Abstract
Twelve dogs dead as consequence of natural infection caused by canine parvovirus (CPV) type 2a (n = 4), type 2b (n = 4) or type 2c (n = 4) were investigated for determining the viral DNA loads in different tissue samples. By means of a real-time PCR assay, CPV DNA was detected in all tissues examined, with the highest titres observed in the lymphoid tissue and the lowest loads in the urinary tract. Surprisingly, the nervous tissue was found to contain considerable amounts of CPV nucleic acid. Similar patterns of tissue distribution were observed in all the examined dogs irrespective of the antigenic variant causing the disease.
Keywords: Dog, Parvovirus, Real-time PCR, Tissue distribution
1. Introduction
Parvovirus was first described as a clinical entity causing haemorrhagic gastroenteritis in dogs in 1977 (Appel et al., 1979) and the aetiological agent was named Canine parvovirus type 2 (CPV-2) to distinguish it from the antigenically unrelated virus, the Canine parvovirus type 1 (CPV-1), also known as minute virus of canines. Virus infection spread rapidly worldwide causing high rate of mortality in pups (Carmichael and Binn, 1981). The dogs are infected through the oronasal route and after 3–10 days they develop an acute gastroenteritis characterised by loss of appetite, vomiting, fever, diarrhoea (from mucoid to haemorrhagic) and leukopenia.
CPV-2 contains a single-stranded DNA genome of about 5200 nucleotides, enclosed in an icosahedral capsid made up of two proteins, VP1 and VP2. Amino acids substitutions in VP2 sequence are known to cause changes in genetic and antigenic properties (Parrish, 1991, Truyen et al., 1994, Truyen et al., 1995). CPV-2 belongs to the feline parvovirus (FPV) subgroup of the genus Parvovirus, together with feline panleukopenia virus (FPLV), mink enteritis virus (MEV), raccoon parvovirus (RPV), raccoon dog parvovirus (RDPV) and blue fox parvovirus (BFPV) (Berns et al., 2000). Its origin is unknown, but it was suggested that it arose as a mutant from FPLV in dog or cat populations or from related viruses in wild carnivores (Truyen et al., 1995, Truyen et al., 1998, Truyen, 1999, Truyen, 2006).
After the first outbreak of CPV-2 infection, there were consecutive emergences of new antigenic variants of this virus, designated type 2a and 2b (Parrish et al., 1985, Parrish et al., 1991). Starting from 1979 and in a relatively short period, these variants replaced the original type 2 worldwide in dogs (Mochizuki et al., 1993, De Ybanez et al., 1995, Greenwood et al., 1996, Truyen et al., 1996, Truyen et al., 2000, Sagazio et al., 1998, Steinel et al., 1998, Buonavoglia et al., 2000, Pereira et al., 2000). CPV-2a and CPV-2b differ from the original type CPV-2 by amino acid changes affecting the VP2 protein and by their extended host range which includes canine and feline cells in vitro and dogs and cats in vivo. Subsequently, further mutations in the CPV capsid protein have been described in several countries, but there is no evidence for a further spread of these mutants (Ikeda et al., 2000). Analysis of Italian CPV isolates revealed the onset in 2000 of an unusual CPV-2 mutant with a change (Asp → Glu) occurring in the strategic residue 426 of the capsid (Buonavoglia et al., 2001). The Glu-426 mutant, also detected in other countries (Nakamura et al., 2004, Decaro et al., 2006d), is widely distributed in Italy and is currently replacing CPV-2b in the Italian dog population (Martella et al., 2004, Desario et al., 2005, Decaro et al., 2005d, Decaro et al., 2005e, Decaro et al., 2006b). In contrast with other mutations previously reported, such as Asp-300 and Pro-265, the mutation at residue 426 has probably provided the mutant with an evolutionary benefit. We are currently investigating whether this change really represents an advantage for viral spread and whether it has biological consequence (Decaro et al., 2005c). Since the mutation Asp426Glu affects the major antigenic region, that has been taken into account for classification of the variants 2a and 2b (Parrish et al., 1985, Parrish et al., 1991), the Glu-426 mutant has been referred to as new antigenic variant 2c (Decaro et al., 2005c, Decaro et al., 2006b).
