Abstract
Persistence was established after most of the SARS-CoV-infected Vero E6 cells died. RNA of the defective interfering virus was not observed in the persistently infected cells by Northern blot analysis. SARS-CoV diluted to 2 PFU failed to establish persistence, suggesting that some particular viruses in the seed virus did not induce persistent infection. Interestingly, a viral receptor, angiotensin converting enzyme (ACE)-2, was down-regulated in persistently infected cells. G418-selected clones established from parent Vero E6 cells, which were transfected with a plasmid containing the neomycin resistance gene, were infected with SARS-CoV, resulting in a potential cell population capable of persistence in Vero E6 cells. Our previous studies demonstrated that signaling pathways of extracellular signal-related kinase (ERK1/2), c-Jun N-terminal protein kinase (JNK), p38 mitogen-activated protein kinase (MAPK), and phosphatidylinositol 3′-kinase (PI3K)/Akt were activated in SARS-CoV-infected Vero E6 cells. Previous studies also showed that the activation of p38 MAPK by viral infection-induced apoptosis, and a weak activation of Akt was not sufficient to protect from apoptosis. In the present study, we showed that the inhibitors of JNK and PI3K/Akt inhibited the establishment of persistence, but those of MAPK/ERK kinase (MEK; as an inhibitor for ERK1/2) and p38 MAPK did not. These results indicated that two signaling pathways of JNK and PI3K/Akt were important for the establishment of persistence in Vero E6 cells.
Keywords: SARS-CoV, Persistent infection, c-Jun N-terminal protein kinase, Phosphatidylinositol 3′-kinase/Akt
Severe acute respiratory syndrome (SARS) is a newly found infectious disease caused by a novel coronavirus, SARS coronavirus (SARS-CoV) [11], [17]. SARS spread from Guangdong Province in China to more than 30 countries in late 2002, causing severe outbreaks of atypical pneumonia. SARS-CoV is an enveloped, single-stranded positive-sense RNA virus with an RNA genome of approximately 30,000 nucleotides encoding at least 15 open reading frames [11]. As the viral virulence and mortality rate of the patients are very high, understanding the mechanisms of the pathogenicity of SARS-CoV is important for the prevention of SARS.
The gene organization of SARS-CoV is similar to those of other coronaviruses. Coronaviruses exhibit a unique ability to establish persistent infections in vivo and in vitro. Mouse hepatitis virus (MHV), a prototype coronavirus, causes central nervous system diseases in rodents. Astrocytes are the predominant cells that harbor persistent viruses in the central nervous system (CNS) [16]. A previous in vitro study also indicated that the astrocytoma cell line DBT has a potential to establish persistent infection after MHV infection [10]. However, the mechanism of establishment of coronavirus persistence has not been yet well understood. Recently, the down-regulation of the pro-apoptotic protein BNip-3 was found in MHV-infected DBT cells by DNA microarray methodologies [2]. In MHV-infected cells, BNip3 levels were significantly decreased at the transcriptional level, and this down-regulation was mediated by the fusion between the viral envelope and the cell membrane. This observation suggested that the anti-apoptotic effect by the down-regulation of BNip3 in MHV-infected DBT cells allows persistent infection.
Recently, a human intestinal cell line, LoVo cells, was shown to permit SARS-CoV infection, resulting in the establishment of persistent infection [4]. However, the mechanism of persistence in LoVo cell is still unclear. The monkey kidney cell line, Vero E6, is widely used in the SARS-CoV research because of a high sensitivity to the virus infection. Vero E6 cells express a viral receptor, angiotensin converting enzyme (ACE)-2 [9], at high levels, and SARS-CoV infection of Vero E6 causes cytopathic effects after 24 h [13]. We showed that SARS-CoV infection of Vero E6 cells induced apoptosis via caspase-3 [13]. Several signaling pathways are activated in SARS-CoV-infected Vero E6 cells. One of the mitogen-activated protein kinases (MAPKs), p38, was shown to be phosphorylated and to have pro-apoptotic roles, including tyrosine dephosphorylation of signal transducer and activator of transcription (STAT) 3 [13], [15]. Although Akt, which is known to act as an anti-apoptosis factor, was also phosphorylated in virus-infected cells, the activity of Akt is not enough to prevent apoptosis in the SARS-CoV-infected cells [14].
