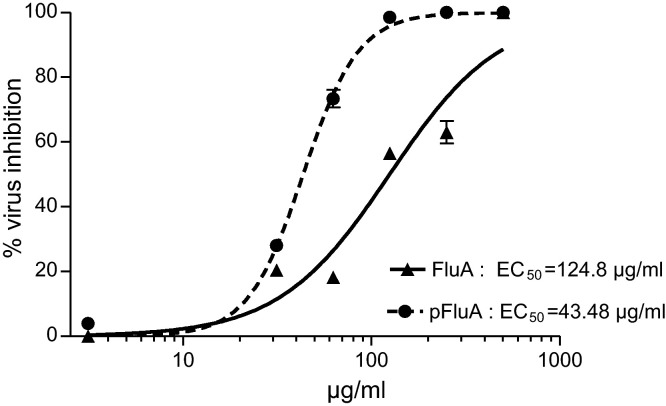
Fig. 3.
Antiviral activity of a Sinupret® preparation against human and porcine influenza virus. To test the efficacy of one Sinupret® preparation – dry extract – on virus replication, virus susceptible cells (MDCK) were infected with a multiplicity of infection (M.O.I.) of 0.0004 (FluA) or 0.0008 (pFluA), without or in presence of six descending non-cytotoxic concentrations of the test substance dry extract. The antiviral activity (y-axis, % virus inhibition) of the test candidate (x-axis, concentration in μg/ml) was determined in plaque-reduction assays (PFU) for FluA (closed triangles) and pFluA (closed circles). The relative inhibitions (% inhibition, ordinate) were calculated by analysing the number of plaques of the respective groups and standardised by the virus control representing 100% infectivity (0% inhibition). Positive controls confirmed the procedure (FluA, amantadine 5 μg/ml, 58% inhibition; pFluA, amantadine 6 μg/ml, 65% inhibition). All data represent means and SEM from two independent experiments with two to three replicates.