on cultured rat cerebellar granule neurons. Although we indeed found some antagonistic activity, amine 11 was 2.5 fold less potent (IC50 = 258 ± 93 μM, n = 3) than amantadine (IC50 = 92 ± 29 μM, n = 3).11
Conclusions
In conclusion, we have synthesized a novel heteroanalog of amantadine. Although 11 does not behave as a bioisostere of amantadine against two of its well-known targets, the potential of this novel amine in medicinal chemistry may still be very high, since adamantane-derived compounds can have activity against a broad and diverse panel of biological targets. Thus, we are currently further exploring the chemistry and biology of several derivatives of 11 with potential activity against several targets, such as the 11β-HSD1 or the soluble epoxide hydrolase enzymes.
Experimental
Chemistry: general
Melting points were determined in open capillary tubes. NMR spectra were recorded in the following spectrometers: 1H NMR (400 MHz), 13C NMR (100.6 MHz). Chemical shifts (δ) are reported in ppm related to internal tetramethylsilane (TMS) and coupling constants are reported in Hertz (Hz). Accurate mass measurements were obtained using ESI technic. Absorption values in the IR spectra [using the Attenuated Total Reflectance (ATR) technique] are given as wave-numbers (cm− 1). Only the more intense bands are given. For the thin layer chromatography (TLC) aluminum-backed sheets with silica gel 60 F254 were used and spots were visualized with UV light and/or 1% aqueous solution of KMnO4.
N-(2-Oxaadamantan-5-yl)-2-chloroacetamide, 9
-
(a)
From 5-bromo-2-oxaadamantane, 8: A solution of 5-bromo-2-oxaadamantane, 8, (593.5 mg, 2.74 mmol) in chloroacetonitrile (0.18 mL, 2.74 mmol) and glacial acetic acid (1.5 mL) was cooled to 0 °C. Then concentrated sulfuric acid (0.22 mL) was added dropwise. The reaction mixture was stirred at 50 °C for 40 h. The solution was added to ice (8 g) and the mixture stirred for few minutes. CH2Cl2 (10 mL) was added, the layers separated and the aqueous layer extracted with further CH2Cl2 (2 × 10 mL). The combined organics were dried over anhyd sodium sulfate and filtered. Evaporation in vacuo of the organics gave an orange oil (894.3 mg). Column chromatography (hexane/ethyl acetate mixture) gave N-(2-oxaadamantan-5-yl)-2-chloroacetamide, 9, and N-(2-oxaadamantan-5-yl)-2-bromoacetamide, 10, as a white solid (167.8 mg, approx. 26.7% yield).
-
(b)
From 2-oxaadamantan-5-ol, 12: A solution of 2-oxaadamantane-5-ol, 12,8a (186 mg, 1.21 mmol) in chloroacetonitrile (0.08 mL, 1.21 mmol) and glacial acetic acid (0.8 mL) was cooled to 0 °C. Then concentrated sulfuric acid (0.1 mL) was added dropwise. The reaction mixture was stirred at room temperature for 70 h. The solution was added to ice (2 g) and the mixture stirred for few minutes. CH2Cl2 (5 mL) was added, the layers separated and the aqueous layer extracted with further CH2Cl2 (2 × 5 mL). The combined organics were dried over anhyd sodium sulfate and filtered. Evaporation in vacuo of the organics gave the N-(2-oxaadamantan-5-yl)-2-chloroacetamide, 9, as a white solid (239.9 mg, 86.5% yield), mp 105–106 °C. IR (ATR) ν, 3272, 3082, 2937, 1661, 1556, 1441, 1414, 1358, 1315, 1271, 1234, 1193, 1150, 1107, 1076, 1017, 975, 961, 924, 817, 799, 777, 685 cm− 1. 1H NMR (400 MHz, CDCl3) δ: 1.60 [dm, 2H, 8′(10′)-Ha], 1.96–2.22 [complex signal, 8H, 4′(9′)-Ha, 4′(9′)-Hb, 8′(10′)-Hb, 6′-H2], 2.27 [m, 1H, 7′-H], 3.94 [s, 2H, CH 2Cl], 4.20 [br s, 2H, 1′(3′)-H], 6.27 (s, 1H, NH). 13C NMR (100.6 MHz, CDCl3) δ: 27.2 (CH, C7′), 34.8 [CH2, C8′(10′)], 39.3 (CH2, C6′), 40.2 [CH2, C4′(9′)], 42.7 (CH2, C2), 51.1 (C, C5′), 69.0 [CH, C1′(3′)], 164.9 (C, C1). HRMS-ESI+ m/z [M+H]+ calcd for [C11H16ClNO2+H]+: 230.0942, found: 230.0951.
