Abstract
Context
In 2012, the French Infectious Diseases Society (French acronym SPILF) initiated the “Coordination of epidemic and biological risk” (SPILF-COREB - Emergences [SCE]) group to support the readiness and response of healthcare workers (HCWs) to new alerts.
Objective
To present the SCE group, its functioning, and the main support it provided for frontline HCWs.
Methods
A multidisciplinary group of heads of infectious disease departments from reference hospitals was created to build a network of clinical expertise for care, training, and research in the field of epidemic and biological risk (EBR). The network developed a set of standardized operational procedures (SOPs) to guide interventions to manage EBR-suspect patients.
Results
A working group created the SOP aimed at frontline HCWs taking care of patients. Priority was given to the development of a generic procedure, which was then adapted according to the current alert. Five key steps were identified and hierarchized: detecting, protecting, caring for, alerting, and referring the EBR patient. The interaction between clinicians and those responsible for the protection of the community was crucial. The SOPs validated by the SPILF and its affiliates were disseminated to a wide range of key stakeholders through various media including workshops and the SPILF's website.
Conclusion
SPILF can easily adapt and timely mobilize the EBR expertise in case of an alert. The present work suggests that sharing and discussing this experience, initiated at the European level, can generate a new collective expertise and needs to be further developed and strengthened.
Keywords: Clinical network, Emerging infectious diseases, Epidemic and biological risk, Standardized operating procedures
Résumé
Contexte
En 2012, la Société de pathologie infectieuse de langue française (SPILF) a créé un groupe « Coordination du Risque Épidémique et Biologique (REB) » pour préparer la réponse des soignants à une nouvelle alerte (SPILF–COREB - Émergences [SCE]).
Objectif
Présenter le groupe SCE, son fonctionnement et les productions mises à disposition des soignants de première ligne.
Méthodes
Avec les responsables des services de maladies infectieuses des établissements de santé de référence, un groupe multidisciplinaire SCE s’est structuré en partenariat avec les sociétés savantes impliquées. Il devait organiser en réseau l’expertise clinique pour soin, formation, et recherche. Il élaborait des procédures opérationnelles pour guider les premières actions de prise en charge des patients suspects REB.
Résultats
Un groupe dédié rédigeait les procédures destinées aux premiers soignants prenant en charge les premiers patients. La priorité a été l’élaboration d’une procédure générique, déclinée ensuite selon l’alerte en cours. Cinq étapes clés ont été identifiées et hiérarchisées : dépister, protéger, prendre en charge, alerter et orienter le patient REB. L’interaction entre cliniciens et acteurs de la protection de la collectivité était essentielle. Les procédures validées par la SPILF et ses partenaires concernés ont été largement diffusées, notamment sur le site web des infectiologues (infectiologie.com).
Conclusion
La SPILF peut mobiliser rapidement en réseau l’expertise REB en cas d’alerte. Ce travail suggère que le partage et les échanges de cette expérience, amorcés avec d’autres pays européens, peuvent générer une nouvelle expertise et méritent d’être développés et consolidés.
Mots clés: Réseau clinique, Maladies infectieuses émergentes, Risque épidémique et biologique, Procédures opérationnelles standardisées
1. Introduction
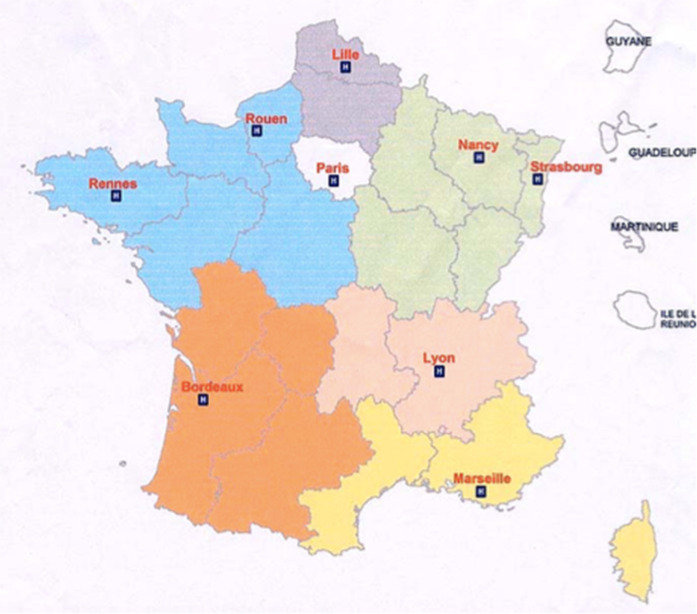
Since the 2001 anthrax alert in the United States (23 American case patients, 5 deaths, and approximately 2500 anthrax-suspect patients in France) and the international severe acute respiratory syndrome (SARS) outbreak in 2003, an organization has been developed in France to address the clinical management of patients suspected of having an infectious disease (ID) related to a natural epidemic or an intentional biological risk (“Epidemic and Biological Risk”–EBR). The response to nuclear, radiological, biological, and chemical risks in France is organized through 12 civil zones of defense (2003 application decree). In each zone 1–2 hospitals are identified as a reference for