Abstract
A single dose of vaccine for Mycoplasma bovis pneumonia, inactivated with saponin, was inoculated subcutaneously into 3–4 week-old calves. The calves were challenged 3 weeks later with a virulent strain of M. bovis on two occasions within 24 h using the aerosol route. The calves were monitored for clinical signs and serological responses then post mortemed 3 weeks after challenge. The vaccine was shown to be highly immunogenic in calves and did not cause adverse effects. Vaccinated calves showed few clinical signs while all unvaccinated calves developed signs of pneumonia. There was a significant decrease in body weight gain in unvaccinated calves compared to vaccinates and a significant increase in lung lesions and rectal temperatures in unvaccinated calves. The vaccine also reduced the spread of M. bovis to internal organs. In conclusion the M. bovis vaccine produced a significant level of protection against a large virulent challenge.
Keywords: Mycoplasma bovis, Calf pneumonia, Saponin, Arthritis
1. Introduction
Mycoplasma bovis is a primary cause of calf pneumonia, arthritis, mastitis, keratoconjunctivitis and other conditions [1] and has been estimated to cost the US cattle industry at least 32 million dollars in mortality and set back losses annually [2]. Few countries are free of disease because of the worldwide movement of cattle. In Europe, M. bovis is believed to be responsible for 25–33% of outbreaks of calf pneumonia and control is largely restricted to management practises such as improving ventilation or reducing stocking densities and chemotherapy. However, it has long been known that M. bovis, like all mycoplasmas, are naturally resistant to many antibiotics because of their lack of a cell wall. Furthermore, over the last decade evidence has accumulated that M. bovis is becoming resistant to antibiotics which have traditionally been effective including the tetracyclines, tilmicosin and spectinomycin [3]. As a result of this increasingly poor response to treatment, the use of vaccines would seem to be a rational approach to the control of disease caused or exacerbated by M. bovis.
The first attempt to develop vaccines against calf pneumonia was reported by Howard et al. in 1987 [4] who showed that a quadrivalent formalised killed vaccine incorporating M. bovis with other respiratory pathogens comprising respiratory syncytial virus, parainfluenza type 3 virus (PI3) and Mycoplasma dispar was effective in protecting against natural outbreaks of bovine respiratory disease. However, no further work continued on this vaccine. A vaccine prepared with formalin-inactivated strains of M. bovis and Mannheimia haemolytica taken from the target herd reduced losses from pneumonia and cost of treatment in newly introduced feedlot calves [5]. Other attempts again using formalin as an inactivant for mycoplasma infections have been largely unsuccessful or produce only transient protection probably because of the damaging effect of this chemical on the antigenic composition of the mycoplasma [6]. In one report the use of a formalin inactivated M. bovis vaccine actually led to an increase in disease following challenge in vaccinated calves compared to sham-vaccinated animals [7]. More modern approaches to vaccination have also been disappointing as an experimental vaccine composed of partially purified membrane proteins from M. bovis also increased pneumonic pathology [8].
The use of saponin, extracted from the bark of the South American tree Guillaia saponaria, as an adjuvant is well known and has been shown to boost the immune response to immunogens [9]; however, it can also very effectively and rapidly lyse the cholesterol rich membranes of mycoplasmas [10]. A vaccine against the serious goat disease, contagious caprine pleuropneumonia (CCPP), in which the causative mycoplasma was inactivated with saponin immediately prior to inoculation, provided significant protection against experimental infection [11]. A saponised vaccine for the small ruminant mycoplasma infection, contagious agalactia, proved more protective than heat killed or formalin inactivated vaccines [6].
We report here the results of an experimental vaccine containing saponised M. bovis cells in preventing infection against a large challenge of virulent and geographically different strain of M. bovis.
2. Materials and methods
2.1. Animals
Three to four-week-old male calves were obtained from a commercial dairy herd of a Holstein/Friesian breed consisting of 600 cows and a similar number of growing heifers. No cattle had been introduced for 7 years. All newborn calves had received colostrum from their dams within 2 h of birth. During the first 4 days, calves were fed with milk from their dams after which they received milk replacer and were placed in individual boxes in the open air. After 2 weeks calves were given premix and lucerna hay quantum satis and had free access to water.
The parental herd was routinely vaccinated against infectious bovine rhinotracheitis virus and bovine viral diarrhoea. No overt clinical signs of pneumonia were seen in the herd. There was no evidence of M. bovis in the nasal cavities or sera of the calves as measured by serological, cultural and PCR techniques (see 2.5, 2.6). M. bovirhinis and Pasteurella multicoda, which represent the normal bovine flora, were detected in the nasal cavities of several calves.
