Abstract
Analysis of virus–host interactions has revealed a variety of ways in which viruses utilize and/or alter host functions in an effort to facilitate efficient replication. Recent work has suggested that certain RNA viruses that replicate in the cytoplasm disrupt the normal trafficking of cellular RNAs and proteins within the host cell. This review will examine the recent evidence showing that poliovirus and vesicular stomatitis virus (VSV) can inhibit nucleo-cytoplasmic transport within cells. Interestingly, the data indicate that inhibition by both viruses involves targeting components of the nuclear pore complex (NPC). Following this, several possible explanations for why viruses might disrupt nucleo-cytoplasmic transport are discussed. Finally, the possibility that disruption of nucleo-cytoplasmic trafficking may be a more common feature of RNA virus–host interactions than previously thought is examined.
Keywords: Vesicular stomatitis virus, Nuclear pore complex, Nucleo-cytoplasmic transport, Poliovirus, Rhinovirus
1. Introduction
Nucleo-cytoplasmic trafficking is critical for many cellular processes including transcription, splicing, translation and the control of cell growth (reviewed in Nakielny and Dreyfuss, 1999) Cargos to be transported across the nuclear envelope contain a variety of different sequence motifs that are collectively called nuclear localization signals (NLS) for cargos that are to be imported into the nucleus, and nuclear export signals (NES) for cargos that are to be exported out of the nucleus. Each NLS (or NES) is recognized by a specific cellular receptor, and this NLS:receptor pair is said to represent a unique transport pathway (Strom and Weis, 2001). Transport receptors are classified as importins or exportins depending upon whether they mediate nuclear import or export.
To deliver cargos to the appropriate cellular compartment, all transport pathways must traverse a large, macromolecular structure called the nuclear pore complex (NPC) that is found embedded in the nuclear envelope (Fig. 1 ). The vertebrate NPC is composed of at least 30 different proteins and has a molecular weight of over 125 MDa (Cronshaw et al., 2002, Stoffler et al., 1999). Many of the proteins that make up the NPC contain a repeating FG amino acid motif and are thus defined as a family of proteins called nucleoporins (Nups) (Ryan and Wente, 2000). The directionality of nucleo-cytoplasmic transport is thought to be provided by the small GTPase Ran, which is predicted to be in a GDP bound state in the cytoplasm, and in a GTP bound form in the nucleus (reviewed in Bischoff et al., 2002). Ran-GTP destabilizes import receptor:cargo complexes in the nucleus resulting in the release of cargo into the nucleoplasm. Conversely, binding of export receptors to cargos is stabilized by Ran-GTP and release of cargo occurs following hydrolysis of Ran-GTP in the cytoplasm.
Fig. 1.
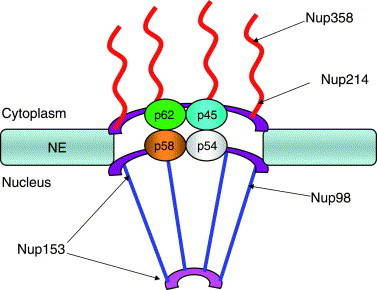
Schematic representation of the NPC. Major structural features discussed in the text are illustrated, including cytoplasmic fibrils and the nuclear basket. Approximate location of various Nups are shown. NE, nuclear envelope.
Many viruses that replicate in the nucleus are ultimately dependent upon nucleo-cytoplasmic transport for successful replication. Following entry, viral genomes must be imported into the nucleus for transcription to take place. Viral mRNAs must then be exported to the cytoplasm for protein synthesis to occur, and viral proteins are then imported into the nucleus where they can participate in viral replication and assembly. Finally, the replicated viral genomes must be exported to the cytoplasm. As would be expected for a process so critical to successful replication, a number of strategies employed by viruses to utilize this machinery have been described (reviewed in Knipe et al., 2001).
In contrast, only a few examples exist of viruses inhibiting nucleo-cytoplasmic trafficking. For example, at late times during infection adenovirus mRNAs are efficiently exported to the cytoplasm while the export of cellular mRNAs is inhibited (Beltz and Flint, 1979). The export of viral mRNA appears to be due to the shuttling ability of the E4 34 kDa protein in combination with the RNA binding activity of the E1B-55 kDa protein (Dobbelstein et al., 1997). While these same two proteins are responsible for the inhibition of cellular mRNA export, the underlying mechanisms responsible for this are not known (Babiss and Ginsberg, 1984, Babiss et al., 1985, Halbert et al., 1985, Pilder et al., 1986, Weinberg and Ketner, 1986). The influenza virus NS1 protein also selectively inhibits export of host mRNAs to the cytoplasm (Alonso-Caplen et al., 1992, Fortes et al., 1994). Inhibition of transport by NS1 appears to be due to its ability to impair 3′ end formation of cellular mRNAs through interaction with CPSF and PAB II (Chen et al., 1999, Nemeroff et al., 1998). Two other examples of viral inhibition of nucleo-cytoplasmic trafficking have been reported (Gustin and Sarnow, 2001, Her et al., 1997). Interestingly, both of these cases involve RNA viruses that replicate entirely within the cytoplasm and involve interactions with the NPC.
2. Poliovirus induced inhibition of nuclear import
Poliovirus is a positive stranded RNA virus that replicates in the cytoplasm of infected cells. Despite the cytoplasmic location of the viral replicative cycle, numerous reports suggest that host nuclear factors may play a role in picornavirus replication. For example, the cellular proteins La, Sam68, nucleolin and polypyrimidine tract binding protein (PTB) have all been shown to interact with poliovirus RNA or proteins (Borman et al., 1993, Hellen et al., 1993, McBride et al., 1996, Meerovitch et al., 1993, Waggoner and Sarnow, 1998). La, Sam68, nucleolin and PTB are predominantly nuclear in uninfected cells, but following infection with poliovirus they accumulate in the cytoplasm (Table 1 and Back et al., 2002, McBride et al., 1996, Meerovitch et al., 1993, Waggoner and Sarnow, 1998). The accumulation of these proteins in the cytoplasm of cells could be brought about by a variety of mechanisms, however, recent data suggests that poliovirus inhibits the nuclear import of these cellular factors, resulting in their accumulation in the cytoplasm of cells.
Table 1.