these risks (16 reference hospitals, among which the military reference center of the Paris - Île-de-France zone and the Guadeloupe-associated reference hospital were included) as presented in Fig. 1 [1]. For biological risks, each of these hospitals has a dedicated ID department, reference microbiology laboratory, zonal prehospital emergency medical service unit (SAMU center 15) including the medical regulation of emergency calls, and departments which include infection control unit, intensive care unit, and a pharmacy involved in the care of these patients. EBR alerts might lead to disruption within the public health sector and the society related to fear of transmission and to the potential negative socio-economic impacts [2]. Immediate access to ID expertise was requested by frontline healthcare workers (HCWs), especially SAMU Center 15 which is responsible for centralized medical calls in France. It is unlikely that EBR-suspect patients would present themselves directly to one of the reference centers identified by the health authorities; they would rather first consult other closer health facilities within the national health system. Therefore, the need for a multidisciplinary network to deliver the operational expertise to frontline HCWs for initial patient management (mainly SAMU Center 15, in-hospital emergency departments, and family physicians [FPs]) was urgent. The 2009 H1N1 influenza pandemic highlighted the importance for timely delivery of appropriate care to patients and for informing the general population about means of protection. This reinforced the concept that the mid/long-term outcome of an epidemic depends heavily on adequate and coherent coordination of individual care and collective measures, decisions and actions undertaken during the initial phase of the alert. In 2009, a dedicated regional EBR Coordination unit (Coordination opérationnelle du risque épidémique et biologique [COREB]) was created in Paris - Île-de-France. In 2012, the French Infectious Diseases Society (SPILF) established a national SPILF–COREB–Emergences (SCE) group to coordinate ID specialists’ response to EBR in collaboration with other key stakeholders, such as other specialists and institutions.
Fig. 1.
Defense and security areas for organization of nuclear, radiological, biological (epidemic and biological risks included), and chemical risks in metropolitan France and overseas departments, with localization of the reference hospitals, March 2016.
Zones de défense civile pour l’organisation du risque Nucléaire-Radiologique-Biologique-Chimique (NRBC) incluant le risque épidémique et biologique, en France métropolitaine et d’outre-mer, avec localisation des établissements de santé de référence, mars 2016
The aim of the present article was to describe the SPILF–COREB - Emergences group functioning and the standard operational procedures (SOPs) that were delivered to frontline HCWs, particularly following the recent alerts of Middle East respiratory syndrome coronavirus (MERS-CoV) infections and Ebola virus disease (EVD).
2. Methods
2.1. Composition of the SCE group
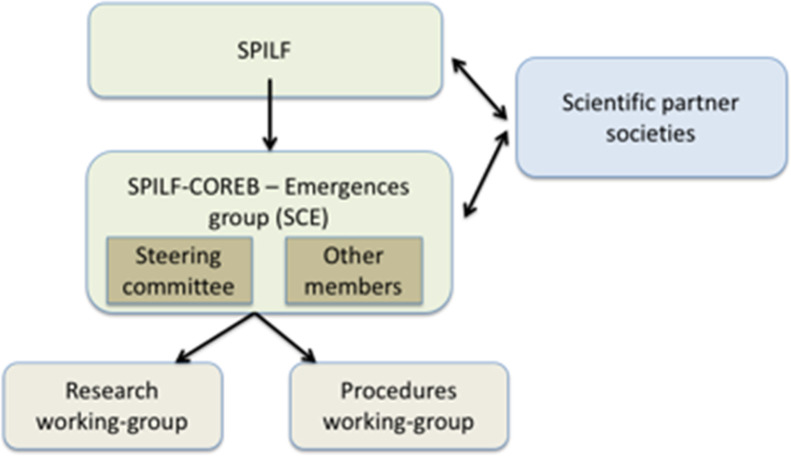
The SCE group initially gathered motivated and experienced ID specialists from national university and general hospitals, involved in field operations. Within the SCE group, a regularly convened steering committee provided a strategic focus to the group, quick decision-making in case of alerts, and inter-crisis vigilance. Specialists in the fields of microbiology and infection control were also convened to participate in the SCE group. Two working groups (WG) were created: Research WG responsible for the preparation of epidemiological and clinical research and Procedure WG responsible for editing SOPs for clinicians (see Fig. 2 for organizational structure). The Procedure WG involved various healthcare professionals including ID, infection control, emergency care specialists, FPs, and nurse managers. The Procedure WG also had regular interactions with microbiology and epidemiology experts; all links were established before an alert crisis period to enhance the relevance and the rapid adaptation of the response. This WG collected and coordinated information and knowledge with the other concerned professionals and stakeholders, and summarized expertise when an EBR alert occurred.
