Abstract
In the past decade, studies into the way in which intracellular bacterial pathogens hijack and subvert their hosts have provided many important insights into regulation of the actin cytoskeleton and cell motility, in addition to increasing our understanding of the infection process. Viral pathogens, however, may ultimately unlock more cellular secrets as they are even more dependent on their hosts during their life cycle.
Keywords: Cytoskeleton, Virus, Actin, Microtubules, Transport, Motility
Nomenclature
Abbreviations
- Ad2
adenovirus serotype 2
- GFP
green fluorescent protein
- HSV-1
herpes simplex virus 1
- IEV
intracellular enveloped virus (vaccinia)
- IMV
intracellular mature virus (vaccinia)
- MAPs
microtubule-associated proteins
- MP
movement protein
- MTOC
microtubule organising centre
- TGN
trans-Golgi network
1. Introduction
Imagine you are a tourist who is trying to get across central London. Not so easy but not impossible given the ‘wonderful’ London transport system. Now imagine a slightly easier scale problem: you are a virus that has just got into a cell and you want to get to your site of replication, which may well be in the nucleus (Fig. 1 ). Finally, if you manage to replicate, your progeny will later have the reverse problem, that is to find a way to get out of the host. How do you do it? Easy, you use the transport system of the cell and hop on the cytoskeleton — but is it as easy as catching the bus or tube?
Fig. 1.
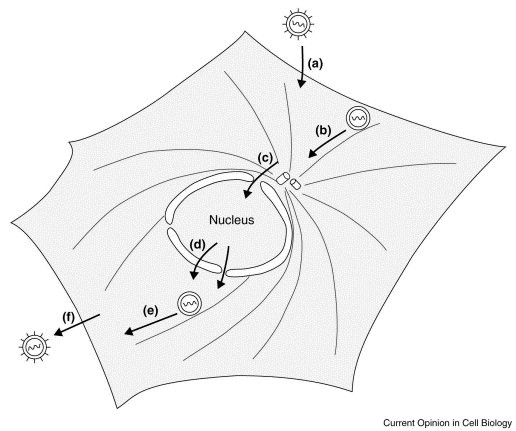
A schematic representation of the possible transport steps, indicated by arrows, faced by incoming and outgoing virus particles during infection. (a) Entry of the virus particle across the plasma membrane into the cell. (b) Retrograde transport of a viral capsid along microtubules from the cell periphery to a perinuclear region. (c) Entry into the nucleus. (d) Exit of newly assembled viral particles from the nucleus. (e) Anterograde transport of assembled viral capsids or capsid components to the cell periphery. (f) Budding at the plasma membrane.
As early as the 1970s, electron micrographs of infected cells showed virus particles in close association with cytoskeletal elements (reviewed [1]), and disruption of the cytoskeleton often resulted in reduced virus yield (for example, see [2], [3]). In addition, in vitro experiments with purified viral preparations demonstrated that some virus particles can interact with microtubules [4], [5], [6], [7]. It was also widely recognized that virus infection often resulted in disruption of the cytoskeleton (reviewed in [8], [9], [10]). However, for many years these initial, tantalising observations were not followed up and the functional role or the molecular basis of these interactions and disruptions remained largely a mystery. Studies with viruses often tended to focus on understanding viral infection mechanisms, including how entry, replication and assembly occur, but not on how viral particles move within and between cells, although this is an essential aspect of the infection cycle.
Over the last ten years, the emergence of bacterial pathogens, such as Listeria and Shigella, as model systems to study actin-based motility has resulted in an increased interest and awareness of pathogens as tools to study cytoskeletal function (reviewed [11], [12]). In addition, the impact of green fluorescent protein (GFP) and better imaging systems have facilitated the study of virus movements in living cells. Consequently a number of studies in recent years have begun to use viruses as tools both to dissect cytoskeletal function and to understand the role of the cytoskeleton during infection. This review is organized around the main cellular transport issues for the virus. In addition, we also discuss virus-induced disruption of the microtubule cytoskeleton.
2. Transport from the cell periphery to the centre of the cell (retrograde transport)
Viruses enter the cell by fusion of their viral envelope with the plasma membrane or via endocytosis followed by their subsequent escape from the endosome by membrane fusion or lysis [13], [14]. These processes result in delivery of the viral capsid to the cytoplasm. The problem is how to get to the site of replication, which may be in the nucleus for some viruses. The size of virus particles, which can be as big as 200–400 nm in the case of vaccinia, is such that they are unlikely to move in the viscous cytoplasm by diffusion alone [14•]. Furthermore, random diffusion is unlikely to result in arrival at a specific cellular destination. The obvious method is to use the normal transport system of the cell — the actin or microtubule cytoskeleton.
The entry of many different viruses, including adenovirus, HIV and vaccinia, is actin dependent [15], [16], [17], [18], [19], [20]. However, the organization of the actin cytoskeleton is not suited to achieving a perinuclear localization. In contrast, the microtubule cytoskeleton, with microtubules radiating from the centrosome (which is conveniently situated near the nucleus) to the periphery lends itself to achieving the desired localization. Moving is one thing but going in the right direction is also essential. Microtubules are also organized in a polarized fashion with minus ends at the MTOC (microtubule organising centre) and plus ends at the cell periphery, thus enabling directed movements to take place.
A number of viruses appear to have evolved to use a common retrograde transport mechanism that involves recruitment of cytoplasmic dynein, a microtubule minus-end-directed motor, to move towards the centre of the cell. Studies with herpes simplex virus 1 (HSV-1) first implicated both microtubules and a microtubule motor in retrograde viral movements [21], [22], [23]. Further experiments showed that dynein was involved in this transport: dynein colocalises with incoming capsids and depolymerisation of microtubules delays retrograde transport and subsequent viral protein synthesis [24]. More recently, video microscopy, combined with functional disruption of the dynein–dynactin complex, showed that adenovirus serotype 2 (Ad2) uses dynein and microtubules for its retrograde transport to the nucleus [25••]. Although Ad2 has an overall net movement towards the nucleus at a speed of 2.2μm/sec, capsids exhibit bidirectional plus- and minus-directed microtubule motility [25••]. The anterograde plus-end-directed motility is attributed to the action of an unidentified microtubule plus-end-directed motor. Curiously, adenovirus serotype 5 (Ad5) is reported to have only dynein-dependent minus-end microtubule directed movement at the same speed as Ad2 [26]. Consistent with this, microinjection of two different anti-kinesin antibodies had no effect on viral translocation [26]. Nevertheless, it is unclear whether dynein is the only motor involved in this movement because blocking dynein function via antibody microinjection only gave a partial phenotype. So, given the large size of the kinesin superfamily we cannot rule out that this motor is also involved, for movement in either direction, as only two anti-kinesin antibodies were microinjected to block movement.
