Many events occurring after vaccination have been attributed to vaccines, when in fact the association was often due to chance.1 However, as with any medical intervention, there are times when adverse events are caused by immunizations.2 Distinguishing which events are causally related to vaccine, rather than coincidental events, is a challenge for the pediatrician and a major focus of vaccine safety science. Consider a child who presents with aseptic meningitis after immunization. Because of the temporal relationship, one may suspect the immunizations as the cause, yet subsequent isolation of enterovirus from cerebrospinal fluid implicates the enteroviral infection instead.3 The term adverse event following immunization (AEFI) is defined as any untoward event that occurs after immunization, regardless of causal association.4 AEFI is the preferred notation to describe such clinical events because the term is free from implications regarding causal relationship and favors an open mind about the role of immunizations. AEFIs are a common part of routine clinical practice.5, 6 The Clinical Immunization Safety Assessment (CISA) network has reviewed many individual cases of AEFIs7, 8, 9 and found that when a comprehensive investigation for alternative etiologies of the AEFI is completed, other causes for the event can often be identified. Yet, such comprehensive evaluations are rarely performed.8 We describe a stepwise approach to the comprehensive assessment of serious AEFIs by health care providers. The main objective is to highlight the important role that health care providers play in this effort by actively evaluating for the most likely causes of serious events when they occur after immunization.
General Approach to Evaluating Serious AEFI
Step 1: Establish a Clear Diagnosis
Many AEFIs can be categorized using the Brighton Collaboration,10 an independent global network of scientists who have developed specific case definitions for select AEFIs to assign levels of diagnostic certainty. Brighton Collaboration case definitions are particularly useful for comparing AEFIs across individuals, regions, and countries, and we encourage providers to use Brighton definitions for AEFIs whenever possible. The application of the Brighton case definition for Guillain-Barré syndrome was used by CISA investigators to classify cases of demyelinating polyneuropathy reported to the Vaccine Adverse Event Reporting System (VAERS) after receipt of the 2009 monovalent H1N1 influenza vaccine.7
Step 2: Consider Whether the Timing of the AEFI Is Consistent with Prior Knowledge and Known Biological Mechanisms
If “risk intervals” for AEFIs are known, it is important to apply these intervals in the evaluation of AEFIs. For example, if a child experiences a febrile seizure 3 days after the receipt of a measles, mumps, and rubella (MMR) vaccine, a parent might consider the immunization to be the cause of the seizure. However, peak vaccine virus replication occurs 1-2 weeks after vaccination,11, 12 and the period of elevated risk for fever and febrile seizures after an MMR vaccine is usually 7-10 days (range 5-12 days)13 after immunization. Thus, it is improbable that a febrile seizure occurring 3 days after immunization was caused by an MMR vaccine.
However, for many serious AEFIs, the period of increased risk after immunization is unclear. In these cases, we encourage providers to carefully document the time course of the AEFI in relation to the vaccination. The natural history of this adverse event should also be reported to VAERS so that this information can be compiled and lead to a better understanding of the risk interval for similar events in the future. The temporal relationship is also useful to CISA investigators if the event is evaluated in this format.
Step 3: Conduct a Thorough Assessment for All Potential Nonvaccine Causes of the AEFI and Seek Evidence that the Vaccine May Be Causally Related to the Event
This step is critical in determining the relationship of the AEFI to the immunization and needs to be completed at the time of the AEFI by the pediatrician or health care provider. Comprehensive etiologic evaluations often are not performed for a variety of reasons, including: (1) the perception that defining the cause may not affect patient management; (2) excessive costs are associated with such evaluations; (3) provider belief that the vaccine was the likely cause; or (4) the provider was not aware of how to conduct such an evaluation. CISA reviewed serious neurologic adverse events reported to VAERS after the pandemic H1N1 influenza vaccine7 and found that when etiologic investigations were conducted, alternate (more likely) causes of the AEFI were often identified (eg, the occurrence of Campylobacter, Mycoplasma, or cytomegalovirus infections before Guillain-Barré syndrome).14, 15 Although identification of an infectious agent at the time of the event cannot completely rule out any possibility that the vaccine was related to the event, this finding lessens the likelihood of a causal association with vaccine.
It is vitally important to uncover other potential and more likely causes for serious AEFIs for 2 reasons: (1) the investigation ensures that providers and patients have complete clinical information on which to make informed decisions regarding current management and future immunizations; and (2) such assessments will enhance our collective knowledge of the true risk of an event after receipt of specific vaccines, thus helping to clarify whether these AEFIs are likely “causal” or “coincidental.” The Table provides a list of many serious AEFIs, a list of potential causes for these disorders, and proposed comprehensive diagnostic evaluations.
Table.
