Abstract
Point-of-care testing (POCT) is a laboratory-medicine discipline that is evolving rapidly in analytical scope and clinical application. In this review, we first describe the state of the art of medical-laboratory tests that can be performed near the patient. At present, POCT ranges from basic blood-glucose measurement to complex viscoelastic coagulation assays. POCT shortens the time to clinical decision-making about additional testing or therapy, as delays are no longer caused by transport and preparation of clinical samples, and biochemical-test results are rapidly available at the point of care. Improved medical outcome and lower costs may ensue.
Recent, evolving technological advances enable the development of novel POCT instruments. We review the underlying analytical techniques. If new instruments are not yet in practical use, it is often hard to decide whether the underlying analytical principle has real advantage over former methods. However, future utilization of POCT also depends on health-care trends and new areas of application. But, even today, it can be assumed that, for certain applications, near-patient testing is a useful complement to conventional laboratory analyses.
Keywords: Bench-top POCT analyzer, Continuous measurement POCT system, Hemostaseological coagulation analyzer, Molecular-biology-based POCT device, POCT, POCT data-manager software, POCT in developing countries, Point-of-care testing, Strip-based POCT, Unit-use analyzer
1. Introduction
A large number of laboratory analyses support correct diagnosis in over 50% of all diseases, in addition to aiding the monitoring of drug therapy in many other cases. Laboratory medicine is therefore a vital component in differential diagnosis in the clinic and in local general practice.
An affordable, competent system of laboratory diagnostics, in the doctor’s office and hospital, has been made possible through centralization of analysis in purpose-built laboratories or in facilities provided by large hospitals. In contrast to this centralization and increased efficiency in laboratory diagnostics, there has been a recent trend towards a more decentralized diagnostic analysis, so-called point-of-care testing (POCT), which occurs directly at patients’ beds, in operating theatres or outpatient clinics, or at sites of accidents. This modern variety of laboratory medicine is characterized by minimizing instrument size and procedures and the increasing use of current information technology. There are analytical devices available that make it possible to process a whole blood sample in a simple manner, allowing untrained staff to carry out laboratory diagnostics. It is clear that the use of POCT shortens the time between sample acquisition and analysis (turnaround time). However, this type of diagnosis is only useful if the results produced lead to immediate therapeutic decisions [1]. However, there is still a paucity of evidence supporting the use of POCT and improved patient outcomes [2].
Nevertheless, it is not difficult to predict that there will be growth in the development of POCT diagnostics for parameters that are at present available only in central laboratories. This growth is supported by new analytical technologies amalgamating issues (e.g., miniaturization, nanoparticle techniques, multiplexing, wireless connectivity, and novel biomarkers). Clinical pathology as a discipline needs to be responsible for this field, since adherence to quality-management systems ensures accurate, reliable biochemical-test results for optimal patient care and safety, regardless of whether the individual test is performed in a central laboratory or as POCT at the bedside [2].
POCT is mainly characterized by proximity to the patient, quantitative or semi-quantitative single measurements, short turnaround time, no sample preparation, no pipetting, use of pre-made reagents, user-friendly dedicated analytical instruments and instant, result-deduced therapeutic action [3], [4]. All outpatient or ward personnel will be expected to be able to use these analyzers. No previous knowledge in sample analysis should be required. However, there is a continual transition between POCT and laboratory-based methods. A generally accepted definition of POCT is still under debate. For example, viscoelastic coagulation tests, described below in this article as “Type 4” POCT, are to be performed near the patient in cases of active bleeding. The results have to be available quickly to allow instant therapeutic action. However, the caregiver is mostly overstrained when operating the viscoelastic apparatus, so often these analyses are performed in a central laboratory with online transmission of the sensorgrams to the operating room; so these analyzers can be called “pseudo-POCT”.
Currently, the annual turnover for POCT in vitro diagnostics in Europe stands at approximately €3bn, Germany contributing €0.9bn of this; the majority comes from blood-sugar-test strips and devices for home testing in diabetes. In the past few years, the market for POCT systems grew yearly by more than 10%, but this rapid growth has been slightly dampened since 2007. Nevertheless, a marked increase is expected mid-term and long-term [1]. Table 1 gives insight into the variety of parameters available at present.
Table 1.
List of laboratory parameters currently available using point-of-care testing (POCT)
| Clinical application | Parameter |
|---|---|
| Acid-base balance, blood gases | pH, pCO2, pO2 |
| Electrolytes | Na+, K+, Cl-, Ca++ion., Mg++ion |
| Metabolites | Cholesterol, HDL-cholesterol, triglycerides, creatinine, urea, uric acid, bilirubin, lactate, ammonia |
| Enzymes | Amylase, alkaline phosphatase, CK, AST, ALT, γ-GT |
| Coagulation | Activated clotting-time (ACT), activated partial thrombo-plastin time (aPTT), prothrombin time (PT, INR), D-dimer, platelet function tests, ex-vivo bleeding time |
| Hematology | Hemoglobin, hematocrit, erythrocytes, leukocytes, thrombo-cytes |
| Hemoglobin fractions | CO-Oximetry |
| Cardiac markers | TnT, TnI, myoglobin, CK-MB, BNP/NT-pro-BNP |
| Diabetes mellitus | Glucose, HbA1c, microalbumin, minimal invasive continuous glucose monitoring |
| Acute-phase proteins | CRP |
| Allergy in-vitro diagnostics | Allergy specific IgE |
| Rheumatology | Antibodies against mutated citrullinated vimentin (anti-MCV) |
| Therapeutic drug monitoring, drugs-of-abuse screening | Therapeutic drugs, alcohol, amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine, methadone, opiates |
| Infectious agents | HIV, infectious mononucleosis, Chlamydia trachomatis, Trichomonas vaginalis, Plasmodium falciparum and vivax, Influenza A and B, Steptococcus A and B |
| Fertility | hCG, LH and FSH, sperm count |
| Urine diagnostics | Urine strips (pH, protein, glucose, ketones, bilirubin, uro-bilinogen, nitrite, leukocytes, erythrocytes), microalbumin, NMP22 bladder carcinoma check |
| Stool diagnostics | Blood |
2. Current techniques: categorization of the state-of-the-art POCT devices
POCT devices often employ biosensors (Fig. 1 ). According to the IUPAC definition, a biosensor is an analytical device for the detection of analytes that combines a biological component with a physicochemical-detector component. This generally occurs through the use of miniaturized analysis systems, where biological components are immobilized on a solid-state surface, which, in turn, interacts with the analyte [5]. These interactions may be detected by using either electrochemical or optical methods. In the latter case, a large role is played by fluorescence or reflection spectroscopy. Different parameters, being characteristic for certain diseases, can be detected by using specific biomolecules.
