Abstract
The Alberta Johne's Disease Initiative (AJDI) is a Johne's disease (JD) control program with the goal of reducing the spread of Mycobacterium avium ssp. paratuberculosis (MAP) through implementation of best management practices. The objective was to estimate the economic benefit of participation in the AJDI. A decision tree was constructed in which disease prevalence, test characteristics, and probabilities for implementation of best management practices suggested by herd veterinarians were implemented. Analysis was performed using a Markov analysis, and input data were assigned using estimates from the AJDI and published data. A cost-effectiveness analysis was performed and the net benefit of participation (from the perspective of a dairy farmer) in the AJDI compared with no participation was calculated. A series of 1-way sensitivity analyses were used to control for uncertainty. Farms participating in the AJDI were estimated to have a net benefit of Can$74 per cow over the course of 10 yr. If project costs were covered by the participating farm, the net benefit was Can$27. In addition to the effects on MAP infection, a reduction in calf diarrhea was modeled for farms that improved their calf management through the use of pasteurizers. In that case, the additional costs outweighed additional revenues compared with the baseline analysis, resulting in a reduced net benefit of Can$19. Participation would not be cost effective if cows in early stages of MAP infection did not have decreased production and if prevalence of MAP infection did not increase on farms with poor management. A limitation of the study, despite high uncertainty in some input parameters, was the lack of knowledge regarding changes in prevalence on farms with various management strategies. In conclusion, participation in the AJDI was cost effective for the average Alberta dairy farm.
Key words: Johne's disease, management practices, economic benefit, Alberta
Introduction
Johne's disease (JD) is a chronic progressive enteritis caused by Mycobacterium avium ssp. paratuberculosis (MAP). In cattle, infection usually occurs in young calves by ingestion of infectious feces. The incubation period is typically 2 to 5 yr, but can be as long as 10 yr after initial infection. Cattle that develop clinical symptoms suffer from a chronic untreatable diarrhea that leads to cachexia and ultimately culling or death (Fecteau and Whitlock, 2010). Direct losses for the dairy industry are due to decreased milk production, premature culling, and decreased slaughter value of infected animals (McKenna et al., 2006). Annual losses due to JD were estimated at Can$2,472 for a 50-cow herd with a mean MAP within-herd prevalence of 7% (Chi et al., 2002). However, in addition to direct losses, an unproven association exists between MAP infection in cattle and Crohn's disease in humans (Barkema et al., 2010; Behr, 2010). Should this association be proven, consumers would reduce consumption of cattle products, which would decrease prices for both dairy and beef products (Groenendaal and Zagmutt, 2008). These factors motivate producers to participate and decision makers to give JD control programs a high priority. In countries with endemic MAP infection, the focus of almost all control programs is to promote implementation of best management practices on dairy farms, with the aim of reducing transmission of MAP and therefore reducing the within-herd prevalence to a low level, or keeping the herd uninfected (McKenna et al., 2006; Bakker, 2010; Kennedy and Citer, 2010; Whitlock, 2010). Knowing the expected costs and benefits due to participation in a JD prevention and control program is essential for farmers to make an informed decision whether to participate or not.
In previous studies, changes in management were cost effective but estimates varied widely (Appendix). Most of the studies were conducted in the United States, where herds are larger and production costs and revenues are lower than in Canada. In addition, these studies did not include detailed information on management strategies used and expected changes in management available to accurately estimate all expected costs and benefits that arise through participation for a whole population of farmers. However, the large amount of data collected by the Alberta Johne's Disease Initiative (AJDI), with participation exceeding 50% of the approximately 580 Alberta dairy farms, provided a great opportunity to assess accurate data on management, changes in management, and the prevalence of the disease in a simulation model. The objective of the study was therefore to determine whether participation in a JD prevention and control program such as the AJDI is cost effective for a dairy farm. As implementation of best hygiene management practices will also reduce the transmission of other diseases (Johnson et al., 2011), expected additional benefits through reduction of losses caused by other fecal-orally transmitted diseases were also incorporated.
Materials and methods
Alberta Johne's Disease Initiative
In 2010, Alberta Milk and the Department of Production Animal Health of the University of Calgary (Calgary, AB, Canada) launched the AJDI. The aims of the program were to increase awareness of JD among dairy farmers and to decrease the prevalence of MAP infection in the province through implementation of best management practices (BMP). The program has 3 components: (1) collection of 6 environmental samples each year to assess the infection status of a herd. These are processed using a commercial liquid culture protocol (Trek Diagnostic Systems, Cleveland, OH) and subsequent IS900 PCR for detection of the MAP-specific insertion sequence 900. The case definition used is positive for IS900 PCR; (2) a risk assessment to analyze strengths and weaknesses in farm management; and (3) a management plan that includes implementation of a maximum of 3 changes in management, agreed upon by the herd veterinarian and the farmer(s), which should reduce the risk of MAP transmission. In contrast to many other programs, the AJDI does not include individual cow testing. Procedures are conducted by specially trained herd veterinarians and the costs for veterinarians’ time and sample processing are covered by the project. However, the participating farm is responsible for costs associated with changes in management.
Design
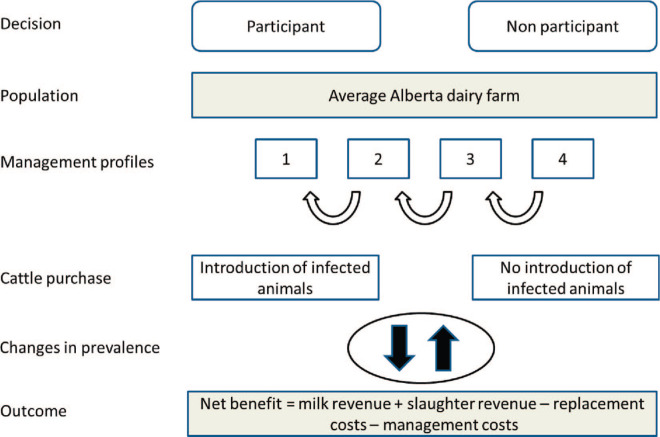
This economic analysis was conducted following Canadian guidelines for economic evaluation of health technologies (CADTH, 2006). TreeAge Pro (TreeAge Software Inc., Williamstown, MA) was used to construct a decision tree to evaluate the cost effectiveness of participation in the AJDI compared with no participation, from the perspective of an Alberta dairy farmer (Dijkhuizen et al., 1995). The calculation used farm characteristics and economic input data that were preferably recently estimated in Canada (Table 1 ). Farms entered the tree in 1 of 4 management profiles (Figure 1 ). Management profiles reflected the risk of horizontal transmission of MAP between adult infectious and young susceptible animals, with profiles 1 and 4 having, respectively, the best and worst within-herd prevention of MAP transmission. Assignment to the 4 management profiles considered management in 3 important areas, using evidence from previous randomized controlled clinical trials (Stabel, 2008; Pithua et al., 2013) and conditions similar to those reported in previous simulation studies (Groenendaal et al., 2002; Dorshorst et al., 2006). Conditions for assignment included the following: (1) calving—only 1 cow present in the calving pen at least 75% of the time, <10% of the calves born outside the calving pen, and <50% of the calves nurse the cow; (2) diet—calves are not regularly fed unpasteurized pooled colostrum, unpasteurized bulk tank milk, or nonsaleable milk; and (3) housing—calves do not have any direct or indirect contact with cows or cow manure. Farms that met the criteria in all 3 areas were assigned to management profile 1 (low risk), farms that met the criteria in 2 of 3 areas were assigned to profile 2, farms that met the criteria in 1 of 3 areas were assigned to profile 3, and farms that did not meet the criteria in any of the 3 management areas were assigned to profile 4 (high risk). A total of 369 first-year AJDI risk assessments, from 64% of the Alberta dairy farms, were used to assess the distribution of management profiles on Alberta dairy farms (Table 2 ).
