Abstract
Studies were undertaken to investigate the antiviral effects of comestible juices, especially cranberry juice, on non-related viral species. After exposure of bacteriophage T2 to a commercially available cranberry (Vaccinium macrocarpon) juice cocktail (CJ), virus infectivity titer was no longer detectible. After a 60-min exposure to orange (OJ) and grapefruit juices (GJ), phage infectivity was reduced to 25–35% of control, respectively. Similar data were observed for the bacteriophage T4. CJ inactivation of phage T4 was rapid, dose-dependent, and occurred at either 4 or 23 °C. Neither pH nor differences in sugar/carbohydrate levels among the juices may be ascribed to the recognized antiviral effects. Further studies were performed to identify the occurrence of antiviral activity by CJ to a mammalian enteric virus. The treatment of the simian rotavirus SA-11 with a 20% CJ suspension was sufficient to inhibit hemagglutination. Under scanning and transmission electron microscopy, CJ was observed to inhibit the adsorption of phage T4 to its bacterial host cells and prevented the replication of rotavirus in its monkey kidney (MA-104) host cells, respectively. The data suggest, for the first time, a non-specific antiviral effect towards unrelated viral species (viz., bacteriophages T2 and T4 and the simian rotavirus SA-11) by a commercially available cranberry fruit juice drink.
Keywords: Cranberry juice, Antiviral activity, Bacteriophages, Rotavirus
Introduction
The existence of anti-microbial agents in comestible plants and their products (e.g., fruit juices) has been of interest to biomedical and nutrition researchers for decades. Studies investigating the effects of naturally occurring anti-microbial and, to a lesser extent, antiviral agents in food and food products, have been performed in both in vivo and in vivo settings (Jassim and Naji, 2003).
Kontiokari et al. (2003), for example, reported a reduction in urinary tract infections (UTIs) among women following the addition of cranberry juice (CJ) to their diets. Cinatl et al. (2003) reported that glycyrrhizin, a component of licorice roots, inhibited the in vivo replication of the severe acute respiratory syndrome (SARS)-associated corona virus and alluded to the use of glycyrrhizin as a possible therapeutic agent. These and similar findings have broad implications, as consumption of defined foods containing antiviral/anti-microbial agents may benefit the health and well-being of diverse populations including, but not limited to infants, geriatric, and the immunocompromised.
Public interest in nutrition and food science has surged within the last two decades. A plethora of professional organizations now address the interests of researchers and lay persons in this growing field. Numerous organizations on both the national and international levels support divisions dedicated to the field of food science and nutrition (Food and Nutritional Information Center, 2005).
Scientific interest in naturally occurring antiviral activity in foods was bolstered during the late 1970s by the studies of Konawalchuk and Spears (1978). Using poliovirus as a model system, selected juices were found to reduce in vivo poliovirus infectivity significantly. More recent studies showed in vivo and/or in vivo antiviral activity against human immunodeficiency virus type-I and Freund's leukemia virus by the plant extracts hypericin and pseudohypericin, from Hypericum perforatum (St. John's wort) (Degar et al., 1992; Meruelo et al., 1988). New classes of antiviral compounds in the edible gypsy mushroom Rozities caperata, were reported recently. Specifically, in vivo antiviral activity by extract RC-183 from this mushroom was deemed effective in inhibiting the replication of herpes simplex virus types 1 and 2, varicella-zoster virus (chicken pox, zoster), influenza, and respiratory syncytial virus (Piraino and Brandt, 1999). In the mouse model, intranasal and intraperitoneal administration of flavonoids (water-soluble plant pigments) inhibited the replication of influenza virus types A and B (Nagai et al., 1995).
It was proposed that flavonoids may be responsible for the in vivo and in vivo (mouse model) antiviral effects of various herbs and comestible plant products (Middleton et al., 2000).
Most studies investigating the effects of naturally occurring antimicrobial agents have been performed on bacterial species using plant extracts or isolated/synthesized plant components. Relatively little work has been reported on the antiviral effects of commercially available juices. Moreover, few if any studies have addressed the antiviral effects of juices on enteric virus infectivity. The purpose of this study was to identify the occurrence and extent of antiviral activity by comestible juices (viz., orange, grapefruit, and especially CJs) on diverse viral species. Studies were performed using the bacteriophages T2 and T4 of Escherichia coli C and B, respectively, with subsequent testing using the simian enteric virus, rotavirus SA-11.