Figure 1.
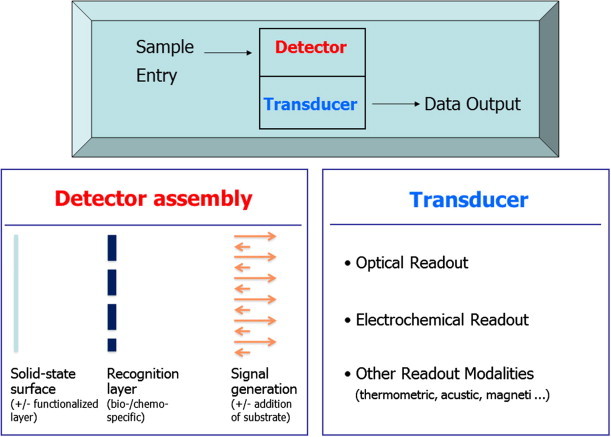
The biosensor as the basis of analysis in many point-of-care testing (POCT) instruments. Note that the recognition layer may comprise attached recognition elements (antibodies, receptors, aptamers …) or immobilized enzymes. In the latter case, the addition of substrates is essential. Not illustrated is the fact that the amplified signal is finally processed by microelectronics and displayed.
POCT analyzers currently available can be separated into various groups. We attempt to categorize the instruments according to their practical use in POCT and moreover by the following attributes: sensor characteristics, complexity, measuring mode, underlying detection principle and/or sample matrix.
2.1. Type 1 – Qualitative strip-based POCT methods
These qualitative tests discriminate plus and minus results and are mostly strip-based. The signaling is often performed by simple visualization or by optical detection using a simple readout device. The detection principles span from chemical-indicator reactions to immunological reactions [e.g., immunochromatography (performed as lateral flow assays)]. The strips are made of a porous matrix mixed with dried reagents onto a carrier element. The sample (e.g., urine, blood, stool or swab material) is deposited onto the matrix and starts the reaction while penetrating and soaking the stick layer. Applications are urinary pregnancy testing, detection of blood in stool, urine dipstick analyses, detection of infectious agents in swab material and others, listed in Table 2 . An interesting new product is the lateral-flow test for penicillin-binding protein 2a (PBP2a) from Alere Health (Atlanta, GA, USA) that detects PBP2a found in methicillin-resistant Staphylococcus aureus (MRSA) directly from bacterial isolates. The key determinant of the broad-spectrum β-lactam resistance in MRSA strains is this PBP2a [6].
Table 2.
Parameters available by strip-based point-of-care testing (POCT) methods
| POCT application | Parameter | Sample |
|---|---|---|
| Pregnancy testing | Human Chorionic Gonadotropin (hCG) | Urine, serum |
| Urine dipstick analyses | Ascorbic acid, glucose, bilirubin, ketone, specific gravity, blood, pH, protein, urobilinogen, leukocytes, microalbumin (MA), anti-VC, nitrite | Urine |
| Microalbumin screening | Albumin | Urine |
| Infectious agents detection | Group A Streptococcus, respiratory syncytial virus (RSV), influenza A + B, HIV, Chlamydia trachomatis antigen, Helicobacter pylori-specific IgG-antibody, MRSA | Swab, serum |
2.2. Type 2 – So-called unit-use analyzers
Here is the simplest form of quantitative POCT device, with most of the analysis taking place on the respective test strips. The reader is used only to read the result from the strips where the reaction has already taken place. These test strips are one-use articles. Examples include glucometers for home and the hospital POCT stations, as well as the i-STAT Abbott (Abbott Park, IL, USA), a multi-parameter unit-use POCT instrument [7].
Blood-sugar analysis is, both historically and financially, the most commonly used POCT technique. This is valid for in-patient and out-patient care, and for home testing by patients. Although generally either capillary or venous blood is used, the results can be presented as blood-glucose or plasma-glucose level, depending on the calibration by the manufacturers. This can make a relevant difference of over 11%, potentially resulting in false therapeutic measures. To minimize the risk of confusion between whole-blood and plasma-glucose results, the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) suggested in 2005 that glucose values should be given as plasma levels only, independent of sample type or measuring method. The USA and other countries have already followed this recommendation, and it is also now being implemented in the German-speaking regions [8], [9].
The strong correlation between the international normalized ratio (INR) and the clinical outcome of oral anticoagulation with vitamin K antagonists (warfarin, marcumar) has led to a tight therapy-monitoring regime using INR POCT devices. INR testing may be performed at the doctor’s office or by the patient (“home testing”). For the most popular devices see Table 3 . All these systems use unit-use test strips and whole blood obtained by finger prick, making them most convenient for the patient [10].
Table 3.
Important point-of-care testing (POCT) devices for testing international normalized ratio (INR)
| POCT device | Detection principle | |
|---|---|---|
| Abbott Laboratories | CoaguSense | Micromechanical (microcoagulation) |
| Alere Health | INRatio 2 (developed by HemoSense, now distributed by Alere Health) | Electrochemical (amperometric) |
| ITC Medical | ProTime InRhythm | Micromechanical (microcoagulation) |
| Roche Diagnostics | CoaguChek XS/XS Pro | Electrochemical (amperometric) |
Different types and sensitivities of the thromboplastins used and the different signaling types (micromechanical versus electrochemical) cause systematic differences between results obtained with the different INR devices [11]. However, the between-day precision of the devices seems to be satisfactory [12], so the monitoring should be performed with one single type of device.
2.3. Type 3 – So-called bench-top POCT analyzers
These instruments are generally more complex than unit-use machines and use different analytical principles [1]:
-
•
spectrophotometric substrate and enzyme-activity measurement;
-
•
hematological particle counting;
-
•
immunoassay; and,
-
•
sensor-based blood-gas analysis
2.3.1. Spectrophotometry/reflectrometry
This is usually applied for clinical-chemistry parameters. The analyzers use different test formats [e.g., centrifugal disks for the Piccolo from Abaxis (Union City, CA, USA), test strips for the Triage Meter Pro (Alere Health) or cassette analyzers for the Reflotron (Roche Diagnostics, Mannheim, Germany)].
2.3.2. Hematological multichannel analyzers
These use conventional techniques, but are tailored for POCT needs. An example is the PocH-100i from Sysmex (Kobe, Japan).