In the present study, Vero E6 cells were subcultured routinely in 75-cm3 flasks in Dulbecco's modified Eagle's medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 0.2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% (v/v) fetal bovine serum (FBS) and maintained at 37 °C in an atmosphere of 5% CO2. For use in the experiments, the cells were split once they reached 90% confluence and were seeded onto 24-well tissue culture plate inserts or 25-cm3 (T-25) flasks. The 100% confluent cells were used in the present and previous studies [13], [14], [15]. The culture medium was changed to 2% FBS containing DMEM prior to virus infection. We used a SARS-CoV isolate, Frankfurt 1, kindly provided by Dr. J. Ziebuhr.
Our recent studies indicated that SARS-CoV induces apoptosis in Vero E6 cells after 24-h post infection (h.p.i.) [13]. Although almost all of the virus-infected cells showed morphological changes indicative of cell death until 48 h.p.i., very few surviving cells were observed, and these cells grew and formed colonies on 4 days p.i. (d.p.i.). To investigate whether SARS-CoV replicates in these surviving cells, indirect immunofluorescence (IF) staining was performed to detect intracellular viral antigens. As shown in Fig. 1 , all the cells showed viral antigens in the cytoplasm at 10 d.p.i. The persistently infected cell culture at 7 passages produced infectious viral particles at 1.26 × 105 PFU/ml. These results indicated that the persistently infected Vero E6 cells produce infectious viruses.
Fig. 1.
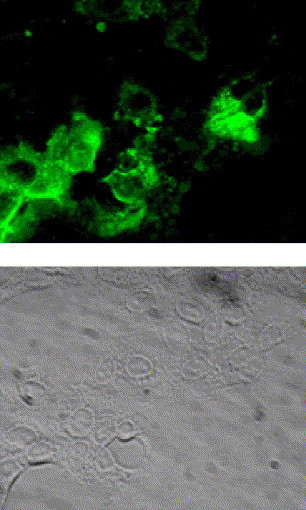
Indirect immunofluorescence staining of SARS-CoV proteins. For the establishment of persistent infection, the virus was infected to Vero E6 cells at 10 m.o.i. At 10 days p.i., intracellular viral antigens were detected by indirect immunofluorescence staining using rabbit anti-SARS-CoV antibody, which recognizes SARS-CoV proteins (unpublished data). Briefly, cells were spotted onto a 12-well Teflon-coated slideglass, air dried in a biosafety cabinet under UV irradiation, and fixed with 100% pre-chilled acetone. Aliquots of 25 μl of 1:160 diluted anti-SARS-CoV antibody were placed on the coated wells and incubated at 37 °C for 30 min in a moist chamber. After washing with PBS, a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG antibody was added at a dilution of 1:40, and incubated for 30 min at 37 °C.
Persistent infection of many RNA viruses in cell culture has been studied. In these studies, it has been demonstrated that the establishment of RNA virus persistent infection in a cell culture is often involved in the generation of defective interfering (DI) particles and reduction of interferons. In the case of measles virus, persistent viral infected Vero cells produced DI particles [3]. Non-cytopathic (ncp) bovine viral diarrhea virus (BVDV) does not induce type I interferon (IFN), whereas cytopathic (cp) BVDV is able to induce type I IFN [1], [6], suggesting that the different capabilities of cp and ncpBVDV to establish persistent infections relates to the difference in their ability to induce IFN [1]. Because Vero E6 cells are known to lack type I interferon genes [5], the involvement of interferon was unlikely in the establishment of SARS-CoV persistence in Vero E6 cells. To investigate whether DI RNAs exist in the persistent cells, Northern blot analysis was performed using a probe complementary to mRNA9 for detecting virus standard genome and all (m)RNAs of SARS-CoV. As shown in Fig. 2 , we could not detect any signals of additional viral mRNA in the viral persistent cells at 11 days p.i. This result suggested that the DI virus was not involved in the establishment of persistence in the case of SARS-CoV. However, we cannot rule out a possibility that a small amount of DI RNAs appeared in the persistent cells. We measured the densities of mRNA8, 9 and 4, 5 using the LAS-3000 mini system (Fuji Photo Film Co. Ltd, Tokyo, Japan). The amount of mRNA8 and 9 of the persistent cells are 81.4% of the acute infected cells. On the other hand, mRNA4 and 5 of the persistent cells were 12.29% of acute infected cells. In addition, a new round of infection did not induce cell death into persistent infected cells (data not shown). Therefore, we investigated whether persistent cells express a functional SARS-CoV receptor, ACE-2. On Western blotting analysis, ACE-2 was not detected in the persistent cell line (Fig. 3 ). Interestingly, ACE-2 was also decreased in the acute infection of SARS-CoV. This result suggested that virus particles produced by persistently infected cells could not infect other cells due to a lack of the receptor.
Fig. 2.