Although no evidence for dynein motor activity in the transport of nucleocapsids of pseudorabies virus (an alphaherpes virus closely related to HSV-1) has been documented, they have been shown to be associated with and dependent on microtubules for their movement to the nucleus [27]. Microtubules are also required for accumulation of human foamy virus [28] and adeno-associated virus [29] at the centre of the cell during establishment of infection.
Interestingly, in the case of herpes, adeno-associated and vaccinia viruses, depolymerization of the microtubule cytoskeleton reduces the efficiency of infection but does not inhibit it [24], [29], [30]. Thus, the microtubule cytoskeleton is not essential, but its viral use (or abuse?) certainly facilitates the infection process. This however, raises the question of how do viruses get from the cell periphery to the nucleus in the absence of microtubules. In the case of the adeno-associated virus, actin has been reported to have an unknown role in virus movements toward the nucleus [29]. The only clear example of a role for the actin cytoskeleton in capsid movements towards the nucleus, during the initial establishment of infection, is that of baculovirus [10], [31]. The mechanism by which baculovirus capsids induce so called ‘actin cables’ remains to be established, but they are similar in appearance to vaccinia-induced actin tails [12].
3. Viral molecules involved in retrograde transport
The loss of the viral envelope or exit from the endosomal compartment is a pre-requisite for movement of HSV and adenovirus towards the nucleus. This suggests that caspid or tegument proteins (which are exposed when the envelope or endosomal membrane is removed) are responsible for recruiting the transport machinery [16], [24]. Bearer et al. [32•] have recently confirmed using the squid giant axon as a model transport system that enveloped HSV cannot undergo retrograde motility [32•]. This study also showed that HSV capsids move at 2.2 μm/sec in the retrograde direction, which is comparable to the speeds observed for endogenous organelles. So which viral proteins recruit or interact with the dynein–dynactin complex and other factors required for retrograde transport?
Pull-down experiments show that the UL34 protein of HSV, which is associated with the incoming viral capsids, interacts with the intermediate chain of cytoplasmic dynein [33]. Furthermore, transiently expressed UL34 partly associates with microtubules [33]. UL25, a minor but essential component of the pseudorabies capsid, may play a similar role, as it colocalises with microtubules when expressed in a non-viral background [27]. Further support for the interaction of herpes virus tegument proteins with microtubules comes from Murata et al. [34] who report that perinuclear accumulation of the HSV tegument proteins, UL41 and UL46, is microtubule dependent [34]. Interaction of capsid proteins with components of the dynein complex is not restricted to herpes virus family members. The cytoplasmic dynein light chain (LC8) was recently isolated as a strong interacting partner of the P phosphoprotein of two lyssaviruses, rabies and Mokola, in a yeast two-hybrid screen [35], [36]. This interaction was confirmed by coimmunoprecipitation experiments, and the two proteins colocalize in infected cells. Interestingly, in the LC8-binding site of the rabies P phosphoprotein (residues 138–172 [36]), serine 162 is phosphorylated [37]. It is possible that phosphorylation of the P protein regulates interaction with and/or activity of the dynein motor complex. The recruitment of the dynein complex provides an ideal means to achieve retrograde axonal transport in neurons during rabies infections. The fact that LC8 is also associated with the myosin V motor, which is involved in vesicular traffic, indicates that additional actin-based motility of rabies may also take place, especially near the plasma membrane [35].
We can expect that further experiments will be aimed at understanding the molecular basis of the interaction between viral capsid proteins and the dynein–dynactin motor complex. For instance, it still remains to be demonstrated whether these viral proteins bind directly to components of the dynein–dynactin motor complex. The other important point that needs to be addressed is that of regulation of the motor complex by viral capsid proteins. As with the dissection of cell motility using bacterial pathogens, viruses are likely to provide powerful and manipulable systems for studying regulation of the dynein–dynactin motor complex.
4. Short range transport or anchoring in the vicinity of the nucleus
Having reached the centre of the cell, some viruses are still faced with the small but important transport problem of getting into the nucleus (Fig. 1). This problem, which will not be discussed here, is one of associating with the nucleus and subsequently being transported across the nuclear pore (reviewed in [38•]).
Vaccinia does not need to get into the nucleus for its replication and assembly as these events take place in the cytoplasm in a perinuclear region in the vicinity of the MTOC [30]. These data, together with the observation that disruption of the microtubule cytoskeleton or the dynein–dynactin complex results in dispersed localization of new virus particles [30], would support the notion that either vaccinia moves inwards on microtubules after entry or that newly formed particles are anchored on microtubules in the perinuclear region. Nevertheless, the fact that incoming vaccinia cores begin to disassemble after entry [39] would tend to suggest that, although they may interact with microtubules upon entry [30], they may not actually move. Thus, it remains to be determined whether the dynein–dynactin complex and microtubules are required for virus particle movements or, alternatively, for organization of the intermediate compartment and trans-Golgi network (TGN), which are used for viral wrapping during morphogenesis. It may, however, be virtually impossible to answer this question as both virus particle movements and Golgi membrane organization seem to depend on microtubules. We know that depolymerization of microtubules results in a three-fold reduction in assembly of the first form of the virus, the so called IMV (intracellular mature virus), whereas disruption of the TGN completely inhibits formation of the intracellular enveloped form of vaccinia (IEV) [30]. Thus, there might be three roles for microtubules and the dynein–dynactin complex during vaccinia morphogenesis. First, to keep IMV anchored near the TGN; second, to maintain TGN organization; and third, to facilitate movement of IMVs from their site of assembly in the virus factory towards the TGN where they are wrapped and form the IEV.
Recent data from Sanderson et al. [40] have suggested that the vaccinia protein A27L is involved in microtubule-based movement of IMV. Deletion of A27L results in clustering of IMV particles in viral factories and prevents them being enveloped by Golgi-derived membranes to form IEV. However, it is not clear from the data presented whether this is due to inhibition of IMV transport to IEV wrapping sites, as Sanderson et al. suggest, or due to a functional defect that prevents IMV lacking A27L from wrapping to form IEV or due to disruption of the TGN. Further work is required to determine precisely at which stage during vaccinia infection the dynein–dynactin complex is used.