2.3.3. Immunological multi-channel devices
For example, the Pathfast from Mitsubishi Chemical (Tokyo, Japan) or the Radiometer AQT90 (see below)] are and tailored for special POCT applications. They use antibody-based immunoassay methodologies.
2.3.4. Blood gas analyzers (BGAs)
BGAs use potentiometric/amperometric or optical sensors for pH, pO2 and pCO2. Additional ion-sensitive electrodes for the measurement of electrolytes and other substrates are available. A selection of systems from the seven market-dominating manufacturers is given in Table 4 .
Table 4.
Benchtop blood-gas analyzers on the in vitro diagnostic market
| Supplier | Point-of-care testing (POCT) device |
|---|---|
| Instrumentation Laboratory (IL) | IEM Premier 3500 & 4000, |
| ITC Medical | Irma TRUpoint |
| Nova Biomedical | Stat Profile pHOx/pHOx Plus/pHOx Plus L, Nova CRT, Stat Profile Critical Care Xpress |
| OPTI Medical Systems | AVOXimeter 4000, OPTI CCA-TS und OPTI R |
| Radiometer | ABL5, ABL800 FLEX, ABL800 BASIC, ABL90 |
| Roche Diagnostics | cobas b 123, cobas b 221 |
| Siemens Medical Solutions Diagnostics | Rapidlab, 248/348, 800, 1200; Rapidpoint 400/405 |
Optional is the configuration of a BGA with a CO-oximetry unit. This is a most remarkable technical development. The CO-oximetry unit [4] is a miniaturized multi-wavelength spectro-photometer, which measures the typical absorption spectra of the various hemoglobin (Hb) species in order to distinguish O2-Hb (oxy-Hb) from other Hb species and to determine the O2-Hb saturation [i.e. the percentage of O2-Hb compared to the total amount of Hb, including CO-Hb, O2-Hb, deoxygenated Hb (HHb with Fe2+), and Met-Hb (HHb with Fe3+)]. All leading companies on the market have sophisticated oximetry units on board their blood-gas analyzers, which have no counterpart in the central medical laboratory. The only technological diversification is the factor that the Hb species are found only inside the erythrocytes. Hence, some systems use a cell-lysis step prior to spectrophotometry, whereas others eliminate the erythrocyte-caused light scattering by applying matrix-assisted algorithms.
2.4. Type 4 – Hemostaseological coagulation analyzers
These POCT compatible machines show a high degree of complexity. Although they are valid for use in POCT, only qualified personnel should operate them (e.g., a laboratory physician or a trained technical assistant). The combined analysis of plasma clotting, thrombocyte function and fibrinolysis is termed viscoelastic coagulation testing [13]. Examples include the ROTEM (TEM International, Munich, Germany) [14] or the Sonoclot from Sienco Inc (Arvada, CO, USA). It is also possible to analyze platelet function in terms of in vitro bleeding time or via optical aggregometry [15]. Examples here are the PFA 100 from Siemens Healthcare Diagnostics (Eschborn, Germany) or the VerifyNow from Accumetrics (San Diego, CA, USA) [16] and [17].
As described in the Introduction, these analyzers have to be called pseudo-POCT.
2.5. Type 5 – Continuous measurement with POCT systems
The most common example here is continuous glucose monitoring [18]. Such analyzing and application systems are already available commercially. They are likely to replace the invasive, intravenous electrode by the minimally invasive location of a microdialysis catheter in subcutaneous tissue (Fig. 2 ). Conversely, other non-invasive methods (e.g., microporation or optical techniques in direct transcutaneous measurement of metabolic parameters) are at least unlikely to prevail. This is mainly due to the broad range of human-skin characteristics concerning thickness, pigmentation and hairiness as well as physiological phenomena (e.g., humidity and salt content).
Figure 2.
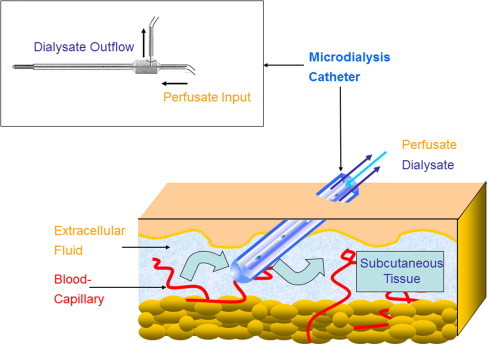
The principle of microdialysis for measuring analyte levels in the subcutaneous tissue. This “alternate-site monitoring” implicates divergent concentrations compared with blood/plasma.
Currently available systems for continuous glucose monitoring are the Guardian RT from Medtronics (Minneapolis, MN, USA), the Glucoday from A. Menarini (Florence, Italy) or the Abbott Navigator. A further example (not currently available in Germany) is the Glucowatch from Cygnus Inc (Petoskey, MI, USA). The Pendra, developed by now-dissolved Swiss company Pendragon Medical, did not reach a market placement, probably because of accuracy problems in the lower range of <50 mg/dL glucose.
2.6. Type 6 – Molecular biology-based POCT devices to detect infectious agents
At present on the market, there are many qualitative test strips to detect infectious pathogens. The basic principle in most systems is immunochromatography of a specific microbial antigen (or, more rarely, antibody) in the patient sample (urine, swab, or whole blood). There have also been some attempts to use molecular biological methods [mostly the polymerase chain reaction, (qRT)-PCR] for POCT, although these are technically demanding (with a DNA/RNA-extraction step required), so that they are no rapid tests in the strict sense (yet). Due to the complexity of the test procedures and the challenging interpretation of results, future rapid nucleic-acid testing (NAT) is more likely to be located in the central laboratory rather than at the bed-side [19]. Nevertheless, rapid quantification of DNA/RNA from various infectious agents will be beneficial for clinical diagnostics.
At present, the GeneXpert system from Cepheid (Sunnyvale, CA, USA) is the cutting-edge device to automate and to integrate all real-time PCR-based NAT steps: sample preparation, DNA amplification and detection. The system is a 1–16-site, random-access instrument, integrating real-time cycler, amplification and fluorescence detection. The use of multiple fluorescence dyes permits multiplex assays for detection of several targets within a single sample. The system provides PCR-test results from a raw clinical sample in about 1 h, enabling time-critical NAT at the point of need. There is a series of different unit-use microfluidic cartridges to be inserted into the analyzer {e.g., the “Xpert SA Nasal Complete” cartridge is designed to detect S. aureus and MRSA colonization from nasal pharyngeal swabs Flu A-panel cartridge is already under evaluation [20]}.