2.2. Vaccine
A strain of M. bovis designated 86B/96 and kept at −70 °C, isolated in the UK from the lung of a calf was grown in Eaton’s medium [12] for 72 h at 38.5 °C and then subcultured in fresh medium for a further 48 h. The mycoplasmas were centrifuged at 10,000×g for 30 min, resuspended and washed once in 0.1 M phosphate buffered saline (pH 7.2). Cells were centrifuged again and resuspended in 1/50th of the original volume. To the washed cells was added 2 mg/ml of filter sterilised saponin (Sigma, Poole) and incubated for 1 h at 37 °C. The saponised cells were then placed at 4 °C. The titre of the washed cells was 108 colony forming units (CFU)/ml and protein content estimated at approximately 2 mg/ml. The vaccine was plated onto blood agar to check for bacterial contamination and into Eaton’s medium to ensure inactivation of mycoplasmas. Three subcultures every 3–4 days of the inactivated cells were performed with plating at every subculture. No mycoplasmas or bacteria were detected.
2.3. Experimental challenge with virulent M. bovis
M. bovis strain 5063, isolated from a calf in Hungary, was propagated in Medium B [13] for 48 h; the titre was 1.2×109 (CFU)/ml.
2.4. Experimental design
Calves were randomly allocated to four groups. One week after vaccination, three groups were transported to experimental accommodation at the Institute, Budapest. Each group was housed separately. During the experiment calves were offered 4 l of milk replacer, 0.7 kg premix and 2 kg of hay twice a day. The fourth (vaccinated) group remained unchallenged on the farm where they were observed for adverse reactions and monitored serologically for 6 months.
Three weeks after vaccination, calves in groups A and B were challenged with a broth culture of M. bovis strain 5063 (see Section 2.3) by aerosol infection on two successive days. The calves were kept for a further 3 weeks after which, they were examined post mortem.
2.5. Microbiology
At the beginning and end of the experiment, nasal swabs were taken from each calf for detection of mycoplasmas in Medium B and for PCR for M. bovis [14]. Screening for P. multocida, M. haemolytica and Haemophilus somnus was carried out using conventional bacteriological techniques [15]. At post mortem nasal cavities, trachea, lungs, lung washes, peribronchial lymph nodes, livers, spleens, kidneys, carpal and tarsal joints were cultured for mycoplasmas. Bacteriological examination was performed on lungs and lung fluids as above.
2.6. Serology
Before vaccination, at challenge and at 7, 14 and 21 days after challenge, sera from each calf were tested for IgG antibodies to M. bovis using an ELISA (Hoechst Roussel Veterinary Diagnostics, Liebefeld-Bern, Switzerland). Sera taken from all calves at the beginning and end of the experiment were also tested for antibodies to bovine viral diarrhoea, infectious bovine rhinotracheitis virus, bovine respiratory virus, adenovirus types I and II, coronavirus and parainfluenza virus type 3.
2.7. Clinical assessment
Rectal temperature of all calves was measured for periods before and after vaccination and sites of vaccination were examined. Calves were also scored for general clinical appearance, respiratory signs, nasal discharge, severity and duration of cough, and for arthritic lesions. Calves were weighed and average body weight gain calculated. Details of the clinical assessment are given in Table 1 .
Table 1.
Quantitative clinical and pathological assessment
| Score |
||||
| 0 | 1 | 2 | 3 | |
| General clinical condition | Normal | Subdued | Depressed | Unresponsive |
| Respiratory signs | Normal | Hyperpnoea | Dyspnoea | Distressed |
| Nasal discharge | Absent | Mild | Purulent | |
| Cough severity | Absent | Mild | Severe/frequent | |
| Arthritis | Absent | Enlarged | Significant exudates/lame | |
| Pleurisy | Absent | Mild | Moderate | Severe |
| Histological lung lesions | Normal | Focal lesions | Extended | Diffuse |
2.8. Pathological examination
The severity of the gross pathological lesions of the inner organs was scored. The percentage of the lung with pneumonic lesions was also estimated. Details of the pathological assessment are given in Table 1. In addition lung lesions were scored macroscopically as follows: 0=no lesions, 1–10 for degree of consolidation in left and right apical, in left and right cardiac and in accessory lobes, 1–5 scores in cranial lobes, left and right diaphragmatic lobes and 1–5 scores in intermediate lobe.