Clinical evaluation of selected AEFIs
| Diagnosis/AEFI | Possible causes temporally related to AEFI other than immunization | Clinical evaluation to consider |
|---|---|---|
| Guillain-Barré syndrome14, 15, 39, 51 | Viral: CMV,∗ EBV,∗ influenza A and B, varicella, HIV, HSV, adenovirus, parainfluenza, WNV | CSF, NP, serum, stool studies for listed viral and bacterial organisms of suspicion |
| Other infectious causes: Campylobacter jejuni,∗Mycoplasma,∗Haemophilus influenzae, Borrelia | Consider saving pretreatment serum for acute and convalescent titer evaluation as IVIG or plasmapharesis is frequently used for treatment. | |
| Other: Surgery, head trauma | ||
| Transverse myelitis52, 53 | Viral: Enterovirus (coxsackievirus A and B, poliovirus), hepatitis A and C, CMV, VZV, EBV, influenza, MMR | CSF, NPS, serum, stool studies for listed viral, bacterial, and parasitic organisms of suspicion |
| Other infectious causes: Campylobacter, Mycoplasma, Brucella melitensis, Enterobius, Schistosoma | Evaluation for systemic autoimmune disorders | |
| Other diagnoses to consider: Systemic autoimmune disorders (MS exacerbation, SLE, systemic sclerosis, mixed connective tissue disorder) | ||
| ADEM31, 54, 55 | Viral: MMR, VZV, EBV, CMV, HSV, hepatitis A and B, coxsackievirus, influenza A or B, HIV, HTLV-1, HHV6, vaccinia, human coronavirus | CSF, NPS, serum, stool studies for listed viral and bacterial organisms of suspicion |
| Bacterial: Mycoplasma, Borrelia, Campylobactor, Leptospira, Chlamydia, Legionella, group A Streptococcus, Rickettsia | Consider saving pretreatment serum for acute and convalescent titer evaluation as IVIG or plasmapharesis is often used for treatment. | |
| Other: Paraneoplastic disorder, organ transplantation | Evaluation for systemic autoimmune disorders | |
| Other diagnoses to consider: Systemic autoimmune disorders | ||
| Encephalitis56 | Viral: HSV, VZV, CMV, EBV, HHV6, La Crosse, Toscana, EEE, WEE, VEE, Chikungunya, JE, St. Louis, WNV, tick-borne encephalitis, Powassan/deer tick, Dengue, Reoviridae, Colorado tick fever, Picornaviridae, echovirus, coxsackievirus, poliovirus, enterovirus, HIV, Papovaviridae, JCV, BKv, influenza A and B, measles, mumps, Nipah, adenovirus, LCM, rabies, parvovirus B19 | CSF, NPS, serum, stool studies for listed viral, bacterial, and parasitic organisms of suspicion. |
| Other infectious causes: B. burgdorferi, B. henselae, Rickettsia/Ehrlichia/Anaplasma spp, M. pneumoniae, Toxoplasma gondii, Plasmodium spp, B. procyonis, Angiostrongylus/Gnathostoma spp, N. fowleri, Acanthamoeba spp, B. mandrillaris, Ameba, cysticercosis, fungi, meningitis, brain abscess, parameningeal abscess | Evaluation for acute and convalescent titers for infectious agents | |
| Other: Venous sinus thrombosis, autoimmune, Reye syndrome, ADEM, acute necrotizing encephalopathy, neoplasm, paraneoplastic disease, cerebrovascular, ischemic stroke, subdural/epidural hematoma, vasculitis, systemic conditions, metabolic conditions, connective tissue disorders, drug intoxication, epilepsy, head injury, confusion migraine | Evaluation for systemic autoimmune disorders, cerebrovascular disease, paraneoplastic disorder, or neoplasm | |
| Aseptic meningitis57 | Viral: Enteroviruses,∗ SLE, JE, WNV, Murry Valley, La Crosse, Jamestown Canyon, Snowshoe hare, HSV 1 and 2, VZV, EBV, CMV, HHV6, Colorado tick fever, mumps, LCM, measles, HIV, adenovirus, parainfluenza, influenza A and B, rotavirus, encephalomyocarditis, parvovirus B19 | CSF, NPS, serum, stool studies for listed viral and bacterial agents |
| Other: Toxins, C. pneumoniae | Serologic evaluation of acute and convalescent titers of infection | |
| Afebrile seizure58, 59, 60, 61 | Epilepsy, severe childhood epilepsies syndromes (Dravet, West, Doose, Lennox-Gastaut), cerebral dysgenesis | MRI (superior to CT unless need for urgent clinical management) |
| Afebrile seizure associated with infection (rotavirus gastroenteritis) | EEG | |
| Neoplasm, trauma, nonaccidental trauma | Genetic analysis | |
| Rotavirus serology | ||
| Cerebellar ataxia61, 62, 63 | Viral: VZV,∗ WNV, rubella, poliovirus type I, influenza A and B, mumps, EBV, parvovirus B19, hepatitis A, echovirus type 9, coxsackievirus type B | CSF analysis for listed agents |