Molecular methods have been developed to quantify the viral loads in the faeces of dogs infected with CPV-2 (Decaro et al., 2005e), characterise the CPV variants (Decaro et al., 2006b) and discriminate between vaccine and field strains (Decaro et al., 2006a, Decaro et al., 2006c).
In this study, by using real-time PCR, we investigated the pattern of distribution of the CPV variants in different tissues of dogs infected naturally.
2. Materials and methods
2.1. Dogs
The carcasses of twelve 3–4-month-old dogs, that had died as a consequence of single CPV infection within 4–5 days after the onset of the clinical signs, were selected from a previous study (Decaro et al., 2006b). All dogs were mixed-bred and had been housed in different animal shelters of Italy until the occurrence of disease or death. By using real-time PCR assays based on minor groove binder (MGB) probe technology (Decaro et al., 2006b), the dogs were found to be infected by CPV-2a (n = 4), CPV-2b (n = 4) or CPV-2c (n = 4). Traditional and molecular methods ruled out co-infections by other common pathogens of dogs, such as Bordetella bronchiseptica, Pasteurella multocida, Leptospira interrogans (Gravekamp et al., 1993), reoviruses (Leary et al., 2002, Decaro et al., 2005b), rotaviruses (Gouvea et al., 1994), caliciviruses (Jiang et al., 1999, Marsilio et al., 2005), canine adenoviruses (Hu et al., 2001), canine distemper virus (Elia et al., 2006), canid herpesvirus (Schulze and Baumgartner, 1998), canine coronavirus (CCoV) (Decaro et al., 2004).
2.2. Sampling and sample preparation
Faecal samples from the rectal ampulla and tissue samples were collected from the dead dogs, including brain, cerebellum, cerebral bulb, tonsils, retropharyngeal and mesenteric lymph nodes, thymus, myocardium, lungs, liver, spleen, kidneys, bladder, bone marrow, jejunum, colon, rectum. Each tissue sample was withdrawn using disposable sterile scalpels.
Faecal samples were homogenised (10%, w/v) in phosphate buffered saline (PBS, pH 7.2) and subsequently clarified by centrifuging at 1500 × g for 15 min. DNA was extracted from faecal homogenates by boiling for 10 min and chilling on ice (Schunck et al., 1995, Uwatoko et al., 1995), whereas nucleic acid purification from tissue samples was achieved by using the DNeasy Tissue Kit (Qiagen S.p.A., Milan, Italy), followed by final elution of DNA with 200 μl of AE buffer and storage at −70 °C until use. To reduce residual inhibitors of DNA polymerase activity to ineffective concentrations, the DNA extracts from both faecal and tissue samples were diluted 1:10 in distilled water (Decaro et al., 2005e).
2.3. Real-time PCR with TaqMan probe for quantitation of CPV-2 DNA
The DNA extracts were tested by a TaqMan assay able to recognise all the CPV-2 strains (Decaro et al., 2005e). The assay is internally controlled by using as exogenous DNA the nucleic acid extracted from ovine herpesvirus type 2 (Decaro et al., 2003). The reaction of real-time PCR (25 μl) contained 12.5 μl of master mix (Bio-Rad Laboratories Srl, Milan, Italy), 600 nM of primers (5′-AAACAGGAATTAACTATACTAATATATTTA-3′) and CPV-Rev (5′-AAATTTGACCATTTGGATAAACT-3′), 200 nM of probe CPV-Pb (5′-TGGTCCTTTAACTGCATTAAATAATGTACC-3′) and 10 μl of standard or template DNA. All standard dilutions and unknown samples were tested in duplicate. The following thermal protocol was used: activation of iTaq DNA polymerase at 95 °C for 10 min and 40 cycles consisting of denaturation at 95 °C for 15 s, primer annealing at 52 °C for 30 s and extension at 60 °C for 1 min.