2-Oxaadamantan-5- amine hydrochloride, 11·HCl
-
(a)
From a mixture of 9 and 10: To a solution of a mixture of N-(2-oxaadamantan-5-yl)-2-chloroacetamide, 9, and N-(2-oxaadamantan-5-yl)-2-bromoacetamide, 10, (113.6 mg, 0.49 mmol) in absolute ethanol (9.6 mL) was added thiourea (44.9 mg, 0.59 mmol) and glacial acetic acid (0.34 mL). The reaction mixture was stirred and heated at reflux overnight. The resulting mixture was allowed to reach room temperature and evaporated in vacuo. The residue was partitioned between CH2Cl2 (10 mL) and water (10 mL) and the layers were separated. The aqueous layer was basified with 10 N NaOH to basic pH and extracted with CH2Cl2 (3 × 10 mL). The combined organics were dried over anhyd sodium sulfate, filtered, and HCl/dioxane was added to form the hydrochloride salt. Evaporation in vacuo of the organics gave 2-oxaadamantan-5-amine hydrochloride, 11·HCl, as a white solid (55.8 mg, 70.0% yield).
-
(b)
From pure 9: To a solution of N-(2-oxaadamantan-5-yl)-2-chloroacetamide, 9, (239.9 mg, 1.05 mmol) in absolute ethanol (20.3 mL) was added thiourea (95.2 mg, 1.25 mmol) and glacial acetic acid (0.72 mL). The reaction mixture was stirred and heated at reflux overnight. The resulting mixture was allowed to reach room temperature and evaporated in vacuo. The residue was partitioned between CH2Cl2 (20 mL) and water (20 mL) and the layers were separated. The aqueous layer was basified with 10 N NaOH to basic pH and extracted with CH2Cl2 (3 × 20 mL). The combined organics were dried over anhyd sodium sulfate, filtered, and HCl/dioxane was added to form the hydrochloride salt. Evaporation in vacuo of the organics gave 2-oxaadamantan-5-amine hydrochloride, 11·HCl, as a white solid (117.4 mg, 64.0% yield), mp 240 °C (sublimation). IR (ATR) ν, 3361, 2893, 2609, 1657, 1625, 1525, 1443, 1383, 1364, 1322, 1197, 1172, 1120, 1095, 1062, 1009, 984, 933, 912, 896, 831, 811, 779, 717, 625 cm−1. 1H NMR (400 MHz, CD3OD) δ: 1.65 [dm, 2H, 8(10)-Ha], 1.89 [d, J = 12 Hz, 2H, 4(9)-Ha], 1.98–2.12 [complex signal, 6H, 6-H2, 8(10)-Hb, 4(9)-Hb], 2.35 [m, 1H, 7-H], 4.23 [br s, 2H, 1(3)-H], 4.86 (s, 3H, NH3). 13C NMR (100.6 MHz, CD3OD δ: 28.4 (CH, C7), 35.0 [CH2, C8(10)], 39.6 (CH2, C6), 40.2 [CH2, C4(9)], 51.8 (C, C5), 69.9 [CH, C1(3)]. Anal. Calcd for C9H16ClNO: C, 56.99; H, 8.50; N, 7.38; calcd for C9H16ClNO·0.75H2O: C, 53.20; H, 8.68; N, 6.89. Found: C, 53.26; H, 8.40; N, 6.58.
Antiviral activity
The antiviral activity of amine 11 was determined in established cell culture assays using a selection of DNA and RNA viruses, including four subtypes of influenza virus [A/Puerto Rico/8/34 (H1N1); A/Virginia/ATCC3/2009 (H1N1); A/Hong Kong/7/87 (H3N2) and B/Hong Kong/5/72].12 The compound’s inhibitory effect on virus replication (antiviral EC50 >100 μM) as well as its cytotoxicity (CC50 >100 μM) were monitored by microscopic inspection, and confirmed by the colorimetric MTS cell viability assay.
Plasmid, mRNA synthesis, and microinjection of oocytes
The cDNA encoding the influenza A/Udorn/72 (A/M2) was inserted into pSUPER vector for expression in Xenopus oocyte plasma membrane. A/M2 S31N and A/M2 V27A mutants were generated by QuikChange site-directed mutagenesis kit (Agilent Technologies). The synthesis of cRNA and microinjection of oocytes have been described previously.13
Two-electrode voltage clamp analysis
Macroscopic membrane current was recorded 24–72 h after injection as described previously.14 Oocytes were perfused at room temperature in Barth’s solution containing (in mM) 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.3 NaNO3, 0.71 CaCl2, 0.82 MgCl2, and 15 HEPES for pH 8.5 or 15 MES for pH 5.5 at a rate of 2 mL/min. The tested compound was dissolved in DMSO and applied (100 μM) at pH 5.5 when the inward current reached a maximum. The compound was applied for 2 min, and residual membrane current was compared with the membrane current before the application of compounds. Currents were recorded at −20 mV and analyzed with pCLAMP 8 software package (Axon Instruments, Sunnyvale, CA).