| Other infectious agents: Bacterial abscess, Mycoplasma, malaria, Legionella, meningococcal meningitis, typhoid | Serologic evaluation of acute and convalescent titers of suspected agents | |
| Other causes: Toxin (alcohol, insecticides, barbiturates, thallium, benzodiazepines, heavy metals, solvents), cerebrovascular (hemorrhage, thrombosis), multiple sclerosis, trauma, neoplasm, paraneoplastic syndrome, hereditary ataxia (Friedrich, ataxia telangiectasia, congenital cerebellar ataxia, Wilson disease, episodic ataxia, spinocerebellar ataxia, other inherited ataxias), cerebral palsy, heat stroke, metabolic disorders (mitochondrial, Hartnup disease, intermittent forms of maple syrup urine disease), hyponatremia, other autoimmune disorders (SLE) | Urine analysis for toxins | |
| MRI, CT | ||
| Genetic analysis | ||
| Evaluation for systemic autoimmune disorders | ||
| Optic neuritis64, 65, 66, 67 | Viral: Measles, mumps, VZV, HHV6 | CSF, NPS, serum studies for listed agents |
| Other: Borrelia, Bartonella, Treponema pallidum | Serologic evaluation of acute and convalescent titers of infectious agents | |
| Other diagnoses to consider: Often the first presentation of multiple sclerosis, neuromyelitis optica, SLE, sarcoidosis, Sjögren syndrome | MRI for accurate diagnosis, extent of lesions | |
| Evaluation for systemic autoimmune disorders | ||
BKv, BK virus; CMV, cytomegalovirus; CSF, cerebrospinal fluid; CT, computed tomography; EBV, Epstein-Barr virus; EEE, Eastern equine encephalitis; EEG, electroencephalography; HHV6, human herpes virus 6; HTLV-1, human lymphotropic virus-1; HSV, herpes simplex virus; IVIG, intravenous immunoglobulin; JCV, Jamestown Canyon virus; JE, Japanese encephalitis; LCM, lymphocytic choriomeningitis; MRI, magnetic resonance imaging; MS, multiple sclerosis; NPS, nasopharyngeal swab; SLE, systemic lupus erythematosus; VEE, Venezuelan equine encephalitis; VZV, varicella zoster virus, WEE, Western equine encephalitis; WNV, West Nile virus.
Most commonly reported associations supported by biological evidence.
Step 4: Providers Are Encouraged to Report Any Clinically Significant or Unexpected AEFIs to the VAERS
Several events are reportable by law (http://vaers.hhs.gov/resources/VAERS_Table_of_Reportable_Events_Following_Vaccination.pdf). VAERS16 is the spontaneous reporting system for AEFIs in the United States. Although VAERS has limitations inherent to any passive surveillance system,17 reports to VAERS have generated hypotheses that can be tested using population-based databases such as the Vaccine Safety Datalink.18 For example, in 1998, a cluster of VAERS reports noting intussusception in infants after receipt of the tetravalent rhesus-based rotavirus vaccine19, 20, 21 led to further studies, resulting in the pharmaceutical company ultimately removing the vaccine from the market.
Step 5: Assess the Causal Association of the AEFI with the Vaccine(s) Using All Clinical Information Collected as Discussed Earlier
Even with complete clinical information, if the provider is concerned the AEFI is causally associated with vaccination, the assessment can be challenging and may require consultation with subspecialists or experts in vaccine safety, such as the CISA network. One primary purpose of CISA is to review clinically complex AEFIs. CISA investigators review all data related to the AEFI, discuss the case with subspecialty experts and ideally the requesting provider, and answer specific questions, typically related to causality and future immunizations. Providers can contact the CISA network through the CISA website (http://www.cdc.gov/vaccinesafety/Activities/CISA.html). CISA has also developed a causality assessment tool for use by health care providers22 that guides providers through an algorithm for causality determination. Because information regarding diagnosis, timing, and evaluation of other known causes is intrinsic to the algorithm, it is necessary to complete steps 1 through 3 to assess causality using this tool.
Comprehensive Evaluations of Case Studies of AEFIs
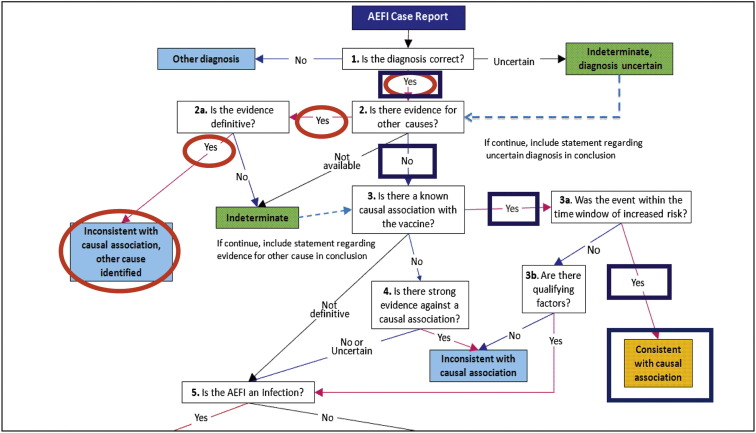
To illustrate the complexities involved with comprehensive AEFI assessments, 2 examples of clinical cases of AEFIs are discussed. The CISA causality algorithm is applied for the 2 examples in the Figure .
Figure.