Fig. 2.
Organization of the SPILF–COREB–Emergences (SCE) group.
Organisation du groupe SPILF–COREB–Émergences (SCE).
2.2. Writing of a SOP
During both inter-crisis periods and alerts, the SCE group had to update available knowledge on emerging IDs and provided relevant information through professional networks. In addition to academic scientific publications, websites were monitored and reviewed to validate international data through documentation from well-established networks such as the World Health Organization (WHO), the Centers for Disease Control and Prevention (CDC), the European Center for Diseases Control (ECDC) [3], and the Promed daily webmail alerts (a program from the International Society for Infectious Diseases).
The first task of the SCE group was to create a generic procedure which would provide a framework for EBR clinical situations that HCWs might encounter [4]. The group then wrote specific response alert SOPs. Each time an alert was triggered at an international or national level, with potential problems for French frontline HCWs, the Procedure WG in collaboration with the steering committee, decided whether or not to generate a specific SOP. A request to write a SOP could come from the SCE group (in agreement with SPILF), from SPILF itself, or from health authorities represented by the General Directorate of Health of the Ministry of Health, or their operational partners. The decision on how to respond to an alert was then discussed with national experts from two fields: epidemiologists from Santé publique France responsible for monitoring and implementing the epidemiological investigations, and microbiologists from the National Reference Center for the corresponding pathogen. Additionally, a brief literature review undertaken by the SCE group of current information preceded each decision. Several types of responses to an alert could be generated, such as a warning by email to a mailing list (short time response), the creation of a ‘frequently asked questions’ document about a new pathogen, or a SOP concerning the care to be given to an EBR-suspect patient (if the alert seemed to be of national interest). The timeframe for response varied between a few days to months depending on the severity and complexity of the situation.
2.3. Validation and diffusion of a SOP
Each SOP or document written by the Procedure WG was first submitted for review to the steering committee and the whole SCE group. It was also submitted to the epidemiologists from Santé publique France and to representatives from each of the relevant partner scientific societies (for example, emergency, intensive care, hygiene, microbiologists, and pediatric specialists). Following feedback, the SOP was modified and the revised version was re-submitted for approval to the steering committee and SPILF chair. It was then sent to national health authorities and their operational partner, the High Council for Public Health (French acronym HCSP), the SCE group, and the corresponding affiliate of each partner society for validation or information, depending on the type of referral. Once the SOP was approved it was published online on the SPILF's website, http://www.infectiologie.com/fr/coreb.html. A press release was also sent to wider stakeholders, such as FPs and the media if appropriate.
3. Results
3.1. SOPs for management of EBR-suspect patients
Frontline HCWs needed a generic procedure in case of an EBR alert, which could guide their initial actions independently of the pathogen. This generic procedure was established in 2009 by the regional COREB group in Paris–Île-de-France. Five hierarchical principles were identified; each could be tailored to specific situations:
-
•
how to detect an EBR-suspect patient (compatible manifestations and exposure). This first step is conducted closely with the ID specialist and the epidemiologist responsible for monitoring at a national level in order to be as coherent and sensitive as possible;
-
•
how to protect HCWs, other patients, and the environment with a rapid and effective application of individual and collective hygiene measures;
-
•
how to care for the patient characterizing the clinical presentation and looking for severe symptoms or risk comorbidities, prescribing symptomatic and when possible specific anti-infective treatments;
-
•
how to alert the primary specialists, lead ID specialist, lead infection control, SAMU Center 15 practitioner, epidemiologists, and health authorities to classify the patient as an excluded or possible case patient, and adjust and apply appropriate protective measures (e.g. follow-up of contacts);
-
•
how to refer the patient towards the appropriate healthcare department, for example isolation unit, common hospitalization ward, or FP consultation.