Cellular nuclear proteins that accumulate in the cytoplasm of poliovirus and rhinovirus-infected cells
| Nuclear protein | Cellular functionsa | Viral targets | Function in viral replication | Referencesb |
|---|---|---|---|---|
| Nucleolin | rRNA transcription and processing, Pol II transcription, mRNA stability | 3′ NCR of poliovirus genome | RNA synthesis? | Waggoner and Sarnow, 1998, Gustin and Sarnow, 2002 |
| hnRNP K | Translational silencing, mRNA stability | ? | ? | Gustin and Sarnow, 2001, Gustin and Sarnow, 2002 |
| PTBc | Splicing, polyadenylation | IRES of entero, rhino and cardiovirus genomes | Translation | Back et al. (2002) |
| La | Pol III transcription, processing | IRES of entero, rhino and cardiovirus genomes | Translation | Meerovitch et al., 1993, Gustin and Sarnow, 2002 |
| hnRNP A1 | mRNA transport, splicing | ? | ? | Gustin and Sarnow, 2001, Gustin and Sarnow, 2002 |
| hnRNP C | Splicing, mRNA stability | ? | ? | Gustin and Sarnow, 2001, Gustin and Sarnow, 2002 |
| Sam68 | cell cycle control?, mRNA transport | 3Dpol of poliovirus | ? | McBride et al., 1996, Gustin and Sarnow, 2002 |
Reported functions of indicated protein in uninfected cells.
Reference demonstrating cytoplasmic accumulation of nuclear protein in infected cells.
PTB has not been shown to accumulate in the cytoplasm of rhinovirus-infected cells.
The initial observation that poliovirus might inhibit nuclear import came from the analysis of a green fluorescent protein (GFP) fused to a classical nuclear localization signal (GFP-NLS). The “classical” NLS is so called because it was one of the first identified and is the best characterized. Nuclear import via the classical pathway is mediated by a heterodimer, consisting of the NLS-binding adapter importin α1 and the receptor importin β1 that mediates transport of the cargo-receptor complex through the NPC (reviewed in Izaurralde and Adam, 1998, Mattaj and Englmeier, 1998, Nakielny and Dreyfuss, 1999). Fusion of the classical NLS with GFP results in a protein that is predominantly nuclear in uninfected cells (Fig. 2 A). Following infection with poliovirus, however, this protein was observed to accumulate in the cytoplasm, similar to what was observed for La, Sam68, nucleolin and PTB (Fig. 2B and Belov et al., 2000, Gustin and Sarnow, 2001). These findings indicated that poliovirus infection inhibited the nuclear accumulation of GFP-NLS and suggested that the classical nuclear import pathway was impaired.
Fig. 2.
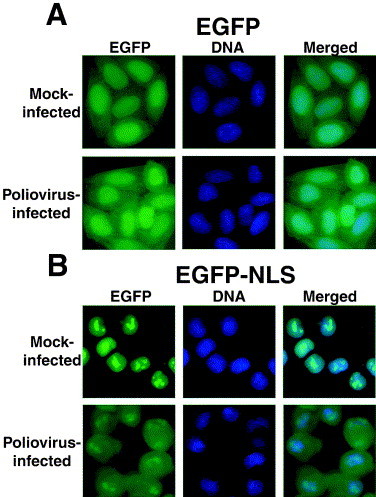
Relocalization of EGFP-NLS molecules in poliovirus-infected cells. (A) HeLa cells stably expressing EGFP were mock-infected or infected with poliovirus as indicated. Cells were processed and examined by fluorescent microscopy at 4.5 h after infection. EGFP fluorescence was visualized using a FITC filter. DNA: Hoechst-stained nuclei were examined with a UV filter. Merged: shows the FITC and Hoechst images merged. (B) HeLa cells stably expressing EGFP-NLS fusion proteins were examined as described in (A). Adapted from Gustin and Sarnow (2001), with permission.
Using a similar approach, the status of the transportin import pathway was evaluated in poliovirus-infected cells. Transportin is the import receptor utilized by a number of different hnRNPs and was identified through its interaction with the M9 NLS of hnRNP A1 (Pollard et al., 1996). The M9 NLS is a glycine-rich sequence that confers both nuclear import and export activity (Michael et al., 1995). Cargos containing the M9 NLS are imported to the nucleus via interaction with transportin, while the receptor responsible for export has not been identified (Izaurralde and Adam, 1998, Nakielny and Dreyfuss, 1999). Fusion of the M9 NLS to GFP (GFP-M9) results in a protein that is predominantly nuclear in uninfected cells. When the distribution of GFP-M9 was examined in poliovirus-infected cells, however, a significant amount was observed to accumulate in the cytoplasm (Gustin and Sarnow, 2001). Relocalization required a functional NLS, as a GFP-M9 molecule containing a single amino acid substitution that disrupts interaction with transportin failed to redistribute in infected cells (Gustin and Sarnow, 2001). In addition, endogenous cargos of the transportin pathway, such as hnRNP A1 relocalized to the cytoplasm of infected cells (Gustin and Sarnow, 2001). Cumulatively, these results indicated that a second import pathway, that of transportin, was inhibited in poliovirus-infected cells.
Analysis of the distribution of hnRNP K in poliovirus-infected cells suggested that a third import pathway might be disrupted. Like hnRNP A1, hnRNP K is a nuclear protein that shuttles between the nuclear and cytoplasmic compartment. Shuttling of hnRNP K is mediated by an amino acid motif termed the K nuclear shuttling (KNS) signal (Michael et al., 1997). The import and export receptors that recognize the KNS signal have not been identified but competition experiments indicate that they are distinct from those used by the classical or transportin import pathways (Michael et al., 1997). The trafficking of hnRNP K was examined in infected cells by indirect immunofluorescence. The results of this analysis demonstrated that hnRNP K relocalized from the nucleus to the cytoplasm of cells following infection with poliovirus, and suggested that, in addition to the classical and transportin pathways, the KNS import pathway was disrupted during poliovirus infection (Gustin and Sarnow, 2001).
The findings described above suggest that the classical, transportin and KNS import pathways are disrupted during poliovirus infection and provide a possible explanation for the cytoplasmic accumulation of nuclear factors. For example, nucleolin has a classical NLS (Schmidt-Zachmann and Nigg, 1993) and the import of La has been recently shown to require components of the classical pathway (Rosenblum et al., 1998). Thus, disruption of the classical import pathway would be predicted to result in the accumulation of nucleolin and La in the cytoplasm. The import of Sam68 (Ishidate et al., 1997) and PTB (Romanelli et al., 1997), on the other hand, appears to involve novel import pathways. This raises the interesting possibility that other import pathways, in addition to the ones mentioned above, may be disrupted during poliovirus infection.