Materials and methods
Bacteriophages and plaque assay
Bacteriophages T2 and T4 of E. coli strains C and B, respectively, were obtained from Ward's Natural Science (Rochester, NY) and Carolina Biological Supply Company (Burlington, NC). Bacteriophage titrations were performed by the double agar layer technique (Lipson and Alsmadi, 1989). Briefly, 0.1 ml virus was inoculated into 10 ml overlay medium (0.75% tryptic soy agar containing log growth phase E. coli strains B or C). Underlay consisted of the same medium but contained 1.5% agar. Plates were incubated at 37 °C for 24–48 h. Plaques were counted on a bacteriologic colony counter and recorded as plaque-forming units (PFU)/ml. The bacteriophage strains were temporarily stored at 4 °C.
Effect of juices on bacteriophage T2 and T4 infectivity titers
Equal volumes of undiluted orange juice (OJ; Tropicana Pure Premium Original Orange Juice, Bradenton, FL), grapefruit juice (GJ; Florida Natural Premium Grapefruit Juice, Florida Nature Flavors-Beverage Manufacturer, Casselberry, FL) and CJ (Cranberry Juice Cocktail, Ocean Spray Cranberry, Inc., Lakeville-Middleboro, MA), were added to equal volumes of bacteriophage T2 suspensions. The suspensions were titered within several minutes of preparation (this time period is designated T 0), and then after 30 (T 30) and 60 (T 60) min. The controls, performed at each time period, were identical to experimentals except that PBS was used in place of each juice. Identical experiments were performed using bacteriophage T4, except that infectivity titers were performed only at T 0. All experiments were performed in triplicate or quadruplicate. Data are graphically expressed as percent of control at each titration period.
Dose response
A 0.1-ml quantity of stock suspensions of bacteriophage T4 was added in triplicate to 0.9 ml of CJ. The CJ was diluted in PBS to 50%, 30%, 10%, 0.01%, 0.005%, 0.001%, and 0.0005% of the commercial product. The suspensions were incubated for 30 min at 37 °C followed by titration by plaque assay. The control was identical to experimentals, except that PBS was substituted in place of either juice.
Effect of temperature
The effects of 4 and 23 °C were tested to determine any effect of these temperatures on the antiviral activity of the bacteriophage T4 by CJ. A total of 0.1 ml bacteriophage T4 was added in triplicate to 0.9 ml of non-diluted CJ, followed by incubation for a period of 60-min at 4 and 23 °C. The control was treated as the experimentals, except PBS was used in place of the CJ.
Time course
A total of 0.l-ml bacteriophage T4 suspension was added to 0.9 ml of a 30% CJ solution followed by incubation at 23 °C. Titrations were performed at time zero (T 0), and after (T 0.5), T 1, T 6, and T 18. The control was identical to experimentals except that the virus was added to PBS and infectivity titers were determined after T 0, T 6 and T 18.
Significance of sugar and carbohydrate content on CJ inactivation of bacteriophage T2
Experiments were performed to determine whether differences in sugar and carbohydrate concentrations between each of the comestible juices might be associated with differences in antiviral activity. As identified on product labels, CJ contains higher sucrose levels than either OJ or GJ. Accordingly, the CJ was diluted in PBS (without Ca+2 or Mg+2) to effect a sugar concentration equal to that specified on the labels of the OJ and GJ packages. Antiviral activity of the CJ was assessed by adding equal volumes of phage T2 to volumes of sucrose-equilibrated CJ to equal those of the OJ and GJ. After a 60-min incubation period at room temperature (23 °C), viral titers were determined.
Simian rotavirus SA-11
The simian rotavirus SA-11, an etiologic agent of gastroenteritis in primates, was kindly supplied by Dr. Mark D. Sobsey (University of North Carolina at Chapel Hill). Briefly, the virus was activated by incubation with trypsin, and then inoculated into MA-104 cell culture monolayers (media containing trace concentrations of trypsin: Lipson and Zelinsky-Papez, 1989). Monolayers were incubated at 37 °C until the appearance of a 90% [monolayer] degeneration [3+ to 4+ cytopathic effect (CPE). Cells and supernatants were then harvested, frozen/thawed twice, centrifuged, and followed by concentration of the supernatant using polyacrylamide absorbent gel (Sigma, Prod. No. P-7651). A total of 0.2 ml aliquots of rotavirus SA-11 (viral stock preparations) were temporarily stored at −20 °C.