3. Novel technological developments and future perspectives
3.1. Goals of the developments
The currently available novel POCT systems are in part forerunners of a new generation of laboratory systems, which will dominate the market in 10–20 years’ time (fourth-generation laboratory systems). These methods are characterized through miniaturization, parallel analyses and networking via information technology (IT) [1].
3.1.1. Miniaturization
Microfluidics, chip technology and electrochemical detection have enabled the construction of miniaturized systems, with which even DNA may be directly analyzed in a few microliters of sample. Immunoassays and other methods of protein determination may also be carried out by “micro-total analysis systems” (μTAS). The advantages of miniaturization are obvious: fluid volumes in the nL and pL range reduce not only the amount of reagents required but also the time needed for basic analytical processes (e.g., mixing and equilibrating) [21].
3.1.2. Parallelization
Chip technology opens up the possibility of measuring multiple channels or multiple time points in the smallest space; photolithographic techniques allow reaction reservoirs and liquid channels to be etched on a wafer to enable 100 or more measurements to be taken simultaneously. An even higher density of reactions has already been realized in gene analysis with the use of microarrays. Appropriate micro-automation systems allow multiple laboratory profiles to be established rapidly and cost effectively, thereby opening up the possibility of obtaining an overview of a patient’s situation in just a few minutes. The use of a pattern of laboratory values – in contrast to single parameters – may, at least in principle, open up new diagnostic and prognostic avenues. However, the attendant problems in evaluating and interpreting the massive amounts of data thus obtained remain unsolved.
3.1.3. Networking
Next-generation laboratory systems will generally be networked via IT, and an international communication standard (POCT1-A) already exists for this purpose. However, current point-to-point communications (e.g., that between decentralized blood-glucose monitors and the laboratory IT system) show only a modest beginning. For the future, there have been discussions on individual electronic patient files, in which laboratory values from various sources can be directly input and linked to other results.
3.2. Novel POCT devices
At present, a wave of novel analytical principles and instruments can be envisioned for the near future, including alternative biological detection elements (scaffolds, aptamers, or anticalins in place of antibodies), sophisticated applications of optical-signal technologies (total internal reflection fluorescence, surface plasmon resonance, reflectometric interference spectroscopy) [22] and new dedicated microarrays for inflammation, malignancies and autoantibody diagnostics. This process will be encouraged through innovation from the communication industry, particularly via wireless networking (WiFi) technology. The following details refer to new instruments that are on the market or will shortly to be released. In many cases, the important clinical evaluation of these devices under routine conditions is still lacking. The list given is not comprehensive.
3.2.1. Type 1 – Qualitative strip-based POCT methods
There are no novel technologies in this category. However, there is an interesting new application [e.g., the DrugWipe (Securetec AG, Brunntal, Germany) drugs-of-abuse tests (amphetamines, benzodiazepines, cannabis, cocaine and opiates), which can be performed on the subject (sweat, saliva, blood) or as a swab test for suspicious surfaces].
3.2.2. Type 2 – Unit-use analyzers
3.2.2.1. Epocal (Ottawa, Canada) EPOC SmartCard
This system uses a thin, credit-card-like format, which contains the biosensors and the relevant microfluidics required. All assay methods are designed as unit-use systems, delivering a result within 30 s. It is also possible to measure blood gases, electrolytes, lactate, and hematocrit.
3.2.2.2. Åmic (Uppsala, Sweden) 4cast-Chip & Reader
This product, taken over from Johnson & Johnson, presents as a microstructured artificial chip, which takes the form of a microscope slide. Capillary action – driven by μ-pillar arrays hydrophilized by dextran – transports the probe over the surface to the reactants coating the slide. It should thereby be possible to replace conventional lateral flow membranes. Immunoassays with fluorescence detection have been developed, the relevant parameters including cTnI, NT-proBNP, CRP and TSH.
3.2.2.3. BioRad (Hercules, CA, USA) In2It HbA1c analyzer
This appears to be a very useful POCT system. It processes the traditionally applied boronate affinity chromatography for HbA1c in a very small table-top device.
3.2.2.4. Axis-Shield (Dundee, Scotland, UK) Afinion AS100 Analyzer
This cartridge-based instrument allows the determination of HbA1c, albumin/creatinine ratio (ACR), and CRP by reflectometry. For testing, whole blood, plasma and urine samples can be used. The advantages of this device are short assay times, all-in-one reagent cartridges and its ease of application.
3.2.2.5. Philips (Amsterdam, Netherlands) Magnotech
This is a hand-held immunoassay machine, which carries out immunological reactions using magnetizable nanoparticles. The particles are labeled with antibodies and exposed to a variable magnetic field. Detection is performed with a “bound-from-free” separation of reaction components, currently using a frustrated total internal reflection method. Nanoparticles can also be used in the so-called giant magnetoresistance effect in signal generation [23]. We expect that the instrument will provide a multi-analyte detection mode, with a spectrum of parameters (e.g., cardiac markers, parathormone and sepsis parameters).
3.2.2.6. Vivacta (Sittingbourne, UK)
This company is developing a new signal-generation and detection technology. The POCT instrument uses a piezo film made from polyvinyl fluoride, which acts as a pyroelectric film, thereby enabling an ultrasensitive non-separation assay design for immunoassays (e.g., cardiac markers, thyroid hormones). When LED light strikes the piezo film, it causes thermal disturbance with subsequent changes in the charge (signal). However, this is only the case when a carbon particle-labeled immuno complex was previously bound. A labeled antibody, free in the solution and not bound to the surface, does not cause a change in the charge of the piezo film.
3.2.2.7. Nanoentek (Seoul, S. Korea) HeArt2
This POCT instrument works via membrane-free immunoassay technology with fluorescence detection (FREND – fluorescence response enhanced nanodrop diagnosis). The Korean company has managed to incorporate a fluorometer in a hand-held machine. Infarct markers will be primarily determined in this case.
3.2.2.8. LifeSign (Somerset, NJ, USA) DXpress Reader
This POCT product is a rapid immunochromatography machine. Qualitative test results are obtained with “StatusFirst” tests (cTnI, myoglobin and CK-MB, alone or in combination).