Despite this possibility, poliovirus infection does not inhibit all trafficking pathways between the nucleus and the cytoplasm. This was demonstrated by monitoring the transport of a fusion protein consisting of the glucocorticoid hormone binding domain (GC), the HIV-1 Rev protein and GFP (REV–GC–GFP) (Love et al., 1998). The GC confers hormone dependent nuclear import upon heterologous proteins (Picard and Yamamoto, 1987). Consequently, in the absence of hormone ligand the REV–GC–GFP fusion protein is restricted to the cytoplasm, but following the addition of hormone it is rapidly (within 30 min) imported into the nucleus (Fig. 3 A and Love et al., 1998). Although the receptor responsible for nuclear import of the GC has not been identified, import does not require importin α, suggesting that a non-classical pathway is used (Savory et al., 1999). The HIV-1 REV protein mediates nuclear export of REV–GC–GFP due to the presence of a leucine-rich NES that interacts with the cellular export receptor, Crm1 (Fischer et al., 1995). The hormone induced import of REV–GC–GFP is reversible and following the removal of hormone, REV–GC–GFP is rapidly exported to the cytoplasm due to the Rev NES (Fig. 3B and Love et al., 1998).
Fig. 3.
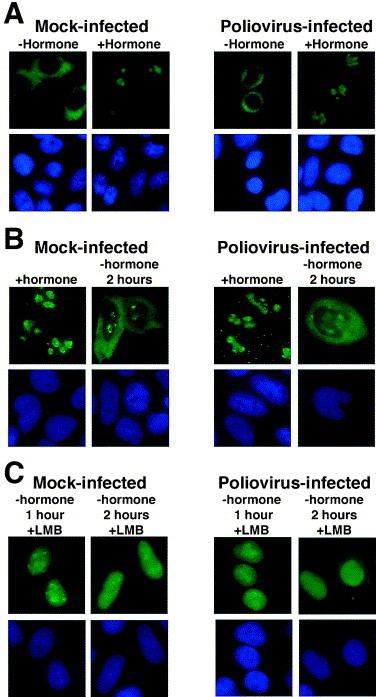
Nucleo-cytoplasmic trafficking of a glucocorticoid receptor-HIV REV fusion protein proceeds normally in poliovirus-infected cells. (A) HeLa cells were transiently transfected with a plasmid that encodes the REV–GC–GFP fusion protein; after 40 h, cells were either mock-infected (Mock-infected) or infected with poliovirus (Poliovirus-infected). Four hours after infection cells were untreated (−hormone) or treated with dexamethasone (+hormone) at 1 μM for 30 min prior to fixation. (B) Cells were transfected as described above and 40 h later were either mock-infected or infected with poliovirus and dexamethasone was added. Two hours later the cells were either fixed (+hormone), or dexamethasone was removed by washing once with PBS and adding fresh medium. Cells were processed for fluorescent microscopy 2 h after removal of dexamethasone (−hormone 2 h). (C) Same as in (B) except that leptomycin B (5 ng/ml, +LMB) was added following removal of dexamethasone. Top panels show GFP using a FITC filter and bottom panels show Hoechst staining of the same field using a UV filter. Adapted from Gustin and Sarnow (2001), with permission.
When hormone was added to poliovirus-infected cells expressing REV–GC–GFP it was rapidly imported into the nucleus at a rate indistinguishable from that seen in uninfected cells (Fig. 3A). Importantly, import of REV–GC–GFP occurred at time when endogenous cellular proteins had relocalized to the cytoplasm (Gustin and Sarnow, 2001). These results demonstrated conclusively that the glucocorticoid receptor import pathway was functional in poliovirus-infected cells. Since REV–GC–GFP was imported into the nucleus it was now possible to determine the status of the Crm1 export pathway in poliovirus-infected cells. For this analysis, REV–GC–GFP was allowed to accumulate in infected cell nuclei, then hormone was removed, and the appearance of REV–GC–GFP in the cytoplasm monitored. Again, the appearance of REV–GC–GFP in the cytoplasm was indistinguishable from that of uninfected cells (Fig. 3B). Accumulation of REV–GC–GFP could be blocked by the addition of leptomycin B, a specific inhibitor of Crm1 dependent export (Fig. 3C and Wolff et al., 1997), demonstrating that the export of REV–GC–GFP was mediated by Crm1 in poliovirus-infected cells. These results demonstrated that the Crm1 export pathway remained functional and suggest a certain level of specificity in the disruption of nucleo-cytoplasmic trafficking pathways in poliovirus-infected cells.
To specifically demonstrate that nuclear import was inhibited in poliovirus-infected cells, an in vitro import assay was employed. This assay utilizes digitonin-permeabilized cells that have been washed extensively to remove the soluble components required for nuclear import such as import receptors and Ran (Adam et al., 1990). The import assay is initiated by the addition of a source of soluble components such as rabbit reticulocyte lysate, GTP and an import cargo that can be easily detected in the cell nucleus (Adam et al., 1992). When poliovirus-infected cells were permeabilized with digitonin and incubated with an import cargo containing a classical NLS, RRL and GTP, very little of the import cargo accumulated in the infected cell nucleus, demonstrating that poliovirus-infected cells do not support the classical nuclear import pathway (Gustin and Sarnow, 2001).
Translocation of receptor:cargo complexes through the NPC can be prevented by carrying out import reactions in the absence of energy or at non-physiological temperatures. Under these conditions translocation through the NPC is blocked, but energy independent interactions between the NPC and receptor:cargo complexes occurs, resulting in the accumulation of import cargo at the nuclear rim (Adam et al., 1990). This is thought to represent ‘docking’ of receptor:cargo complexes at the NPC and is one of the earliest steps in the translocation process. When digitonin-permeabilized poliovirus-infected cells were assayed in the absence of energy, very little of the import cargo accumulated at the nuclear rim, indicating that docking of receptor:cargo complexes at the NPC was inhibited (Gustin and Sarnow, 2001). These results demonstrated convincingly that poliovirus infection results in an inhibition of the classical nuclear import pathway that may be caused, at least in part, by an inability of receptor:cargo complexes to successfully dock at the NPC.
The observation that receptor:cargo complexes do not dock at the NPC of poliovirus-infected cells suggested that infection disrupted this structure in some fashion. Analysis of a subset of Nups in poliovirus-infected cells revealed that two members of this family, Nup153 and p62, were degraded during the course of infection (Fig. 4 A). In contrast, the levels of soluble components of the transport machinery, such as the small GTPase Ran, importin-β, transportin or NTF2 remained unchanged (Fig. 4A and Gustin and Sarnow, unpublished). Importantly, the degradation of Nup153 and p62 coincided with the relocalization of nuclear proteins to the cytoplasm and inhibition of import in vitro (Gustin and Sarnow, 2001).