3. Results
The viral DNA titres found in the different tissues of the dogs infected naturally by types 2a, 2b and 2c are reported in Fig. 1 . CPV-2 DNA was demonstrated in all tissues analysed, showing a very wide distribution of the virus in the organism. The highest viral loads were detected in the lymphoid tissues, with maximal titres observed in the tonsils of dogs infected with CPV-2c (median titre = 8.98 × 108 DNA copies/10 μl of template) and in the spleen of dogs infected with CPV-2b (median titre = 7.33 × 108 DNA copies/10 μl of template). Very high DNA titres were observed in the bone marrow (median titre of 4.39 × 108 in dogs infected with CPV-2a). The urinary tract was found to contain the lowest CPV-2 DNA amounts, with median titres of about 105 DNA copies/10 μl of template in the bladder of all examined dogs. Surprisingly, viral DNA was detected also in the nervous tissues, including brain, cerebellum and cerebral bulb, with median titres above 106 DNA copies/10 μl of template in all dogs. Faecal samples contained lower viral quantities than internal organs, with titres ranging between 3.71 × 107 and 8.10 × 107 DNA copies/10 μl of template for types 2c and 2a, respectively.
Fig. 1.
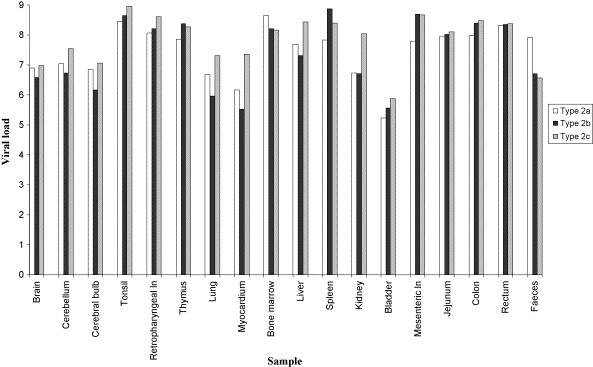
Viral DNA loads detected by real-time PCR in tissues of dogs infected naturally by the antigenic variants of CPV-2. Viral titres are expressed as median log10 DNA copy numbers/10 μl of template. Ln, lymph node.
4. Discussion
CPV infects dogs through the oronasal route and reaches the intestinal mucosa after an initial spread to lymphoid tissues (Appel and Parrish, 1987). Viraemia may reach very high titres of viral DNA and persist for several weeks, even after the virus has disappeared from the intestinal content (Decaro et al., unpublished data). In this study, all tissues analysed were shown to contain CPV DNA, probably as a consequence of viral spread in the organism through the blood. The number of dogs was limited, with only four dogs analysed per each of the three CPV variants. In fact, in order to limit the variability of viral titres due to different ages, breeds and clinical courses of the disease, only pups were chosen that were mixed-bred, had approximately the same age and had died after a similar duration of disease (4–5 days) as a consequence of single CPV infection. Moreover, it was difficult to recruit dogs dead due to infection with CPV-2b, since this variant is disappearing progressively from the Italian dog population (Martella et al., 2004, Martella et al., 2005, Decaro et al., 2005c, Decaro et al., 2005e, Decaro et al., 2006a), and only one type 2b strain has been detected in Italy in 2006 (Decaro et al., unpublished data).
Tissue distribution of CPV was found to have similar patterns in dogs infected by types 2a, 2b and 2c, revealing that the variants have the same biological behaviour. Parvovirus replication in dogs and cats takes place mainly in highly mitotically active tissues, such as bone marrow, lymphoid organs and intestinal crypts (Appel and Parrish, 1987). Involvement of the nervous tissues has been described in cats (Csiza et al., 1972, Wilcox et al., 1984, Url et al., 2003), whereas in dogs CPV antigen has been never detected in neurons, despite the presence of neurodegeneration (Agungpriyono et al., 1999, Url and Schmidt, 2005).