3.2.3. Type 3 – Bench-top analyzers
3.2.3.1. MagnaBioSciences (San Diego, CA, USA) MICT Benchtop System
The technology of the magnetic immunochromatographic tests (MICTs) is based on the detection of magnetic nanoparticles in a lateral immunochromatography membrane chip. The advantage lies in the detection of the complete capture region inside the 200-μm thick layer. The nanoparticles are stimulated in an oscillating magnetic field, built up by a C-shaped electromagnet. The magnetic measurement of the immunochromatography membrane is then carried out via a matrix of inductive thin-film spools. Once again, the parameters in question are heart-attack markers, infective agents and hormone levels.
3.2.3.2. Radiometer (Bionshoj, Denmark) AQT 90 Flex
This is a random-access immunoassay analyzer. It offers various sandwich immunofluorometry assays for troponin I, CKMB, myoglobin, NTproBNP, CRP, β-HCG and D-dimer, which are detectable in heparin plasma. All the reagents are available as “all-in-one dry reagents”. The capture antibodies are biotinylated and bind to the streptavidin-coated cup surface.
3.2.3.3. Sysmex (Kobe, Japan) Eurolyser Smart 546
This is a POCT-capable immuno-turbidimetry analyzer for CRP, hsCRP, Hb and ferritin. Latex enhancement allows for the highly sensitive measurement of CRP. Aside from this device, there is another larger instrument (Smart 700/340) for the measurement of further standards.
3.2.3.4. T2. Biosystems (Cambridge, MA, USA) Nano DX
This company favors magnetic-resonance detection of superparamagnetic and antibody-labeled nanoparticles via spin-relaxation signaling. The specific antigen-antibody reaction leads to nanoparticle clusters. These, in turn, cause changes in the spin characteristics of the hydrogen atoms. Using conventional NMR technology, it is possible to detect the T2 signal. The standards to be measured in serum, urine, saliva or other body fluids have yet to be determined.
3.2.3.5. Randox (Crumlin, Co. Antrim, UK) Biochip Array Technology
The method used for this device is based on the well-known enzyme-linked immunosorbent assay (ELISA) principle. They developed a platform that makes it possible to test multiple analytes simultaneously on a single biochip with a single set of reagents, controls and calibrators. For detection, a CCD camera and image-processing software are used. At present, there are different biochips available (e.g., cardiac, thyroid, fertility, cerebral, cytokine and adhesion protein arrays, and two drugs-of-abuse arrays). Further, there are arrays for anti-microbial drugs, growth promoters and synthetic steroids on the market.
3.2.4. Type 4 – Hemostaseological coagulation analyzers
An innovative technology based on an established detection principle is realized in the new Multiplate analyzer (Dynabyte, Munich, Germany). The device uses the impedance aggregometry principle [24] for the assessment of platelet function in whole blood. Blood thrombocytes are non-thrombogenic in their resting state, but expose receptors on their surface when being activated. This allows them to attach on vascular injuries and artificial surfaces (e.g., the sensor wires). When the platelets aggregate on the Multiplate wires, they enhance the electrical resistance between them, which can be continuously recorded. In order to enhance the resistance on the sensor wires, tight attachment of the platelets is required. A number of platelet-stimulating test reagents are available for specific, comprehensive platelet diagnostics.
3.2.5. Type 5 – Continuous measurement with POCT systems
For a continuous mode of POCT measurements, minimally-invasive microdialysis systems have become accepted as best approach. Nevertheless, the most desired clinical access for such probes, the intravenous line, was, for a long time, an unfulfilled clinical desire. Only recently, the MicroEye system of Probe Scientific Ltd. (Coventry, UK) enabled continuous, automated monitoring of substances in the circulating blood stream without withdrawing blood from the patient by applying an intravenous microdialysis catheter (http://www.probescientific.com/microeye/).
3.2.6. Type 6 – Molecular biology-based POCT devices for the detection of infectious agents
Many companies are currently working on different POCT-suitable DNA-amplification techniques (e.g., the quantitative detection of the Influenza A panel or of enterovirus strains).
3.2.6.1. Nanosphere (Northbrook, IL, USA)
The Verigene system uses 50-nm gold-nanoparticle-probe technology for the detection of infectious DNA or RNA. The probe technology uses the change in optical properties of these gold nanoparticles that occurs when two or more nanoparticles are brought into close proximity. The wavelength of light scattered from the surface of the particles is within the visible range and can easily be detected. Details of the distance-dependent optical properties of gold nanoparticles are portrayed in Fig. 3 .
Figure 3.
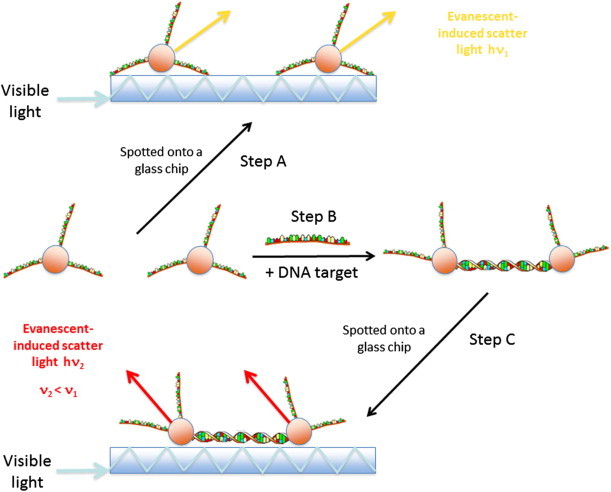
The principle of colorimetric detection of nucleic-acid sequences. When single-stranded DNA-modified gold nanoparticles are attached to a glass slide and white light is coupled into the glass chip (Step A), the evanescent induced scatter is observed. The non-hybridized gold nanoparticles scatter with h × ν1. Step B: the DNA probes are hybridized to a DNA target in solution. When they are spotted onto the glass slide (Step C) these hybridized gold nanoparticles scatter with h × ν2. The plasmon efffect induces a red light shift with ν2 < ν1. The scheme presented is in accordance with [48].
3.2.6.2. Enigma (Salisbury, Wiltshire, UK)
This company is working on providing the next generation of integrated and automated real-time PCR-based diagnostics systems.
3.2.6.3. DxNA (St. George, UT, USA)
A mobile POCT technology (GeneSTAT) provides RT-PCR-based detection of severe acute respiratory syndrome (SARS) or highly pathogenic avian influenza.
3.2.6.4. Idaho Technology (Salt Lake City, UT, USA)
The FilmArray system is a user-friendly automated multiplex PCR.
3.2.6.5. IQuum (Marlborough, MA, USA)
The Liat analyzer offers an innovative lab-in-a-tube platform for detection and genotyping of DNA/RNA viruses and bacteria.