Fig. 4.
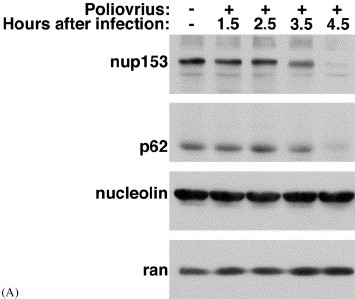
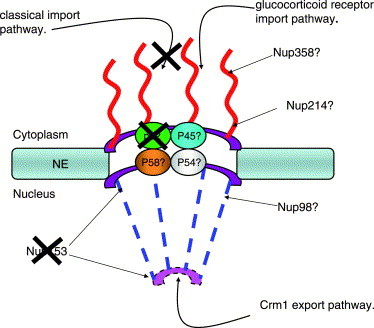
Degradation of NPC proteins in poliovirus-infected HeLa cells. (A) Fifty μg of whole cell lysates prepared from mock-infected cells or cells that had been infected with rhinovirus for the indicated length of time were analyzed by immunoblotting with monoclonal antibody 414 to detect Nup153 and p62, or MS3 to detect nucleolin. Adapted from Gustin and Sarnow (2001), with permission. (B) Changes to the NPC and transport pathways caused by poliovirus infection. X indicates transport pathway disrupted or Nup degraded. Question marks indicate that the status of these Nups in the NPC has not been determined. NE, nuclear envelope.
In vitro binding studies have shown that Nup153 and p62 can interact with components of the classical import pathway (Moroianu et al., 1997, Nakielny et al., 1999, Percipalle et al., 1997, Shah et al., 1998). Thus, the loss of either of these components could result in an inhibition of nuclear import via the classical pathway (Fig. 4B). The loss of Nup153 and p62 could also have effects that extend to other transport pathways such as those used by the cellular proteins shown to relocalize in poliovirus-infected cells (Table 1). For example, Nup153 has been shown to bind directly to Ran-GDP (Nakielny et al., 1999), while p62 is known to interact with NTF2, a protein required for the nuclear import of Ran (Paschal and Gerace, 1995, Ribbeck et al., 1998, Smith et al., 1998). Thus, the loss of Nup153 or p62 could disrupt the normal trafficking of Ran within the cell and result in the defects described above. In addition, Nup153 can interact in-vitro with transportin (Nakielny et al., 1999), and disruption of this interaction could impair the nuclear import of transportin cargos, such as hnRNP A1. Recently, however, Walther et al. (2001) reported the de novo assembly of NPCs using Xenopus oocyte extracts from which Nup153 had been depleted. These Nup153-deficient NPCs supported the import of a cargo bearing an M9 NLS, but not that of a cargo containing a classical NLS (Walther et al., 2001). Interestingly, depletion of Nup153 from Xenopus extracts resulted in the formation NPCs lacking many components of the nuclear basket (Walther et al., 2001). Thus, the inhibition of the transportin import pathway in poliovirus-infected cells may be attributable to loss of p62 or other, as yet unidentified NPC components.
While the loss of Nup153 and p62 could have effects that extend to multiple trafficking pathways, it is difficult to imagine how the loss of these two proteins could directly prevent docking of receptor:cargo complexes at the NPC (Fig. 4B). Docking of receptor:cargo complexes is thought to occur at fibrils that extend into the cytoplasm from the NPC (Allen et al., 2000). Nup153 is found on the nucleoplasmic side of the NPC (Sukegawa and Blobel, 1993), while p62 is part of a complex of proteins found in the central channel of the NPC (Guan et al., 1995). As mentioned above, the NPC is composed of over 30 different proteins (Cronshaw et al., 2002), and the status of only two of them have been examined directly in poliovirus infected cells. Thus, it will be interesting to determine if other components of the NPC, perhaps those present in the cytoplasmic fibrils, such as Nup358 are degraded or disrupted in some fashion during picornavirus infection.
3. Inhibition of nucleo-cytoplasmic trafficking by the vesicular stomatitis virus matrix protein
Vesicular stomatitis virus (VSV) is an enveloped virus with a negative stranded RNA genome that replicates in the cytoplasm of cells. The most abundant protein in the VSV virion is the M protein and, as would be expected for a structural component, a variety of data implicate the M protein in the assembly and budding of VSV from infected cells (reviewed in Rose and Whitt, 2001). Interestingly however, the M protein also encodes functions that are distinct from its role in viral assembly. For example, expression of the M protein has been shown to cause the cell rounding (Blondel et al., 1990) and inhibition of transcription (Black and Lyles, 1992) typical of VSV infections. Genetically these different roles of the M protein in VSV infection can be separated. Mutations in M that disrupt assembly do not prevent inhibition of transcription, while mutations that prevent inhibition of host cell gene expression do not affect assembly (Black et al., 1993, Coulon et al., 1990). In addition to these functions, expression of the M protein has been shown to inhibit a variety of nucleo-cytoplasmic trafficking pathways.
The initial observation that the VSV M protein could inhibit transport across the nuclear envelope came from studies in Xenopus laevis oocytes. Expression of the M protein in oocytes resulted in a dramatic inhibition in the export of U snRNAs, rRNAs and mRNAs to the cytoplasm and the import of proteins and snRNPs to the nucleus (Fig. 5 and Her et al., 1997). The M protein did not block all transport through the NPC, as the export of tRNAs proceeded normally (Her et al., 1997). Analysis of M proteins from different vesiculoviruses revealed that all maintained the ability to inhibit transport, indicating that this was a conserved feature of the M protein within the Vesiculovirus genus (Petersen et al., 2001).
Fig. 5.
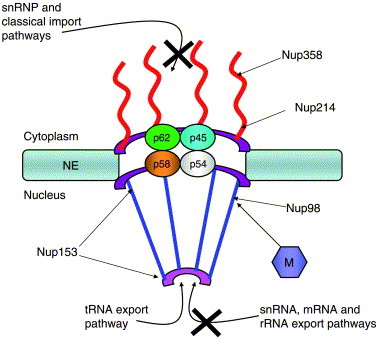
Changes to the NPC and transport pathways caused by the VSV M protein. VSV M is thought to associate with Nup98 on the nucleoplasmic side of the NPC, but may also associate with other NPC components. The precise location of VSV M or the number of molecules present at the NPC is not known. X indicates transport pathway disrupted. NE, nuclear envelope.