Additional dedicated propositions for the most probable infectious agents were enclosed in an appendix section added at the end of this generic procedure: hemorrhagic viral fevers, anthrax, and potentially epidemic pneumonia [4].
Four other specific SOPs were written between 2009 and 2012 (Table 1 ).
Table 1.
Table of procedures written by the SCE group, 2009-2016.
Tableau des procédures rédigées par le groupe SCE, 2009-2016.
| Initiative of the referral | Year | Pathogen | Type of SOP | Date of diffusion/publication |
|---|---|---|---|---|
| COREB Île-de-France, endorsed by SPILF | 2009 | All EBR agents | Standardized generic procedure for SAMU and emergency departments focusing on care of EBR-suspect patients | January 2011 |
| 2010 | Seasonal flu | Care of patients suspected of seasonal flu in emergency departments and initial patient management | January 2010 | |
| 2011 | Measles | Care of patients suspected of measles in emergency departments and initial patient management | May 2011 | |
| 2012 | Hantavirus | Care of patients suspected of Hantavirus pulmonary syndrome | September 2012 | |
| 2013 | MERS CoV | How to manage a patient suspected of MERS Co-V infection | May 2013 | |
| SPILF-COREB-Emergences | 2013 | MERS CoV | Questionnaire for reception and referral of patients suspected of MERS Co-V infection in ambulatory or outpatient care | Never published |
| 2014 | HxNy flu | How to detect and manage a HxNy-suspect patient | February 2014 | |
| 2014 | Ebola | How to detect and manage an Ebola-suspect patient | Never published | |
| 2014 | Ebola | How to detect and manage an Ebola-suspect patient - Abstract | April 2014 | |
| 2014 | Ebola | Questionnaire for frontline workers for reception of a patient suspected of EVD infection | November 2014 | |
| 2015 | MERS CoV | UPDATE: How to manage a patient suspected of MERS Co-V infection | October 2015 | |
| 2016 | Seasonal flu | UPDATE: Global care for seasonal flu-suspect patients | February 2016 | |
| 2016 | Zika | Frequently Asked Questions “Zika virus” - Information document for SAMU medical regulation | April 2016 | |
| 2016 | Lassa | Fever among travelers returning from West Africa - Information note to emergency departments and SAMU practitioners | May 2016 | |
| Ministry of Health | 2014 | Ebola | Indications and modalities for managing biological samples of an Ebola-infected patient in France - PMOR | August 2014 |
| 2014 | Ebola | Care of an Ebola-infected patient in France - PMOR | Ongoing update | |
| 2015 | All EBR agent | Management of an exposed person to an EBR-infected patient (suspect, probable, or confirmed) in a non-healthcare context | Submitted to the HCSP, September 2016 |
SAMU: prehospital emergency medical service unit; EBR: epidemic and biological risk; PMOR: professional multidisciplinary operational recommendation; HCSP: High Council for Public Health.
Eleven additional SOPs have been written and published online on the SPILF's website (http://www.infectiologie.com) since 2012 – when the SCE Procedure WG was set up. They focused on various pathogens according to ongoing alerts, including MERS-CoV infection (March 2013) and EVD (April 2014) (Table 1).