In contrast with previous studies, we have demonstrated the presence at high titres of CPV nucleic acid in all tissues examined including brain, cerebellum and bulb. Whether the presence of viral DNA is associated to effective replication and expression of viral proteins in neurons should be assessed by identification of CPV antigens using supplementary techniques, such as immunohistochemistry.
CPV DNA titres detected in the faeces were generally lower than those observed in lymphoid organs. It has been shown previously that shedding of CPV DNA in the faeces reaches maximal loads in the first days after infection (with a peak at 7–8 days post-infection), with a rapid decrease already at 10–11 days post-infection (Decaro et al., 2005a, Elia et al., 2005). Moreover, in the late stage of infection the high antibody levels in the gut lumen may sequestrate most of the virions, so that traditional tests, such as haemagglutination and virus isolation, give frequently false negative results (Decaro et al., 2005e, Desario et al., 2005). The results of the present work indicate that in laboratories where molecular methods are not employed routinely, traditional diagnostic methods for detection of CPV in dead dogs should be carried out on the internal organs rather than on the faeces or intestinal contents.
References
- Agungpriyono D.R., Uchida K., Tabaru H., Yamaguchi R., Tateyama S. Subacute massive necrotizing myocarditis by canine parvovirus type 2 infection with diffuse leukoencephalomalacia in a puppy. Vet. Pathol. 1999;36:77–80. doi: 10.1354/vp.36-1-77. [DOI] [PubMed] [Google Scholar]
- Appel M.J.G., Scott W.F., Carmichael L.E. Isolation and immunization studies of canine parvo-like virus from dogs with haemorrhagic enteritis. Vet. Rec. 1979;105:156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Appel M.J.G., Parrish C.R. Canine parvovirus type 2. In: Appel M.J., editor. vol. 1. Elsevier; New York: 1987. pp. 69–92. (Virus Infections of Carnivores). [Google Scholar]
- Berns K.I., Bergoin M., Bloom M., Lederman M., Muzyczka N., Siegl G., Tal J., Tattersall P. Family parvoviridae. In: van Regenmortel M.H.V., Fauquet C.M., Bishop D.H.L., Carstens E.B., Estes M.K., Lemon S.M., Maniloff J., Mayo M.A., McGeoch D.J., Pringle C.R., Wickner R.B., editors. Virus Taxonomy, Classification and Nomenclature of Viruses. Academic Press; New York: 2000. pp. 311–323. [Google Scholar]
- Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L.E. Evidence for evolution of canine parvovirus type-2 in Italy. J. Gen. Virol. 2001;82:1555–1560. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- Buonavoglia D., Cavalli A., Pratelli A., Martella V., Greco G., Tempesta M., Buonavoglia C. Antigenic analysis of canine parvovirus strains isolated in Italy. New Microbiol. 2000;23:93–96. [PubMed] [Google Scholar]
- Carmichael L.E., Binn L.N. New enteric viruses in the dog. Adv. Vet. Sci. Comp. Med. 1981;25:1–37. [PubMed] [Google Scholar]
- Csiza C.K., Scott F.W., De Lahunta A., Gillespie J.H. Respiratory signs and central nervous system lesions in cats infected with panleukopenia virus. A case report. Cornell Vet. 1972;62:192–195. [PubMed] [Google Scholar]
- De Ybanez R.R., Vela C., Cortes E., Simarro I., Casal J.I. Identification of types of canine parvovirus circulating in Spain. Vet. Rec. 1995;136:174–175. doi: 10.1136/vr.136.7.174. [DOI] [PubMed] [Google Scholar]
- Decaro N., Tinelli A., Pratelli A., Martella V., Tempesta M., Buonavoglia C. First two confirmed cases of malignant catarrhal fever in Italy. New Microbiol. 2003;26:339–344. [PubMed] [Google Scholar]