Subsequent injection experiments in Xenopus oocytes revealed that VSV M acted from within the nucleus to inhibit transport (Petersen et al., 2000, von Kobbe et al., 2000). When M was expressed in oocytes it inhibited transport and was detected in both the cytoplasm and the nucleus (Her et al., 1997, Petersen et al., 2000). Injection of anti-M antibodies into the cytoplasm of oocytes expressing M prevented inhibition of transport and depleted the nuclear pool of M (Petersen et al., 2000). Injection of these same antibodies into the nucleus blocked the M inhibitory activity but did not deplete M from the cytoplasm (Petersen et al., 2000). As the antibody was restricted to the nucleus in this experiment, the result indicated that ‘free’ M in the cytoplasm could not inhibit transport. In agreement with this, Von Kobbe et al., demonstrated that a C-terminal truncation mutant of M (aa1-77) could still block transport, but only if it was injected into the nucleus of oocytes (von Kobbe et al., 2000), indicating that VSV M present in the nucleus was responsible for inhibition of transport.
Analysis of the distribution of M protein in HeLa cells demonstrated that a fraction of M localized to the nuclear envelope and colocalized with NPC proteins (Petersen et al., 2000, von Kobbe et al., 2000). Mutational analysis identified a short stretch of amino acids located in the N-terminus of M (aa51-59) that is required for inhibition of transport (Petersen et al., 2000, von Kobbe et al., 2000). Mutations within this region block the ability of M to inhibit transport and prevent association with the NPC. Using bacterially expressed VSV M and soluble extracts from HeLa cell nuclei, von Kobbe et al. (2000) identified Nup98 as an NPC component that interacted with the M protein. The lectin wheat germ agglutinin could compete with M for binding to Nup98, suggesting that interaction involved O-linked N-acetylglucosamine moieties present on Nup98 (von Kobbe et al., 2000). The inability to detect interaction of M with bacterially expressed or in vitro translated Nup98 supported the interpretation that M interacted with a glycosylated form of Nup98 (von Kobbe et al., 2000). Importantly, a mutant form of M that did not inhibit transport or localize to the NPC did not interact with Nup98 in this assay (von Kobbe et al., 2000). Thus, there appears to be a functional correlation between interaction with Nup98 and disruption of nucleo-cytoplasmic trafficking.
To further characterize the significance of the M-Nup98 interaction, the distribution of VSV M in murine Nup98 knockout cells was examined. In wild-type mouse cells, as in HeLa cells, a significant fraction of a GFP-M fusion protein was found at the nuclear rim (von Kobbe et al., 2000). In cells lacking Nup98, however, much less VSV M was associated with the nuclear rim, suggesting that interaction with Nup98 directed M to the NPC (von Kobbe et al., 2000). It should be pointed out, however, that a small amount of VSV M was detected at the nuclear envelope in Nup98 knockout cells, and this may be due to an inherent ability of M to associate with membranes (von Kobbe et al., 2000, Ye et al., 1994). Alternatively, inhibition of transport by M may involve interaction with as yet unidentified NPC components. Because of this, it would be interesting to determine if M can still inhibit transport across the NPC in cells lacking Nup98.
Nup98 is an FG repeat containing nucleoporin that has been immunolocalized to the basket structure on the nucleoplasmic side of the NPC (Radu et al., 1995). In addition, Nup98 is also found associated with intranuclear filaments that extend from the NPC into the nucleus (Fontoura et al., 2001). Inactivation of Nup98 by injection of antibodies into Xenopus oocytes resulted in defects in snRNA, rRNA and mRNA export but did not inhibit tRNA export or protein import (Powers et al., 1997). Interestingly, these defects in RNA transport are very similar to those seen in Xenopus oocytes expressing the VSV M protein. Photobleaching studies revealed that Nup98 is mobile and moves between the nuclear filaments and the NPC in a transcription dependent manner, consistent with its role in RNA export (Griffis et al., 2002). The observations that Nup98 interacts with transportin, the import receptor for hnRNP A1, suggests that it may have roles in nuclear import as well (Fontoura et al., 2000). Thus, disruption of Nup98 function by VSV M could account for the defects in export and import pathways observed in Xenopus oocytes expressing M (Fig. 3). As mentioned above, however, antibodies to Nup98 did not inhibit protein import in Xenopus oocytes, indicating that VSV M may target additional components of the NPC to bring about inhibition of this transport pathway.
4. Why do viruses inhibit nucleo-cytoplasmic trafficking?
Inhibition of nuclear transport by RNA viruses could provide several advantages for virus replication and pathogenesis. For example, preventing the export of host mRNAs to the cytoplasm should reduce competition for the translation machinery and consequently increase viral protein synthesis. For RNA viruses that replicate in the cytoplasm, such as poliovirus and VSV, inhibition of nuclear import may contribute to the accumulation of nuclear factors in the cytoplasm were they could contribute to viral replication. In the case of poliovirus and rhinovirus infections, nuclear proteins known to accumulate in the cytoplasm have roles in a diverse array of activities pertaining to RNA metabolism including; transcription, processing, transport, translation and stability (Table 1). Clearly, these are activities that an RNA virus replicating in the cytoplasm might find useful.
Inhibition of nucleo-cytoplasmic transport could also impair the ability of cells to defend against viral invasion. For example, the interferon response pathway requires that signal transducers and activators of transcription (STATs) be imported into the nucleus to activate expression of genes encoding anti-viral functions (reviewed in Stark et al., 1998). Inhibition of nucleo-cytoplasmic transport could prevent the import of activated STATs or the export of target gene mRNAs, thus reducing the effectiveness of the interferon response. Interestingly, a recent study has revealed a link between the interferon response and the NPC. Enninga et al. (2002) found that expression of two NPC proteins, Nup98 and Nup96, was induced by interferon-γ treatment. Remarkably, induction of Nup98/96 expression by interferon-γ relieved the block to mRNA export caused by the VSV M protein (Enninga et al., 2002). While the exact function of Nup98 in the antiviral response is not known, these findings suggest that rhabdoviruses, such as VSV, may target Nup98 in an effort to inhibit its antiviral activity. The finding that certain picornaviruses also target components of the NPC suggest that this could be a common mechanism by which RNA viruses inhibit the host response to infection. In support of this possibility, analysis of whole cell lysates has revealed that Nup98 is rapidly degraded following infection with poliovirus (Gustin and Sarnow, unpublished).