Use of the causality algorithm22 for assessing causal relationship of 2 clinical examples of AEFIs. Example 1: The algorithm is applied for a case of varicella rash after varicella vaccine if vaccine strain virus is identified using advanced molecular techniques (blue squares). Example 2: The algorithm is applied for a case of ADEM after 2009 monovalent H1N1 vaccine if concurrent parainfluenza infection is identified in the patient (red circles). Note: The causality algorithm includes a different order of “steps”; however, the information obtained through comprehensive assessment can be inserted into the algorithm regardless of the order in which it was obtained.
Varicella
A 1-year-old child presents with a vesicular eruption after receipt of the varicella vaccine. The first step is to accurately characterize the lesions and clinical presentation as consistent with varicella. Step 2 is to consider whether the lesions and symptoms occurred during a plausible risk interval after vaccination. The reported risk interval for varicella rash after the varicella vaccine is 5-42 days,23 and the usual incubation period after wild-type varicella infection is typically 14-16 days.24 To establish the actual cause of the rash (ie, vaccine vs wild-type varicella) with the greatest level of certainty (step 3), a provider should: (1) obtain biological samples to confirm the presence of varicella; and (2) use molecular methods to determine whether it is wild-type or vaccine strain.25 A consultation with an infectious disease specialist would likely facilitate the logistics of this evaluation. Confirmation of cause (ie, wild-type or vaccine strain varicella virus) results in a clear causality assessment (step 5; Figure).
If the rash were disseminated and associated with the vaccine strain, further investigation would be necessary, because disseminated vaccine-type infections usually occur in the setting of immunodeficiency.26, 27, 28, 29
Acute Disseminated Encephalomyelitis
Consider a 5-year-old child who develops symptoms of altered mental status and gross motor abnormalities 3 weeks after receiving routine immunizations. The evaluation starts with establishing the diagnosis of acute disseminated encephalomyelitis (ADEM) (step 1) with appropriate neurologic examinations and magnetic resonance imaging. The Brighton Collaboration has developed an ADEM case definition to help determine the level of diagnostic certainty.10 Step 2 requires the provider to consider carefully whether the symptoms began during an evidence-supported postvaccination risk interval. CISA has recently proposed a risk interval of 2-48 days for ADEM.30 Step 3 is the comprehensive laboratory evaluation for other possible causes for the event or evidence of vaccine association. Identification of suspected viral and bacterial organisms would require the collection of: (1) cerebrospinal fluid; (2) nasopharyngeal swab; (3) serum; and (4) stool samples (Table). ADEM may be managed with intravenous immunoglobulin; thus, it is important to obtain and save sera for later testing before intravenous immunoglobulin administration because this treatment will alter the patient's serologic status.31 An evaluation for systemic autoimmune disorders may also be appropriate. This thorough evaluation allows for the most informed causality assessment (step 5; Figure). CISA reviewed 8 cases of ADEM reported to VAERS after receipt of the 2009 H1N1 vaccine; of these, 2 patients were found to have concurrent parainfluenza virus infection.7
Discussion
For some AEFIs, evidence exists supporting a causal relationship with one or more vaccines based on biological plausibility, epidemiologic, mechanistic, or pathologic factors. Examples include extensive limb swelling after diphtheria, tetanus and acellular pertussis vaccine,32 large local reactions after several vaccines,33, 34, 35 sterile abscesses after vaccines containing alum,36 febrile seizures occurring after measles-containing vaccines,13, 37 and anaphylaxis or other immediate hypersensitivity reactions after gelatin-containing vaccines.12, 38, 39, 40, 41 These relationships have been reviewed extensively in prior publications and the CISA network has developed guidelines to evaluate and manage hypersensitivity reactions occurring after immunizations.42
The rare occurrences of serious or life-threatening AEFIs are of greatest concern for patients, providers, and public health stakeholders, and their evaluation poses a challenge. For the majority of serious AEFIs, the evidence has either been contradictory or inconclusive as to whether a causal relationship exists between the AEFI and specific vaccines. The Institute of Medicine recently reviewed 158 specific AEFIs temporally associated with 1 of 8 vaccines38 according to a strict causal methodology that evaluated both mechanistic and epidemiologic evidence. “Mechanistic evidence” was clinical or biological evidence that a vaccine could cause the specific event, and “epidemiologic evidence” was assessed according to the precision of results and methodologic limitations of peer-reviewed epidemiologic studies.38 The committee found that there was inadequate evidence to determine whether a causal link exists for the majority (135) of AEFIs, concluding that the current “evidence is inadequate to accept or reject a causal relationship.” Systematic reviews of AEFIs by CISA with comprehensive evaluations may provide additional data to assist in these causal determinations when reassessed in the future.43 With so much uncertainty regarding causal associations of serious AEFIs, health care providers can play an essential role in enhancing our vaccine safety knowledge by fully evaluating for all likely cauess at the time these events are diagnosed.
Because immunizations have been so effective at greatly reducing vaccine preventable diseases, vaccine adverse effects have become more evident than the consequences of the diseases that vaccines prevent. When AEFIs are not thoroughly evaluated for causal association, affected patients may believe the vaccine is the only potential culprit. Such unsubstantiated beliefs may ultimately result in public distrust of vaccines and reduced vaccine uptake.