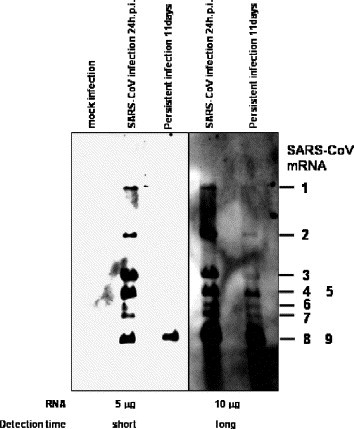
Northern blot analysis of viral mRNAs. Vero E6 cells were inoculated with SARS-CoV at 10 m.o.i. Total RNA was extracted with Isogen (Nippon Gene, Tokyo, Japan) from SARS-CoV-infected cells at 24 h and 11 days p.i. and from mock-infected cells. RNAs were electrophoresed on 1% agarose gels in the presence of formaldehyde, blotted onto the nitrocellulose membrane. The probe was constructed based on the PCR product of mRNA9. Briefly, viral RNA was reverse-transcribed using SuperScriptIII (Invitrogen, Carlsbad, CA, USA) and the reverse primer, 5′-AGTCAGTCTAATACGACTCACTATAGGGTCCTAAGAAGCTATTAAAATTAGC-3′(29,687 to 29,721 nt of GenBank accession numberNC_004718). The underline indicates the promoter sequence of T7 RNA polymerase. PCR amplification was performed using High Fidelity Platinum Taq DNA polymerase (Invitrogen) using the reverse primer and forward primer, 5′-TGGCTAGCGGAGGTGGTGAAACTGCCC-3′ (28,751 to 28,777 nt). The RNA probe was transcribed from the PCR product using T7 RNA polymerase (Ambion, Austin, TX, USA) under incorporating digoxigenin (DIG RNA labeling kit, Boehringer Mannheim GmbH, Mannheim, Germany). Hybridization and wash were performed as described previously [12]. Left and right panels indicated short and long detection times using the LAS-3000 mini system (Fuji Photo Film Co. Ltd, Tokyo, Japan), respectively.
Fig. 3.
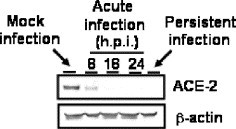
Down-regulation of ACE-2 by SARS-CoV infection. Western blot of acute infection (8, 18, and 24 h.p.i.) and persistent infection (passage 7) were performed using anti-ACE-2 antibody. Whole-cell extracts were electrophoresed in 10–20% gradient polyacrylamide gel and transferred onto PVDF membranes (Immobilon-P, Millipore, Bedford, MA, USA). We applied two sets of samples to the polyacrylamide gels, and the blots were divided into two sheets for detection by a ProtoBlot II AP system (Promega Co., Madison, WI, USA), as described previously [13], [14], [15]. Mouse anti-human ACE-2 ectodomain monoclonal antibody was purchased from R and D systems Inc (Minneapolis, MN, USA) and used at a dilution of 1:1000. Mouse anti-beta actin antibody was purchased from Sigma (St. Louis, MO, USA) and used at a dilution of 1:5000.
We were interested in determining whether the virus or the host cell is responsible for the establishment of persistent SARS-CoV infection. As shown above, small numbers of Vero E6 cells survived and then continued to grow after SARS-CoV infection. These cells were infected and produced cytopathic viruses (data not shown). The majority of the virus in a seed could induce the apoptotic cell death of Vero E6 cells [13]. If some species of the seed SARS-CoV are involved in an establishment of persistence, seed virus with a limited dilution should not permit persistent infection. The seed SARS-CoV in the present study contained 2 × 108 plaque forming unit (PFU)/ml on Vero E6 cells. The seed virus was diluted from 2 × 102 to 2 × 10−1 PFU, and these virus suspensions were used to inoculate aliquots of 1.5 × 105 cells in T-25 flasks. As shown in Fig. 4 , no cell death was observed in cells infected with virus at a dilution of 2 × 10−1 PFU, indicating that viral dilution had been performed correctly. We found that 2 × 100 (=2) PFU-infected cells had fewer plaques than did the 2 × 102 (=200) PFU-infected cells at 36 h.p.i. However, all flasks other than those infected at 2 × 10−1 PFU showed death of almost all cells at 4 d.p.i. and surviving colonies at 7 d.p.i. These colonies became larger at 13 d.p.i. From these results, it seems most likely that some particular viruses in the seed virus did not induce persistent infection.
Fig. 4.
Establishment of persistent SARS-CoV-infected cells by diluted viruses. Aliquots of 1.5 × 105 Vero E6 cells were inoculated with 2 × 10−1, 2 × 100, 2 × 101, and 2 × 102 PFU of SARS-CoV. The cells were stained with 0.05% crystal violet after fixing with 20% formaldehyde.