5. Inhibition of nucleo-cytoplasmic trafficking by other RNA viruses
Recent studies have revealed that other picornaviruses can also disrupt nucleo-cytoplasmic trafficking. Belov et al. (2000) reported that a classical NLS-GFP fusion protein accumulated in the cytoplasm of cells infected with coxsackievirus B3. Although nuclear import or NPC components were not examined in this study, these results are consistent with coxsackievirus B3 infection disrupting nucleo-cytoplasmic trafficking. More recently, the analysis of rhinovirus type 14-infected cells has shown that other picornaviruses inhibit nuclear import and target Nup153 and p62 for degradation (Gustin and Sarnow, 2002). These results demonstrate that viruses from two different genera in the Picornaviridae family have a similar inhibitory effect upon nucleo-cytoplasmic trafficking, and suggest that this may be common feature of picornavirus–host interactions.
Other RNA viruses may also disrupt trafficking between the nucleus and the cytoplasm. As discussed above, the influenza virus NS 1 protein has been shown to inhibit export of host mRNAs (Alonso-Caplen et al., 1992, Fortes et al., 1994). In this case, however, the inhibition appears to be a consequence of defective mRNA processing and not due to a disruption in nucleo-cytoplasmic trafficking per se or interaction with NPC components (Chen et al., 1999, Nemeroff et al., 1998). The cytoplasmic accumulation of hnRNP A1 in cells infected with mouse hepatitis virus suggests that the transportin import pathway may be disrupted in coronavirus-infected cells (Li et al., 1997). The hepatitis C virus NS5A protein has been reported to interact with karyopherin β3, a member of the importin β family of proteins that has been implicated in ribosomal protein import (Chung et al., 2000, Jakel and Gorlich, 1998). Expression of karyopherin β3 can functionally complement the loss of two yeast importin β family members, PSE1/KAP121 and KAP123 (Chung et al., 2000). Interestingly, expression of NS5A in yeast blocks this complementation, suggesting that NS5A may inhibit the function of karyopherin β3 (Chung et al., 2000). Cumulatively, these findings suggest that inhibition of nuclear import may be a more common feature of RNA virus cytopathology than previously thought.
6. Conclusions
Viruses frequently disrupt cellular processes such as transcription, translation and DNA synthesis (to name a few) in an effort to augment viral replication. It is intriguing that two unrelated RNA viruses disrupt nucleo-cytoplasmic trafficking and appear to do so by targeting components of the NPC. For VSV this requires association of M with the NPC mediated at least in part through interaction with Nup98. In the case of poliovirus, inhibition of transport correlates with the degradation of two NPC components, Nup153 and p62. As mentioned above however, both viruses may target as yet unidentified NPC proteins to bring about inhibition of transport. Identifying additional targets at the NPC will help to determine if common mechanisms may be responsible for the inhibition of nuclear transport mediated by these viruses. Exactly how widespread inhibition of nucleo-cytopasmic trafficking is and its role in viral replication remains to be determined.
Acknowledgements
Work in the author's laboratory is supported by the National Institutes of Health and the National Center for Research Resources Center of Biomedical Research Excellence (COBRE). Grant #P20 RR15587.
References
- Adam S.A., Marr R.S., Gerace L. Nuclear protein import in permeabilized mammalian cells requires soluble cytoplasmic factors. J. Cell. Biol. 1990;111:807–816. doi: 10.1083/jcb.111.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam S.A., Sterne-Marr R., Gerace L. Nuclear protein import using digitonin-permeabilized cells. Methods Enzymol. 1992;219:97–110. doi: 10.1016/0076-6879(92)19013-v. [DOI] [PubMed] [Google Scholar]
- Allen T.D., Cronshaw J.M., Bagley S., Kiseleva E., Goldberg M.W. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell. Sci. 2000;113(Pt 10):1651–1659. doi: 10.1242/jcs.113.10.1651. [DOI] [PubMed] [Google Scholar]
- Alonso-Caplen F.V., Nemeroff M.E., Qiu Y., Krug R.M. Nucleocytoplasmic transport: the influenza virus NS1 protein regulates the transport of spliced NS2 mRNA and its precursor NS1 mRNA. Genes Dev. 1992;6:255–267. doi: 10.1101/gad.6.2.255. [DOI] [PubMed] [Google Scholar]
- Babiss L.E., Ginsberg H.S. Adenovirus type 5 early region 1b gene product is required for efficient shutoff of host protein synthesis. J. Virol. 1984;50:202–212. doi: 10.1128/jvi.50.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiss L.E., Ginsberg H.S., Darnell J.E., Jr Adenovirus E1B proteins are required for accumulation of late viral mRNA and for effects on cellular mRNA translation and transport. Mol. Cell. Biol. 1985;5:2552–2558. doi: 10.1128/mcb.5.10.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S.H., Kim Y.K., Kim W.J., Cho S., Oh H.R., Kim J.E., Jang S.K. Translation of polioviral mRNA is inhibited by cleavage of polypyrimidine tract-binding proteins executed by polioviral 3C(pro) J. Virol. 2002;76:2529–2542. doi: 10.1128/jvi.76.5.2529-2542.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov G.A., Evstafieva A.G., Rubtsov Y.P., Mikitas O.V., Vartapetian A.B., Agol V.I. Early alteration of nucleocytoplasmic traffic induced by some RNA viruses. Virology. 2000;275:244–248. doi: 10.1006/viro.2000.0427. [DOI] [PubMed] [Google Scholar]
- Beltz G.A., Flint S.J. Inhibition of HeLa cell protein synthesis during adenovirus infection. Restriction of cellular messenger RNA sequences to the nucleus. J. Mol. Biol. 1979;131:353–373. doi: 10.1016/0022-2836(79)90081-0. [DOI] [PubMed] [Google Scholar]
- Bischoff F.R., Scheffzek K., Ponstingl H. How ran is regulated. In: Weis K., editor. Vol. 1. Springer; Berlin: 2002. pp. 49–66. (Nuclear Transport). [Google Scholar]
- Black B.L., Lyles D.S. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 1992;66:4058–4064. doi: 10.1128/jvi.66.7.4058-4064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black B.L., Rhodes R.B., McKenzie M., Lyles D.S. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 1993;67:4814–4821. doi: 10.1128/jvi.67.8.4814-4821.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel D., Harmison G.G., Schubert M. Role of matrix protein in cytopathogenesis of vesicular stomatitis virus. J. Virol. 1990;64:1716–1725. doi: 10.1128/jvi.64.4.1716-1725.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman A., Howell M.T., Patton J.G., Jackson R.J. The involvement of a spliceosome component in internal initiation of human rhinovirus RNA translation. J. Gen. Virol. 1993;74(Pt 9):1775–1788. doi: 10.1099/0022-1317-74-9-1775. [DOI] [PubMed] [Google Scholar]