Because serious AEFIs occur very infrequently, international collaboration may be helpful to assess the risk of an AEFI after a particular vaccination. Other countries have systems in place to address provider questions related to vaccine safety, such as the Green Channel in Italy44 and InfoVac in Switzerland.45 Global collaborations can also provide larger sample sizes to better assess AEFI risk through epidemiologic studies.46
For AEFIs potentially due to either wild-type or live attenuated vaccine strains, advances in molecular techniques that correctly characterize agents as wild-type or vaccine strain should be used in consultation with subspecialists to improve causal assessments. Advancing scientific techniques have also led to new explanations for AEFIs. One landmark discovery was reported by Berkovic et al, in 2006 in which investigators used genetic analyses to identify de novo mutations in the sodium channel gene SCN1A in patients with alleged vaccine-induced encephalopathy.47 By uncovering the mechanisms for this AEFI, which prior to this recent finding was believed by many to be causally related to vaccination, these researchers made a significant contribution to the scientific understanding of this rare event. The findings were recently replicated in 5 additional cases of alleged vaccine-induced encephalopathy.48 It is possible that some individuals experience a greater immunogenic response due to vaccine compared with the general population; understanding the genetic rationale for such events will likely be an important area of future research.49, 50
Many AEFIs have insufficient evidence, both mechanistically and epidemiologically, to assess a causal relationship with vaccines, and there is a great need for additional vaccine safety research designed to further examine hypothesized associations. Most serious AEFIs are rare and are not causally related to vaccine(s). However, when serious AEFIs occur, it is important for health care providers to perform comprehensive clinical assessments that include accurate diagnosis, consideration of biological plausibility, comprehensive evaluation for all potential causes, and reporting to the VAERS. The CISA network is available to assist providers regarding complex cases of AEFIs and has recently developed a causality assessment algorithm for health care providers.22 Complete evaluations will improve clinical care by providing accurate risk information to affected patients, will provide a greater understanding of the risk of these rare events, and comprise a key component of postmarketing vaccine safety monitoring.
Acknowledgments
We would like to thank Dr Ellen Wright Clayton (chair of the Institute of Medicine Committee to Evaluate Vaccine Safety) for her expert review and advice.
Footnotes
Financial support and conflict of interest information is available at www.jpeds.com (Appendix).
Appendix.
Supported by the CISA network through a subcontract with America's Health Insurance Plans (contract 200-2002-00732 from the Centers for Disease Control and Prevention). C.D. is a member of the Scientific Advisory Board of PharmaJet and is on Safety Monitoring Committees for HVTN and Pfizer. N.H. has received compensation for serving on Safety Monitoring Committees for Novartis and Merck, receives grant support from Merck, and received an honorarium for attending a meeting with Pfizer. N.K. and R.B. receive research grants from GlaxoSmithKline, Merck, Sanofi Pasteur, MedImmune, Novartis, and Pfizer. C.M. has served as an investigator for Merck, Pfizer, Novartis, MedImmune, Sanofi, and GSK, a consultant for GSK, Sanofi, and Novartis, and a speaker for Sanofi, Novartis, and Pfizer. P.L. has served on the Data Safety Monitoring Board for Novartis and receives limited funding from Merck. K.E. receives funding from the National Institutes of Health (Contract HHSN272200800007C) and CDC (Contracts U01 IP000488-02 and 200-2012-50430). M.B. is a salaried employee for CSL Behring and receives consulting fees for CISA and Sanofi-Aventis. P.D. is on the advisory board of DIME, CSL, Talecris, and Caridian. The other authors declare no conflicts of interest.
References
- 1.Halsey N.A. The science of evaluation of adverse events associated with vaccination. Semin Pediatr Infect Dis. 2002;13:205–214. doi: 10.1053/spid.2002.125864. [DOI] [PubMed] [Google Scholar]
- 2.Chen R.T. Vaccine risks: real, perceived and unknown. Vaccine. 1999;17(Suppl 3):S41–S46. doi: 10.1016/s0264-410x(99)00292-3. [DOI] [PubMed] [Google Scholar]
- 3.Dulek D.E., Donofrio P.D., Sejvar J.J., Edwards K.M. Enteroviral meningitis and concurrent peripheral motor axonal polyneuropathy. Pediatr Infect Dis J. 2012;31:206–208. doi: 10.1097/INF.0b013e31823a0d6e. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Immunization safety: adverse events following immunization. 2009. http://www.who.int/immunization_safety/aefi/en/. Accessed November 7, 2011.