Table 1.
Farm characteristics and baseline economic data of the average Alberta dairy farm.
| Parameter | Estimate | Reference | Model input1 |
|---|---|---|---|
| Annual milk production per cow (kg/305-d lactation) | 10,126 | Government of Canada, 2011 | Normal (10,126; 100) |
| Milk price (Can$/kg)2 | 0.8 | Alberta Milk, 2012 | Normal (0.8; 0.1) |
| Heifer raising costs | 2,500 | OMAFRA, 2011 | Normal (2,312.5; 93.75) |
| (Can$/heifer) | 2,125 | Mohd Nor et al., 2012 | |
| Live weight of a slaughter cow (kg) | 700 | Holstein Canada, 2013 | Normal (700; 88) |
| Slaughter value (Can$/kg of live weight) | 0.87 | Alberta Beef, 2012 | Normal (0.87; 0.1) |
| Annual culling rate (%) | 38 | Government of Canada, 2011 | Beta (39.3; 64.12) |
| Herd size (milking cows) | 145 | Government of Canada, 2011 | 145 |
| Calving interval (d) | 422 | Norman et al., 2009 | 422 |
| Annual purchase rate (%/cow present) | 0.3 | Weber et al., 2006 | Beta (63.8; 21,204.53) |
| Labor costs (Can$/h) | 17.33 | Lang, 2010 | 17.33 |
Normal=normal distribution (mean; SD); Beta = beta distribution (α; β).
Can$=Canadian dollars.
Figure 1.
Decision tree to assess the economic impact for dairy farms participating in the Alberta Johne's Disease Initiative from a farmer's perspective. Color version available in the online PDF.
Table 2.
Baseline management and changes in management profiles of farms participating in the Alberta Johne's Disease Initiative (AJDI)1
| Parameter2 | Estimate | Input distribution3 |
|---|---|---|
| Farms in management profile 1 (%) | 3 | Beta (62.05; 2,006.28) |
| Farms in management profile 2 (%) | 15 | Beta (54.25; 307.42) |
| Farms in management profile 3 (%) | 40 | Beta (38; 57) |
| Farms in management profile 4 (%) | 42 | (100 − profile 1+2+3)4 |
| Farms improving at least 1 management profile (%) | 26 | Beta (12.31; 35.05) |
| Among those, farms improving 2 profiles (%) | 19 | Beta (9.85; 41.99) |
| Farms downgrading at least 1 management profile (%) | 11 | Beta (6.27; 50.69) |
| Among those, farms downgrading 2 profiles (%) | 4 | Beta (2.46; 58.98) |
Data obtained through review of 369 first- and 227 second-year AJDI risk assessments.2Management profiles reflected the risk of horizontal transmission of Mycobacterium avium ssp. paratuberculosis (MAP) between adult infectious and young susceptible animals, with profile 1 having the best within-herd prevention of MAP transmission and profile 4 having the poorest within-herd prevention of MAP transmission. These profiles were assigned according to the management in 3 areas: A: calving, B: diet, C: housing.3Beta distribution (α; β).4To avoid cumulative percentage >100 through random sampling of all percentages in parallel.
The probability of farms changing management profiles was assessed through comparison of management profiles in yr 1 with management profiles in yr 2 on 227 farms participating in the AJDI for 2 consecutive years. Management costs and changes in within-herd MAP prevalence were dependent on the management profile. The tree also incorporated the risk of introduction of MAP infection into previously uninfected herds through purchase of MAP-infected animals. The tree was populated using real-time data from the AJDI and published data. The databases Scopus (Elsevier, Amsterdam, the Netherlands) and Medline (Atlanta, GA) were used to search the scientific literature. Variables were entered in form of distributions to enable probabilistic sensitivity analysis. The weighted averages of estimates from different input sources were used as means of the assigned distributions. The standard deviations were approximated using 25% of the difference between highest and lowest input estimate. If only 1 source was available, the upper and lower limit of the 95% confidence interval was used as basis for the calculation. If no confidence interval was reported, a conservative range was assigned according to the authors’ opinions (R. Wolf, K. Orsel, and H. W. Barkema have a major MAP research focus, whereas F. Clement is a health economist). Normal distributions were used for normally distributed unrestricted input data, β distributions were used for proportions, and a log normal distribution was used for the apparent within-herd prevalence at the start of the study.
Comparators
The tree compared farms participating in the AJDI to farms not participating in the AJDI. The tree design was identical for AJDI-participating and nonparticipating farms, except that AJDI-participating farms changed their management profile, whereas nonparticipating farms did not. As no information is available on changes in management on farms not participating in a control program, this assumption was necessary.
Benefits
Benefits included revenues through sale of milk and slaughter cows minus replacement costs. Revenues were reduced by production losses caused by MAP infection. Herd and within-herd prevalence estimates were chosen from 2 peer-reviewed studies (Sorensen et al., 2003; Scott et al., 2006). Environmental sample results from the AJDI were used as an additional source for herd prevalence data. A log normal distribution was used to implement variability of within-herd prevalence among Alberta dairy farms; 45% of the farms were recoded as uninfected. An animal-level MAP prevalence of 14% was chosen as the mean of the distribution (Table 3 ). This resulted in a right-skewed distribution of MAP within-herd prevalence, which represents a high proportion of farms either uninfected or infected with a low within-herd prevalence and a small proportion of “problem farms” with a high within-herd prevalence, similar to previous reports regarding Alberta dairy farms (Sorensen et al., 2003; Scott et al., 2006).
Table 3.
Estimates for prevalence, test accuracy, and direct costs associated with Mycobacterium avium ssp. paratuberculosis (MAP) infection.
| Parameter | Estimate (95% CI) | Reference | Model input1 |
|---|---|---|---|
| Prevalence of infected herds (%) | 402 (36.4–53.6) | Sorensen et al., 2003 | 55 |
| 58.82 (42.2–75.4) | Scott et al., 2006 | ||
| 57 (NA)3 | AJDI4 | ||
| True adult cow prevalence (%) | 8.12 (7.3–9.0) | Sorensen et al., 2003 | 14.23 |
| 17.52 (NA) | Scott et al., 2006 | ||
| Losses in milk production (%) | 6.2 (1.9–10.4) | Hendrick et al., 2005 | Beta (10.95; 113.72) |
| 2.2 (NA) | Wilson et al., 1993 | ||
| 12 | Raizman et al., 2009 | ||
| Increase in risk of culling(hazard ratio) | 3.2 (2.5–4.2) | Hendrick et al., 2005 | Normal (3.08; 0.425) |
| 3.0 (1.6–5.8) | Raizman et al., 2009 | ||
| Reduced slaughter weight (kg) | 59 (NA) | Whitlock et al., 1985 | Normal (59; 10) |
| Sensitivity of fecal culture (%) | 38 (NA) | Whitlock et al., 2000 | Beta (26.58; 66.67) |
| 19.4 (13.3–25.5) | McKenna, 2005 | ||
| Percentage of production loss associated with fecal culture-negative, MAP-infected cows5 | 50 (0–100) | Assumption | Beta (1.5; 1.5) |
Beta=beta distribution (α; β); Normal=normal distribution (mean; SD).2Based on serum ELISA (herds with 2 or more test-positive cows).3NA=not assessed.4Based on results of 2 consecutive years of environmental sampling on 227 farms participating in the Alberta Johne's Disease Initiative.5The proportion of these animals in a herd was calculated using the within-herd prevalence and the sensitivity of fecal culture.