3.3. Future parameters
A number of novel parameters for POCT applications are under discussion. Potentially interesting − but still clinically unevaluated − new markers for POCT applications are as follows.
3.3.1. NGAL
Neutrophil gelatinase-associated lipocalin (NGAL) tests for acute renal failure [25], [26].
3.3.2. Galectin-3
This β-galactoside-binding protein tests for fibrosis and adverse cardiac remodeling [27], [28].
3.3.3. Copeptin
This C-terminal residue of proAVP is for early diagnosis of myocardial infarction [29], [30].
3.3.4. Preeclampsia
The combination of placental growth factor (PIGF), soluble fms-like tyrosine kinase 1 (sFLT-1) [31], [32], [33], and Endoglin are for early diagnosis of preeclampsia [32], [34].
4. Future organizational challenges
Many of the possibilities of extending the application of POCT depend on the wishes, the needs and the practicalities arising from using POCT, as well as the advantages and the disadvantages for the patient. Many people should be involved in this discussion: clinical and laboratory medical personnel, doctors in practice, political and regulatory authorities, state and private health insurers, industry and, not least, the patient. Furthermore, applications of POCT must be scientifically grounded, evidence based {e.g., via outcome analysis, which has only partly been the case to date [9]} and financially viable. Financial viability is strongly associated with the respective national reimbursement system for the various tests, which is, in most countries, mainly a question of healthcare politics.
4.1. POCT management – Quality assessment
The quality of POCT can be assured only through a POCT-coordination system, in which the clinical laboratory plays a pivotal role. All POCT activities should be discussed and agreed upon between the clinics and the clinical laboratory [35]. There are many examples of fruitful cooperation even today. However, a future necessity will be the more structured search protocol for a new POCT method with agreement on the core laboratory method so that the clinical colleagues can assume clinical acceptability of the result variations caused by the POCT method. New rules are needed for assessing agreement on a certain analyte result between two different methodologies [36]. In some analytical cases (e.g., blood-glucose or prothrombin-time monitoring), error-grid analysis can be used to determine cut-offs where differences between central laboratory and POCT results may impact clinical decisions [37]. Novel statistical approaches are yet to be defined.
Data management of the POCT results is also fundamental to quality [38]. Tight surveillance of POCT data can show error trends before they affect the results. However, a prerequisite for this is comprehensive connectivity of all POCT systems used within a hospital-wide network. Newer POCT devices have data-storage functions that can collect key information at the time the test is performed and later transmit the result together with the internal quality-control data to a server-based POCT data manager software linked to the information system of the hospital. Recently developed connectivity standards (e.g., POCT1-A) enable different POCT devices to share a common interface.
POCT data-management software programs can be categorized into proprietary and non-proprietary. Examples of the first category are Cobas IT 1000 from Roche, Radiance from Radiometer or RapidComm from Siemens Healthcare. Non-proprietary software solutions in the EU are POCelerator from Conworx (Berlin, Germany), or, in the USA, Quick-Linc from Telcor (Lincoln, NE, USA) [39]. Wireless real-time communication of test results and quality data will become a reality in the near future [40].
4.2. Trends in healthcare
The number of patients requiring inpatient treatment in a hospital, together with the length of their stay, is likely to decline further in the near future – to the advantage of outpatient treatment and patient observation in their home environment. The importance of POCT will increase in these areas. In outpatient care, it is clear what advantage immediate diagnostic and therapeutic decisions offers: the patient may not need to be further investigated. An enlargement of the current parameter spectrum is also important for the doctor in practice; here, in addition to the tests now available [41], a means of identifying infectious agents would be required.
Home-care will be extended – apart from glucose and INR monitoring – when new tests for chronic diseases become readily available. The most important trend in this context is termed “personalized medicine”. In this case, chronic diseases (e.g., diabetes or atherosclerosis) would be diagnosed earlier than by traditional clinical diagnosis, which is focused on the patient’s clinical signs and symptoms. The therapeutic strategies would then be tailored to each patient individually, thus improving the results of treatment (Fig. 4 ).
Figure 4.
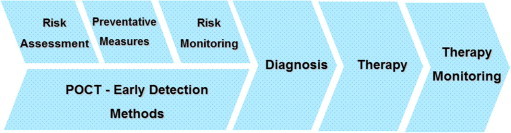
Preventative medicine via early detection of chronic disease (e.g., the multigenetic diseases arteriosclerosis or diabetes mellitus type 2) with the help of simple-to-use point-of-care testing (POCT) methods.
“Telemedicine” is increasingly important in this area. Instruments used to monitor the patient can, in the right circumstances, send the data obtained directly to the relevant medical service point, which can intervene (e.g., when critical values are exceeded). The effectiveness of home monitoring and thus patient safety are improved.
4.3. New areas of use
Though POCT may be used in principle at “any” locale, its use today is mainly centered on various areas of the hospital, general practitioners and patient self-monitoring of glucose and INR. Above and beyond this, other potential uses have been discussed, or have indeed been, albeit mostly in small numbers, already realized. These include:
-
•
mobile emergency paramedical care (blood mobiles, mobiles for public events);
-
•
transport vehicles (e.g., ambulances and helicopters);
-
•
sport medicine or competitive sport;
- •
-
•
remote areas, “third world” countries;
-
•
military use;
-
•
at the pharmacy;
-
•
prisons;
-
•
alternative-medicine practitioners;
-
•
corporate healthcare operations;
-
•
nursing homes;
-
•
veterinary medicine;
-
•
fitness studios; and,
-
•
home healthcare
In many cases, the equipment currently available, as well as the spectrum of parameters, is only partly suitable; but, future developments will raise the acceptance level and therefore the use of POCT in these specific areas.
4.4. POCT in developing countries
The availability of medical treatment to the population as a whole is particularly important in developing countries. The current western systems (e.g., structured around a large central hospital) are largely unsuitable; rather a decentralized health system offers the most effective means. In this case, a decentralized diagnostic process with POCT systems can play a very useful part. There have been a few successful movements in this direction (e.g., in reserves of the Australian aborigines) [44]. However, widespread use of the currently available systems is hindered by costs, complexity and unreliability under extreme conditions of heat or humidity. The parameters offered often do not meet those required in these situations. The American National Institute of Biomedical Imaging and Bioengineering (NIBIB) has therefore begun co-operation with India, the aim of which is to develop devices, which are suitable for use at the point of need [45]. The International Council for Standardization in Hematology (ICSH) is working on a set of guidelines, which will be applicable worldwide for POCT standardization in hematology and which can also be used in developing countries [46].