5. Transport from the cell centre to the periphery (anterograde transport)
Anterograde transport, following viral replication, can be roughly divided into two categories: either movement of assembled viral particles or movement of capsid components (for viruses that assemble at the plasma membrane) and movement of nucleoprotein complexes (in the case of plant viruses).
6. Anterograde transport of assembled viral capsids/particles
As with retrograde movements, it is unlikely that a large virus particle, such as HSV or vaccinia, which assemble in the nucleus or perinuclear region, respectively, would ever get out of the cell, assuming that there is no cell lysis, without the help of the cytoskeleton. The same consideration is also likely to apply for smaller viruses [14•].
The best documented example of anterograde transport of intact virus particles is that of herpes virus translocation to the periphery of neurons [41], [42], [43], [44], [45]. Only non-enveloped nucleocapsids translocate from the cell body to the periphery of neurons, although enveloped and non-enveloped viruses are found in the soma of neurons [44]. Further studies from Cunningham and colleagues, using confocal and electron microscopy, suggest that herpes nucleocapsids are indeed transported when fully assembled by a microtubule-dependent mechanism to the cell periphery [45]. However, viral envelope glycoproteins are transported separately to the plasma membrane via conventional transport vesicles [45]. Therefore the herpes nucleocapsid uses the microtubule cytoskeleton during both retrograde and anterograde transport, but it is neither known how it regulates which way to go nor which motor is involved in anterograde movements.
In the case of vaccinia virus, anterograde motility is better understood — or is it? IEV (which is formed when IMV becomes enwrapped within a membrane cisterna derived from the TGN) is able to nucleate actin tails that are similar to those formed by some species of Listeria and Shigella (reviewed in [11], [12]). IEVs are propelled through the cytoplasm of infected cells on the tips of actin tails, using the power of actin polymerization, until they contact the plasma membrane and extend into neighbouring cells. Vaccinia strains that are unable to induce actin tails have a small plaque phenotype suggesting they have reduced cell to cell spread [46], [47], [48]. Recent work has begun to identify the proteins involved in vaccinia-induced actin polymerization [49], [50], [51], [52]. The integral IEV membrane protein A36R, which has a cytoplasmic domain of ∼195 residues, is responsible for initiating the cascade of events that leads to actin tail formation [51]. Although deletion and mutant analyses have demonstrated that A36R is required for actin tail assembly, it still remains to be established whether it is sufficient. Vaccinia actin tail formation is dependent on tyrosine phosphorylation of A36R by Src family kinases [51]. Phosphorylation of A36R results in recruitment of the adaptor molecule Nck, WASP-interacting protein (WIP) and N-WASP, which can activate the actin nucleating activity of the Arp2/3 complex [51], [52]. Thus, it appears that vaccinia achieves actin-based motility by mimicking and hijacking components of tyrosine kinase receptor signaling pathways that normally occur at the plasma membrane. It remains to be determined which Src family kinase is responsible for A36R phosphorylation, how the virus activates the kinase and where in the cell phosphorylation of A36R occurs.
Although the ability to form actin tails is beneficial to the spread of vaccinia, it is not essential. Vaccinia virus particles are able to reach the cell periphery in the absence of actin tail formation (see images in [49]), suggesting that viral particles can also move out on microtubules. Indeed, microtubule-based anterograde movements of vaccinia virus particles do occur and are essential, as actin tails only form at the plasma membrane (JM Rietdorf, M Way, unpublished data). It appears that A36R-dependent actin tail formation is not common to all poxviruses. Myxoma, Shope fibroma and fowlpox viruses do not encode A36R homologues in their genomes, although variola virus, the causative agent of smallpox, does. However, actin colocalizes with fowlpox virus close to the plasma membrane and depolymerization of the actin cytoskeleton with cytochalasin D treatment reduces production of extracellular fowlpox virus [53].
7. Anterograde transport of capsid components for viruses that assemble at the plasma membrane
Many different viruses assemble at the plasma membrane of the cell, including influenza and retroviruses [13••]. Viral membrane proteins destined for the envelope around the viral particle get to the plasma membrane via the secretory pathway of the host. The problem is how do the other viral proteins and nucleic acids, which may well exist as a ribonucleic-acid–protein complex, get to the site of virus assembly. Once again, unfortunately for the host, it appears that the cytoskeleton comes to the rescue, at least in the case of retroviruses. The Gag protein of murine leukemia virus interacts with KIF4, a microtubule plus-end-directed kinesin motor, both in vitro and in vivo [54], [55]. The same in vitro interaction has also been demonstrated for Mason–Pfizer monkey, Rous sarcoma, human and simian immunodeficiency viruses, implicating microtubules and kinesin motors in the anterograde movements of retroviral components to the plasma membrane [55]. HIV Gag can also associate with actin in vivo and in vitro [56], [57]. The role of actin during infection remains to be established in vivo, but it probably functions during viral budding. Indeed, HIV virions contain actin and the actin-binding proteins erzin and moesin (which are associated with the plasma membrane) [58], [59]. Furthermore, disruption of the actin cytoskeleton with cytochalasin D reduces the release of HIV-1 from infected cells by 40% [60]. One can envisage a situation where retroviral Gag moves out to the plasma membrane on microtubules, there it associates with viral membrane proteins and actin during particle assembly and budding.
8. Plant viruses and movement proteins
Plant viruses encode one or more specialised molecules, called ‘movement proteins’ (MPs), which bind to the viral nucleic acid. The resulting nucleoprotein complex is transported to, and subsequently through, intercellular connections called plasmodesmata into neighbouring cells to spread the infection (reviewed [61], [62]). MP–nucleic-acid interactions are well documented for many plant viruses, thus much of the research in the past year has focused on MP–cytoskeleton interactions and the mechanism of transport.