- 5.Miller E., Batten B., Hampton L., Campbell S.R., Gao J., Iskander J. Tracking vaccine-safety inquiries to detect signals and monitor public concerns. Pediatrics. 2011;127(Suppl 1):S87–S91. doi: 10.1542/peds.2010-1722M. [DOI] [PubMed] [Google Scholar]
- 6.Gust D.A., Gangarosa P., Hibbs B., Pollard R., Wallach G., Chen R.T. National Immunization Information Hotline: calls concerning adverse events, 1998-2000. J Health Commun. 2004;9:387–394. doi: 10.1080/10810730490503487. [DOI] [PubMed] [Google Scholar]
- 7.Williams S.E., Pahud B.A., Vellozzi C., Donofrio P.D., Dekker C.L., Halsey N., et al. Causality assessment of serious neurologic adverse events following 2009 H1N1 vaccination. Vaccine. 2011;29:8302–8308. doi: 10.1016/j.vaccine.2011.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams S.E., Klein N.P., Halsey N., Dekker C.L., Baxter R.P., Marchant C.D., et al. Overview of the Clinical Consult Case Review of adverse events following immunization: Clinical Immunization Safety Assessment (CISA) network 2004-2009. Vaccine. 2011;29:6920–6927. doi: 10.1016/j.vaccine.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaRussa P., Edwards K., Dekker C., Klein N., Halsey N., Marchant C., et al. Understanding the role of human variation in vaccine adverse events: the Clinical Immunization Safety Assessment (CISA) network. Pediatrics. 2011 doi: 10.1542/peds.2010-1722J. 127-3 (Vaccine Safety Supplement) [DOI] [PubMed] [Google Scholar]
- 10.The Brighton Collaboration. 2010. https://brightoncollaboration.org/internet/en/index.html. Accessed October 23, 2010.
- 11.Plotkin S, Orenstein W, editors. Vaccines. 4th ed. Philadelphia, Saunders 2004.
- 12.Centers for Disease Control and Prevention. Possible side effects from vaccines. 2011. http://www.cdc.gov/vaccines/vac-gen/side-effects.htm#dtap. Accessed January 20, 2011.
- 13.Klein N.P., Fireman B., Yih W.K., Lewis E., Kulldorff M., Ray P., et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126:e1–e8. doi: 10.1542/peds.2010-0665. [DOI] [PubMed] [Google Scholar]
- 14.Hadden R.D., Karch H., Hartung H.P., Zielasek J., Weissbrich B., Schubert J., et al. Preceding infections, immune factors, and outcome in Guillain-Barre syndrome. Neurology. 2001;56:758–765. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- 15.van Doorn P.A., Ruts L., Jacobs B.C. Clinical features, pathogenesis, and treatment of Guillain-Barre syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. VAERS: Vaccine Adverse Event Reporting System. 2010. http://vaers.hhs.gov/index. Accessed December 23, 2010.
- 17.Iskander J.K., Miller E.R., Chen R.T. The role of the Vaccine Adverse Event Reporting system (VAERS) in monitoring vaccine safety. Pediatr Ann. 2004;33:599–606. doi: 10.3928/0090-4481-20040901-11. [DOI] [PubMed] [Google Scholar]
- 18.Baggs J., Gee J., Lewis E., Fowler G., Benson P., Lieu T., et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011;127(Suppl 1):S45–S53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- 19.Intussusception among recipients of rotavirus vaccine: United States, 1998-1999. MMWR Morb Mortal Wkly Rep. 1999;48:577–581. [PubMed] [Google Scholar]
- 20.Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48:1007. [PubMed] [Google Scholar]
- 21.Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Jumaan A.O., Okoro C.A., et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 22.Halsey N.A., Edwards K.M., Dekker C.L., Klein N.P., Baxter R., Larussa P., et al. Algorithm to assess causality after individual adverse events following immunizations. Vaccine. 2012;30:5791–5798. doi: 10.1016/j.vaccine.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 23.Sharrar R.G., LaRussa P., Galea S.A., Steinberg S.P., Sweet A.R., Keatley R.M., et al. The postmarketing safety profile of varicella vaccine. Vaccine. 2000;19:916–923. doi: 10.1016/s0264-410x(00)00297-8. [DOI] [PubMed] [Google Scholar]
- 24.American Academy of Pediatrics . In: Red Book: 2006 Report of the Committee on Infectious Diseases. 27th ed. Pickering L.K., Baker C.J., Long S.S., McMillan J.A., editors. American Academy of Pediatrics; Elk Grove Village, IL: 2006. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Chicken pox (varicella): interpreting laboratory tests. 2012. http://www.cdc.gov/chickenpox/hcp/lab-tests.html. Accessed October 22, 2012.