The 3 main components of losses due to MAP infection considered in the analysis were (1) loss in milk production, (2) increased risk of being culled, and (3) decreased slaughter value (Table 3). Only studies that used fecal culture as their test method were included as sources for production loss estimates (Whitlock et al., 1985; Wilson et al., 1993; Hendrick et al., 2005; Raizman et al., 2009). These losses were assigned to a proportion of MAP-infected cattle equivalent to the sensitivity of fecal culture. Proportionate disease losses (50%) were assigned to infected cattle that were negative by fecal culture (Table 3).
Costs
Costs were management costs that depended on farm management profile. Changes in management suggested by herd veterinarians as part of the AJDI procedures were used to assign costs for various management areas (Table 4 ). As veterinarians suggest different solutions to meet the criteria for each area, a commonly suggested low-cost solution and a commonly suggested high-cost solution were chosen for each area. No costs were assigned to farms in management profile 4 (high risk). The sum of the costs of all 3 areas (calving, diet, housing) was assigned to profile 1 (low risk). As not all farms in profiles 2 and 3 met the criteria in the same areas, weighted averages according to criteria met in first-year AJDI risk assessments were used to assign costs for profiles 2 and 3 (Table 4).
Table 4.
Costs (Canadian dollars; Can$) for changes in management in 3 areas important for the control of Mycobacterium avium ssp. paratuberculosis transmission on Alberta dairy farms.
| Management area and suggested changes1 | Annual costs(Can$/cow) | Model input2 |
|---|---|---|
| Calving | Normal (10.17; 2.59) | |
| Build additional calving pens | 10.353 | |
| Remove calves immediately after birth | 5.004 | |
| Diet | Normal (26.86; 10.31) | |
| Pasteurize colostrum and milk before feeding to calves | 47.495 | |
| Feed only dams colostrum or colostrum replacement and milk replacer to heifers | 6.236 | |
| Housing | Normal (3.50; 0.44) | |
| Keep young stock and cows separated | 3.57 |
Calving: only 1 cow present in the calving pen at least 75% of the time and <10% of the calves born outside the calving pen, and <50% of the calves nurse the cow; diet: calves are not regularly fed unpasteurized pooled colostrum, unpasteurized bulk tank milk or nonsaleable milk; housing: calves do not have any direct or indirect contact to cows or cow manure.2Normal=normal distribution (mean; SD).3Increase the number of calving pens from 2 pens per 100 cows to 4 pens per 100 cows using existing buildings. The costs for installation of 1 calving pen were assumed to be Can$5,000 on material and 10 h of labor; projected life time: 10 yr.4Assuming 20 min extra work per cow and calving.5Initial investment Can$12,250; projected life time: 6 yr; daily operating costs (energy, maintenance, cleaning): $4.73; waste milk production per cow and lactation: 42 kg; waste milk assumed to be free; extra labor: 0.5 h/d.6Extra work for feeding dams colostrum: 5 min per calving; heifer calves fed colostrum replacer: 25%; costs for colostrum replacer per calf: Can$19.70; daily costs for milk replacer: Can$1.20.7Minor investment into separating housing facilities: material: Can$5,000; labor: 5 h.
Effectiveness
Simulation studies and observational studies were used to estimate the longitudinal change in MAP prevalence dependent on management in the 3 areas. A recent review comparing outcomes of the Dutch JD simulation model JohneSSim with the Danish simulation model PTB-Simherd was used to retrieve estimations on the expected change in MAP within-herd prevalence for management profiles 1 and 4 (Nielsen et al., 2011). Additionally, 2 longitudinal studies were considered for estimates on prevalence changes in profile 1 (Benedictus et al., 2008; Collins et al., 2010). To avoid bias in these studies by wrong assumptions in simulations and by communication of test results to participating producers in observational studies, input studies were considered to have equal weight, and very conservative estimates (including zero prevalence increase or decrease) were chosen for subsequent 1-way sensitivity analysis. As no estimates were available for management profiles 2 and 3, 50% of the prevalence decrease in profile 1 was assigned to profile 2, and 50% of the prevalence increase in profile 4 was assigned to profile 3 (Table 5 ). The change in within-herd prevalence was incorporated as a factor of the starting prevalence, which was added to the stage-specific prevalence; this resulted in a linear increase or decrease of the within-herd MAP prevalence, at a magnitude dependent on the starting prevalence. For farms changing their management profile, the factor for the prevalence change was adjusted after 2 yr, mimicking a delayed response in adult cow within-herd prevalence (due to the nature of the disease).
Table 5.
Expected change in within-herd prevalence of Mycobacterium avium ssp. paratuberculosis on dairy farms, depending on the management profile.
| Parameter | Estimate | Reference | Input distribution1 |
|---|---|---|---|
| Annual prevalence reduction for herds in profile 1 (%) | 102 | Nielsen et al., 2011 | Normal (0.08; 0.009) |
| 83 | Collins et al., 2010 | ||
| 6.53 | Benedictus et al., 2008 | ||
| 102 | Nielsen et al., 2011 | ||
| Proportionate prevalence reduction in profile 2 (%) | 50 | Assumption | Beta (1.5; 1.5) |
| Annual prevalence increase for herds in profile 4 (%) | 202 | Nielsen et al., 2011 | Normal (−0.19; 0.007) |
| 172 | Nielsen et al., 2011 | ||
| Proportionate prevalence increase in profile 3 (%) | 50 | Assumption | Beta (1.5; 1.5) |
Normal=normal distribution (mean; SD); Beta=beta distribution (α; β). 2Source reviewed 2 simulation studies with similar outcomes.3Intervention in source study defined as changes in management and testing and culling of test-positive animals.
Modeling
Data were analyzed using a Markov simulation on the herd level (Dijkhuizen et al., 1995). The chosen time horizon of the dynamic simulation was 10 yr, with a stage interval of 1 yr. Costs and effectiveness were discounted on a value of 5%. The analysis used 5,000 iterations with 500 samples. The apparent within-herd prevalence was resampled per individual simulated farm (sample) as it was used as a parameter of individual variation among farms. All other distributions were resampled per group of iterations, as they were used as parameters of uncertainty. Calculation outputs were exported into Excel (2010; Microsoft Corp., Redmond, WA). The net benefit; namely, the incremental effectiveness minus the incremental costs, was calculated for each iteration. The net benefit was reported per cow over the duration of 10 yr. The mean and confidence intervals of incremental costs, incremental effectiveness, and net benefit were calculated using Microsoft Excel functions (AVERAGE, CONFIDENCE.NORM). Means and confidence ellipses were presented using the “ellip” command in Stata 11 (Stata Corp., College Station, TX).
Uncertainty and Variability
To identify sources of uncertainty, 1-way sensitivity analyses were performed around estimates of all input variables. Results were ranked in accordance to their effect on the mean net benefit, and the most important sources of uncertainty were presented in a tornado diagram designed in Excel (Microsoft Corp.). The effect of variability in apparent MAP fecal culture within-herd prevalence was assessed through 1-way sensitivity analysis using prevalence values between 0 and 3% (in increments of 1 percentage unit).
