Abstract
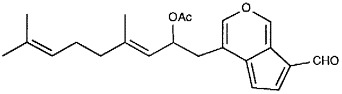
Halituna(1), a novel diterpene aldehyde possessing a unique cyclopentadieno[c]pyran ring system, has been isolated from the marine alga Halimeda tuna. The structure of 1 was elucidated by spectroscopic techniques. Halitunal shows antiviral activity against murine coronavirus A59 in-vitro.
Halitunal, a novel diterpene aldehyde, has been isolated from the marine alga Halimeda tuna, and its structure elucidated by spectroscopic methods. Halitunal bears a unique cyclopentadieno[c]pyran ring system and shows activity against mouse coronavirus.
References
- 1.Fenical W., Paul V.J. Science. 1983;221:747. doi: 10.1126/science.221.4612.747. [DOI] [PubMed] [Google Scholar]
- 2.Paul V.J., Fenical W. Tetrahedron. 1984;40(16):3053. [Google Scholar]
- 3.Tillekeratne L.M.V., Schmitz F.J. Phytochemistry. 1984;23(6):1331. [Google Scholar]
- 4.Paul V.J., Van Alstyne K.L. J. Exp. Mar. Biol. Ecol. 1988;119:15. [Google Scholar]
- 5.Murine coronavirus strain A59 inhibitions were measured by reduction in cell fusion and cytopathic effects in NCTC 1469 mouse liver cells. Readings in these assays range from 0 for no observable inhibition of virus to 3+ for complete viral inhibition by the test compound. Halitunal(1) showed 2+ (ca. 50%) inhibition of viral replication at a dose of 20 μg per test well.
- 6.Halitunal(1): HREIMS m/z meas. 354.18228, 19.7%, calc. for C20H26O4, Δ 0.8 mmu, UV (EtOH): λmax=426, 310, 287, 240, 227 nm; ϵ = 4640, 3920, 6180, 7860, 8500; IR (microKBr) 1720, 1695, 1625, 1455, 1385, 1230, 1015 cm−1; 1H NMR (360 MHz, C6D6): δ 9.97 (1H, s, H16), 9.05 (s, 1H, H1), 7.41 (1H, d, J= 3.3 Hz, H4)), 6.89 (1H, s, H17), 6.68 (1H, dd, J= 3.3, 0.8 Hz, H5), 6.03 (1H, ddd, J= 9.1, 7.7, 5.6 Hz), 5.12 (1H, dq, J= 9.1, 1.2 Hz, H10), 5.02 (1H, m, H14), 2.88 (1H, ddd, 13.7, 5.6, 0.6 Hz, H8), 2.43 (1H, dd, J= 13.7, 7.7 Hz, H8), 1.87 (2H, m, H13), 1.83 (2H, m, H12), 1.64 (3H, s, H22), 1.63 (3H, d, J= 0.9 Hz, H20), 1.46 (3H, s, H19), 1.33 (3H, s, H18); 13C NMR (90 MHz, C6D6): δ 184.2 (s, C16), 169.5 (s, C21), 150.4 (d, C1), 145.0 (d, C4), 141.8 (s, C11), 141.4 (d, C17), 131.8 (s, C15), 135.3 (s, C6), 125.4 (s, C2), 124.0 (d, C14), 122.9 (s, C3), 122.9 (d, C10), 120.2 (s, C7), 109.4 (d, C5), 69.6 (d, C9), 39.7 (t, C12), 34.7 (t, C8), 26.5 (t, C13), 25.7 (d, C20), 20.7 (q, C22), 17.6 (q, C19), 16.6 (C18).
- 7.Bendall M.R., Pegg D.T., Doddrell D.M., Williams D.H. J. Org. Chem. 1982;47:3021. [Google Scholar]
- 8.Marion D., Wüthrich K. Biochem. Biophys. Res. Comm. 1983;113:967. doi: 10.1016/0006-291x(83)91093-8. [DOI] [PubMed] [Google Scholar]
- 9.Bax A., Freeman R. J. Magn. Res. 1981;44:542. [Google Scholar]
- 10.Bax A., Subramanian S. J. Magn. Res. 1986;67:565. [Google Scholar]
- 11.Bax A., Summers M.F. J. Am. Chem. Soc. 1986;108:2093. [Google Scholar]
- 12.Breitmaier E., Voelter W. VCH; New York: 1987. p. 141. (Carbon-13 NMR Spectroscopy). [Google Scholar]
- 13.Bax A., Marion D. J. Magn. Res. 1988;78:186. While apparent JCH values in HMBC experiments are frequently inaccurate due to homonuclear coupling, nonabsorptive lineshapes and associated magnitude processing, in cases where there is no homonuclear coupling, large long range JCH values (20 Hz) can be reliably identified. For a further discussion see. [Google Scholar]