Antagonist activity against NMDA receptors
The functional assay for antagonist activity against NMDA receptors was performed using primary cultures of rat cerebellar granule neurons that were prepared according to established protocols.11 Cells were grown on 10 mm poly-l-lysine coated glass cover slips, and used for the experiments after 6–10 days in vitro culture. Cells were loaded with 6 μM Fura-2 AM (Invitrogen-Molecular Probes) for 30 min. Then, the coverslip was mounted on a quartz cuvette containing a Locke–Hepes buffer using a special holder. Measurements were performed using a PerkinElmer LS-55 fluorescence spectrometer equipped with a fast-filter accessory, under mild agitation and at 37 °C. Analysis from each sample was recorded real-time during 1400 s. After stimulation with NMDA (100 μM, in the presence of 10 μM glycine), increasing cumulative concentrations of the compound to be tested were added. The percentages of inhibition at every tested concentration were analyzed using a non-linear regression curve fitting (variable slope) by using the software GraphPad Prism 5.0.
Acknowledgments
R.L. thanks the Generalitat de Catalunya for a PhD Grant (FI). S.V. thanks financial support from Ministerio de Ciencia e Innovación (Project CTQ2011-22433) and the Generalitat de Catalunya (Grant 2014-SGR-00052). L.N. acknowledges the financial support from the KU Leuven Geconcerteerde Onderzoeksacties (GOA/15/019/TBA), and the technical assistance from W. van Dam.
Footnotes
Supplementary data (1H and 13C NMR spectra of 9 and 11·HCl) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.tetlet.2015.01.160.
Supplementary data
1H and 13C NMR spectra of 9 and 11·HCl.
References and notes
- 1.(a) Lundgren R.J., Peters B.D., Alsabeh P.G., Stradiotto M. Angew. Chem., Int. Ed. 2010;49:4071–4074. doi: 10.1002/anie.201000526. [DOI] [PubMed] [Google Scholar]; (b) Lundgren R.J., Stradiotto M. Angew. Chem., Int. Ed. 2010;49:8686–8690. doi: 10.1002/anie.201003764. [DOI] [PubMed] [Google Scholar]; (c) Lundgren R.J., Stradiotto M. Aldrichim. Acta. 2012;45:59–65. [Google Scholar]; (d) Su M., Buchwald S.L. Angew. Chem., Int. Ed. 2012;51:4710–4713. doi: 10.1002/anie.201201244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Zapf A., Ehrentraut A., Beller M. Angew. Chem., Int. Ed. 2000;39:4153–4155. doi: 10.1002/1521-3773(20001117)39:22<4153::aid-anie4153>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]; (f) Zapf A., Jackstell R., Rataboul F., Riermeier T., Monsees A., Fuhrmann C., Shaikh N., Dingerdissen U., Beller M. Chem. Commun. 2004:38–39. doi: 10.1039/b311268n. [DOI] [PubMed] [Google Scholar]; (g) Rataboul F., Zapf A., Jackstell R., Harkal S., Riermeier T., Monsees A., Dingerdissen U., Beller M. Chem. Eur. J. 2004;10:2983–2990. doi: 10.1002/chem.200306026. [DOI] [PubMed] [Google Scholar]
- 2.(a) Shibuya M., Tomizawa M., Suzuki I., Iwabuchi Y. J. Am. Chem. Soc. 2006;128:8412–8413. doi: 10.1021/ja0620336. [DOI] [PubMed] [Google Scholar]; (b) Iwabuchi Y. Chem. Pharm. Bull. 2013;61:1197–1213. doi: 10.1248/cpb.c13-00456. [DOI] [PubMed] [Google Scholar]