- 26.Jean-Philippe P., Freedman A., Chang M.W., Steinberg S.P., Gershon A.A., LaRussa P.S., et al. Severe varicella caused by varicella-vaccine strain in a child with significant T-cell dysfunction. Pediatrics. 2007;120:e1345–e1349. doi: 10.1542/peds.2004-1681. [DOI] [PubMed] [Google Scholar]
- 27.Addition of severe combined immunodeficiency as a contraindication for administration of rotavirus vaccine. MMWR Morb Mortal Wkly Rep. 2010;59:687–688. [PubMed] [Google Scholar]
- 28.Monafo W.J., Haslam D.B., Roberts R.L., Zaki S.R., Bellini W.J., Coffin C.M. Disseminated measles infection after vaccination in a child with a congenital immunodeficiency. J Pediatr. 1994;124:273–276. doi: 10.1016/s0022-3476(94)70318-3. [DOI] [PubMed] [Google Scholar]
- 29.Hayes E.B. Acute viscerotropic disease following vaccination against yellow fever. Trans R Soc Trop Med Hyg. 2007;101:967–971. doi: 10.1016/j.trstmh.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Rowhani-Rahbar A., Klein N.P., Dekker C.L., Edwards K.M., Marchant C.D., Vellozzi C., et al. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine. 2012;31:271–277. doi: 10.1016/j.vaccine.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Murthy S.N., Faden H.S., Cohen M.E., Bakshi R. Acute disseminated encephalomyelitis in children. Pediatrics. 2002;110:e21. doi: 10.1542/peds.110.2.e21. [DOI] [PubMed] [Google Scholar]
- 32.Rennels M.B., Black S., Woo E.J., Campbell S., Edwards K.M. Safety of a fifth dose of diphtheria and tetanus toxoid and acellular pertussis vaccine in children experiencing extensive, local reactions to the fourth dose. Pediatr Infect Dis J. 2008;27:464–465. doi: 10.1097/INF.0b013e31816591f7. [DOI] [PubMed] [Google Scholar]
- 33.Ebo D.G., Bridts C.H., Stevens W.J. IgE-mediated large local reaction from recombinant hepatitis B vaccine. Allergy. 2008;63:483–484. doi: 10.1111/j.1398-9995.2007.01618.x. [DOI] [PubMed] [Google Scholar]
- 34.Quinn P., Gold M., Royle J., Buttery J., Richmond P., McIntyre P., et al. Recurrence of extensive injection site reactions following DTPa or dTpa vaccine in children 4-6 years old. Vaccine. 2011;29:4230–4237. doi: 10.1016/j.vaccine.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 35.Jackson L.A., Yu O., Nelson J.C., Dominguez C., Peterson D., Baxter R., et al. Injection site and risk of medically attended local reactions to acellular pertussis vaccine. Pediatrics. 2011;127:e581–e587. doi: 10.1542/peds.2010-1886. [DOI] [PubMed] [Google Scholar]
- 36.Klein N.P., Edwards K., Sparks R.C., Dekker C.L. Recurrent sterile abscesses following aluminium adjuvant-containing vaccines. BMJ Case Rep. 2009 doi: 10.1136/bcr.09.2008.0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vestergaard M., Hviid A., Madsen K.M., Wohlfahrt J., Thorsen P., Schendel D., et al. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004;292:351–357. doi: 10.1001/jama.292.3.351. [DOI] [PubMed] [Google Scholar]
- 38.Institute of Medicine . National Academies Press; Washington, DC: 2011. (Adverse effects of vaccines: Evidence and causality). 6. [PubMed] [Google Scholar]
- 39.Jacobs R.L., Lowe R.S., Lanier B.Q. Adverse reactions to tetanus toxoid. JAMA. 1982;247:40–42. [PubMed] [Google Scholar]
- 40.Kattan J.D., Konstantinou G.N., Cox A.L., Nowak-Wegrzyn A., Gimenez G., Sampson H.A., et al. Anaphylaxis to diphtheria, tetanus, and pertussis vaccines among children with cow's milk allergy. J Allergy Clin Immunol. 2011;128:215–218. doi: 10.1016/j.jaci.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 41.Johann-Liang R., Josephs S., Dreskin S.C. Analysis of anaphylaxis cases after vaccination: 10-year review from the National Vaccine Injury Compensation Program. Ann Allergy Asthma Immunol. 2011;106:440–443. doi: 10.1016/j.anai.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Wood R.A., Berger M., Dreskin S.C., Setse R., Engler R.J., Dekker C.L., et al. An algorithm for treatment of patients with hypersensitivity reactions after vaccines. Pediatrics. 2008;122:e771–e777. doi: 10.1542/peds.2008-1002. [DOI] [PubMed] [Google Scholar]
- 43.Strom B.L., Kimmel S.E., Hennessy S., editors. Pharmacoepidemiology. 5th ed. John Wiley & Sons; Oxford, UK: 2012. [Google Scholar]
- 44.Zanoni G., Nguyen T.M., Valsecchi M., Gallo G., Tridente G. Prevention and monitoring of adverse events following immunization: the “Green Channel” of the Veneto region in Italy. Vaccine. 2003;22:194–201. doi: 10.1016/s0264-410x(03)00566-8. [DOI] [PubMed] [Google Scholar]
- 45.InfoVac. 2010. http://www.infovac.ch/index.php?Itemid=95. Accessed December 7, 2010.
- 46.World Health Organization. Global Advisory Committee on Vaccine Safety. 2012. http://www.who.int/vaccine_safety/initiative/detection/en/. Accessed February 28, 2012.