Specialized, novel rapid microbiological tests must be developed to recognize the common infective agents in these countries. The Bill and Melinda Gates Foundation has sponsored research in this direction in order to establish a simple POCT application for AIDS patients. This so-called CD4 initiative should make it possible to count the CD4 positive T-lymphocytes in HIV-infected patients, with a view to better control of anti-retroviral therapy.
The value of POCT in epidemic disease in developing countries can be demonstrated in the following example. Tuberculosis treatment is hampered by slow, insensitive diagnostic methods, particularly for the detection of drug-resistant forms. An early diagnosis is essential to reduce mortality and to interrupt transmission, but the insufficient healthcare infrastructure in poor countries limits their accessibility and effect.
Boehme et al. [47] assessed the performance of the automated molecular identification of Mycobacterium tuberculosis (MTB) and the testing of resistance to rifampin (RIF) on the GeneXpert MTB/RIF system (mentioned above), applying sputum samples of patients from Peru, Azerbaijan, South Africa, and India. As a result, the authors stated that the MTB/RIF test provided sensitive detection of tuberculosis and rifampin resistance directly from untreated sputum in less than 2 h. The test was specific in 604 of 609 patients without tuberculosis (99.2%). As compared with phenotypic drug-susceptibility testing, MTB/RIF testing correctly identified 200 of 205 patients (97.6%) with rifampin-resistant bacteria and 504 of 514 (98.1%) with rifampin-sensitive bacteria. The device is definitely applicable in rural areas without being compromised by a lack of analytical quality.
5. Obstacles
All future projects should take into account that POCT can in principle be brought simply to the patient, but not necessarily the expertise of the laboratory doctor. It is difficult to imagine how complex panels of analyses (e.g., tumor markers or genetic susceptibility tests), supplied via POCT, could be interpreted without adequate technical expertise and knowledge. It is therefore vital, in the interest of the patient, that POCT is employed by laboratory medical personnel who can suggest appropriate tests and carry them out satisfactorily.
A specific problem involves the problem of quality assessment. The wide use of POCT in areas outside of the standard hospital environment or by general practitioners can lead to new problems in quality assurance. It is clear today, that use of POCT without relevant trained personnel leads to unsatisfactory results and that the end-user has to rely on a competent laboratory medical advisory service, at least when difficulties arise.
Further development of the relevant equipment is likely to result in inbuilt quality control, so that the current procedures for internal and external controls will become obsolete. However, the problems of end use by untrained or unsupervised personnel will increase, particularly with respect to pre-analysis (patient preparation, sample acquisition, recognition of invalid samples) and post-analysis (documentation, data protection) [1]. This is notably the case where POCT is used in areas far from a laboratory (e.g., developing countries). It is an important task for laboratory medicine as a whole to make it clear that POCT analysis that meets the medical requirements rests on more than reliable analysis systems.
Acknowledgements
This work was gratefully funded in part by the European Commission under the Sixth Framework Program within the integrated research project CARE-MAN (HealthCARE by Biosensor Measurements and Networking), NMP4-CT-2006-017333.
References
- 1.Luppa P.B., Schlebusch H. Springer Medizin Verlag; Heidelberg, Germany: 2008. POCT-Patientennahe Labordiagnostik. [Google Scholar]
- 2.Clinical and Laboratory Standards Institute, CLSI document POCT07-P, 2009 (http://www.clsi.org/source/orders/free/poct07-p.pdf).
- 3.Kost G.J. Lippincott Williams Wilkins; Philadelphia, USA: 2002. Principles and Practice of Point-of-Care Testing. [Google Scholar]
- 4.Price G.J., St. John A., Kricka L.L. AACC Press; Washington DC, USA: 2010. Point-of-Care Testing. [Google Scholar]
- 5.Stöcklein W. Chemie in unserer Zeit. 2006;40:32. [Google Scholar]
- 6.Berger-Bächi B. S. Rohrer. Arch. Microbiol. 2002;178:165. doi: 10.1007/s00203-002-0436-0. [DOI] [PubMed] [Google Scholar]
- 7.Shibasaki M., Ibuki T., Tanaka Y. J. Anesth. 2010;24:643. doi: 10.1007/s00540-010-0933-2. [DOI] [PubMed] [Google Scholar]
- 8.D’Orazio P., Burnett R.W., Fogh-Andersen N., Jacobs E., Kuwa K., Külpmann W.R., Larsson L., Lewenstam A., Maas A.H.J., Mager G., Naskalski J.W. Clin. Chem. 2005;51:1573. doi: 10.1373/clinchem.2005.051979. [DOI] [PubMed] [Google Scholar]
- 9.Nichols J.H., Christenson R.H., Clarke W., Gronowski A., Hammett-Stabler C.A., Jacobs E., Kazmierczak S., Lewandrowski K., Price C., Sacks D.B., Sautter R.L., Shipp G., Sokoll L., Watson I.D., Winter W., Zucker M.L. Clin. Chim. Acta. 2007;379:14. doi: 10.1016/j.cca.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Torreiro E.G., Fernandez E.G., Rodriguez R.M., Lopez C.V., Nunez J.B. Thromb. Hemost. 2009;101:969. [PubMed] [Google Scholar]
- 11.Solvik U.O., Petersen P.H., Monsen G., Stavelin A.V., Sandberg S. Clin. Chem. 2010;56:1618. doi: 10.1373/clinchem.2010.146233. [DOI] [PubMed] [Google Scholar]
- 12.Christensen T.D., Larsen T.B., Jensen C., Maegaard M., Sorensen B. Thromb. Hemost. 2009;101:563. doi: 10.1160/th08-09-0601. [DOI] [PubMed] [Google Scholar]