Scenario Analyses
The fecal-oral pathway is the most important transmission pathway for MAP (Fecteau and Whitlock, 2010). As management-based JD prevention and control programs aim to reduce transmission by this route, it is reasonable to assume that participation in the AJDI reduces the incidence of other fecal-orally transmitted pathogens; for example, Cryptosporidium spp., Escherichia coli, rotavirus and coronavirus, coccidia, and Salmonella spp. (Johnson et al., 2011). Scenario analysis 1 estimated the additional effect of changes in the 3 management areas on the incidence of other fecal-orally transmitted diseases. Estimates on effectiveness of immediate separation of cow and calf after birth, use of individual calving pens versus multi-cow calving pens, and the effect of colostrum pasteurization on the incidence of calf diarrhea (management areas 1 and 2) were based on results of 3 randomized controlled trials (Quigley et al., 1994, 1995; Pithua et al., 2009; Godden et al., 2012; Table 6 ). The cost of calf diarrhea was included as a reduction of the benefits in our model. This reduction was composed of treatment costs and animal losses. However, losses in future performance were not considered, because a previous study reported lower first-lactation milk production for cows with a history of mild calfhood diarrhea, but did not report lower milk production for cows with a history of severe diarrhea (Svensson and Hultgren, 2008). The costs for focus area “diet” were assumed to be Can$47.49/cow per year to simulate the situation that all farms meeting the “diet” criterion would use on-farm milk pasteurizers.
Table 6.
Relationship between management practices suggested for control of transmission of Mycobacterium avium ssp. paratuberculosis and the incidence of calf diarrhea and its associated costs (Canadian dollars; Can$).
| Parameter | Estimate | Reference | Model input1 | ||
|---|---|---|---|---|---|
| Hazard ratio for scour treatment for calves fed non-heat-treated versus heat-treated pooled colostrum | 1.32 (1.14–1.53) | Godden et al., 2012 | Normal (1.32; 0.39) | ||
| Effectiveness of immediate cow-calf separation | Not significant | Quigley et al., 1994, 1995 | — | ||
| Effectiveness of individual calving pens | Not significant | Pithua et al., 2009 | — | ||
| Cumulative incidence of preweaning diarrhea (%) | 20.48 | Waltner-Toews et al., 1986b | Beta (269; 965) | ||
| 24.7 | Wells et al., 1997 | ||||
| Age at first occurrence (d) | 16 | Waltner-Toews et al., 1986b | Normal (16; 2) | ||
| Duration (d) | 3 | Waltner-Toews et al., 1986b | Normal (3; 1) | ||
| Case fatality rate (%) | 5.5–7.1 | Waltner-Toews et al., 1986a | Beta (14.46; 215.13) | ||
| Percentage of total heifer rearing costs before weaning | 12.3 | Gabler et al., 2000 | Beta (4.77; 33.81) | ||
| Daily treatment costs for diarrhea (Can$); light/severe case | 40/2002 | Expert opinion3 | Normal (45.33; 5.66) |
Normal=normal distribution (mean; SD); Beta = beta distribution (α; β). 2Assuming 10% of the patients would require intensive treatment for 1 d.3Personal communication with an Alberta dairy practitioner and an ex-practitioner currently employed by a major pharmaceutical company.
The second scenario analysis simulated the situation in which project costs were covered by the participating farm instead of by the project. Annual project costs of Can$200 for conducting the risk assessment and sample collection, Can$360 for sample processing (liquid culture and subsequent IS900 PCR), and Can$45 for administrative work were added to the costs for participating farms.
RESULTS
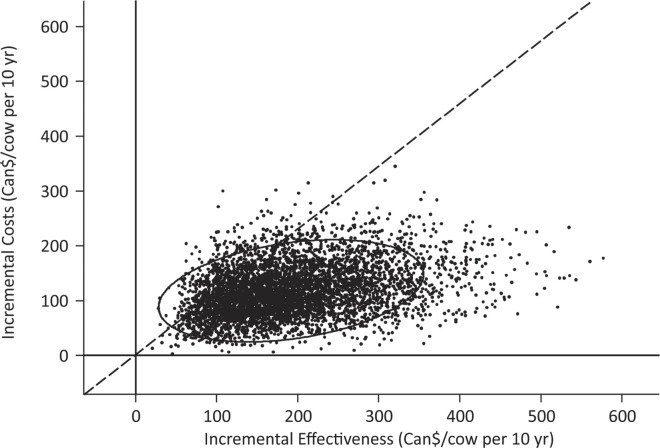
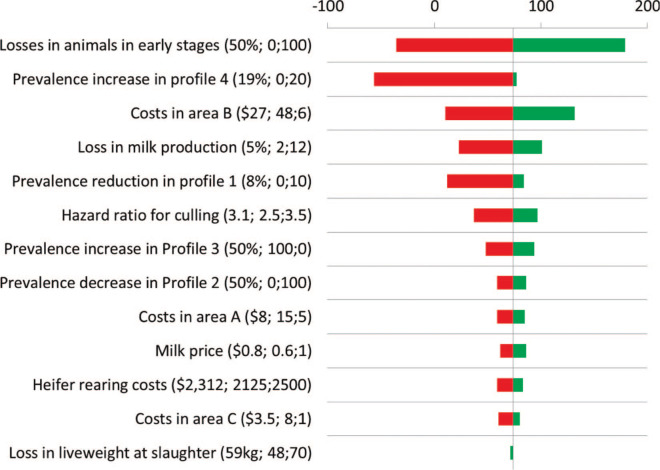
Mean incremental costs for participation were Can$117 (95% CI: $117–119) and mean incremental effectiveness was Can$191 (95% CI: $190–194) per cow per 10 yr (Figure 2 ). Overall, participating farms had a Can$74 (95% CI: $72–76) higher net benefit per cow per 10 yr compared with nonparticipating farms (Figure 2). The most important sources of uncertainty were proportional losses in fecal culture-negative MAP-infected cattle and magnitude of the MAP prevalence increase in management profile 4 (Figure 3 ). Extreme values in those input parameters yielded a negative net benefit for producers participating in the AJDI. However, net benefit increased with increasing within-herd MAP prevalence upon initiation of the program (Figure 4 ).
Figure 2.
Incremental costs and incremental effectiveness for participation in the Alberta Johne's Disease Initiative versus no participation. Iterations below the dashed line resulted in a positive net benefit.
Figure 3.
Tornado diagram displaying sources of uncertainty of a simulation model analyzing the economic impact of participation in the Alberta Johne's Disease Initiative (default value; lower limit;upper limit). Color version available in the online PDF.
Figure 4.
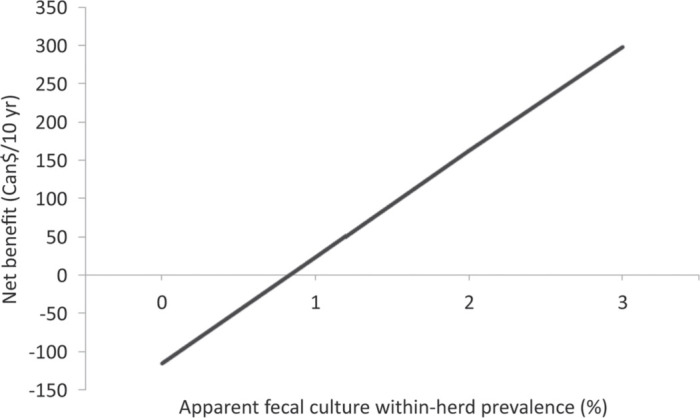
Effect of apparent within-herd prevalence on the net benefit for participation in the Alberta Johne's Disease Initiative from the perspective of an Alberta dairy farmer (fecal culture sensitivity mean: 28%; SD: 5%; fecal culture specificity: 100%).