Cells and cell culture
African green monkey kidney cell cultures (MA-104) were used throughout the animal virus experiments. Growth medium consisted of Earle's minimal essential medium (E-MEM) supplemented with 1% l-glutamine, 100 units penicillin, 100 μg/ml streptomycin, 1 μg/ml amphotericin B, and 10% fetal bovine serum (FBS). Cultures were maintained in the same formulation but containing 2% FBS. The cell cultures were grown and maintained in T25 cm2 vented cell culture flasks in a 5% CO2 atmosphere at 37 °C. Monolayers were passaged by trypsinization (0.025% trypsin) and seeded at a concentration of approximately 1×105 cells/ml (Lipson and Stotzky, 1983; Lipson, 1992). Seed MA-104 tube cultures were obtained from ViroMed Laboratories, Minneapolis, MN.
Micro-hemagglutination assay
Hemagglutination (micro-hemagglutination) testing was performed in order to investigate the effect of CJ on the attachment of rotavirus [antigen] to RBCs. Guinea pig RBC's (RBC) were used in the hemagglutination assay. Working RBC suspensions were prepared by standard procedures (Kalica et al., 1978), with modification to the assay as follows: Briefly, 0.7 ml of packed RBC's (400×g for 10 min) were added to 9.3 ml PBS without Ca+2 or Mg+2. Fifty-microliter volumes of RBC preparations were then added to equal volumes of rotavirus containing CJ concentrations of 1.3%, 2.5%, 5%, 10%, 12%, 20%, 33%, and 50% in 96-well, round-bottom microtiter plates. Plates were subjected to several seconds of mixing on a microtiter plate mixer, sealed with adhesive paper to prevent evaporation, and then incubated for 30 min at 23 °C.
Rotavirus-induced hemagglutination was identified microscopically at magnifications of 100× and 400×. Inocula, which did not affect RBC clumping (hemagglutination) were deemed free of rotavirus, or contained rotavirus antigen levels below the concentration detectible by the micro-hemagglutination assay. Inocula, which produced RBC aggregation (hemagglutination), were deemed reactive/positive. The positive control consisted of equal volumes of rotavirus and PBS (without Ca2+ or Mg2+). The negative control was identical to that of the experimentals, except that PBS was substituted for the rotavirus. Experiments were performed in quadruplicate.
Time response
Ten microliter volumes of SA-11 were added to equal volumes of CJ and incubated for 0, 10, 30, and 60 min. After each time period, the CJ-virus preparations were added to 50 μl of the prepared GP-RBC suspensions and incubated for 30 min, followed by inspection using light microscopy.
Electron microscopy
Scanning electron microscopy (SEM)
SEM was employed to determine whether CJ-treated bacteriophage T4 was able to adsorb/attach onto the surface of its bacterial host. Briefly, 0.3 ml of a 1×109 PFU/ml phage T4 suspension was each incubated for 30 min at RT with equal volumes of CJ or PBS (control). After the 30-min incubation period, both preparations were inoculated into equal volumes of log phage E. coli B. The preparations were incubated at 37 °C for 30 min. The experimental and control were centrifuged at 8000×g for 10 min, followed by decanting of the supernatant, and then fixation of the bacteria for 5 min in 1% glutaraldehyde/4% paraformaldehyde. Five microliter of the fixed bacterial preparation were placed on formvar-coated, 300-mesh grids for 5 min followed by staining with phosphotungstic acid for 15 s. Excess fluid was removed with absorbent paper (Schiffenbauer and Stotzky, 1982). Specimens were examined using an Amray 1850-FE scanning electron microscope. Experiments were performed in duplicate.