The colocalization of tobacco mosaic virus (TMV) MP with microtubules in vivo and in vitro indicated early on that microtubules had an important role in viral spread [63], [64]. However, there was no evidence that the MP–microtubule complexes were associated with viral nucleic acids. Recently in situ hybridisation methods have provided the missing link to demonstrate that viral RNA (vRNA) colocalises with MPs and microtubules [65•]. Nevertheless, it remained to be shown whether this colocalisation reflected a functional interaction that was important during infection. Three parallel studies have now addressed this issue [66], [67], [68]. First, it was demonstrated that increased association of MP with microtubules correlates with a higher rate of vRNA cell to cell spread [66]. Furthermore, both events take place more efficiently at higher temperature [66]. These observations are consistent with data from the second study of the distribution of GFP-labelled MP during temperature shifts that depolymerise/repolymerise microtubules [67]. MP association with filamentous structures (presumably microtubules) was lost upon microtubule depolymerisation by cold treatment; this effect was reversible when microtubules were repolymerised [67]. Further indirect evidence for a role of microtubles in vRNA transport comes from a mutant of MP that colocalises with vRNA but not with microtubules. This mutation results in MP and associated vRNA accumulation in a perinuclear region, presumably due to its inability to interact and move on microtubules [67]. The third study provided the most definitive answer to this issue (i.e. does colocalisation of microtubules with MP reflect a functional interaction important in infection spread) using a different elegant approach [68••]. Boyko et al. [68••] identified a short amino-acid sequence motif conserved among MPs of different TMVs that was also present in α-, β- and γ-tubulin. Mutagenesis of this motif in MP resulted in inhibition of vRNA transport and prevented association of MP with microtubules at the restrictive temperature. However, this movement-deficient phenotype was readily reversed at the permissive temperature, indicating that intercellular spread of vRNA depends on the association of MP with microtubules [68••]. What is even more interesting is how MP associates with microtubules. Experiments with purified MP–microtubule complexes reveal that the two proteins are very tightly associated and can only be separated by chaotropic reagents. This is in stark contrast to microtubule-associated proteins, which bind to the outside of microtubules and are readily dissociated by salt. Although no direct evidence is provided, Boyko et al. [68••] suggest that MP co-assembles with tubulin and becomes an integral part of the microtubule lattice. Interestingly, expression of MP in mammalian cells results in loss of centrosomal γ-tubulin staining, although there was no obvious association with the centrosome.
There are clearly many interesting questions that remain to be answered concerning the properties of MP, including how it associates with microtubules and whether this interaction is dependent on the presence of vRNA. There are also the questions of how MP actually moves on or with microtubules and how this movement is regulated. So far we know that phosphorylation of TMV MP regulates its movement through plasmodesmata, but not nucleoprotein complex formation, only in Nicotiana tabacum plants[69•].
In contrast to microtubules, the role of actin in transport of MP is unclear. On the basis of the partial colocalisation of the TMV MP with actin in vivo and an interaction in vitro, it has been proposed that the actin cytoskeleton is involved in the transport of the MP–nucleic-acid complexes through plasmodesmata [64]. However, disruption of the actin cytoskeleton has little effect on the localisation of MP [63], [64]. Thus, it has been suggested that the actin cytoskeleton may regulate the plasmodesmata size-exclusion-limit, rather than active transport of the nucleoprotein within and between cells (reviewed [61], [62]). An alternative or additional role for actin during infection has been proposed [65], [70]. During infection vRNA colocalises with MP and replicase in close proximity to the ER, suggesting that viral replication and translation take place on this membrane compartment. Colocalization of viral proteins and replication complexes with the ER have also been reported for tobacco etch potyvirus [71] and brome mosaic bromovirus [72], [73]. Disruption of the ER with brefeldin A, actin with cytochalasin D or microtubules with oryzralin all result in redistribution of vRNA and MP [65•]. Thus, it is possible that the actin cytoskeleton imparts structural stability and motor activity to the plant cell ER, which is in turn required to anchor the replicase–MP–vRNA structures. Once replication is completed, microtubules take over and transport MP with the bound newly synthesised vRNA over long distances [65], [70]. This suggestion that actin provides structural and motor activity might explain the actin–MP in vitro interaction [64]. Whether the ER itself plays a role in viral trafficking remains to be seen. It also remains to be examined whether host proteins, other than actin and tubulin, have a role in the transport of the nucleoprotein complexes within and between plant cells.
9. Disruption of the microtubule cytoskeleton during infection
Oncogenic transformation by a number of different viruses results in severe changes in organization and regulation of the cytoskeleton. In fact, during virtually every viral infection, cell morphology is often drastically affected, along with changes in the actin and microtubule cytoskeleton (Fig. 2 ; reviewed in [8], [9], [10], [13]). These changes are usually referred to as ‘cytopathic’ effects, and their extent varies with different viruses and cell types but tends to take place many hours after initial infection. Cytopathic effects have not been explored, except to avoid them when developing viral expression systems used to express proteins easily and efficiently in cells. The questions then arise, what causes this phenomenon and what is its function, if any?
Fig. 2.
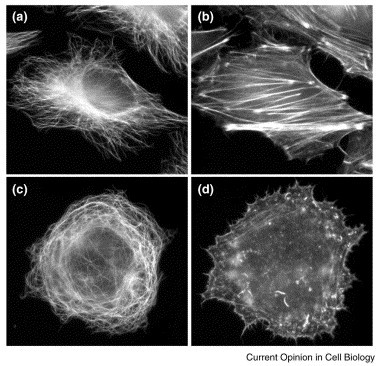
Immunofluorescence microcopy images of uninfected (a,b) and vaccina-infected (c,d) HeLa cells, double labelled to visualise the microtubule (a,c) and actin (b,d) cytoskeleton. Vaccina infection disrupts microtubule and actin overlay. (See cover for microtubule/actin overlay.)
Formation of vaccinia actin tails disrupts the actin cytoskeleton (Fig. 2) and is obviously useful in enhancing viral spread. However, in the absence of actin tail formation the actin cytoskeleton is still disrupted, suggesting that the vaccinia genome contains additional proteins that regulate or modify the actin cytoskeleton. Vaccinia infection also disrupts the microtubule cytoskeleton (Fig. 2 [30]). This disruption is probably the consequence of overexpression of vaccinia-encoded microtubule-associated proteins (MAPs) and destruction of centrosome function. What is the advantage for vaccinia in disrupting microtubule cytoskeleton organization? The vaccinia MAP-like proteins, A10L and L4R, are essential components of the vaccinia core (the nucleocapsid), which is released into the cytoplasm during virus entry [19], [39]. In vitro, A10L and L4R mediate the interaction of vaccinia cores with microtubules [30], suggesting that vaccinia is likely to interact with microtubules via these proteins immediately after entry. The association of newly synthesised A10L and L4R with microtubules does not take place until later during infection, after extensive viral protein synthesis and particle assembly has occurred. Therefore, this association may play no functional role during infection and may merely represent the unavoidable side effect of accumulation of excess viral proteins with MAP properties in the cytoplasm of infected cells.