Inclusion of the effect on other fecal-orally transmitted diseases (scenario analysis 1) resulted in a net benefit of Can$19 ($17–22) per cow per 10 yr. Mean incremental effectiveness was Can$200 (95%CI: 198–203) and mean incremental costs were Can$180 (95%CI: 179–182). If AJDI costs were covered by producers (scenario analysis 2), the net benefit was Can$27 ($25–30).
Discussion
Participation in the AJDI was cost effective for an average Alberta dairy farm. Additional costs through implementation of BMP were outweighed by additional benefits through lower disease costs. Only a small number of iterations resulted in a negative net benefit, which means that there is a small chance that participation would result in a negative net benefit for the average Alberta dairy farmer. This is apparently the first study that incorporated extensive data on baseline management and changes in management observed within an existing JD prevention and control program. As in all simulations, the outcome depends on the model design, its assumptions, and parameter estimates. Parameter uncertainty was addressed using 1-way sensitivity analysis on all input parameters. A high uncertainty in magnitude was present for several parameters, and this uncertainty affected results (Figure 3). This uncertainty was due to limited knowledge regarding pathogenesis of the disease but also to the conservatively chosen ranges around estimates of input parameters. Consequently, analysis precision was relatively low (Figure 3). Regardless, the most important aspect of the information, from a farmer's perspective, is knowing whether participation results in a positive or in a negative net benefit.
The uncertainty around the estimates of only 2 parameters in the model affected farmers’ decisions. The first of these parameters was proportional production losses in MAP-infected animals in early stage of the disease; that is, the fecal culture-negative cattle in this simulation. If these cattle produced the same amount of milk as healthy cattle and had no greater risk of being culled, as well as no reduction in slaughter value, participation in the AJDI would not be cost effective. However, this was very unlikely, especially because an infection trial recently conducted at the University of Calgary reported that 18-mo-old steers infected with MAP weighed, on average, 39 kg less than similarly housed uninfected controls [R. Mortier, H. W. Barkema, K. Orsel, R. Wolf, and J. De Buck (all from Department of Production Animal Health, Faculty of Veterinary Medicine, University of Calgary, Calgary, Alberta, Canada), unpublished data; Mortier et al., 2013]. Not all exposed animals had positive test results, which gives evidence that animals are affected by the disease although they do not consistently test positive. Nielsen et al., 2009 reported decreased milk production starting 300 d before the first positive milk ELISA test result in cows previously negative by ELISA (Nielsen et al., 2009). Those authors also reported higher milk production in cows with fluctuating antibody responses, which was regarded as indicative of possible misclassification of cattle caused by imperfect test sensitivity and specificity (Nielsen et al., 2009). Therefore, production losses due to MAP infection are generally underestimated due to nondifferential misclassification (Dohoo et al., 2003), suggesting that estimates used in the present study are rather conservative. The second parameter affecting farmers’ decisions was the increase in within-herd MAP prevalence on farms with poor management. In simulation studies, within-herd prevalence increased over time if BMP were not implemented (Groenendaal et al., 2002; Kudahl et al., 2008; Nielsen et al., 2011). In addition, 2 randomized controlled clinical trials provide evidence for an association between calving pen design and colostrum source with the risk of MAP infection (Stabel, 2008; Pithua et al., 2013). Nevertheless, apparently no observational study has been published in the peer-reviewed literature on the longitudinal change in within-herd prevalence on farms not implementing any MAP control strategy. Although the mainly fecal-oral transmission of the disease implies that within-herd prevalence increases if BMP are not implemented, the lack of proof was the reason for the use of a very conservative approach in sensitivity analysis, simulating a constant within-herd MAP prevalence if BMP were not implemented (management profile 4). In that unlikely case, participation in the AJDI would not be cost effective.
Results of a previous study (Dorshorst et al., 2006), which indicated that the within-herd MAP prevalence in the first year was the parameter with the greatest effect on the economic results, were confirmed by this study. As it is a parameter of variability among farms rather than a parameter of uncertainty, it was not included in the tornado diagram, but a separate graph was constructed that enables farmers with test results available to estimate their expected benefit of participation in the AJDI (Figure 4). The apparent fecal culture prevalence was used as a basis to show the results because it is more often available to farmers than the true within-herd prevalence. We concluded that farms with an apparent fecal culture MAP prevalence <0.8% should not expect to derive a positive net benefit from participation in the AJDI.
Based on the economic simulation model, uninfected herds had a negative net benefit, as MAP-associated production losses could not be reduced. For a test-and-cull control program, this finding might be close to reality, because such a program would only influence the prevalence of MAP. However, for a control program based on reduction of disease transmission through management changes, it is an oversimplification. A program that reduces the fecal-oral transmission pathway of MAP should also reduce fecal-oral transmission of other pathogens. This was the rationale for the first scenario analysis, which included additional benefits through reduction of other fecal-orally transmitted diseases. Although the scientific literature has many reports estimating the association between young stock health and management (Johnson et al., 2011), most of the studies could not be used as sources of estimates on the effectiveness of management changes on the risk of fecal-orally transmitted diseases. In that regard, most studies were observational, and their quantitative estimates, which varied widely among studies, could be biased by confounding through other uncontrolled factors affecting the study participants. Therefore, only estimates from 3 randomized controlled clinical trials were included in the analysis (Quigley et al., 1994, 1995; Pithua et al., 2009; Godden et al., 2012). Surprisingly, immediate separation of calves from their dams and the use of individual calving pens versus multi-cow calving pens were not described as effective in reducing calfhood disease, although an observational study reported decreased risk of mortality for calves immediately removed from their dams (Wells et al., 1996). Because we aimed to conduct a conservative analysis, we did not simulate a reduction in calfhood diseases for these management practices, again probably underestimating the true net benefit. In contrast, strong evidence was found for the effectiveness of heat treatment of calves’ liquid diet for control of calfhood diseases (Godden et al., 2012). To simulate this effectiveness, we assumed that all farms controlling management area “milk” would purchase an on-farm pasteurizer, which increased the annual costs in this area from Can$27 in the baseline analysis to Can$47 in the scenario analysis. Although the incremental effectiveness increased through additional revenues (Can$199 to Can$210) caused by increased calf health, the net benefit decreased (Can$73 to Can$17) as a result of increased costs (Can$126 to Can$191). Moreover, this scenario analysis was conducted conservatively, as it did not include any losses in milk production or lower fertility for cows with a history of diarrhea. This decision was made because a previous publication estimated a 344-kg lower milk production in cows with a history of mild diarrhea but no significant losses in production for cows with a history of severe diarrhea (Svensson and Hultgren, 2008). Assuming cows with a history of diarrhea would have a 344-kg reduction in milk production during their first lactation would result in a net benefit of Can$46 per cow over 10 yr (results not shown). Based on the analysis shown, increased investment costs led to a significant reduction in expected net benefit, emphasizing the need for governmental support or funding from producer organizations to support investments into biosecurity. The second scenario analysis estimated the net benefit after expiration of AJDI project funding in mid-2013. In that case, participation would still be cost effective.