Lesions were also assessed histologically for interstitial pneumonia, lympho-histiocytic bronchitis, cattarrhal pneumonia.
3. Results
3.1. Microbiology
No M. bovis was detected in the nasal cavities of calves at any time before challenge. At post mortem, with the exception of the nasal cavity, M. bovis was isolated from the organs of significantly fewer vaccinated calves than non-vaccinated calves (Table 2 ). M. bovis was also isolated from the joints of two lame non-vaccinated calves.
Table 2.
Isolation of M. bovis from the organs of vaccinated and non vaccinated challenged calves
| Isolation site | Group A (n = 7), vaccinated/challenge | Group B (n =7), challenge only | Group C (n = 8), control |
| Nasal cavity | 7 | 7 | 0 |
| Trachea | 3* | 7 | 0 |
| Bronchial washing | 2** | 7 | 0 |
| Lung | 2** | 7 | 0 |
| Peribronchial lymph node | 2* | 6 | 0 |
| Spleen | 0* | 3 | 0 |
| Liver | 0 | 1 | 0 |
| Kidney | 0 | 0 | 0 |
| Joint | 0*** | 2*** | 0*** |
P<0.05 by χ2 analysis.
P<0.01 by χ2 analysis.
Four joints sampled from each calf.
There was no evidence of infection with M. haemolytica or H. somnus in the lungs of calves throughout the experiment. P. multocida was isolated from several calves in groups A–C at the beginning of the experiment and from most calves at the end. Corynebacterium species were also found sporadically at the beginning and end (data not shown). Neither of these two species were found in the lungs of any calves.
3.2. Serology
The results of ELISA for M. bovis antibodies are shown in Fig. 1 . No antibodies were detected in the non vaccinated controls (group C) throughout the experiment. In the two vaccinated groups A and D antibodies were detected after 2 weeks. Mean antibody levels, as detected by ELISA, were still high (155% of positive control) 6 months later in group D which remained on the farm; there was no clinical evidence of natural respiratory disease in vaccinated or non-vaccinated calves on this farm. Group B seroconverted rapidly after challenge and reached the same level as group A by the end of the experiment.
Fig. 1.
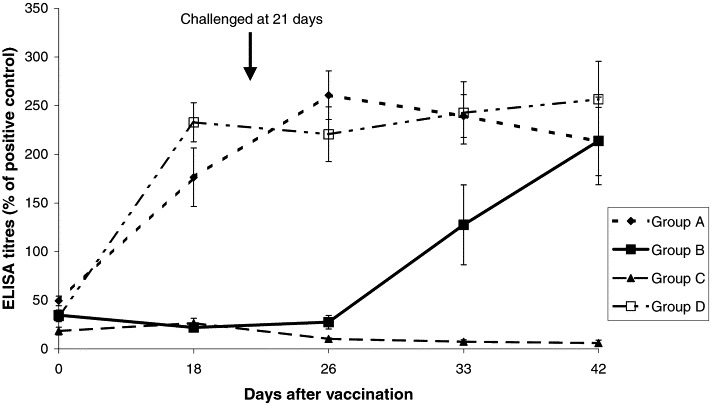
Serological response of vaccinated and challenged (A), challenged only (B), control (C) and vaccinated groups of calves left on the farm (D) as detected by M. bovis ELISA.
Most of the calves were serologically positive for infectious bovine rhinotracheitis and parainfluenza virus 3 but this did not increase in titre during the experiment. There was no evidence of the presence other viruses.
3.3. Clinical assessment
No adverse signs of the vaccine were seen at the site of inoculation. Palpation revealed swelling of tissue in three calves in the two vaccinated groups which disappeared after 4 days. Clinical signs of pneumonia were seen in group B, 5–6 days after challenge followed shortly by coughing and nasal discharge; a few calves in group A showed mild clinical signs. There was statistically differences in all subjective scoring between the vaccinated (A) and non vaccinated (B) groups (Table 3 ). No clinical disease was seen in the control group (C) at any time.
Table 3.
Clinical assessment of calves: total scores for each group
| Group | Respiratory signs | Nasal discharge | Cough | Feed refusal |
| A (vaccinated/challenge) | 17* | 33* | 15* | 16* |
| B (challenge only) | 111 | 90 | 67 | 49 |
| C (control) | 6 | 3 | 1 | 3 |
P<0.001 between groups A and B using χ2 analysis.