3.2. Experience with MERS-CoV and Ebola virus alerts
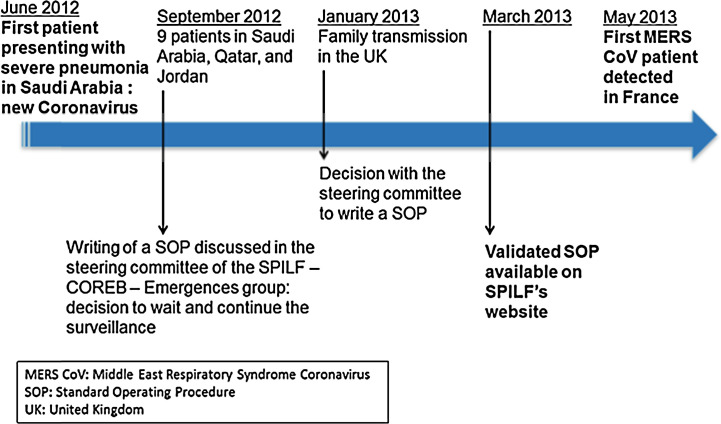
The workflow to prepare a new SOP for MERS-CoV alert is highlighted in Fig. 3 . On March 2012, a first case patient due to a new coronavirus infection was mentioned in the Promed mail. On September 2012, a total of nine patients infected with the novel coronavirus in Saudi Arabia, Qatar, and Jordan were reported to public health authorities and WHO. Two patients were referred to Europe for specialist care; this new coronavirus was also characterized by genome sequencing at the Erasmus Medical Center [5]. Following discussion at the steering committee, the SCE group decided to wait and continue to pay particular attention to the evolution of this new infection as recommended by the epidemiology experts from Santé publique France who had established a system for international surveillance. The SARS SOP (first published in 2003) was republished on the ‘News’ section of the SPILF's website. In January 2013, the British Health Protection Agency announced that an additional case patient of the novel coronavirus infection was imported and confirmed in a resident. This led to the SCE steering committee deciding to provide a specific SOP for care of MERS-CoV-suspect patients, which was prepared within two months and informally shared with health authorities and their operator HCSP. The HCSP used this work to finalize its own recommendations on March 19, 2013. On April 5, 2013 the SOP was circulated to frontline emergency specialists and ID practitioners, and published online on the SPILF's website. The first patient presenting with MERS-CoV was diagnosed in France in May 2013 [6].
Fig. 3.
Initiation of a specific standard operating procedure (SOP): example of the Middle East Respiratory Syndrome (MERS)-coronavirus Standard Operating Procedure (SOP); UK: United Kingdom.
Initiation de la rédaction d’une procédure opérationnelle spécifique : exemple de la procédure Middle East Respiratory Syndrome (MERS)-coronavirus.
More recently, the SCE group has created documentation covering the EVD preparation in France. Once the public health emergency of international concern was declared by WHO in August 2014, French hospitals had to prepare for the possible arrival of patients suspected of EVD infection. The SCE group was asked by the relevant health authorities to rapidly prepare a specific SOP. The first step was to republish, on the ‘News’ page of the SPILF's website, the specific viral hemorrhagic fever appendix related to the 2011 generic procedure. Then, during the first two months of the alert (April–June 2014) a specific SOP for clinical care of an Ebola virus-suspect patient and a questionnaire for all professionals in specific hospital facilities for such patients were prepared. A summary of the SCE EVD clinical SOP was added to the Alert Response Bulletin sent by the French Ministry of Health to all concerned HCWs on September 2, 2014. From May to July 2014, the first formal Professional Multidisciplinary Operational Recommendation (PMOR) was written following a request from the Ministry of Health, to define the appropriate and feasible technical conditions for manipulations of biological samples potentially containing Ebola virus by the microbiological laboratories in the EBR reference hospitals. This led to the publication of a decree by the Health authorities adapting the legal framework for the management of Ebola-containing biological samples on August 6, 2014. The PMOR raised questions about the appropriate personal protective equipment (PPE) against EVD in the reference hospitals, and a dedicated working group was formed to cooperate with the National Institute of Research and Safety for Prevention of Occupational Accidents and Diseases (French acronym INRS) to address the issue. Much of the preparation focused on the use of PPE and, while waiting for national guidelines, each hospital created a contingency plan using their own equipment and clothing to use in local environment. In parallel, the SCE group and the INRS collected, analyzed, and synthesized procedures from each reference hospital and harmonized recommendations were proposed for the donning and doffing of the PPE in case of Ebola-infected patient care [7]. This document was also disseminated to the HCSP and health authorities, and published on the INRS website.
3.3. Other achievements
Following the above-mentioned alerts, the inter-crisis sharing of information was embedded through regular newsletters (two times a year) to primary care physicians. They aimed to facilitate the sharing of information and experiences between HCWs facing EBR through the national network of 16 reference hospitals. The newsletter was made of three parts: an editorial written by the SCE group on recent actions undertaken by the group, an open forum article alternately written by the ID experts from one of the 16 reference national hospitals, and an update of potentially operational epidemiological information and scientific articles [8].
Finally, a yearly meeting with the 16 reference hospitals and all the main stakeholders of the EBR preparation and management (for example, physicians, microbiologists, hygiene specialists, nurses, managers, hospital administrators, and health authorities) has been convened since 2015. This meeting provides an effective opportunity to share best practice and experience to develop growing know-how and insight into addressing key issues related to the management of EBR-associated ID treatment in France.