Major limitations of this study were the extensive knowledge gaps on MAP transmission and changes in prevalence over time. The objective was to construct a simple model and add complexity only in the case of sufficient knowledge available to support it. Consequently, longitudinal changes in within-herd MAP prevalence were modeled as a linear change dependent on the starting prevalence. An alternative approach would be to model various transmission pathways through the use of contact structures and estimates for intrauterine transmission, as well as transmission through contaminated environment. The approach used could be regarded as an oversimplification; nevertheless, it answered the research question and was less vulnerable to incorrect assumptions than an extensive simulation model. Another limitation was the insufficient knowledge regarding the effectiveness of the implementation of specific management practices for control of MAP. Consequently, the effect of specific management practices on MAP within-herd prevalence was not assessed. When assigning costs for changes in management practices, we assumed that extra labor would be available on farm and no additional personnel had to be hired. This assumption was valid for management practices suggested in the AJDI, as veterinary practitioners are instructed to suggest changes that can be implemented easily with low financial burden and limited extra work, as this will maximize the probability of implementation. No information was available on baseline management and management dynamics on farms not participating in the control program. The assumption that the baseline on AJDI participating farms is representative of dairy farms in the province poses only a minor risk for bias in the analysis because sensitivity analysis showed that variations in baseline management did not have a major effect on net benefit. Assumptions that nonparticipating farms will keep their management constant over 10 yr, whereas participating farms change their management repeatedly throughout the years may seem inappropriate. Nevertheless, it still represents a conservative assumption: management changes on participating farms were modeled bidirectionally. Therefore, farms not only progressed to a better (lower risk) management profile, but also downgraded to a worse (higher risk) management profile according to observations made within the AJDI. Therefore, progress on management profiles on participating versus nonparticipating farms was limited to the observed progress minus the observed downgrading, which represented a rather conservative approach.
Results of this study were comparable with those of previous studies in the sense that all studies reported a positive net benefit if BMP were implemented (Appendix). With a net benefit of Can$7 per cow per year, the present study resulted in higher estimates than previous simulation studies (Groenendaal et al., 2002; Cho et al., 2013) and lower estimates than an observational study (Groenendaal and Wolf, 2008). Differences of that magnitude can be expected, as all studies considered different populations with different cost and revenue estimates and differences in disease prevalence. In addition, studies varied significantly in their designs and assumptions.
This study was conducted from the perspective of an Alberta dairy farmer. Results are most generalizable to other parts of Canada in which the within-herd prevalence and cost and revenue estimates are similar. It is expected that the net benefits would be slightly lower for eastern Canada due to a tendency toward lower herd prevalence and within-herd prevalence (Tiwari et al., 2006). It is more challenging to generalize results to herds outside Canada. The milk price in the United States is lower, thereby reducing the expected net benefit (Geuss, 2013). Conversely, a higher herd prevalence of MAP increases the net benefit (Lombard et al., 2013). Herd size is another important factor that should be considered. Although average herd sizes are similar between Alberta (145 cows in 2011) and the United States (172 cows in 2010), some areas in the United States, such as California or New Mexico, include an increasing number of very large dairy operations with >1,000 cows (Hoard's Dairyman, 2010; Government of Canada, 2011). For those herds, some investments into biosecurity, as well as project costs, could be amortized across more cows, which would reduce the burden of these costs. In addition, management of those operations is very different from the management on farms in Alberta, making generalizability of results more challenging. Generalization to Europe is not feasible, as the dairy industry (management and structure) varies significantly among countries. Another challenge is that knowledge on MAP prevalence is limited (Nielsen and Toft, 2009). All of these issues in generalizability led to the conclusion that input of region-specific parameters is required to use the current model as a support tool for dairy farmers and decision makers worldwide. Regardless, a major advantage of the presented model is that most area-specific input parameters can be studied easily and are available online for most dairy populations (the model operated with TreeAgePro is available upon request from the authors).
To fill persistent knowledge gaps, an extensive longitudinal study estimating the association between management and changes in MAP within-herd prevalence, as well as estimating production losses of MAP-infected animals, should be conducted. Such a study should be done on several herds representing various management strategies, and test results should not be communicated with producers.
APPENDIX.
Table A1.
Review of the economic effect of changes in management to control transmission of Mycobacterium avium ssp. paratuberculosis (MAP) on dairy farms.
| Reference;Study location;Study design | Losses due to MAP infection | Analyzed interventions | Economic outcome1 |
|---|---|---|---|
| Groenendaal et al., 2002;Netherlands;Simulation | Lower milk productionDiagnosis and treatment costsReduced slaughter valueIncreased risk of being culled | Better calving hygiene and immediate removal of newborn calves from the dam, colostrum from own dam followed by milk replacer, separation of cows and calves, and test and cull | Net benefit: €1,183 for a 50-cow herd over 20 yr |
| Groenendaal et al., 2002;United States; Simulation | Lower milk production Diagnosis and treatment costs Reduced slaughter value Increased risk of being culled | Rearing of heifers off site from d 1. Simulations were conducted with and without improvements in management before the calves were sent to the rearing facility | Net benefit: US$29,905 without improved management and US$ 43,917 with improved management for a 100-cow herd over 20 yr |
| Dorshorst et al., 2006; United States; Simulation | Lower milk production Decreased fertility Reduced slaughter value Increased risk of being culled | Better calving hygiene and immediate removal of newborn calves from the dam, colostrum from only 1 dam followed by milk replacer, separation of cows and calves | Although improved colostrum hygiene and feeding only milk replacer yielded a positive net benefit, improved maternity pen hygiene was not cost effective |
| Cho et al., 2013; United States; Simulation | Lower milk production Reduced slaughter value Increased risk of being culled | Improvements in calf liquid diet management, separation of cows and calves | Net benefit: US$165,621 for a 100-cow herd with an initial prevalence of 10% over 50 yr |
| Groenendaal and Wolf, 2008; United States; Observational | Lower milk production Reduced slaughter value Increased risk of being culled | Variety of changes in management implemented on 40 farms; testing of animals (testing costs either included or not included) | Net benefit: US$34 per cow-year if testing costs were excluded, and −US$14 if testing costs were included |
| Kudahl et al., 2008; Denmark; Simulation | Lower milk production Decreased fertility Reduced slaughter value Increased risk of being culled | Better calving hygiene and immediate removal of newborn calves from the dam, colostrum from own dam or colostrum replacer followed by milk replacer, and separation of cows and calves | Farms implementing the intervention had a higher net benefit than farms that did not implement the intervention |
Net benefit = economic outcome for farms implementing the intervention – economic outcome for farms not implementing the intervention.