Two calves in group B developed clinical arthritis after 2 weeks showing an accumulation of fluid in the tarsal or carpal joints accompanied by pain and increase in joint temperature. No other calves in any group became affected.
Feed refusal was noticed in groups A and B but was statistically higher in group B than in A (Table 3).
Statistically higher rectal temperatures were seen in group B than in A, 5 days after challenge and this continued from 12 to 20 days (data not shown).
The average body weights of groups A, B and C did not differ from each other significantly before or for the 2 weeks following challenge. However, after 3 weeks a significantly lower weight gain (P<0.05) was recorded in group B compared with groups A and C (Fig. 2 ).
Fig. 2.
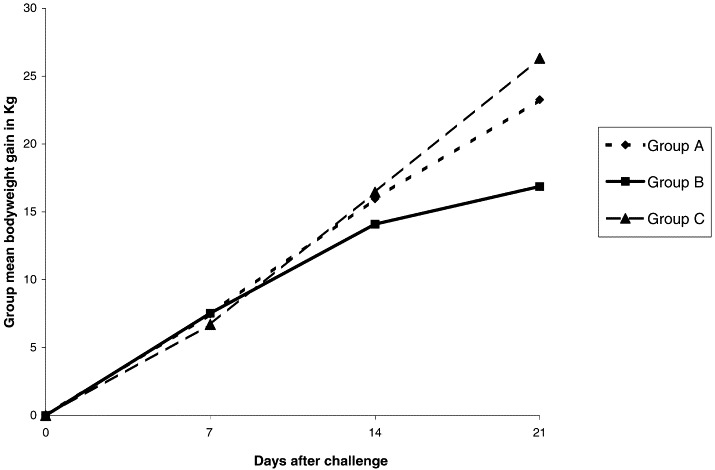
Gain in mean bodyweight (kgs) for vaccinated and challenged (A), challenged only (B) and control groups of calves (C).
3.4. Pathological examination
Lung damage, as assessed by the percentage of the lung affected, was significantly higher in the challenged only group (B) than in the vaccinated group (A) (Table 4 ); there was a smaller significant difference between group A and the controls (C). Further, lung lesion scores were significantly higher in group B (125) than in either group A (68) (P<0.001) and group C (52) (P<0.001); there was a smaller significant difference between groups A and C (P<0.05).
Table 4.
Macroscopic examination of lungs of calves: combined percentage of lobes showing pneumonic lesions
| Lung | A (vaccinate/challenge) | B (challenge only) | C (controls) |
| Apical | 17.3 | 52.3 | 8.7 |
| Caudal | 13.4 | 35.8 | 3.7 |
| Cranial | 5.9 | 28.1 | 0.5 |
| Accessory | 1.3 | 20.8 | 2.5 |
| Inter | 0 | 3.1 | 0 |
| Total | 37a | 140.1a, b | 15.4b |
P<0.01 between groups A and B.
P<0.5 between groups A and C.
Histological lesion scores of interstitial pneumonia (thickening of septa by lymphoid cells and macrophages), lympho-histiocytic bronchitis, catarrhal bronchopneumonia accompanied by accumulation of intra-alveolar and intrabronchila exudate consisting of neutrophils, macrophages and lymphocytes and coagulative necrosis and pleurisy as well as total histological score was significantly lower in vaccinated challenged group (A) than those in non-vaccinated challenge group (B) (Table 5 ). There was no significant difference between groups A and C apart from the lympho-histiocytic bronchitis score.
Table 5.
Histological examination of lungs of calves: total scores for each group
| Lesions | A (vaccinate/challenge) | B (challenge only) | C (controls) |
| Interstitial pneumonia | 7 | 19 | 8 |
| Lympho-histiocytic bronchitis | 13 | 22 | 1 |
| Catarrhal broncho-pneumonia | 4 | 10 | 0 |
| Others (e.g. atelectasia) | 5 | 7 | 2 |
| Total | 29a, b | 58a | 11b |
P<0.001 between groups A and B.
P not significant between groups A and C.