Vaccinia is not the only virus to encode MAPs. The VP22 tegument protein of HSV-1 colocalizes with microtubules in infected cells and induces extensive stable microtubule bundles when expressed in non-infected cells [74]. Other viral proteins that associate with microtubules in vivo or in vitro are listed in Table 1 . Interestingly, a large number of these viral MAPs are structural components of viral capsids/particles, suggesting that although they probably associate with microtubules at the later stages of infection because of overexpression, they may in fact have a functional role in viral transport at earlier stages of infection. Furthermore, the presence of MAPs in virus capsids/particles may be required for viral movements on microtubules. For example, the combined action of a MAP and a microtubule motor are required for the movement of phagosomes on microtubules [75], [76].
Table 1.
Viral proteins that associate with the microtubule cytoskeleton or bind microtubules in vitro
| Virus | Protein | Reference |
|---|---|---|
| Vaccinia virus | A10L and L4R | [30] |
| Herpes simplex virus | VP22 | [74] |
| Herpes simplex virus | UL34 | [33] |
| Murine coronavirus | N | [79] |
| Tobacco mosiac virus | MP | [80] |
| Cauliflower mosaic virus | Aphid transmission factor | [81] |
| Pseudorabies virus | UL25 | [27] |
| Simian virus 40 | Large T antigen | [82] |
| Vesicular stomatitis virus | M | [83] |
| Rotavirus | VP4 spike protein | [84] |
| Beet yellows virus | HSP70-related p65 | [85] |
| Kunjin virus | NS3 | [86] |
| Baculovirus | P10 | [87] |
| Adenovirus-2 | E3/19K | [88] |
In the case of vaccinia virus infection it is clear that disruption of centrosome function contributes to disorganization of the microtubule cytoskeleton [30]. Recent electron microscopy studies showed that cytomegalovirus infection also destabilises the centrosome [77], [78]. The lack of MTOC-organized microtubules in HSV-infected cells may also be indicative of a loss of centrosome function [74]. Understanding the mechanism by which vaccinia and cytomegalovirus disrupt centrosome function remains to be elucidated, but it is likely to provide some exciting insights into the regulation of this mysterious organelle. But why do viruses destroy the microtubule cytoskeleton organization that is so useful for viral transport? In the case of the vaccinia virus, disruption of microtubule cytoskeleton organization does not take place until after extensive virus assembly and transport to the periphery has taken place. Furthermore, the formation of long cellular extensions, up to 200μm in length, full of stable microtubles, which may not be possible in the presence of a functioning centrosome, provides a convenient way for the virus to travel long distances [30]. Although it should be possible to identify the viral proteins involved, we may never know whether cytopathic effects are truly useful to the virus or merely the consequence of viral protein side effects.
10. Conclusions
A clear pattern is now emerging for how viral transport involves the host cytoskeleton. As with vesicular membrane traffic, microtubules are the cytoskeletal element of choice for directed long range retrograde and anterograde transport of viral capsids or nucleoprotein complexes. The role of actin, on the other hand, largely appears to be concerned with events at the plasma membrane. These include viral entry and exit, and actin may provide an anchoring mechanism to assist virus particle assembly at the plasma membrane in addition to its role in budding or in mediating active outward transport, as with vaccinia virus. In the next few years we can expect to see more studies filling in our knowledge of cellular virus movements, which are usually vaguely eluded to in virology textbooks. Ultimately however, viruses may provide convenient and manipulable systems to dissect and understand motor protein function. They also provide a system to study cooperation and crosstalk between actin and microtubule cytoskeletons during transport.
11. Acknowledgements
We would like to thank Eric Karsenti and Bodo MH Lange for comments on the manuscript and Urs Greber for discussions concerning Adenovirus motility.
12. References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
•of special interest
• •of outstanding interest
References
- 1.Luftig RB. Does the cytoskeleton play a significant role in animal virus replication? J Theor Biol. 1982;99:173–191. doi: 10.1016/0022-5193(82)90397-6. [DOI] [PubMed] [Google Scholar]
- 2.Satake M, Luftig RB. Microtubule-depolymerizing agents inhibit Moloney murine leukaemia virus production. J Gen Virol. 1982;58:339–349. doi: 10.1099/0022-1317-58-2-339. [DOI] [PubMed] [Google Scholar]
- 3.Payne LG, Kristensson K. The effect of cytochalasin D and monensin on enveloped vaccinia virus release. Arch Virol. 1982;74:11–20. doi: 10.1007/BF01320778. [DOI] [PubMed] [Google Scholar]
- 4.Weatherbee JA, Luftig RB, Weihing RR. Binding of adenovirus to microtubules. II. Depletion of high-molecular- weight microtubule-associated protein content reduces specificity of in vitro binding. J Virol. 1977;21:732–742. doi: 10.1128/jvi.21.2.732-742.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luftig RB, Weihing RR. Adenovirus binds to rat brain microtubules in vitro. J Virol. 1975;16:696–706. doi: 10.1128/jvi.16.3.696-706.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babiss LE, Luftig RB, Weatherbee JA, Weihing RR, Ray UR, Fields BN. Reovirus serotypes 1 and 3 differ in their in vitro association with microtubules. J Virol. 1979;30:863–874. doi: 10.1128/jvi.30.3.863-874.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Matos AP, Carvalho ZG. African swine fever virus interaction with microtubules. Biol Cell. 1993;78:229–234. doi: 10.1016/0248-4900(93)90134-z. [DOI] [PubMed] [Google Scholar]
- 8.Luftig RB, Lupo LD. Viral interactions with the host-cell cytoskeleton: the role of retroviral proteases. Trends Microbiol. 1994;2:178–182. doi: 10.1016/0966-842x(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 9.Knipe DM. Fields Virology. Raven Press Ltd; 1996. Virus-host cell interactions; pp. 273–299. [Google Scholar]
- 10.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 1997;5:142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 11.Cossart P. Actin-based motility of pathogens: the Arp2/3 complex is a central player. Cell Micriobiol. 2000;2:195–205. doi: 10.1046/j.1462-5822.2000.00053.x. [DOI] [PubMed] [Google Scholar]