- Decaro N., Pratelli A., Campolo M., Elia G., Martella V., Tempesta M., Buonavoglia C. Quantitation of canine coronavirus RNA in the faeces of dogs by TaqMan RT-PCR. J. Virol. Meth. 2004;119:145–150. doi: 10.1016/j.jviromet.2004.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Desario C., Elia G., Martella V., Lorusso E., Buonavoglia C. Maternally-derived antibodies in pups and protection from canine parvovirus infection. Biologicals. 2005;33:261–267. doi: 10.1016/j.biologicals.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Desario C., Ricci D., Camero M., Lorusso E., Elia G., Lavazza A., Martella V., Buonavoglia C. Virological and molecular characterization of a Mammalian orthoreovirus type 3 strain isolated from a dog in Italy. Vet. Microbiol. 2005;109:19–27. doi: 10.1016/j.vetmic.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Desario C., Campolo M., Elia G., Martella V., Ricci D., Lorusso E., Buonavoglia C. Clinical and virological findings in pups naturally infected by canine parvovirus type 2 Glu-426 mutant. J. Vet. Diagn. Invest. 2005;17:133–138. doi: 10.1177/104063870501700206. [DOI] [PubMed] [Google Scholar]
- Decaro N., Elia G., Campolo M., Desario C., Lucente M.S., Bellacicco A.L., Buonavoglia C. New approaches for the molecular characterization of canine parvovirus type 2 strains. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2005;52:316–319. doi: 10.1111/j.1439-0450.2005.00869.x. [DOI] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Desario C., Campolo M., Di Trani L., Tarsitano E., Tempesta M., Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 DNA in the feces of dogs. Vet. Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Decaro N., Elia G., Desario C., Roperto S., Martella V., Campolo M., Lorusso A., Cavalli A., Buonavoglia C. A minor groove binder probe real-time PCR assay for discrimination between type 2-based vaccines and field strains of canine parvovirus. J. Virol. Meth. 2006;136:65–70. doi: 10.1016/j.jviromet.2006.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Elia G., Martella V., Campolo M., Desario C., Camero M., Cirone F., Lorusso E., Lucente M.S., Narcisi D., Scalia P., Buonavoglia C. Characterisation of the canine parvovirus type 2 variants using minor groove binder probe technology. J. Virol. Meth. 2006;133:92–99. doi: 10.1016/j.jviromet.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Decaro N., Martella V., Elia G., Desario C., Campolo M., Buonavoglia D., Bellacicco A.L., Tempesta M., Buonavoglia C. Diagnostic tools based on minor groove binder technology for rapid identification of vaccine and field strains of canine parvovirus type 2b. J. Virol. Meth. 2006;138:10–16. doi: 10.1016/j.jviromet.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Decaro N., Martella V., Desario C., Bellacicco A.L., Camero M., Manna L., D’aloja D., Buonavoglia C. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2006;53:468–472. doi: 10.1111/j.1439-0450.2006.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desario C., Decaro N., Campolo M., Cavalli A., Cirone F., Elia G., Martella V., Lorusso E., Camero M., Buonavoglia C. Canine parvovirus infection: which diagnostic test for virus? J. Virol. Meth. 2005;121:179–185. doi: 10.1016/j.jviromet.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Elia G., Cavalli A., Cirone F., Lorusso E., Camero M., Buonavoglia D., Tempesta M. Antibody levels and protection to canine parvovirus type 2. J. Vet. Med. B. 2005;52:320–322. doi: 10.1111/j.1439-0450.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L., Buonavoglia C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Meth. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Gouvea V., Santos N., Timenetsky M.doC. Identification of bovine and porcine rotavirus G types by PCR. J. Clin. Microbiol. 1994;32:1338–1340. doi: 10.1128/jcm.32.5.1338-1340.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravekamp C., Van de Kemp H., Franzen M., Carrington D., Schoone G.J., Van Eys G.J., Everard C.O., Hartskeerl R.A., Terpstra W.J. Detection of seven species of pathogenic leptospires by PCR using two sets of primers. J. Gen. Microbiol. 1993;139:1691–1700. doi: 10.1099/00221287-139-8-1691. [DOI] [PubMed] [Google Scholar]
- Greenwood N.M., Chalmers W.S.K., Baxendale W., Thompson H. Comparison of isolates of canine parvovirus by monoclonal antibody and restriction-enzyme analysis. Vet. Rec. 1996;138:495–496. doi: 10.1136/vr.138.20.495. [DOI] [PubMed] [Google Scholar]
- Hu R.L., Huang G., Qiu W., Zhong Z.H., Xia X.Z., Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet. Res. Commun. 2001;25:77–84. doi: 10.1023/a:1006417203856. [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Mochizuki M., Naito R., Nakamura K., Myazawa T., Mikami T., Takahashi E. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology. 2000;278:13–19. doi: 10.1006/viro.2000.0653. [DOI] [PubMed] [Google Scholar]
- Jiang X., Huang P.W., Zhong W.M., Farkas T., Cubitt D.W., Matson D.O. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Meth. 1999;83:145–154. doi: 10.1016/s0166-0934(99)00114-7. [DOI] [PubMed] [Google Scholar]
- Leary P.L., Erker J.C., Chalmers M.L., Cruz A.T., Wetzel J.D., Desai S.M., Mushahwar I.K., Dermody T.S. Detection of mammalian reovirus RNA by using reverse transcription-PCR: sequence diversity within the λ3-encoding L1 gene. J. Clin. Microbiol. 2002;40:1368–1375. doi: 10.1128/JCM.40.4.1368-1375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsilio F., Di Martino B., Decaro N., Buonavoglia C. Nested PCR for the diagnosis of calicivirus infections in the cat. Vet. Microbiol. 2005;105:1–7. doi: 10.1016/j.vetmic.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Martella V., Cavalli A., Pratelli A., Bozzo G., Camero M., Buonavoglia D., Narcisi D., Tempesta M., Buonavoglia C. A canine parvovirus mutant is spreading in Italy. J. Clin. Microbiol. 2004;42:1333–1336. doi: 10.1128/JCM.42.3.1333-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martella V., Decaro N., Elia N., Buonavoglia C. Surveillance activity for canine parvovirus in Italy. J. Vet. Med. B: Infect. Dis. Vet. Public Health. 2005;52:312–315. doi: 10.1111/j.1439-0450.2005.00875.x. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Harasawa R., Nakatami H. Antigenic and genomic variabilities among recently prevalent parvoviruses of canine and feline origin in Japan. Vet. Microbiol. 1993;38:1–10. doi: 10.1016/0378-1135(93)90070-n. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Tohya Y., Miyazawa T., Mochizuki M., Phung H.T., Nguyen N.H., Huynh L.M., Nguyen L.T., Nguyen P.N., Nguyen P.V., Nguyen N.P., Akashi H. A novel antigenic variant of canine parvovirus from a Vietnamese dog. Arch. Virol. 2004;149:2261–2269. doi: 10.1007/s00705-004-0367-y. [DOI] [PubMed] [Google Scholar]
- Parrish C.R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991;183:195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- Parrish C.R., O’Connel P.H., Evermann J.F., Carmichael L.E. Natural variation of canine parvovirus. Science. 1985;230:1046–1048. doi: 10.1126/science.4059921. [DOI] [PubMed] [Google Scholar]