4. Discussion
Vaccines exist for most of the major respiratory pathogens of cattle: infectious bovine rhinotracheitis, bovine viral diarrhoea and respiratory syncytial virus and usually provide satisfactory levels of protection. A lack of a commercial vaccine for M. bovis probably reflects the inconclusive results of previous experiments including those in which an exacerbation of disease was seen following vaccination [7], [8]. Furthermore, the need for specialist and expensive facilities for diagnosing mycoplasma infection has led to an under reporting of mycoplasma diseases giving rise to the still pervasive view amongst many veterinarians that the role of M. bovis in the respiratory disease complex is not proven [16]. The importance of M. bovis has been shown conclusively following its introduction into both Northern and the Republic of Ireland in the early 1990s where it rapidly became a major cause of respiratory disease as well as frequent cause of mastitis and arthritis [17], [18]. In Britain and France M. bovis is believed to be responsible for 25–33% of calf pneumonias [19], [20] while in the USA some authors believe it may be involved in up to 50% of chronic calf pneumonias. In Denmark an increase in cases has also been reported [21]. Finally the ease with which M. bovis can cause pneumonia in calves experimentally should demonstrate its importance in the field ([22], present work).
The results from the work reported here indicate that even a single dose of vaccine prepared from saponised M. bovis cells may provide effective control against mycoplasma-induced calf pneumonia and even against geographically diverse strains as in the present case. In addition the vaccine appeared safe and highly immunogenic. Calves tested 6 months after immunisation had high levels of humoral immunity. Evidence was also provided that the vaccine may also protect against arthritis though further work will be needed to confirm this. It would be expected that an M. bovis vaccine would also reduce infection by other ubiquitous bacteria such as M. haemolytica, P multocida and H. somnus where M. bovis was the primary underlying cause impairing the host defence system [23].
The successful use of saponin in vaccines has already been demonstrated for other mycoplasma infections such as CCPP and contagious agalactia. Its effectiveness must be associated with the fact that it apparently preserves the major antigens seen in untreated whole cells [6]. Kensil et al. [9] speculated that the high level of protection seen with the use of saponins with vaccines in mice may be caused by the ability of saponins to induce an isotype profile similar to that seen in natural immunity to bacterial infections. It is not known what the effect of saponin alone would have been on the calves challenged in the present experiment although a brief stimulation of the immune system, in particular the Th1 response, may have been expected (B. Morein, personal communication); however, it is highly unlikely to have led to the levels of protection seen with the saponised vaccine.
Saponin has also been reported to inactivate mycoplasmas rapidly [22] suggesting that further improvements could be made to the vaccine preparation described here. However, it is clear that a balance must be struck between reducing the concentration to prevent adverse reactions, although only mild reactions were seen here, and retaining its effectiveness as an adjuvant.
The reported resistance of M. bovis isolates to commonly used antibiotics over the last 10 years [3] strongly suggests that vaccines may provide a more effective approach to the control of this increasingly important disease. The vaccine should be administered immediately after maternal antibody has waned at about 2–4 weeks of age as this may interfere with vaccination; these antibody levels could be monitored conveniently by ELISA.
Acknowledgements
We acknowledge the financial assistance and support of the Department of Environment, Food and Rural Affairs.
References
- 1.Pfutzner H., Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders. Scientific and Technical Review. Offices International Des Epizooties. 1996;15(4):1477–1494. doi: 10.20506/rst.15.4.987. [DOI] [PubMed] [Google Scholar]
- 2.Rosengarten R, Citti C. The role of ruminant mycoplasmas in systemic infection. In: Stipkovits L, Rosengarten R, Frey J, editors. Mycoplasmas of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics, vol. 3. Brussels: European Commission; 1999. p. 14–17.
- 3.Ayling R.D., Baker S.E., Peek M.L., Simon A.J., Nicholas R.A.J. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet Rec. 2000;146:745–747. doi: 10.1136/vr.146.26.745. [DOI] [PubMed] [Google Scholar]
- 4.Howard C.J., Stott E.J., Thomas L.H., Gourlay R.N., Taylor G. Protection against respiratory disease in calves induced by vaccines containing respiratory syncytial virus, parinfluenza type 3 virus, Mycoplasma bovis and M. dispar. Vet Rec. 1987;121:372–376. doi: 10.1136/vr.121.16.372. [DOI] [PubMed] [Google Scholar]
- 5.Urbaneck D., Leibig F., Forbrig T., Stache B. Erfahrungsbericht zur Anwedung bestandsspezifischer impfstoffe gegen respiratorische infektionen mit beteiligung von Mykoplasma bovis in einem mastrindergrossbestand [Experiences with herd specific vaccines against respiratory infections with M. bovis in a large feedlot] Der praktische Tierarzt. 2000;81:756–763. [Google Scholar]
- 6.Tola S., Manunta D., Rocca S., Rocchiagiani A.M., Idini G., Angioi P.P. Experimental vaccination against Mycoplasma agalactiae using different inactivated vaccines. Vaccine. 1999;17:2764–2768. doi: 10.1016/s0264-410x(99)00070-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbusch R. Test of an inactivated vaccine against Mycoplasma bovis respiratory disease by transthoracic challenge with an abcessing strain. Abstracts of the 12th International Organisation of Mycoplasmology Conference, Sydney, Australia, 22–28 July 1998, p. 185.