Transmission electron microscopy (TEM)
TEM was used to identify the effect of CJ on the penetration of MA-104 cells by simian rotavirus SA-11 particles. Briefly, 1 ml rotavirus stock was added to an equal volume of CJ or PBS followed by incubation for 30 min at 23 °C. CJ-treated and PBS control viral suspensions were added to confluent host cell culture monolayers grown in T25 cm2 flasks. The inocula were incubated at 37 °C for 60 min in a 5% CO2 environment. Monolayers were washed twice with PBS, re-fed maintenance medium, and incubated for 5 days. Monolayers were subsequently washed twice with PBS, fixed with formaldehyde/3% cocadylic acid, scraped from flasks, pelleted, dehydrated in an ethanol series, and embedded en bloc. Thin sections were prepared using a diamond knife, placed on 300-mesh copper grids and stained (Gordon et al., 1986; Lipson et al., 1993). Specimens were observed using a Hitachi 7000 TEM.
Statistical analysis
Experiments were performed in triplicate or quadruplicate. Bacteriophage titrations and rotavirus hemagglutination assays were performed in triplicate or quadruplicate. The arithmetic mean±standard error of the mean (SEM) of control and experimental results were calculated using the Student's t-test. P<0.05 was considered statistically significant.
Results and discussion
Infectivity titers of bacteriophage T2 were reduced by OJ and GJ to 25–35% of the control (P<0.05). CJ, however, reduced bacteriophage T2 infectivity titers at T 0 to undetectable levels (Fig. 1a ). Similar results were obtained for bacteriophage T4, wherein CJ reduced viral titers by more than 1 log10 (i.e., 92% of control). OJ and GJ affected a bacteriophage T4 reduction in titer by ca. 20% (Fig. 1b). Due to the rapidity of these reactions, the marked antiviral effect imparted to bacteriophages T2 and T4 by CJ is suggested to have occurred at an early stage in the virus replication cycle. Based on the similar inactivation patterns identified between the bacterial virus species tested, extended studies were performed using the bacteriophage T4.
Fig. 1.
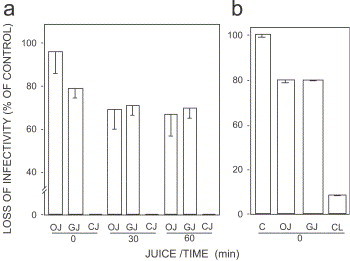
Effect of comestible juices on the loss of infectivity of bacteriophages T2 and T4. Orange (OJ), grapefruit (GJ) and cranberry juices (CJ) were added to equal volumes of bacteriophage T2 or T4. Infectivity titers were expressed as plaque-forming units per ml. Titers were expressed as infectivity loss as percent of control. (a) Effect of juices on bacteriophage T2 loss of infectivity. Experiments were performed at time zero (T0) and then after 30 (T30) and 60 (T60) min of experimental preparations. OJ: T0 vs. T30, P=0.05; T0 vs. T30, P=0.07. GJ: T0 vs. T30, P=0.23; T0 vs. T30, P=0.18. Control (C) titer: 2.2×105 PFU/ml. (b) Effect of juices on bacteriophage T4 loss of infectivity. Experiments were performed at T0. C vs. OJ, P=0.01; C vs. GJ, P=0.005, C vs. CJ, P⩽0.001. Control titer: 1.2×105 PFU/ml. Experiments were conducted in triplicate or quadruplicate. Data are presented as mean±SEM.
Incubation at 4 and 23 °C for 60 min of CJ-treated bacteriophage T4 affected a reduction of infectivity titers to undetectable levels. Under identical assay conditions, no significant differences in titer yield occurred among the bacteriophage T4 controls following inoculation at 4 or 23 °C (; Table 1 ). Variations in temperature are recognized to exert an effect on virus loss of infectivity, with increased temperature associated with increased rate of virus “kill.” However, temperature-induced inactivation rates may differ between viruses (Babich and Stotzky, 1980; Feng et al., 2003). In the current study, temperatures of 4 and 23 °C had no effect on control titer yields. These data not only denote the relative stability of the bacteriophage T4 at 23 °C, but further indicate the significant antiviral effect mediated by CJ.
Table 1.
Effect of refrigeration and room temperature on bacteriophage T4 inactivation by cranberry juice
| Plaque forming units (PFU/ml) | ||
|---|---|---|
| Temperature (°C) | Controla | Experimentalb |
| 4 | 2.2±0.5×109c, d | 0 |
| 23 | 1.9±0.1×109d | 0 |
Virus and PBS.