- 13.Ganter M.T., Hofer C.K. Anesth. Analg. 2008;106:1366. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 14.Leemann H., Lustenberger T., Talving P., Kobayashi L., Bukur M., Brenni M., Brüesch M., Spahn D.R., Keel M.J. J. Trauma. 2010;69:1403. doi: 10.1097/TA.0b013e3181faaa25. [DOI] [PubMed] [Google Scholar]
- 15.Harrison P., Segal H., Blasbery K., Furtado C., Silver L., Rothwell P.M. Stroke. 2005;36:1001. doi: 10.1161/01.STR.0000162719.11058.bd. [DOI] [PubMed] [Google Scholar]
- 16.Marshall P.W., Williams A.J., Dixon R.M., Growcott J.W., Warburton S., Armstrong J., Moores J. Br. J. Clin. Pharmacol. 1997;44:151. doi: 10.1046/j.1365-2125.1997.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee P.Y., Chen W.H., Ng W., Cheng X., Kwok J.Y., Tse H.F., Lau C.P. Am. J. Med. 2005;118:723. doi: 10.1016/j.amjmed.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 18.Corstjens A.M., Ligtenberg J.J., van der Horst I.C., Spanjersberg R., Lind J.S., Tulleken J.E., Meertens J.H., Zijlstra J.G. Crit. Care. 2006;10:R135. doi: 10.1186/cc5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seme K., Mocilnik T., Komlos K.F., Doplihar A., Persing D.H., Poljak M. J. Clin. Microbiol. 2008;46:1510. doi: 10.1128/JCM.01694-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenny S.L., Hu Y., Overduin P., Meijer A. J. Clin. Virol. 2010;49:85. doi: 10.1016/j.jcv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo M.R., Rosenfeld L.G. Malays. J. Pathol. 2007;29:57. [PubMed] [Google Scholar]
- 22.Gauglitz G., Proll G. Adv. Biochem. Eng. Biotechnol. 2008;109:395. doi: 10.1007/10_2007_076. [DOI] [PubMed] [Google Scholar]
- 23.de Boer B.M., Kahlman J.A.H.M., Jansen T.P.G.H., Duric H., Veen J. Biosens. Bioelectron. 2007;22:2366. doi: 10.1016/j.bios.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Cardinal D.C., Flower R.J. J. Pharmacol. Methods. 1980;3:135. doi: 10.1016/0160-5402(80)90024-8. [DOI] [PubMed] [Google Scholar]
- 25.Rosner M.H. Adv. Clin. Chem. 2009;49:73. doi: 10.1016/s0065-2423(09)49004-8. [DOI] [PubMed] [Google Scholar]
- 26.Haase M., Haase-Fielitz A., Bellomo R., Mertens P.R. Curr. Opin. Hematol. 2011;18:11. doi: 10.1097/MOH.0b013e3283411517. [DOI] [PubMed] [Google Scholar]
- 27.de Boer R.A., Yu L., van Veldhuisen D.J. Curr. Heart Fail Rep. 2010;7:1. doi: 10.1007/s11897-010-0004-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lok D.J., Van Der Meer P., de la Porte P.W., Lipsic E., Van Wijngaarden J., Hillege H.L., van Veldhuisen D.J. Clin. Res. Cardiol. 2010;99:323. doi: 10.1007/s00392-010-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgenthaler N.G., Struck J., Alonso C., Bergmann A. Clin. Chem. 2006;52:112. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 30.Morgenthaler N.G. Congest. Heart Fail. 2010;16:S37. doi: 10.1111/j.1751-7133.2010.00177.x. [DOI] [PubMed] [Google Scholar]
- 31.Kita N., Mitsushita J. J. Curr. Med. Chem. 2008;15:711. doi: 10.2174/092986708783885309. [DOI] [PubMed] [Google Scholar]
- 32.Foidart J.M., Schaaps J.P., Chantraine F., Munaut C., Lorquet S. Reprod. Immunol. 2009;82:106. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Stepan H., Schaarschmidt W., Jank A., Verlohren S., Kratzsch J. Z. Geburtshilfe Neonatol. 2010;214:234. doi: 10.1055/s-0030-1262827. [DOI] [PubMed] [Google Scholar]
- 34.Akolekar R., Syngelaki A., Sarquis R., Zvanca M., Nicolaides K.H. Prenat. Diagn. 2011;31:66. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 35.Casagrande I. Clin. Chem. Lab. Med. 2010;48:931. doi: 10.1515/CCLM.2010.191. [DOI] [PubMed] [Google Scholar]
- 36.Gialamas A., Laurence C.O., Yelland L.N., Tideman P., Worley P., Shephard M.D., Tirimacco R., Willson K.J., Ryan P., Gill J., Thomas D.W., Beilby J.J. Clin. Biochem. 2010;43:515. doi: 10.1016/j.clinbiochem.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Petersen J.R., Vonmarensdorf H.M., Weiss H.L., Elghetany M.T. Clin. Chim. Acta. 2010;411:131. doi: 10.1016/j.cca.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Nichols J.H. Expert Rev. Mol. Diagn. 2003;3:563. doi: 10.1586/14737159.3.5.563. [DOI] [PubMed] [Google Scholar]
- 39.Clarke B. Point of Care. 2007;6:148. doi: 10.1097/POC.0b013e318265db42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nichols J.H. Point of Care. 2008;7:271. [Google Scholar]
- 41.Stürenburg E., Junker R. Dtsch. Arztebl. Int. 2009;106:48. doi: 10.3238/arztebl.2009.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freidewald S.F., Fink E.J., Dobler G. J. Lab. Med. 2006;30:211. [Google Scholar]
- 43.Kost G.J., Tran N.K., Tuntideelert M., Kulrattanamaneeporn S., Peungposop N. Am. J. Clin. Pathol. 2006;126:513. doi: 10.1309/NWU5E6T0L4PFCBD9. [DOI] [PubMed] [Google Scholar]
- 44.Shephard M.D.S., Mazzachi B.C., Shephard A.K., Burgoyne T., Dufek A., Kit J.A., Mills D., Dunn D. Point of Care. 2006;5:168. [Google Scholar]
- 45.Kost G.J. Point of Care. 2008;7:103. doi: 10.1097/POC.0b013e318162f3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briggs C., Carter J., Lee S.H., Sandhaus L., Simon-Lopez R., Vives Corrons J.L. Int. J. Lab. Hematol. 2008;30:105. doi: 10.1111/j.1751-553X.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- 47.Boehme C.C., Nabeta P., Hillemann D., Nicol M.P., Shenai S., Krapp F., Allen J., Tahirli R., Blakemore R., Rustomjee R., Milovic A., Jones M., O’Brien S.M., Persing D.H., Ruesch-Gerdes S., Gotuzzo E., Rodrigues C., Alland D., Perkins M.D. N. Engl. J. Med. 2010;363:1005. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Storhoff J.J., Lucas A.D., Garimella V., Bao Y.P., Müller U.R. Nat. Biotechnol. 2004;22:883. doi: 10.1038/nbt977. [DOI] [PMC free article] [PubMed] [Google Scholar]