- 8.Bryson D, Ball HJ, Brice N, Pollock DS, Forster F. Pathology of induced Mycoplasma bovis pneumonia in experimentally vaccinated calves. In: Stipkovits L, Rosengarten R, Frey J, editors. Mycoplasmas of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics, vol. 3. Brussels: European Commission; 1998. p. 128–32.
- 9.Kensil C.R., Patel U., Lennick M., Marciani D. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 1991;146:431–437. [PubMed] [Google Scholar]
- 10.Razin S., Argaman M. Lysis of mycoplasma, bacterial protoplasts, spheroplasts and L-forms by various agents. J. Gen. Microbiol. 1963;30:155–172. doi: 10.1099/00221287-30-1-155. [DOI] [PubMed] [Google Scholar]
- 11.Rurangirwa F.R., McGuire T.C., Kibor A., Chema S. An inactivated vaccine for contagious caprine pleuropneumonia. Vet Rec. 1987;121:397–402. doi: 10.1136/vr.121.17.397. [DOI] [PubMed] [Google Scholar]
- 12.Nicholas RAJ, Baker SE. Recovery of mycoplasmas from animals. In: Miles RJ, Nicholas RAJ, editors. Mycoplasma Protocols, vol. 3. Totowa, USA: Humana Press; 1998. p. 37–44.
- 13.Erno H., Stipkovits L. Bovine mycoplasmas: cultural and biochemical studies. Acta Vet Scand. 1973;14:436–449. doi: 10.1186/BF03547431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey J, Subramaniam S, Bergonier D, Nicolet J. DNA repair genes uvrC as genetic targets for discrimination of closely related mycoplasmas. In: Stipkovits L, Rosengarten R, Frey J, editors. Mycoplasmas of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics, vol. 3. Brussels: European Commission; 1999. p. 40–43.
- 15.Barrow GI, Feltham RKA. Cowan and Steel’s Manual for the Identification of Medical Bacteria. 3rd ed. 1993. p. 250–56.
- 16.Step D.L., Kirkpatrick J.G. Mycoplasma infection in cattle. Pneumonia–arthritis syndrome. The Bovine Practitioner. 2001;35:149–155. [Google Scholar]
- 17.Brice N., Finlay D., Bryson D.G., Henderson J., McConnell W., Ball H.J. Isolation of Mycoplasma bovis from cattle in Northern Ireland 1993–1998. Vet Rec. 2000;146:643–644. doi: 10.1136/vr.146.22.643. [DOI] [PubMed] [Google Scholar]
- 18.Byrne W.J., McCormack R., Brice N., Egan J., Markey B., Ball H.J. Isolation of Mycoplasma bovis from bovine clinical samples in the Republic of Ireland. Vet Rec. 2001;148:331–333. doi: 10.1136/vr.148.11.331. [DOI] [PubMed] [Google Scholar]
- 19.Nicholas R.A.J., Baker S., Ayling R.D., Stipkovits L. Mycoplasma infections in growing cattle. Cattle Practice. 2000;8:115–118. [Google Scholar]
- 20.Grand D le, Phillippe S, Calavalas D, Bezille P, Poumarat F. Prevalence of Mycoplasma bovis infection in France. In: Poveda JB, Fernandez A, Frey J, Johansson K-E, editors. Mycoplasmas of Ruminants: Pathogenicity, Diagnostics, Epidemiology and Molecular Genetics, vol. 5. Brussels: European Commission; 2001. p. 106–9.
- 21.Kusiluka L.J.M., Ojeniyi B., Friis N.F. Increasing prevalence of Mycoplasma bovis in Danish cattle. Acta Vet Scand. 2000;41:139–146. doi: 10.1186/BF03549645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stipkovits L., Ripley P.H., Varga J., Palfi V. Use of valnemulin in the control of Mycoplasma bovis infection under field conditions. Vet Rec. 2001;148:399–402. doi: 10.1136/vr.148.13.399. [DOI] [PubMed] [Google Scholar]
- 23.Rebhun WC. Respiratory diseases. In: Diseases of cattle. Baltimore: Williams and Wilkins; 1995. p. 79–80.