- 47.Berkovic S.F., Harkin L., McMahon J.M., Pelekanos J.T., Zuberi S.M., Wirrell E.C., et al. De-novo mutations of the sodium channel gene SCN1A in alleged vaccine encephalopathy: a retrospective study. Lancet Neurol. 2006;5:488–492. doi: 10.1016/S1474-4422(06)70446-X. [DOI] [PubMed] [Google Scholar]
- 48.Reyes I.S., Hsieh D.T., Laux L.C., Wilfong A.A. Alleged cases of vaccine encephalopathy rediagnosed years later as Dravet syndrome. Pediatrics. 2011;128:e699–e702. doi: 10.1542/peds.2010-0887. [DOI] [PubMed] [Google Scholar]
- 49.Reif D.M., McKinney B.A., Motsinger A.A., Chanock S.J., Edwards K.M., Rock M.T., et al. Genetic basis for adverse events after smallpox vaccination. J Infect Dis. 2008;198:16–22. doi: 10.1086/588670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White O.J., McKenna K.L., Bosco A., van den Biggelaar H.J.A., Richmond P., Holt P.G. A genomics-based approach to assessment of vaccine safety and immunogenicity in children. Vaccine. 2012;30:1865–1874. doi: 10.1016/j.vaccine.2011.12.118. [DOI] [PubMed] [Google Scholar]
- 51.Israeli E., Agmon-Levin N., Blank M., Chapman J., Shoenfeld Y. Guillain-Barre syndrome: a classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol. 2012;42:121–127. doi: 10.1007/s12016-010-8213-3. [DOI] [PubMed] [Google Scholar]
- 52.Frohman E.M., Wingerchuk D.M. Clinical practice. Transverse myelitis. N Engl J Med. 2010;363:564–572. doi: 10.1056/NEJMcp1001112. [DOI] [PubMed] [Google Scholar]
- 53.Bhat A., Naguwa S., Cheema G., Gershwin M.E. The epidemiology of transverse myelitis. Autoimmun Rev. 2010;9:A395–A399. doi: 10.1016/j.autrev.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Pavone P., Pettoello-Mantovano M., Le Pira A., Giardino I., Pulvirenti A., Giugno R., et al. Acute disseminated encephalomyelitis: a long-term prospective study and meta-analysis. Neuropediatrics. 2010;41:246–255. doi: 10.1055/s-0031-1271656. [DOI] [PubMed] [Google Scholar]
- 55.Huynh W., Cordato D.J., Kehdi E., Masters L.T., Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15:1315–1322. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Long S.S. Encephalitis diagnosis and management in the real world. Adv Exp Med Biol. 2011;697:153–173. doi: 10.1007/978-1-4419-7185-2_11. [DOI] [PubMed] [Google Scholar]
- 57.Rotbart H.A. Viral meningitis. Semin Neurol. 2000;20:277–292. doi: 10.1055/s-2000-9427. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh D.T., Chang T., Tsuchida T.N., Vezina L.G., Vanderver A., Siedel J., et al. New-onset afebrile seizures in infants: role of neuroimaging. Neurology. 2010;74:150–156. doi: 10.1212/WNL.0b013e3181c91847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lloyd M.B., Lloyd J.C., Gesteland P.H., Bale J.F., Jr. Rotavirus gastroenteritis and seizures in young children. Pediatr Neurol. 2010;42:404–408. doi: 10.1016/j.pediatrneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 60.von Spiczak S., Helbig I., Drechsel-Baeuerle U., Muhle H., van Baalen A., van Kempen M.J., et al. A retrospective population-based study on seizures related to childhood vaccination. Epilepsia. 2011;52:1506–1512. doi: 10.1111/j.1528-1167.2011.03134.x. [DOI] [PubMed] [Google Scholar]
- 61.Menkes S.M., editor. Child neurology. 6th ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- 62.Asokumar P., Gogate Y.V., Gangadhar P., Bhansali A. Reversible cerebellar ataxia: a rare presentation of depletional hyponatremia. Neurol India. 2011;59:631–632. doi: 10.4103/0028-3886.84356. [DOI] [PubMed] [Google Scholar]
- 63.Chattopadhyay P., Dhua D., Philips C., Saha S. Acute cerebellar ataxia in lupus. Lupus. 2011;20:1312–1315. doi: 10.1177/0961203311403346. [DOI] [PubMed] [Google Scholar]
- 64.Absoud M., Cummins C., Desai N., Gika A., McSweeney N., Munot P., et al. Childhood optic neuritis clinical features and outcome. Arch Dis Child. 2011;96:860–862. doi: 10.1136/adc.2009.175422. [DOI] [PubMed] [Google Scholar]
- 65.Cakmakli G., Kurne A., Guven A., Serdaroglu A., Topaloglu H., Teber S., et al. Childhood optic neuritis: the pediatric neurologist's perspective. Eur J Paediatr Neurol. 2009;13:452–457. doi: 10.1016/j.ejpn.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 66.Ogata N., Koike N., Yoshikawa T., Takahashi K. Human herpesvirus 6-associated uveitis with optic neuritis diagnosed by multiplex PCR. Jpn J Ophthalmol. 2011;55:502–505. doi: 10.1007/s10384-011-0069-4. [DOI] [PubMed] [Google Scholar]
- 67.Bonhomme G.R., Waldman A.T., Balcer L.J., Daniels A.B., Tennekoon G.I., Forman S., et al. Pediatric optic neuritis: brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009;72:881–885. doi: 10.1212/01.wnl.0000344163.65326.48. [DOI] [PubMed] [Google Scholar]