References
- Alberta Beef. 2012. Daily cattle report. Accessed Aug. 26, 2013. http://www.albertabeef.org/producers/dailycattlereport/
- Alberta Milk. 2012. Current milk class price. Accessed Aug. 26, 2013. http://www.albertamilk.com/quotainfo/milkclassprices.aspx?g=&k=Home
- Bakker D. Paratuberculosis control measures in Europe. In: Behr M.A., Collins D.M., editors. Paratuberculosis–Organism, Disease and Control. CABI; Wallingford, UK: 2010. pp. 306–318. [Google Scholar]
- Barkema H.W., Hendrick S., De Buck J., Kaplan G.G., Rioux K. Crohn's disease in humans and Johne's disease in cattle–Linked diseases? In: Krause D., Hendrick S., editors. Zoonotic Pathogens in the Food Chain. CABI; Wallingford, UK: 2010. pp. 197–213. [Google Scholar]
- Behr M.A. Paratuberculosis and Crohn's disease. In: Behr M.A., Collins D.M., editors. Paratuberculosis–Organism, Disease and Control. CABI; Wallingford, UK: 2010. pp. 40–50. [Google Scholar]
- Benedictus A., Mitchell R.M., Linde-Widmann A., Sweeney R., Fyock T., Schukken Y.H., Whitlock R.H. Transmission parameters of Mycobacterium avium subspecies paratuberculosis infections in a dairy herd going through a control program. Prev. Vet. Med. 2008;83:215–227. doi: 10.1016/j.prevetmed.2007.07.008. [DOI] [PubMed] [Google Scholar]
- CADTH (Canadian Agency for Drugs and Technologies in Health). 2006. Guidelines for the economic evaluation of health technologies: Canada. Accessed Aug. 26, 2013. http://www.cadth.ca/media/pdf/186_EconomicGuidelines_e.pdf
- Chi J., VanLeeuwen J.A., Weersink A., Keefe G.P. Direct production losses and treatment costs from bovine viral diarrhoea virus, bovine leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum. Prev. Vet. Med. 2002;55:137–153. doi: 10.1016/s0167-5877(02)00094-6. [DOI] [PubMed] [Google Scholar]
- Cho J., Tauer L.W., Schukken Y.H., Smith R.L., Lu Z., Grohn Y.T. Cost-effective control strategies for Johne's disease in dairy herds. Can. J. Agric. Econ. 2013;61:583–608. [Google Scholar]
- Collins M.T., Eggleston V., Manning E.J.B. Successful control of Johne's disease in nine dairy herds: Results of a six-year field trial. J. Dairy Sci. 2010;93:1638–1643. doi: 10.3168/jds.2009-2664. [DOI] [PubMed] [Google Scholar]
- Dijkhuizen A.A., Huirne R.B.M., Jalvingh A.W. Economic analysis of animal diseases and their control. Prev. Vet. Med. 1995;25:135–149. [Google Scholar]
- Dohoo I., Martin S.W., Stryhn H. Validity in observational studies. In: McPike M.S., editor. Veterinary Epidemiologic Research. Atlantic Veterinary College; Charlottetown, PEI, Canada: 2003. pp. 207–234. [Google Scholar]
- Dorshorst N.C., Collins M.T., Lombard J.E. Decision analysis model for paratuberculosis control in commercial dairy herds. Prev. Vet. Med. 2006;75:92–122. doi: 10.1016/j.prevetmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Fecteau M.E., Whitlock R.H. Paratuberculosis in cattle. In: Behr M.A., Collins D.M., editors. Paratuberculosis–Organism, Disease and Control. CABI; Wallingford, UK: 2010. pp. 144–156. [Google Scholar]
- Gabler M.T., Tozer P.R., Heinrichs A.J. Development of a cost analysis spreadsheet for calculating the costs to raise a replacement dairy heifer. J. Dairy Sci. 2000;83:1104–1109. doi: 10.3168/jds.S0022-0302(00)74975-7. [DOI] [PubMed] [Google Scholar]
- Geuss, J. 2013. US milk prices continue to decline as cheese inventories remain high. Accessed Aug. 26, 2013. http://www.dairyreporter.com/Commodities/US-milk-prices-continue-to-decline-as-cheese-inventories-remain-high?utm_source=copyright&utm_medium=OnSite&utm_campaign=copyright
- Godden S.M., Smolenski D.J., Donahue M., Oakes J.M., Bey R., Wells S., Sreevatsan S., Stabel J., Fetrow J. Heat-treated colostrum and reduced morbidity in preweaned dairy calves: Results of a randomized trial and examination of mechanisms of effectiveness. J. Dairy Sci. 2012;95:4029–4040. doi: 10.3168/jds.2011-5275. [DOI] [PubMed] [Google Scholar]
- Government of Canada. 2011. Statistics of the Canadian dairy industry. Accessed Aug. 26, 2013. http://www.dairyinfo.gc.ca/pdf/publication_2012edition.pdf
- Groenendaal H., Nielen M., Jalvingh A.W., Horst S.H., Galligan D.T., Hesselink J.W. A simulation of Johne's disease control. Prev. Vet. Med. 2002;54:225–245. doi: 10.1016/s0167-5877(02)00027-2. [DOI] [PubMed] [Google Scholar]
- Groenendaal H., Wolf C.A. Farm-level economic analysis of the US National Johne's disease demonstration herd project. J. Am. Vet. Med. Assoc. 2008;233:1852–1858. doi: 10.2460/javma.233.12.1852. [DOI] [PubMed] [Google Scholar]
- Groenendaal H., Zagmutt F.J. Scenario analysis of changes in consumption of dairy products due to a hypothetical causal link between Mycobacterium avium subspecies paratuberculosis and Crohn's disease. J. Dairy Sci. 2008;91:3245–3258. doi: 10.3168/jds.2007-0698. [DOI] [PubMed] [Google Scholar]
- Hendrick S.H., Kelton D.F., Leslie K.E., Lissemore K.D., Archambault M., Duffield T.F. Effect of paratuberculosis on culling, milk production, and milk quality in dairy herds. J. Am. Vet. Med. Assoc. 2005;227:1302–1308. doi: 10.2460/javma.2005.227.1302. [DOI] [PubMed] [Google Scholar]
- Hoard's Dairyman. 2010. A 2010 snapshot of U.S. dairying. Accessed Aug. 26, 2013. http://www.hoards.com/blog_U.S.%20dairying%20in%202010
- Holstein Canada. 2013. Accessed Aug. 26, 2013. https://www.holstein.ca/Public/en/About_Us/The_Canadian_Dairy_Industry#
- Johnson K., Burn C.C., Wathes C.C. Rates and risk factors for contagious disease and mortality in young dairy heifers. Pages 205–214 in Animal Science Reviews. 2011 [Google Scholar]
- Kennedy D., Citer L. Paratuberculosis control measures in Australia. In: Behr M.A., Collins D.M., editors. Paratuberculosis: Organism, Disease and Control. CABI; Wallingford, UK: 2010. pp. 330–343. [Google Scholar]
- Kudahl A.B., Nielsen S.S., Østergaard S. Economy, efficacy, and feasibility of a risk-based control program against paratuberculosis. J. Dairy Sci. 2008;91:4599–4609. doi: 10.3168/jds.2008-1257. [DOI] [PubMed] [Google Scholar]
- Lang, B. 2010. Dairy farm wage rates. Accessed Aug. 26, 2013. http://www.omafra.gov.on.ca/english/livestock/dairy/facts/wagerate.htm
- Lombard J.E., Gardner I.A., Jafarzadeh S.R., Fossler C.P., Harris B., Capsel R.T., Wagner B.A., Johnson W.O. Herd-level prevalence of Mycobacterium avium ssp. paratuberculosis infection in United States dairy herds in 2007. Prev. Vet. Med. 2013;108:234–238. doi: 10.1016/j.prevetmed.2012.08.006. [DOI] [PubMed] [Google Scholar]
- McKenna, S. L. B. 2005. Evaluation of ELISA and fecal culture strategies for diagnosis of Mycobacterium avium ssp. paratuberculosis. PhD Thesis. Faculty of Veterinary Medicine, University of Prince Edward Island, Charlottetown, PEI, Canada.