- Chen Z., Li Y., Krug R.M. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 1999;18:2273–2283. doi: 10.1093/emboj/18.8.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.M., Lee J., Kim J.E., Song O.K., Cho S., Lim J., Seedorf M., Hahm B., Jang S.K. Nonstructural protein 5A of hepatitis C virus inhibits the function of karyopherin beta3. J. Virol. 2000;74:5233–5241. doi: 10.1128/jvi.74.11.5233-5241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon P., Deutsch V., Lafay F., Martinet-Edelist C., Wyers F., Herman R.C., Flamand A. Genetic evidence for multiple functions of the matrix protein of vesicular stomatitis virus. J. Gen. Virol. 1990;71(Pt 4):991–996. doi: 10.1099/0022-1317-71-4-991. [DOI] [PubMed] [Google Scholar]
- Cronshaw J.M., Krutchinsky A.N., Zhang W., Chait B.T., Matunis M.J. Proteomic analysis of the mammalian nuclear pore complex. J. Cell. Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelstein M., Roth J., Kimberly W.T., Levine A.J., Shenk T. Nuclear export of the E1B 55-kDa and E4 34-kDa adenoviral oncoproteins mediated by a rev-like signal sequence. EMBO J. 1997;16:4276–4284. doi: 10.1093/emboj/16.14.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enninga J., Levy D.E., Blobel G., Fontoura B.M. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science. 2002;295:1523–1525. doi: 10.1126/science.1067861. [DOI] [PubMed] [Google Scholar]
- Fischer U., Huber J., Boelens W.C., Mattaj I.W., Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- Fontoura B.M., Blobel G., Yaseen N.R. The nucleoporin Nup98 is a site for GDP/GTP exchange on ran and termination of karyopherin beta 2-mediated nuclear import. J. Biol. Chem. 2000;275:31289–32196. doi: 10.1074/jbc.M004651200. [DOI] [PubMed] [Google Scholar]
- Fontoura B.M., Dales S., Blobel G., Zhong H. The nucleoporin Nup98 associates with the intranuclear filamentous protein network of TPR. Proc. Natl. Acad. Sci. USA. 2001;98:3208–3213. doi: 10.1073/pnas.061014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes P., Beloso A., Ortin J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994;13:704–712. doi: 10.1002/j.1460-2075.1994.tb06310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffis E.R., Altan N., Lippincott-Schwartz J., Powers M.A. Nup98 is a mobile nucleoporin with transcription-dependent dynamics. Mol. Biol. Cell. 2002;13:1282–1297. doi: 10.1091/mbc.01-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan T., Muller S., Klier G., Pante N., Blevitt J.M., Haner M., Paschal B., Aebi U., Gerace L. Structural analysis of the p62 complex, an assembly of O-linked glycoproteins that localizes near the central gated channel of the nuclear pore complex. Mol. Biol. Cell. 1995;6:1591–1603. doi: 10.1091/mbc.6.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.E., Sarnow P. Effects of poliovirus infection on nucleo-cytoplasmic trafficking and nuclear pore complex composition. EMBO J. 2001;20:240–249. doi: 10.1093/emboj/20.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin K.E., Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert D.N., Cutt J.R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J. Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C.U., Witherell G.W., Schmid M., Shin S.H., Pestova T.V., Gil A., Wimmer E. A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proc. Natl. Acad. Sci. USA. 1993;90:7642–7646. doi: 10.1073/pnas.90.16.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Her L.S., Lund E., Dahlberg J.E. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus [see comments] Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- Ishidate T., Yoshihara S., Kawasaki Y., Roy B.C., Toyoshima K., Akiyama T. Identification of a novel nuclear localization signal in Sam68. FEBS Lett. 1997;409:237–241. doi: 10.1016/s0014-5793(97)00455-9. [DOI] [PubMed] [Google Scholar]
- Izaurralde E., Adam S. Transport of macromolecules between the nucleus and the cytoplasm. RNA. 1998;4:351–364. [PMC free article] [PubMed] [Google Scholar]
- Jakel S., Gorlich D. Importin beta, transportin, RanBP5 and RanBP7 mediate nuclear import of ribosomal proteins in mammalian cells. EMBO J. 1998;17:4491–4502. doi: 10.1093/emboj/17.15.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D.M., Samuel C.E., Palese P. Virus-host cell interactions. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincot Williams & Wilkins; Philadelphia: 2001. pp. 133–170. [Google Scholar]
- Li H.P., Zhang X., Duncan R., Comai L., Lai M.M. Heterogeneous nuclear ribonucleoprotein A1 binds to the transcription-regulatory region of mouse hepatitis virus RNA. Proc. Natl. Acad. Sci. USA. 1997;94:9544–9549. doi: 10.1073/pnas.94.18.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D.C., Sweitzer T.D., Hanover J.A. Reconstitution of HIV-1 rev nuclear export: independent requirements for nuclear import and export [In Process Citation] Proc. Natl. Acad. Sci. USA. 1998;95:10608–10613. doi: 10.1073/pnas.95.18.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj I.W., Englmeier L. Nucleocytoplasmic transport: the soluble phase. Annu. Rev. Biochem. 1998;67:265–306. doi: 10.1146/annurev.biochem.67.1.265. [DOI] [PubMed] [Google Scholar]
- McBride A.E., Schlegel A., Kirkegaard K. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA. 1996;93:2296–2301. doi: 10.1073/pnas.93.6.2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K., Svitkin Y.V., Lee H.S., Lejbkowicz F., Kenan D.J., Chan E.K., Agol V.I., Keene J.D., Sonenberg N. La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol. 1993;67:3798–3807. doi: 10.1128/jvi.67.7.3798-3807.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael W.M., Siomi H., Choi M., Pinol-Roma S., Nakielny S., Liu Q., Dreyfuss G. Signal sequences that target nuclear import and nuclear export of pre-mRNA-binding proteins. Cold Spring Harbour Symp. Quant. Biol. 1995;60:663–668. doi: 10.1101/sqb.1995.060.01.071. [DOI] [PubMed] [Google Scholar]