- 3.Davies W.L., Grunert R.R., Haff R.F., McGahen J.W., Neumayer E.M., Paulshock M., Watts J.C., Wood T.R., Hermann E.C., Hoffmann C.E. Science. 1964;144:862–863. doi: 10.1126/science.144.3620.862. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lamoureux G., Artavia G. Curr. Med. Chem. 2010;17:2967–2978. doi: 10.2174/092986710792065027. [DOI] [PubMed] [Google Scholar]; (b) Liu J., Obando D., Liao V., Lifa T., Codd R. Eur. J. Med. Chem. 2011;46:1949–1963. doi: 10.1016/j.ejmech.2011.01.047. [DOI] [PubMed] [Google Scholar]; (c) Wanka L., Iqbal K., Schreiner P.R. Chem. Rev. 2013;113:3516–3604. doi: 10.1021/cr100264t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Banister S.D., Yoo D.T., Chua S.W., Cui J., Mach R.H., Kassiou M. Bioorg. Med. Chem. Lett. 2011;21:5289–5292. doi: 10.1016/j.bmcl.2011.07.028. [DOI] [PubMed] [Google Scholar]; (b) Ye X.-Y., Chen S.Y., Nayeem A., Golla R., Seethala R., Wang M., Harper T., Sleczka B.G., He B., Gordon D.A., Robl J.A. Bioorg. Med. Chem. Lett. 2011;21:6699–6704. doi: 10.1016/j.bmcl.2011.09.055. [DOI] [PubMed] [Google Scholar]; (c) Nelson D.W., Frost J.M., Tietje K.R., Florjancic A.S., Ryther K., Carroll W.A., Dart M.J., Daza A.V., Hooker B.A., Grayson G.K., Fan Y., Garrison T.R., El-Kouhen O.F., Yao B., Pai M., Chandran P., Zhu C., Hsieh G.C., Meyer M.D. Bioorg. Med. Chem. Lett. 2012;22:2604–2608. doi: 10.1016/j.bmcl.2012.01.121. [DOI] [PubMed] [Google Scholar]; (d) Tanaka Y., Gamo K., Oyama T., Ohashi M., Waki M., Matsuno K., Matsuura N., Tokiwa H., Miyachi H. Bioorg. Med. Chem. Lett. 2014;24:4001–4005. doi: 10.1016/j.bmcl.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Duque M.D., Camps P., Profire L., Montaner S., Vázquez S., Sureda F.S., Mallol J., López-Querol M., Naesens L., De Clercq E., Prathalingam S.R., Kelly J.M. Bioorg. Med. Chem. 2009;17:3198–3206. doi: 10.1016/j.bmc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiner P.R., Lauenstein O., Kolomitsyn I.V., Nadi S., Fokin A.A. Angew. Chem., Int. Ed. 1998;37:1895–1897. [Google Scholar]
- 8.(a) Duddeck H., Wagner P. Liebigs Ann. Chem. 1984:1981–1988. [Google Scholar]; (b) Alder R.W., Carta F., Reed C.A., Stoyanova I., Willis C.L. Org. Biomol. Chem. 2010;8:1551. doi: 10.1039/b921957a. [DOI] [PubMed] [Google Scholar]
- 9.(a) Jirgensons A., Kauss V., Kalvinsh L., Gold M.R. Synthesis. 2000:1709–1712. [Google Scholar]; (b) Jiang D., He T., Ma L., Wang Z. RSC Adv. 2014;4:64936–64946. [Google Scholar]
- 10.Duque M.D., Ma C., Torres E., Wang J., Naesens L., Juárez-Jiménez J., Camps P., Luque F.J., DeGrado W.F., Lamb R.A., Pinto L.H., Vázquez S. J. Med. Chem. 2011;54:2646–2657. doi: 10.1021/jm101334y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canudas A.M., Pubill D., Sureda F.X., Verdaguer E., Camps P., Muñoz-Torrero D., Jiménez A., Camins A., Pallàs M. Exp. Neurol. 2003;180:123–130. doi: 10.1016/s0014-4886(02)00029-8. [DOI] [PubMed] [Google Scholar]
- 12.(a) Setaki D., Tataridis D., Stamatiou G., Kolocouris A., Foscolos G.B., Fytas G., Kolocouris N., Padalko E., Neyts J., De Clercq E. Bioorg. Chem. 2006;34:248–273. doi: 10.1016/j.bioorg.2006.05.004. [DOI] [PubMed] [Google Scholar]; (b) Naesens L., Vanderlinden E., Roth E., Jeko J., Andrei G., Snoeck R., Pannecouque C., Illyes E., Batta G., Herczegh P., Sztaricskai F. Antiviral Res. 2009;82:89–94. doi: 10.1016/j.antiviral.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gazzarrini S., Kang M., Abenavoli A., Romani G., Olivari C., Gaslini D., Ferrara G., van Etten J.L., Kreim M., Kast S.M., Thiel G., Moroni A. Biochem. J. 2009;420:295–303. doi: 10.1042/BJ20090095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balannik V., Lamb R.A., Pinto L.H. J. Biol. Chem. 2008;283:4895–4904. doi: 10.1074/jbc.M709433200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1H and 13C NMR spectra of 9 and 11·HCl.