- Parrish C.R., Aquadro C.F., Strassheim M.L., Evermann J.F., Sgro J.-Y., Mohammed H.O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 1991;65:6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C.A., Monezi T.A., Mehnert D.U., D’Angelo M., Durigon E.L. Molecular characterisation of canine parvovirus in Brazil by polymerase chain reaction assay. Vet. Microbiol. 2000;75:127–133. doi: 10.1016/s0378-1135(00)00214-5. [DOI] [PubMed] [Google Scholar]
- Sagazio P., Tempesta M., Buonavoglia D., Cirone F., Buonavoglia C. Antigenic characterization of canine parvovirus strains isolated in Italy. J. Virol. Meth. 1998;73:197–200. doi: 10.1016/s0166-0934(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Schulze C., Baumgartner W. Nested polymerase chain reaction and in situ hybridization for diagnosis of canine herpesvirus infection in puppies. Vet. Pathol. 1998;35:209–217. doi: 10.1177/030098589803500306. [DOI] [PubMed] [Google Scholar]
- Schunck B., Kraft W., Truyen U. A simple touch-down polymerase chain reaction for the detection of canine parvovirus and feline panleukopenia virus in feces. J. Virol. Meth. 1995;55:427–433. doi: 10.1016/0166-0934(95)00069-3. [DOI] [PubMed] [Google Scholar]
- Steinel A., Venter E.H., van Vuuren M., Truyen U. Antigenic and genetic analysis of canine parvoviruses in southern Africa. Ondersteeport J. Vet. Res. 1998;65:239–242. [PubMed] [Google Scholar]
- Truyen U. Emergence and recent evolution of canine parvovirus. Vet. Microbiol. 1999;69:47–50. doi: 10.1016/s0378-1135(99)00086-3. [DOI] [PubMed] [Google Scholar]
- Truyen U. Evolution of canine parvovirus—a need for new vaccines? Vet. Microbiol. 2006;117:9–13. doi: 10.1016/j.vetmic.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Truyen U., Agbandje M., Parrish C.R. Characterization of the feline host range and a specific epitope of feline panleukopenia virus. Virology. 1994;200:494–503. doi: 10.1006/viro.1994.1212. [DOI] [PubMed] [Google Scholar]
- Truyen U., Gruenberg A., Chang S.-F., Obermaier B., Veijalainen P., Parrish C.R. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J. Virol. 1995;69:4702–4710. doi: 10.1128/jvi.69.8.4702-4710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen U., Platzer G., Parrish C.R. Antigenic type distribution among canine parvoviruses in dogs and cats in Germany. Vet. Rec. 1996;138:365–366. doi: 10.1136/vr.138.15.365. [DOI] [PubMed] [Google Scholar]
- Truyen U., Müller T., Heidrich R., Tackmann K., Carmichael L.E. Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus. Epidemiol. Infect. 1998;121:433–440. doi: 10.1017/s0950268898001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truyen U., Steinel A., Bruckner L., Lutz H., Mostl K. Distribution of antigenic types of canine parvovirus in Switzerland, Austria and Germany. Schweiz. Arch. Tierheilkd. 2000;142:115–119. [PubMed] [Google Scholar]
- Url A., Truyen U., Rebel-Bauder B., Weissenbock H., Schmidt P. Evidence of parvovirus replication in cerebral neurons of cats. J. Clin. Microbiol. 2003;41:3801–3805. doi: 10.1128/JCM.41.8.3801-3805.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Url A., Schmidt P. Do canine parvoviruses affect canine neurons? An immunohistochemical study. Res. Vet. Sci. 2005;79:57–59. doi: 10.1016/j.rvsc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Uwatoko K., Sunairi M., Nakajima M., Yamaura K. Rapid method utilizing the polymerase chain reaction for detection of canine parvovirus in feces of diarrhoeic dogs. Vet. Microbiol. 1995;43:315–323. doi: 10.1016/0378-1135(94)00102-3. [DOI] [PubMed] [Google Scholar]
- Wilcox G.E., Flower R.L., Cook R.D. Recovery of viral agents from the central nervous system of cats. Vet. Microbiol. 1984;9:355–366. doi: 10.1016/0378-1135(84)90004-x. [DOI] [PubMed] [Google Scholar]