4. Discussion
This article describes the approach of the scientific medical ID society to create an operational physician-based multidisciplinary network contributing to the preparation and response to an EBR situation.
4.1. Clinical approach in EBR networks
Since the 2001 anthrax and 2003 SARS alerts, response strategies from French public health authorities were primarily based on epidemiological and biological networks. At the international level, a review of the literature in the PubMed database showed the diversity of these networks. Several transitory or virtual physician networks were then established during outbreaks, with the aim of collecting data on patients to improve the clinical response and/or to conduct real-time clinical research (SARS crisis–WHO [9], REVA GRIPPE–SRLF [10], (H1N1)pdm2009 - HPA [11]). The experience of the 2003 SARS crisis and the 2009 pandemic flu suggested that patient care needed to be integrated into the response preparation. This concept was adopted at the international level for the recent MERS-CoV and EVD epidemics, with the creation of the Emerging Disease Clinical Assessment and Response Network (EDCARN) [12] in which the French SCE group participated. Here again, there was strong evidence to suggest that biological and epidemiological networking organizations should better address clinician expertise. Indeed, the main objectives and actions of healthcare system managers needed to focus on ensuring and improving timely and adjusted patient care and appropriate infection control measures [13]. Since then, several European initiatives have created multidisciplinary structures with the aim of sharing information on EBR-associated IDs to enhance preparedness and response (EUNID [14], [15], [16]) or to establish a framework for integrating clinical research into epidemic response (collaborations named PREPARE [17], ISARIC [18], REACTING). Another initiative is the European Training in Infectious Disease Emergencies program (ETIDE), conducted since 2006 which aims to “enhance European capacity to recognize and respond in a coordinated fashion to any infectious disease emergency, whether accidental or deliberate in origin” and which was opened to multidisciplinary professionals including frontline HCWs in Europe [19]. Based on this experience, the French SCE group aimed to contribute to an international approach, keeping at its core patient's care and encouraging operational interaction between ID specialists, epidemiologists, biologists (etc.) with frontline physicians. This field-based validated expertise can be available to physicians, patients and health authority decision-making.
4.2. Operational effectiveness and sustainability of preparedness
Unfortunately, at the end of each recent EBR crisis, network activity vanished and most European projects were not sustained because of a lack of funding. SOPs and actions must be worked out, tested, and revised during inter-crisis periods to prepare for future crises. A new COREB national mission was created in 2014 to share and reinforce the experience of the French SCE group; this new mission was supported and funded concomitantly by the French Ministry of Health and the SPILF. Its main objective was to allow maintenance activity and continuous professional multidisciplinary operational expertise. The COREB mission has been embedded in the National Plan dedicated to the management of Exceptional Health Situations published by the French Ministry of Health in 2014 (ORSAN plan) [20]. Here the main task is, during the inter-crisis time, to maintain the skills – strongly based on training of health professionals – and to stimulate a permanent activity of the EBR clinical network of the 16 reference hospitals for preparation adjusted to the risk level. In parallel, preparing and publishing SOPs using a PMOR methodology is considered essential. A future supplementary strategy to be developed is the preparation and management of EBR-suspect patients by outpatient medical staff, which reflects the reality of a patient pathway. Such an in-depth response strategy would increase frontline practitioners’ involvement, limit the activity overload of reference hospitals, and allow for a more operational, graduated, and adjusted mobilization of the healthcare system if an alert occurs.
4.3. International cooperation
Considering that microorganisms travel across the world as fast as – or even more rapidly than – humans, international networks will benefit from gathering relevant experience from local and regional networks.
In December 2013, WHO and the International Severe Acute Respiratory and Emerging Diseases Consortium (ISARIC) convened physicians treating patients presenting with MERS CoV infection from the five affected countries in the Middle East and other EBR experienced countries, to learn from their first-hand experience, exchange best practice, and discuss potential clinical interventions. The French SCE group participated in this workshop. Similar workshops for active interactions among clinical experts across the world were run when EVD spread in West Africa (2014–2015). These were the basis for developing the EDCARN network.
The development of the French SCE group has been mirrored in other countries such as Germany and Italy. The SCE project started to be developed at the European level in February 2016. Three meetings of physicians from eight European countries close to France were organized in Paris, Berlin, and Madrid to try and settle an EBR clinical operational network.