- 12.Frischknecht F, Way M. Surfing pathogens and the lessons learned for actin polymerization. Trends Cell Biol. 2001;11:30–38. doi: 10.1016/s0962-8924(00)01871-7. [DOI] [PubMed] [Google Scholar]
- 13.Flint SJ, Enquist LW, Krug RM, Racaniello VR, Skalka AM. Principles of virology. Molecular Biology, Pathogenesis and Control. American Society for Microbiology Press; Washington: 2000. An excellent textbook, designed along the lines of the ‘classic’ Molecular Biology of the Cell, that provides first class reference material on all aspects of virology, accompanied by clear and beautiful illustrations. [Google Scholar]
- 14.Sodeik B. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 2000;8:465–472. doi: 10.1016/s0966-842x(00)01824-2. A review covering aspects of viral transport that includes information concerning theoretical diffusion rates of virus particles. [DOI] [PubMed] [Google Scholar]
- 15.Li E, Stupack D, Bokoch GM, Nemerow GR. Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J Virol. 2000;74:7085–7095. doi: 10.1128/jvi.74.15.7085-7095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li E, Stupack DG, Brown SL, Klemke R, Schlaepfer DD, Nemerow GR. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J Biol Chem. 2000;275:14729–14735. doi: 10.1074/jbc.275.19.14729. [DOI] [PubMed] [Google Scholar]
- 18.Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanderpasschen A, Hollinshead M, Smith GL. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J Gen Virol. 1998;79:877–887. doi: 10.1099/0022-1317-79-4-877. [DOI] [PubMed] [Google Scholar]
- 20.Krijnse-Locker J, Kuehn A, Schliech S, Rutter G, Hohenberg H, Wepf R, Griffiths G. Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell. 2000;11:2497–2511. doi: 10.1091/mbc.11.7.2497. During vaccinia infection the more infectious, extracellular enveloped virus (EEV) enters the cell ‘silently’, whereas the less infectious intracellular mature virus (IMV) activates a signaling cascade that involves Rac. It remains to be examined whether entering the cell ‘silently’ gives an advantage to the EEV in infection and whether the IMV-induced signaling cascade is similar to that observed during Shigella entry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristensson K, Lycke E, Roytta M, Svennerholm B, Vahlne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine] J Gen Virol. 1986;67:2023–2028. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- 22.Topp KS, Meade LB, LaVail JH. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994;14:318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topp KS, Bisla K, Saks ND, Lavail JH. Centripetal transport of herpes simplex virus in human retinal pigment epithelial cells in vitro. Neuroscience. 1996;71:1133–1144. doi: 10.1016/0306-4522(95)00497-1. [DOI] [PubMed] [Google Scholar]
- 24.Sodeik B, Ebersold MW, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suomalainen M, Nakano MY, Keller S, Boucke K, Stidwill RP, Greber UF. Microtubule-dependent plus- and minus-end-directed motilities are competing processes for nuclear targeting of adenovirus. J Cell Biol. 1999;144:657–672. doi: 10.1083/jcb.144.4.657. The first video microscopy study showing viral retrograde movement, in this case of adenovirus, along microtubules. Careful and detailed analysis shows that there are in fact two types of ‘competing’ movement, towards the plus and the minus microtubule ends, that result in an overall retrograde movement towards the centre of the cell. The plus-end-directed microtubule motor remains to be identified, but dyenin mediates the minus-end-directed movement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–156. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 27.Kaelin K, Dezelee S, Masse MJ, Bras F, Flamand A. The UL25 protein of pseudorabies virus associates with capsids and localizes to the nucleus and microtubules. J Virol. 2000;74:474–482. doi: 10.1128/jvi.74.1.474-482.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saib A, Puvion-Dutilleul F, Schmid M, Peries J, De The H. Nuclear targeting of incoming human foamy virus gag proteins involves a centriolar step. J Virol. 1997;71:1155–1161. doi: 10.1128/jvi.71.2.1155-1161.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ploubidou A, Moreau V, Ashman K, Reckmann I, Gonzalez C, Way M. Vaccinia virus infection disrupts microtubule organization and centrosome function. EMBO J. 2000;19:3932–3944. doi: 10.1093/emboj/19.15.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanier LM, Volkman LE. Actin binding and nucleation by Autographa california M nucleopolyhedrovirus. Virology. 1998;243:167–177. doi: 10.1006/viro.1998.9065. [DOI] [PubMed] [Google Scholar]
- 32.Bearer EL, Breakefield XO, Schuback D, Reese TS, LaVail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc Natl Acad Sci USA. 2000;97:8146–8150. doi: 10.1073/pnas.97.14.8146. The authors take advantage of the squid giant axon to develop a novel assay to study retrograde movement of herpes simplex virus particles. They show that only non-enveloped herpes particles are motile, indicating that viral tegument/capsid proteins interact with the transport machinery of the cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye G-J, Vaughan KT, Vallee RB, Roizman B. The Herpes simplex virus 1 UL34 protein interacts with a cytoplasmic dynein intermediate chain and targets nuclear membrane. J Virol. 2000;74:1355–1363. doi: 10.1128/jvi.74.3.1355-1363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murata T, Goshima F, Daikoku T, Inagaki-Ohara K, Takakuwa H, Kato K, Nishiyama Y. Mitochondrial distribution and function in herpes simplex virus- infected cells. J Gen Virol. 2000;81:401–406. doi: 10.1099/0022-1317-81-2-401. [DOI] [PubMed] [Google Scholar]