Virus and cranberry juice.
Mean±SEM.
P=0.39.
Infectivity titers of the bacteriophage T4 were undetectable 30 min after virus inoculation when treated with CJ concentrations of 10%, 30%, or 50%. CJ concentrations <0.01% CJ displayed no antivirus activity (Fig. 2 ).
Fig. 2.
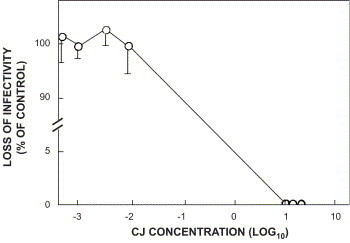
Effect of cranberry juice (CJ) concentration on the loss of bacteriophage T4 infectivity. Bacteriophage T4 was added to CJ concentrations of 50%, 30%, 10%, 0.01%, 0.005%, 0.001%, and 0.0005%. Suspensions were incubated for 30 min at 23 °C. The control consisted of bacteriophage T4 in the presence of PBS. Control titer: 2.4×107 PFU/ml. Data are presented as mean±SEM.
Time-course experiments utilizing a CJ concentration of 30% showed a bacteriophage T4 loss of infectivity after 30 to 60 min of ca. 1 log10. Extended incubation times to 6 and 18 h of CJ-treated virus resulted in a loss of bacteriophage T4 titer to undetectable levels (Fig. 2). These time course studies concur with that described above, wherein CJ effected a phage T4 loss of infectivity >1 log10 of control (Fig. 1b).
Scanning electron microscopy did not show attachment of CJ-treated phage T4 to its host bacterial cell (E. coli B) (Plate 1a ), whereas non-treated phage T4 was seen attached to cells of its bacterial host (Plate 1b). These micrographs suggest an inhibitory effect by CJ on the early attachment phase of the bacteriophage replication cycle. It was not determined whether the CJ-mediated antiviral effect was directed to phage antigenic determinants or to receptor sites of the host cell.
Plate 1.
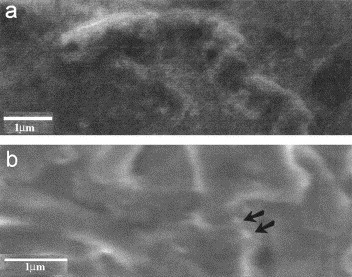
Effect of cranberry juice on the attachment of bacteriophage T4 to its bacterial host. A total of 0.3 ml of a 1×109 PFU/ml phage T4 suspension was mixed for 30 min at RT with equal volumes of cranberry juice or PBS (control). Pelleted cell preparations were fixed, placed on grids, and then examined by scanning electron microscopy. (a) CJ-treated bacteriophage did not attach to its E. coli B host cells (30,400×D). (b) Non-treated bacteriophage T4 (arrows) was observed attached to its bacterial host cells (40,000×D).
Using the simian enteric virus, the rotavirus SA-11, no characteristic particles were observed by TEM in MA-104 host cells, which had been inoculated with CJ-treated virus. Single-shelled or anomalous virus-like particles were observed within the CJ-treated system (Plate 2a ; arrows). Among positive control rotavirus infected MA-104 cell cultures, double shelled, ca. 70 nm, icosahedral “wheel-like” particles were observed in peripherally located cytoplasmic vesicles (Plate 2b). These particles were morphologically characteristic to those virions recognized within the genus Rotavirus of the family Reoviridae (Estes, 1991; Lipson et al., 2001a, Lipson et al., 2001b). These photomicrographs further suggest the broad efficacy of CJ as an antiviral agent, spanning the prokaryotic viral system to that of a mammalian enteric virus.
Plate 2.