5. Conclusion
The need for a clinical network, linked with epidemiological and microbiological networks, is now recognized as a pre-requisite for improving the readiness and response to EBR alerts. The emerging expertise from the French SPILF–COREB - Emergences group can be rapidly mobilized for the main stakeholders: it helps frontline HCWs to manage patients and contributes to integrating field experience into strategic decision-making by health authorities and crisis managers. The SCE group's activity and efficiency for creating SOPs now needs to be evaluated. The present work nevertheless suggested that sharing and discussing this experience initiated at the European level was able to generate new collective expertise; this needs to be further developed and strengthened.
Steering committee
S. Alfandari (Tourcoing), H. Aumaitre (Perpignan), F. Bricaire (Paris), P. Brouqui* (Marseille), J-M. Chapplain (Rennes), M-C. Chopin (Valenciennes), H. Coignard-Biehler (Paris), B. Hoen* (Pointe-à-Pitre), V. Jarlier (Paris), C. Leport (Paris), O. Lortholary* (Paris), A. Mérens (Saint Mandé), D. Peyramond (Lyon), C. Rabaud* (Nancy), C. Rapp* (Saint-Mandé), F. Roblot (Poitiers), J. Salomon (Garches), P. Tattevin (Rennes).
Other ID members
J. Beytout (Clermont-Ferrand), E. Bouvet (Paris), A. Cabié* (Fort-de-France), F. Caron (Rouen), E. Caumes* (Paris), C. Chidiac* (Lyon), D. Christmann (Strasbourg), F. Djossou* (Cayenne), M. Dupont* (Bordeaux), J. Gaillat (Annecy), B. Guéry* (Lille), Y. Hansmann* (Strasbourg), D. Malvy (Bordeaux), B. Marchou (Toulouse), C. Michelet* (Rennes), M-P. Moiton* (Saint-Denis de La Réunion), C. Perronne (Garches), P-M. Roger (Nice), E. Senneville (Tourcoing), J-P. Stahl (Grenoble), R. Verdon (Caen), D. Vittecoq (Kremlin-Bicêtre), Y. Yazdanpanah* (Paris).
*Responsible ID specialists in zonal centers.
Representatives of the scientific partner societies
SUdF: F. Braun, M. Nahon, SF2H: B Grandbastien, P. Parneix, SFP: B Chabrol, R Cohen, SFAR: C. Ecoffay, R Gauzit, SFM: R. Courcol, A. Mérens, SFMU: P-Y. Gueugniaud, Y-E. Claessens, SMV: E. Caumes, P-H. Consigny, SRLF: P-F. Laterre, JL Diehl.
Procedure working group
P. Berthelot (Saint Etienne), T. Blanchon (Paris), M-C. Chopin (Valenciennes), H. Coignard-Biehler (Paris), Y. Kieffer (Paris), M. Lardière (Lyon), A. Mahamat (Cayenne), M. Méchain (Bordeaux), C. Rapp (Saint Mandé), O. Rogeaux (Chambéry), L. Rossignol (Paris).
Contributions of authors
H. Coignard-Biehler wrote the article.
C. Rapp, J.M. Chapplain, B. Hoen, F. Cazenave-Roblot, C. Rabaud, and P. Brouqui reviewed the article.
D. Che was the representative of Santé publique France for the joint work with COREB, and reviewed the article.
P. Berthelot was the representative of the hygiene scientific society for the joint work with COREB, and reviewed the article.
C. Leport wrote the article.
Disclosure of interest
This work was partly supported by SPILF.
The authors declare that they have no competing interest.
Acknowledgments
This work was developed with all the members of the SPILF- COREB–Emergences group (2012, updated in 2016). Kemal Ashon helped with the English revision.
Footnotes
This work was presented as a poster at the European Congress of Clinical Microbiology and Infectious Diseases, on May 10, 2014, Barcelona, Spain.
References
- 1.Ministère de l’Emploi et de Solidarité, Direction de l’hospitalisation et de l’organisation des soins, Haut fonctionnaire de la défense. Circulaire DHOS/HFD no 2002-284 du 3 mai 2002 relative à l’organisation du système hospitalier en cas d’afflux de victimes. SP 3 312, NOR: MESH0230349C. [Internet. Available from: http://www.sante.gouv.fr/IMG/pdf/circulaire_du_2_mai_2003-2.pdf - Accessed on September 28, 2017].