- 35.Jacob Y, Badrane H, Ceccaldi PE, Tordo N. Cytoplasmic dynein LC8 interacts with lyssavirus phosphoprotein. J Virol. 2000;74:10217–10222. doi: 10.1128/jvi.74.21.10217-10222.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raux H, Flamand A, Blondel D. Interaction of the rabies virus P protein with the LC8 dynein light chain. J Virol. 2000;74:10212–10216. doi: 10.1128/jvi.74.21.10212-10216.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta AK, Blondel D, Choudhary S, Banerjee AK. The phosphoprotein of rabies virus is phosphorylated by a unique cellular protein kinase and specific isomers of protein kinase C. J Virol. 2000;74:91–98. doi: 10.1128/jvi.74.1.91-98.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittaker GR, Kann M, Helenius A. Viral entry into the nucleus. Annu Rev Cell Dev Biol. 2000;16:627–651. doi: 10.1146/annurev.cellbio.16.1.627. A comprehensive and well written review that clearly explains the challenges viruses face and the strategies they have developed to gain access to the nucleus. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen K, Snijder EJ, Schleich S, Roos N, Griffiths G, Krijnse Locker J. Characterization of vaccinia virus intracellular cores: Implications for viral uncoating and core structure. J Virol. 2000;74:3525–3536. doi: 10.1128/jvi.74.8.3525-3536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanderson CM, Hollinshead M, Smith GL. The vaccinia virus A27L protein is needed for the microtubule-dependent transport of intracellular mature virus particles. J Gen Virol. 2000;81:47–58. doi: 10.1099/0022-1317-81-1-47. [DOI] [PubMed] [Google Scholar]
- 41.Penfold ME, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc Natl Acad Sci USA. 1994;91:6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun N, Cassell MD, Perlman S. Anterograde, transneuronal transport of herpes simplex virus type 1 strain H129 in the murine visual system. J Virol. 1996;70:5405–5413. doi: 10.1128/jvi.70.8.5405-5413.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garner JA, LaVail JH. Differential anterograde transport of HSV type 1 viral strains in the murine optic pathway. J Neurovirol. 1999;5:140–150. doi: 10.3109/13550289909021996. [DOI] [PubMed] [Google Scholar]
- 44.Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J Virol. 1999;73:8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miranda-Saksena M, Armati P, Boadle RA, Holland DJ, Cunningham AL. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J Virol. 2000;74:1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolffe EJ, Katz E, Weisberg A, Moss B. The A34R glycoprotein gene is required for induction of specialized actin-containing microvilli and efficient cell-to-cell transmission of vaccinia virus. J Virol. 1997;71:3904–3915. doi: 10.1128/jvi.71.5.3904-3915.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolffe EJ, Weisberg AS, Moss B. Role for the vaccinia virus A36R outer envelope protein in the formation of virus-tipped actin-containing microvilli and cell-to-cell virus spread. Virology. 1998;25:20–26. doi: 10.1006/viro.1998.9103. [DOI] [PubMed] [Google Scholar]
- 48.Sanderson CM, Frischknecht F, Way M, Hollinshead M, Smith GL. Roles of vaccinia virus EEV-specific proteins in intracellular actin tail formation and low pH-induced cell-cell fusion. J Gen Virol. 1998;79:1415–1425. doi: 10.1099/0022-1317-79-6-1415. [DOI] [PubMed] [Google Scholar]
- 49.Röttger S, Frischknecht F, Reckmann I, Smith GL, Way M. Interactions between vaccinia virus IEV membrane proteins and their roles in IEV assembly and actin tail formation. J Virol. 1999;73:2863–2875. doi: 10.1128/jvi.73.4.2863-2875.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frischknecht F, Cudmore S, Moreau V, Reckmann I, Röttger S, Way M. Tyrosine phosphorylation is required for actin based motility of vaccinia but not Listeria or Shigella. Curr Biol. 1999;9:89–92. doi: 10.1016/s0960-9822(99)80020-7. [DOI] [PubMed] [Google Scholar]
- 51.Frischknecht F, Moreau V, Röttger S, Gonfloni S, Reckmann I, Superti-Furga G, Way M. Actin based motility of vaccinia virus mimics receptor tyrosine kinase signalling. Nature. 1999;401:926–929. doi: 10.1038/44860. [DOI] [PubMed] [Google Scholar]
- 52.Moreau V, Frischknecht F, Reckmann I, Vincentelli R, Rabut G, Stewart D, Way M. A complex of N-WASP and WIP integrates signalling cascades that lead to actin polymerization. Nat Cell Biol. 2000;2:441–448. doi: 10.1038/35017080. [DOI] [PubMed] [Google Scholar]
- 53.Boulanger D, Smith T, Skinner MA. Morphogenesis and release of fowlpox virus. J Gen Virol. 2000;81:675–687. doi: 10.1099/0022-1317-81-3-675. [DOI] [PubMed] [Google Scholar]
- 54.Kim W, Tang Y, Okada Y, Torrey TA, Chattopadhyay SK, Pfleiderer M, Falkner FG, Dorner F, Choi W, Hirokawa N. Binding of murine leukemia virus Gag polyproteins to KIF4, a microtubule-based motor protein. J Virol. 1998;72:6898–6901. doi: 10.1128/jvi.72.8.6898-6901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang Y, Winkler U, Freed EO, Torrey TA, Kim W, Li H, Goff SP, Morse HC. Cellular motor protein KIF-4 associates with retroviral gag. J Virol. 1999;73:10508–10513. doi: 10.1128/jvi.73.12.10508-10513.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rey O, Canon J, Krogstad P. HIV-1 Gag protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 57.Liu B, Dai R, Tian CJ, Dawson L, Gorelick R, Yu XF. Interaction of the human immunodeficiency virus type 1 nucleocapsid with actin. J Virol. 1999;73:2901–2908. doi: 10.1128/jvi.73.4.2901-2908.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ott DE, Coren LV, Kane BP, Busch LK, Johnson DG, Sowder RC II, Chertova EN, Arthur LO, Henderson LE. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J Virol. 1996;70:7734–7743. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilk T, Gowen B, Fuller SD. Actin associates with the nucleocapsid domain of the human immunodeficiency virus Gag polyprotein. J Virol. 1999;73:1931–1940. doi: 10.1128/jvi.73.3.1931-1940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, Nonomura Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci USA. 1995;92:2026–2030. doi: 10.1073/pnas.92.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Citovsky V. Tobacco mosaic virus: a pioneer of cell-to-cell movement. Philos Trans R Soc Lond B Biol Sci. 1999;354:637–643. doi: 10.1098/rstb.1999.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reichel C, Mas P, Beachy RN. The role of the ER and cytoskeleton in plant viral trafficking. Trends Plant Sci. 1999;4:458–462. doi: 10.1016/s1360-1385(99)01490-9. [DOI] [PubMed] [Google Scholar]