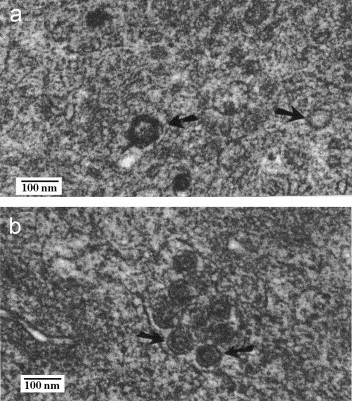
Effect of cranberry juice on the infection of MA-104 cells by rotavirus: transmission electron microscopy (TEM). One microliter rotavirus stock was added to an equal volume of CJ or PBS followed by incubation for 30 min at RT. CJ-treated and control suspensions were each added to MA-104 cell culture monolayers follow by incubation for 120 h at 37 °C. The monolayers were examined by TEM. (a) CJ-treated rotavirus. Micrographs reveal an absence of mature rotavirus particles with morphologically anomalous single shelled virus-like structures (arrows) in the MA-104 cytosol (×50,000). (b) Mature ca. 70 nm double-shelled “wheel-like” rotavirus particles (arrows) observed in cytoplasmic vesicles (×50,000).
CJ-mediated anti-rotavirus activity was further evident in the juice's ability to inhibit the hemagglutination reaction. A CJ concentration ⩾20% resulted in a total inhibition of hemagglutination (Plate 3a ). A reduced concentration of CJ (i.e., 12%) did not inhibit the rotavirus-induced hemagglutination reaction (Table 2 ). Non-CJ-treated rotavirus, as expected, resulted in a hemagglutination reaction (Plate 3b). PBS-treated GP-RBC's alone imparted no aggregative effect (Plate 3a,b). It is proposed that a modification by CJ of rotavirus glycoprotein spike moieties may have occurred, thereby preventing virus binding to its complementary cellular receptor sites. However, alteration or blockage of receptor sites on RBC's may not be excluded as a mechanism ascribed to the CJ-mediated inhibition of the hemagglutination reaction.
Plate 3.
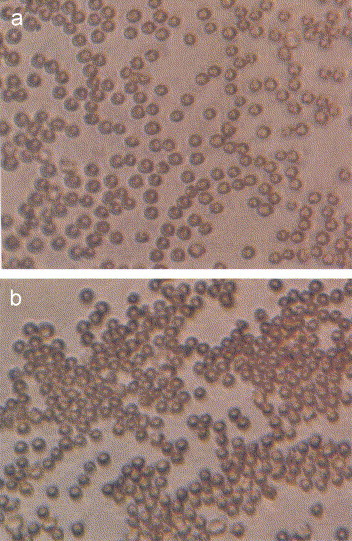
Inhibition of rotavirus-associated hemagglutination by cranberry juice. Rotavirus SA-11 was incubated with varying concentrations of cranberry juice (CJ). CJ-treated virus was added to a 0.7% suspension of guinea pig red blood cells (RBC's) and placed into round bottom microtiter plates. (a) CJ-treated rotavirus did not affect hemagglutinate (×400). (b) Hemagglutination occurred following inoculation of RBCs with untreated rotavirus (×400). Inoculation of the guinea pig RBCs with PBS yielded a pattern identical to that observed with the CJ-treated rotavirus.
Table 2.
Effect of cranberry juice (CJ) concentration on rotavirus SA-11-induced micro-hemagglutination
| Concentration of CJ (%) | Hemagglutination |
|
|---|---|---|
| Experimental | Control | |
| 10 μl (50%) | − − −a | +++b |
| 5.0 μl (33%) | − − − | +++ |
| 2.5 μl (20%) | − − − | +++ |
| 1.3 μl (12%) | +++ | +++ |
−, Negative hemagglutination reaction; virus diluted in PBS.
+, Positive hemagglutination reaction; virus plus cranberry juice.
At neat, the pH of all juices were essentially within one order of magnitude of each other [OJ (pH 3.7), GJ (pH 3.1), CJ (pH 2.9)]. Upon dilution with equal volumes of rotavirus and bacteriophage suspensions, pH readings for each juice were 4.2, 3.8, 3.6, and 3.9, 3.3, and 2.9, respectively. Importantly, rotavirus is stable at pH 3 for ca. 30 min and virtually unaffected at pH 4.0 (Weiss and Clark, 1985). Bacteriophage T4, moreover, is resistant to acid pH values ranging from pH 3.2 to 5.2) (Sabatino and Maier, 1980). Accordingly, system pH is not suggested to be a significant factor in the antivirus activity imparted by CJ to the rotavirus or the bacteriophage T4.