- Michael W.M., Eder P.S., Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroianu J., Blobel G., Radu A. RanGTP-mediated nuclear export of karyopherin alpha involves its interaction with the nucleoporin Nup153. Proc. Natl. Acad. Sci. USA. 1997;94:9699–9704. doi: 10.1073/pnas.94.18.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S., Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- Nakielny S., Shaikh S., Burke B., Dreyfuss G. Nup153 is an M9-containing mobile nucleoporin with a novel Ran-binding domain. EMBO J. 1999;18:1982–1995. doi: 10.1093/emboj/18.7.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff M.E., Barabino S.M., Li Y., Keller W., Krug R.M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3'end formation of cellular pre-mRNAs. Mol. Cell. 1998;1:991–1000. doi: 10.1016/s1097-2765(00)80099-4. [DOI] [PubMed] [Google Scholar]
- Paschal B.M., Gerace L. Identification of NTF2, a cytosolic factor for nuclear import that interacts with nuclear pore complex protein p62. J. Cell. Biol. 1995;129:925–937. doi: 10.1083/jcb.129.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percipalle P., Clarkson W.D., Kent H.M., Rhodes D., Stewart M. Molecular interactions between the importin alpha/beta heterodimer and proteins involved in vertebrate nuclear protein import. J. Mol. Biol. 1997;266:722–732. doi: 10.1006/jmbi.1996.0801. [DOI] [PubMed] [Google Scholar]
- Petersen J.M., Her L.S., Varvel V., Lund E., Dahlberg J.E. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 2000;20:8590–8601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J.M., Her L.S., Dahlberg J.E. Multiple vesiculoviral matrix proteins inhibit both nuclear export and import. Proc. Natl. Acad. Sci. USA. 2001;98:8590–8595. doi: 10.1073/pnas.151240998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Yamamoto K.R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Moore M., Logan J., Shenk T. The adenovirus E1B-55K transforming polypeptide modulates transport or cytoplasmic stabilization of viral and host cell mRNAs. Mol. Cell. Biol. 1986;6:470–476. doi: 10.1128/mcb.6.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard V.W., Michael W.M., Nakielny S., Siomi M.C., Wang F., Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Powers M.A., Forbes D.J., Dahlberg J.E., Lund E. The vertebrate GLFG nucleoporin, Nup98, is an essential component of multiple RNA export pathways. J. Cell. Biol. 1997;136:241–250. doi: 10.1083/jcb.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radu A., Moore M.S., Blobel G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995;81:215–222. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Ribbeck K., Lipowsky G., Kent H.M., Stewart M., Gorlich D. NTF2 mediates nuclear import of Ran. EMBO J. 1998;17:6587–6598. doi: 10.1093/emboj/17.22.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli M.G., Weighardt F., Biamonti G., Riva S., Morandi C. Sequence determinants for hnRNP I protein nuclear localization. Exp. Cell. Res. 1997;235:300–304. doi: 10.1006/excr.1997.3677. [DOI] [PubMed] [Google Scholar]
- Rose J.K., Whitt M.A. Rhabdoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., editors. Fields Virology. fourth ed. Lippincot Williams & Wilkins; Philadelphia: 2001. pp. 1221–1244. [Google Scholar]
- Rosenblum J.S., Pemberton L.F., Bonifaci N., Blobel G. Nuclear import and the evolution of a multifunctional RNA-binding protein. J. Cell. Biol. 1998;143:887–899. doi: 10.1083/jcb.143.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan K.J., Wente S.R. The nuclear pore complex: a protein machine bridging the nucleus and cytoplasm. Curr. Opin. Cell. Biol. 2000;12:361–371. doi: 10.1016/s0955-0674(00)00101-0. [DOI] [PubMed] [Google Scholar]
- Savory J.G., Hsu B., Laquian I.R., Giffin W., Reich T., Hache R.J., Lefebvre Y.A. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol. Cell. Biol. 1999;19:1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann M.S., Nigg E.A. Protein localization to the nucleolus: a search for targeting domains in nucleolin. J. Cell. Sci. 1993;105:799–806. doi: 10.1242/jcs.105.3.799. [DOI] [PubMed] [Google Scholar]
- Shah S., Tugendreich S., Forbes D. Major binding sites for the nuclear import receptor are the internal nucleoporin Nup153 and the adjacent nuclear filament protein Tpr. J. Cell. Biol. 1998;141:31–49. doi: 10.1083/jcb.141.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Brownawell A., Macara I.G. Nuclear import of Ran is mediated by the transport factor NTF2. Curr. Biol. 1998;8:1403–1406. doi: 10.1016/s0960-9822(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Stark G.R., Kerr I.M., Williams B.R., Silverman R.H., Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Stoffler D., Fahrenkrog B., Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr. Opin. Cell. Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- Strom A.C., Weis K. Importin-beta-like nuclear transport receptors. Genome Biol. 2001;2:1–9. doi: 10.1186/gb-2001-2-6-reviews3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukegawa J., Blobel G. A nuclear pore complex protein that contains zinc finger motifs, binds DNA, and faces the nucleoplasm. Cell. 1993;72:29–38. doi: 10.1016/0092-8674(93)90047-t. [DOI] [PubMed] [Google Scholar]
- von Kobbe C., van Deursen J.M.A., Rodrigues J.P., Sitterlin D., Bachi A., Wu X., Wilm M., Carno-Fonseca M., Izaurralde E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleopoin Nup98. Mol. Cell. 2000;6:1243–1252. doi: 10.1016/s1097-2765(00)00120-9. [DOI] [PubMed] [Google Scholar]
- Waggoner S., Sarnow P. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 1998;72:6699–6709. doi: 10.1128/jvi.72.8.6699-6709.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther T.C., Fornerod M., Pickersgill H., Goldberg M., Allen T.D., Mattaj I.W. The nucleoporin Nup153 is required for nuclear pore basket formation, nuclear pore complex anchoring and import of a subset of nuclear proteins. EMBO J. 2001;20:5703–5714. doi: 10.1093/emboj/20.20.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D.H., Ketner G. Adenoviral early region 4 is required for efficient viral DNA replication and for late gene expression. J. Virol. 1986;57:833–838. doi: 10.1128/jvi.57.3.833-838.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B., Sanglier J.J., Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo-cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem. Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- Ye Z., Sun W., Suryanarayana K., Justice P., Robinson D., Wagner R.R. Membrane-binding domains and cytopathogenesis of the matrix protein of vesicular stomatitis virus. J. Virol. 1994;68:7386–7396. doi: 10.1128/jvi.68.11.7386-7396.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