- 63.Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 64.McLean BG, Zupan J, Zambryski PC. Tobacco mosaic virus movement protein associates with the cytoskeleton in tobacco cells. Plant Cell. 1995;7:2101–2114. doi: 10.1105/tpc.7.12.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mas P, Beachy RN. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J Cell Biol. 1999;147:945–958. doi: 10.1083/jcb.147.5.945. Using in situ hybridisation methods, the authors demonstrate that viral RNA colocalizes with microtubules, in infected plant cells, thus adding the missing link to the long postulated hypothesis that microtubules probably play a role in viral RNA transport in plants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boyko V, Ferralli J, Heinlein M. Cell-to-cell movement of TMV RNA is temperature-dependent and corresponds to the association of movement protein with microtubules. Plant J. 2000;22:315–325. doi: 10.1046/j.1365-313x.2000.00740.x. [DOI] [PubMed] [Google Scholar]
- 67.Mas P, Beachy RN. Role of microtubules in the intracellular distribution of tobacco mosaic virus movement protein. Proc Natl Acad Sci USA. 2000;97:12345–12349. doi: 10.1073/pnas.97.22.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boyko V, Ferralli J, Ashby J, Schellenbaum P, Heinlein M: Function of microtubules in intercellular transport of plant virus RNA. Nat Cell Biol. 2000;2:826–832. doi: 10.1038/35041072. Starting from an interesting sequence homology motif between plant movement protein (MP) and tubulins leads to the hypothesis of incorporation of MP molecules within the microtubule polymer (a hypothesis that remains to be tested). Further studies might show that this is the first viral transport system where microtubule treadmilling is used in transport of the vRNA rather than microtubules being used as track for motor driven movements. [DOI] [PubMed] [Google Scholar]
- 69.Waigmann E, Chen MH, Bachmaier R, Ghoshroy S, Citovsky V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. Embo J. 2000;19:4875–4884. doi: 10.1093/emboj/19.18.4875. How is cell to cell spread of viral infection controlled? In the case of plant cells, this can be achieved via regulation of MP activity. Phosphorylation of the MP of tobacco mosaic virus acts as a negative regulatory mechanism, preventing MP cell to cell movement. Although this is a host-specific regulatory mechanism, it opens the way for the study of viral RNA transport regulation in plant cells. It also raises the question whether phosphorylation regulates interaction with microtubules. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heinlein M, Padgett HS, Gens JS, Pickard BG, Casper SJ, Epel BL, Beachy RN. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell. 1998;10:1107–1120. doi: 10.1105/tpc.10.7.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaad MC, Jensen PE, Carrington JC. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. Embo J. 1997;16:4049–4059. doi: 10.1093/emboj/16.13.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Restrepo-Hartwig MA, Ahlquist P. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J Virol. 1996;70:8908–8916. doi: 10.1128/jvi.70.12.8908-8916.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Restrepo-Hartwig M, Ahlquist P. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J Virol. 1999;73:10303–10309. doi: 10.1128/jvi.73.12.10303-10309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elliott G, O'Hare P. Herpes simplex virus type 1 tegument protein VP22 induces stabilization and hyperacetylation of microtubules. J Virol. 1998;72:6448–6455. doi: 10.1128/jvi.72.8.6448-6455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blocker A, Severin FF, Habermann A, Hyman AA, Griffiths G, Burkhardt JK. Microtubule-associated protein-dependent binding of phagosomes to microtubules. J Biol Chem. 1996;271:3803–3811. doi: 10.1074/jbc.271.7.3803. [DOI] [PubMed] [Google Scholar]
- 76.Blocker A, Severin FF, Burkhardt JK, Bingham JB, Yu H, Olivo J-C, Schroer TA, Hyman AA, Griffiths G. Molecular requirements for bi-directional movement of phagosomes along microtubules. J Cell Biol. 1997;137:113–129. doi: 10.1083/jcb.137.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bystrevskaya VB, Lobova TV, Smirnov VN, Makarova NE, Kushch AA. Centrosome injury in cells infected with human cytomegalovirus. J Struct Biol. 1997;120:52–60. doi: 10.1006/jsbi.1997.3897. [DOI] [PubMed] [Google Scholar]
- 78.Gilloteaux J, Nassiri MR. Human bone marrow fibroblasts infected by cytomegalovirus: ultrastructural observations. J Submicrosc Cytol Pathol. 2000;32:17–45. [PubMed] [Google Scholar]
- 79.Kalicharran K, Dales D. The murine coronavirus as a model of trafficking and assembly of viral proteins in neural tissue. Trends Microbiol. 1996;4:264–269. doi: 10.1016/0966-842X(96)10045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of Tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- 81.Blanc S, Schmidt I, Vantard M, Scholthof HB, Kuhl G, Esperandieu P, Cerutti M, Louis C. The aphid transmission factor of cauliflower mosaic virus forms a stable complex with microtubules in both insect and plant cells. Proc Natl Acad Sci USA. 1996;93:15158–15163. doi: 10.1073/pnas.93.26.15158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maxwell SA, Ames SK, Sawai ET, Decker GL, Cook RG, Butel JS. Simian virus 40 large T antigen and p53 are microtubule-associated proteins in transformed cells. Cell Growth Differ. 1991;2:115–127. [PubMed] [Google Scholar]
- 83.Melki R, Gaudin Y, Blondel D. Interaction between tubulin and the viral matrix protein of vesicular stomatitis virus: possible implications in the viral cytopathic effect. Virology. 1994;202:339–347. doi: 10.1006/viro.1994.1350. [DOI] [PubMed] [Google Scholar]
- 84.Nejmeddine M, Trugnan G, Sapin C, Kohli E, Svensson L, Lopez S, Cohen J. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J Virol. 2000;74:3313–3320. doi: 10.1128/jvi.74.7.3313-3320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karasev AV, Kashina AS, Gelfand VI, Dolja VV. HSP70-related 65kDa protein of beet yellows closterovirus is a microtubule-binding protein. FEBS Lett. 1992;304:12–14. doi: 10.1016/0014-5793(92)80578-5. [DOI] [PubMed] [Google Scholar]
- 86.Ng ML, Hong SS. Flavivirus infection: essential ultrastructural changes and association of Kunjin virus NS3 protein with microtubules. Arch Virol. 1989;106:103–120. doi: 10.1007/BF01311042. [DOI] [PubMed] [Google Scholar]
- 87.Cheley S, Kosik KS, Paskevich P, Bakalis S, Bayley H. Phosphorylated baculovirus p10 is a heat-stable microtubule-associated protein associated with process formation in Sf9 cells. J Cell Sci. 1992;102:739–752. doi: 10.1242/jcs.102.4.739. [DOI] [PubMed] [Google Scholar]
- 88.Dahllof B, Wallin M, Kvist S. The endoplasmic reticulum retention signal of the E3/19K protein of adenovirus-2 is microtubule binding. J Biol Chem. 1991;266:1804–1808. [PubMed] [Google Scholar]