CJ has a higher concentration of sodium, carbohydrates, and sugars than OJ and GJ (Table 3 ). To attain the nutrient levels comparable to OJ and GJ, appropriate dilutions of CJ were performed. Reduced sodium, carbohydrate and sugars (by dilution with PBS without Ca2+ and Mg2+) did not modify the antiviral activity of CJ to bacteriophage T2 (data not shown). The higher concentration of solutes in CJ, which could result in an increase in osmotic pressure, does not appear to be a factor in antiviral activity of the CJ.
Table 3.
Nutrition per servinga
| Product | Sodium (mg) | Potassium (mg) | Carbohydrate (g) | Sugars (g) | Protein (g) | Iron (%) | Total fat |
|---|---|---|---|---|---|---|---|
| CJb | 35 | 30 | 33 | 33 | 0 | 2 | 0 |
| OJc | 0 | 450 | 26 | 22 | 2 | 0 | 0 |
| GJd | 0 | 300 | 22 | 20 | 1 | 0 | 0 |
Nutrient levels per serving identified on container packaging at 240 ml per serving. See Materials and methods for product description.
CJ, Cranberry juice cocktail.
OJ, Orange juice.
GJ, Grapefruit juice.
These data show, for the first time, non-specific antiviral activity by CJ upon unrelated viral species. The antiviral effect by CJ on bacteriophage T2 and, to a lesser extent, upon bacteriophage T4 effected a >90% loss of virus infectivity titer. This effect was rapid, dose-dependent, and appeared to be related to the adsorption stage of the viruses’ replication cycle. Antiviral activity by CJ was addressed further by testing with the simian rotavirus SA-11, a pathogenic primate enteric virus. CJ-treated rotavirus failed to effect a productive infection in its primate host cell cultures. In contrast to 5-day-old controls, characteristic ca. 70 nm “wheel-like” rotavirus particles were absent from CJ-treated rotavirus experimental eukaryotic system. CJ-mediated antiviral activity moreover, was observed by the juice's inhibitory effect upon the hemagglutination reaction. Further studies are needed to elucidate the mechanism(s) of our findings and, of equal importance, proceed to animal model systems.
Acknowledgments
We thank H.P. Lipson for proofreading the manuscript. This work was supported by a grant from the Cranberry Institute, Wareham, MA, and by a St. Francis College Faculty Research Award.
References
- Babich H., Stotzky G. Reduction in inactivation rates of bacteriophages by clay minerals in lake water. Water Res. 1980;14:185–187. [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degar S., Prince A.M., Pascual D., Levin B., Mazur Y., Lavie D., Ehrlich L.S., Carter C., Meruelo D. Inactivation of the human immunodeficiency virus by hypercin: evidence for photochemical alterations of p24 and a block is uncoating. AIDS Res. Hum. Retroviruses. 1992;8:1929–1936. doi: 10.1089/aid.1992.8.1929. [DOI] [PubMed] [Google Scholar]
- Estes M.K. Rotaviruses and their replication. In: Fields B.N., Knipe D.M., Chanock R.M., Hirsch M.S., Melnick J.L., Monath T.P., editors. Fundamental Virology. second ed. Raven Press; New York: 1991. pp. 619–642. [Google Scholar]
- Feng Y.Y., Ong S.L., Hu J.Y., Tan X.L., Ng W.J. Effects of pH and temperature on the survival of coliphages MS2 and QB. J. Ind. Microbiol. Biotechnol. 2003;30:549–552. doi: 10.1007/s10295-003-0080-y. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Information Center, 2005. International Resources. USDA and ARS, 〈http://www.nal.usda.gov/fnic/etext/000039.html〉.