- 2.Achonu C., Laporte A., Gardam M.A. The financial impact of controlling a respiratory virus outbreak in a teaching hospital: lessons learned from SARS. Can J Public Health. 2005;96(1):52–54. doi: 10.1007/BF03404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease Prevention and Control. Available from: https://ecdc.europa.eu/en [Internet. Accessed on September 28, 2017].
- 4.Leport C., Vittecoq D., Perronne C., Debord T., Carli P., Camphin P., et al. Infections at risk for epidemic or biological threat. Importance of the initial management of suspect patients. Press Med Paris Fr. 2011;40(4 Pt 1):336–340. doi: 10.1016/j.lpm.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 6.Guery B., Poissy J., el Mansouf L., Sejourne C., Ettahar N., Lemaire X., et al. Clinical features and viral diagnosis of two cases of infection with Middle East Respiratory Syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381(9885):2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institut national de recherche et de sécurité pour la prévention des accidents du travail et des maladies professionnelles . 2015. Maladie à virus Ebola. Tenues et procédures de déshabillage des soignants en établissement de santé de référence. [Internet. Available from: http://www.inrs.fr/dms/inrs/CataloguePapier/ED/TI-ED-6209/ed6209.pdf - accessed on September 28, 2017] [Google Scholar]
- 8.Lettres COREB [Internet. Available from: http://www.infectiologie.com/UserFiles/File/medias/coreb/lettre-coreb-n-5-vf-16-juin-2017.pdf - Accessed on September 28, 2017].
- 9.Knobler S., Mahmoud A., Lemon S., Mack A., Sivitz L., Oberholtzer K., editors. Learning from SARS: Preparing for the Next Disease Outbreak: Workshop Summary. National Academies Press (US); Washington (DC): 2004. [PubMed] [Google Scholar]
- 10.Brun-Buisson C., Richard J-CM, Mercat A., Thiebaut A.C.M., Brochard L. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183(9):1200–1206. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 11.Walunj A. Global clinical networking and pandemic influenza. Intens Care Soc. 2010:165–170. [Google Scholar]
- 12.EDCARN: Emerging Disease Clinical Assessment and Response Network [Internet]. Available from: http://www.who.int/csr/edcarn/en/- Accessed on September 28, 2017.
- 13.Thomson G., Nicoll A. Responding to new severe diseases--the case for routine hospital surveillance and clinical networks in Europe. Euro Surveill. 2010;15(49) doi: 10.2807/ese.15.49.19745-en. [DOI] [PubMed] [Google Scholar]
- 14.Baka A., Fusco F.M., Puro V., Vetter N., Skinhoj P., Ott K., et al. A curriculum for training healthcare workers in the management of highly infectious diseases. Euro Surveill. 2007;12(6) doi: 10.2807/esm.12.06.00716-en. [DOI] [PubMed] [Google Scholar]
- 15.Bannister B., Puro V., Fusco F.M., Heptonstall J., Ippolito G. Framework for the design and operation of high-level isolation units: consensus of the European Network of Infectious Diseases. Lancet Infect Dis. 2009;9:45–56. doi: 10.1016/S1473-3099(08)70304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouqui P., Puro V., Fusco F.M., Bannister B., Schilling S., Follin P., et al. Infection control in the management of highly pathogenic infectious diseases: consensus of the European Network of Infectious Disease. Lancet Infect Dis. 2009;9(5):301–311. doi: 10.1016/S1473-3099(09)70070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.2014. PREPARE Newsletter. [Available from: http://www.prepare-europe.eu/Portals/0/Documents/Newsletters/PREPARE%20Newsletter%20September%202014%20-%20web.pdf - accessed on September 28, 2017] [Google Scholar]
- 18.Dunning J.W., Merson L., Rohde G.G.U., Gao Z., Semple M.G., Tran D., et al. Open source clinical science for emerging infections. Lancet Infect Dis. 2014;14(1):8–9. doi: 10.1016/S1473-3099(13)70327-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bannister B., Prygodzicz A., Ippolito G. Training health care workers to face highly infectious diseases. Clin Microbiol Infect. 2009;15(8):740–742. doi: 10.1111/j.1469-0691.2009.02872.x. [DOI] [PubMed] [Google Scholar]
- 20.Ministère des affaires sociales et de la santé . 2014. Guide d’aide à l’organisation de l’offre de soins en situation sanitaire exceptionnelle. [Internet. Available from: http://solidarites-sante.gouv.fr/systeme-de-sante-et-medico-social/securite-sanitaire/article/le-dispositif-orsan - accessed on September 28, 2017] [Google Scholar]