- McKenna S.L.B., Keefe G.P., Tiwari A., VanLeeuwen J.A., Barkema H.W. Johne's disease in Canada. Part II: Disease impacts, risk factors and control programs for dairy producers. Can. Vet. J. 2006;47:1089–1099. [PMC free article] [PubMed] [Google Scholar]
- Mohd Nor N., Steeneveld W., Mourits M.C.M., Hogeveen H. Estimating the costs of rearing young dairy cattle in the Netherlands using a simulation model that accounts for uncertainty related to diseases. Prev. Vet. Med. 2012;106:214–224. doi: 10.1016/j.prevetmed.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Mortier R., Barkema H.W., Bystrom J.M., Illanes O., Orsel K., Wolf R., Atkins G., De Buck J. Evaluation of age-dependent susceptibility in calves infected with two doses of Mycobacterium avium subspecies paratuberculosis using pathology and tissue culture. Vet. Res. 2013;44:94. doi: 10.1186/1297-9716-44-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S.S., Krogh M.A., Enevoldsen C. Time to the occurrence of a decline in milk production in cows with various paratuberculosis antibody profiles. J. Dairy Sci. 2009;92:149–155. doi: 10.3168/jds.2008-1488. [DOI] [PubMed] [Google Scholar]
- Nielsen S.S., Toft N. A review of prevalences of paratuberculosis in farmed animals in Europe. Prev. Vet. Med. 2009;88:1–14. doi: 10.1016/j.prevetmed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Nielsen S.S., Weber M.F., Kudahl A., Marce C., Toft N. Stochastic models to simulate paratuberculosis in dairy herds. Rev. Sci. Tech. 2011;30:615–625. doi: 10.20506/rst.30.2.2058. [DOI] [PubMed] [Google Scholar]
- Norman H.D., Wright J.R., Hubbard S.M., Miller R.H., Hutchison J.L. Reproductive status of Holstein and Jersey cows in the United States. J. Dairy Sci. 2009;92:3517–3528. doi: 10.3168/jds.2008-1768. [DOI] [PubMed] [Google Scholar]
- OMAFRA (Ontario Ministry of Agriculture, Food, and Rural Affairs). 2011. Investing in your dairy herd's future. Accessed Aug. 26, 2013. http://www.omafra.gov.on.ca/english/livestock/dairy/facts/11-055.htm
- Pithua P., Espejo L.A., Godden S.M., Wells S.J. Is an individual calving pen better than a group calving pen for preventing transmission of Mycobacterium avium subsp paratuberculosis in calves? Results from a field trial. Res. Vet. Sci. 2013;95:398–404. doi: 10.1016/j.rvsc.2013.03.014. [DOI] [PubMed] [Google Scholar]
- Pithua P., Wells S.J., Godden S.M., Raizman E.A. Clinical trial on type of calving pen and the risk of disease in Holstein calves during the first 90 days of life. Prev. Vet. Med. 2009;89:8–15. doi: 10.1016/j.prevetmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J.D., III, Martin K.R., Bemis D.A., Potgieter L.N.D., Reinemeyer C.R., Rohrbach B.W., Dowlen H.H., Lamar K.C. Effects of housing and colostrum feeding on the prevalence of selected infectious organisms in feces of Jersey calves. J. Dairy Sci. 1994;77:3124–3131. doi: 10.3168/jds.S0022-0302(94)77255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley J.D., III, Martin K.R., Bemis D.A., Potgieter L.N.D., Reinemeyer C.R., Rohrbach B.W., Dowlen H.H., Lamar K.C. Effects of housing and colostrum feeding on serum immunoglobulins, growth, and fecal scores of Jersey calves. J. Dairy Sci. 1995;78:893–901. doi: 10.3168/jds.S0022-0302(95)76703-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizman E.A., Fetrow J.P., Wells S.J. Loss of income from cows shedding Mycobacterium avium subspecies paratuberculosis prior to calving compared with cows not shedding the organism on two Minnesota dairy farms. J. Dairy Sci. 2009;92:4929–4936. doi: 10.3168/jds.2009-2133. [DOI] [PubMed] [Google Scholar]
- Scott H.M., Sorensen O., Wu J.T., Chow E.Y., Manninen K., VanLeeuwen J.A. Seroprevalence of Mycobacterium avium subspecies paratuberculosis, Neospora caninum, bovine leukemia virus, and bovine viral diarrhea virus infection among dairy cattle and herds in Alberta and agroecological risk factors associated with seropositivity. Can. Vet. J. 2006;47:981–991. [PMC free article] [PubMed] [Google Scholar]
- Sorensen O., Rawluk S., Wu J., Manninen K., Ollis G. Mycobacterium paratuberculosis in dairy herds in Alberta. Can. Vet. J. 2003;44:221–226. [PMC free article] [PubMed] [Google Scholar]
- Stabel J.R. Pasteurization of colostrum reduces the incidence of paratuberculosis in neonatal dairy calves. J. Dairy Sci. 2008;91:3600–3606. doi: 10.3168/jds.2008-1107. [DOI] [PubMed] [Google Scholar]
- Svensson C., Hultgren J. Associations between housing, management, and morbidity during rearing and subsequent first-lactation milk production of dairy cows in Southwest Sweden. J. Dairy Sci. 2008;91:1510–1518. doi: 10.3168/jds.2007-0235. [DOI] [PubMed] [Google Scholar]
- Tiwari A., VanLeeuwen J.A., McKenna S.L.B., Keefe G.P., Barkema H.W. Johne's disease in Canada: Part I: Clinical symptoms, pathophysiology, diagnosis, and prevalence in dairy herds. Can. Vet. J. 2006;47:874–882. [PMC free article] [PubMed] [Google Scholar]
- Waltner-Toews D., Martin S.W., Meek A.H. Dairy calf management, morbidity and mortality in Ontario Holstein herds. III. Association of management with morbidity. Prev. Vet. Med. 1986;4:137–158. [Google Scholar]
- Waltner-Toews D., Martin S.W., Meek A.H., McMillan I. Dairy calf management, morbidity and mortality in Ontario Holstein herds. I. The data. Prev. Vet. Med. 1986;4:103–124. [Google Scholar]
- Weber M.F., van Roermund H.J.W., Vernooij J.C.M., Kalis C.H.J., Stegeman J.A. Cattle transfers between herds under paratuberculosis surveillance in the Netherlands are not random. Prev. Vet. Med. 2006;76:222–236. doi: 10.1016/j.prevetmed.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Wells S.J., Dargatz D.A., Ott S.L. Factors associated with mortality to 21 days of life in dairy heifers in the United States. Prev. Vet. Med. 1996;29:9–19. [Google Scholar]
- Wells S.J., Garber L.P., Hill G.W. Health status of preweaned dairy heifers in the United States. Prev. Vet. Med. 1997;29:185–199. doi: 10.1016/s0167-5877(96)01078-1. [DOI] [PubMed] [Google Scholar]
- Whitlock R. H. Paratuberculosis control measures in the USA. In: Behr M.A., Collins D.M., editors. Paratuberculosis–Organism, Disease and Control. CABI; Wallingford, UK: 2010. pp. 319–329. [Google Scholar]
- Whitlock R.H., Hutchinson L.T., Merkal R.S. Prevalence and economic considerations of Johne's disease in the northeastern U.S. Proc. US. Anim. Health. Assoc. 1985;89:484–490. [Google Scholar]
- Whitlock R.H., Wells S.J., Sweeney R.W., Van Tiem J. ELISA and fecal culture for paratuberculosis (Johne's disease): Sensitivity and specificity of each method. Vet. Microbiol. 2000;77:387–398. doi: 10.1016/s0378-1135(00)00324-2. [DOI] [PubMed] [Google Scholar]
- Wilson D.J., Rossiter C., Han H.R., Sears P.M. Association of Mycobacterium paratuberculosis infection with reduced mastitis, but with decreased milk production and increased cull rate in clinically normal dairy cows. Am. J. Vet. Res. 1993;54:1851–1857. [PubMed] [Google Scholar]