- Gordon R.E., Solano D., Kleinerman J. Tight junction alterations of respiratory epithelium following long term NO2 exposure and recovery. Exp. Lung Res. 1986;11:l79–l93. doi: 10.3109/01902148609064295. [DOI] [PubMed] [Google Scholar]
- Jassim S.A.A., Naji M.A. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- Kalica A.R., James H.D., Kapikian A.Z. Hemagglutination by Simian rotavirus. J. Clin. Microbiol. 1978;7:314–315. doi: 10.1128/jcm.7.3.314-315.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konawalchuk J., Spears J.I. Antiviral effect of commercial juices and beverages. Appl. Environ. Microbiol. 1978;35:1219–1220. doi: 10.1128/aem.35.6.1219-1220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontiokari T., Laitinen J., Jarvi I., Pokkam T., Sunqvist K., Uhari M. Dietary factors protecting women from urinary tract infection. Am. J. Clin. Nutr. 2003;77:600–604. doi: 10.1093/ajcn/77.3.600. [DOI] [PubMed] [Google Scholar]
- Lipson, S.M., 1992. The neutralization test. In: Isenberg, H.D. (Ed.), Clinical Microbiology Procedures Handbook, vol. 2. American Society of Microbiology, Washington, DC, pp. 8.14.1–8.14.8.
- Lipson S.M., Alsmadi O. Enhancement of bacteriophage phiX-174 plaques by homoionic clay minerals. J. Gen. Microbiol. 1989;135:3497–3503. [Google Scholar]
- Lipson S.M., Stotzky G. Adsorption of reovirus to clay minerals: effect of cation exchange capacity, cation saturation, and surface area. Appl. Environ. Microbiol. 1983;46:673–682. doi: 10.1128/aem.46.3.673-682.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson S.M., Zelinsky-Papez K.A. Comparison of four latex agglutination (LA) and three enzyme-linked immunosorbent assays (ELISA) for the detection of rotavirus in fecal specimens. Am. J. Clin. Pathol. 1989;92:637–643. doi: 10.1093/ajcp/92.5.637. [DOI] [PubMed] [Google Scholar]
- Lipson S.M., Lipson, Poshni I.A., Ashley R.I. Presumptive identification of common adenoviruses by the appearance of differential cytopathic effects in human lung carcinoma (A549) cell cultures. FEMS Microbiol. 1993;113:175–182. doi: 10.1111/j.1574-6968.1993.tb06510.x. [DOI] [PubMed] [Google Scholar]
- Lipson S.M., Shaikh F., David K., Qian L. Detection of precytopathic effect of enteroviruses in clinical specimens by centrifugation-enhanced antigen detection. J. Clin. Microbiol. 2001;39:2755–2759. doi: 10.1128/JCM.39.8.2755-2759.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson S.M., Svenssen L., Goodwin L., Porti D., Danzi S., Pergolizzi R. Evaluation of two current generation enzyme immunoassays and an improved isolation-based assay for the rapid detection and isolation of rotavirus from stool. J. Clin. Virol. 2001;21:17–27. doi: 10.1016/s1386-6532(00)00181-5. [DOI] [PubMed] [Google Scholar]
- Meruelo D., Lavie G., Lavie D. Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: aromatic polycyclic diones hypericin and pseudohypericin. Proc. Natl. Acad. Sci. 1988;85:5230–5234. doi: 10.1073/pnas.85.14.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E., Jr., Kandaswami C., Theoharides T. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharm. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Nagai T., Moriguchi R., Suzuki Y., Tomimori T., Yamada H. Mode of action of the anti-influenza activity of plant flavonoid 5,7,4″-trihyroxy-8- methoxyflavone, from the roots of Scutellaris balcalensis. Antiviral Res. 1995;26:11–25. doi: 10.1016/0166-3542(94)00062-d. [DOI] [PubMed] [Google Scholar]
- Piraino F., Brandt C.R. Isolation and partial characterization of an antiviral, RC-183, from the edible mushroom Rozites caperata. Antiviral Res. 1999;43:67–78. doi: 10.1016/s0166-3542(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Sabatino C.M., Maier S. Differential inactivation of three bacteriophages by acid and alkaline pH used in the membrane adsorption-elution method of virus recovery. Can. J. Microbiol. 1980;26:1403–1407. doi: 10.1139/m80-233. [DOI] [PubMed] [Google Scholar]
- Schiffenbauer M., Stotzky G. Adsorption of coliphages T1 and T7 to clay minerals. Appl. Environ. Microbiol. 1982;43:590–596. doi: 10.1128/aem.43.3.590-596.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C., Clark H.F. Rapid inactivation of rotaviruses by exposure to acid or acidic gastric juice. J. Gen. Virol. 1985;66:2725–2730. doi: 10.1099/0022-1317-66-12-2725. [DOI] [PubMed] [Google Scholar]