If a certain Vero E6 cell has the ability to establish persistence after viral infection, such a cell should survive without cell death following infection. To address this possibility, clones from parent Vero E6 cells were established by geneticin-selection (500 μg/ml) after the transfection of pcDNA3 (Invitrogen). Although a total of 115 clones was obtained, 24 clones showed morphological changes or grew very slowly. Therefore, 91 clones that showed similar growth rates in 24-well plates were infected with SARS-CoV at 10 m.o.i. The parental Vero E6 cells were used as a control. As shown in Fig. 5 , 16 of the 91 clones established persistent infection at 20 d.p.i. Especially, the growth rate of persistently infected clone number 65 was higher than those of the other 15 persistently infected clones. Thus, growth rate was different among the persistently infected clones. This may be due to a difference in a number of survived cells among these 16 cell clones. Interestingly, 75 of the 91 clones failed to establish persistent infection. These results suggested that parental Vero E6 cells always produce a minor population of cells with a potential to allow persistence.
Fig. 5.
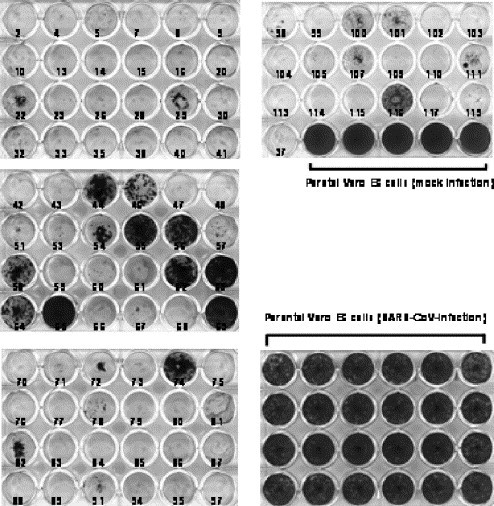
Persistent SARS-CoV infection of cloned Vero E6 cells. A plasmid, pcDNA3, was transfected into Vero E6 cells using FuGene6 (Roche Diagnostics, Penzberg, Germany), and cloned cells were picked after neomycin selection (500 μg/ml). The 91 cloned cells were inoculated with SARS-CoV at 10 m.o.i. At 20 days p.i., the 24-well plates were fixed and stained.
Recently, we reported that extracellular signal-related kinase (ERK1/2), c-Jun N-terminal protein kinase (JNK), p38 MAPK, and Akt are phosphorylated in SARS-CoV-infected Vero E6 cells and that viral replication was not affected by the activation of signaling pathways [13], [14], [15]. To clarify the signaling pathway important for establishing persistent infection, Vero E6 cells were pre-treated with several concentrations of inhibitors of MAPKs and PI3K/Akt, and then infected with 2 m.o.i. of SARS-CoV. As shown in Fig. 6 , SB203580 as an inhibitor of p38 MAPK and PD98059 as an inhibitor of MEK1/2 did not affect the establishment of persistent infection, whereas no surviving cells were observed following treatment with SP600125, an inhibitor of JNK, or LY294002, an inhibitor of PI3K/Akt. This result suggested that JNK and PI3K/Akt are necessary to establish persistence. Our previous study and Fig. 7A indicated that virus-induced Akt phosphorylation was not strong, and it was significantly down-regulated at 24 h.p.i.[14] Therefore, we concluded that the weak Akt activation was not sufficient to protect the viral infected Vero E6 cells from apoptosis. To confirm that the phosphorylation of Akt is necessary to establish persistence, SARS-CoV was infected to clone cell lines of Vero E6, which were showed in Fig. 5. At 20 h.p.i, phosphorylated Akt was not detected in viral infected clone cell lines 43, 48, and 80, which could not establish persistence in Fig. 5, while phosphorylated Akt was detected in viral infected parental Vero E6, clone cell lines 55, 58, and 65 (Fig. 7B). This result suggested that, at least, the phosphorylation of Akt in viral infected cells were necessary to establish persistence. However, Akt was not phosphorylated in persistent cell lines (Fig. 7A). The phosphorylation of Akt may not be necessary to maintain persistence after establishment.
Fig. 6.
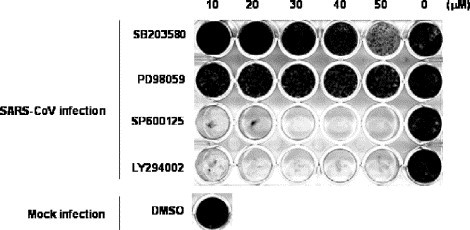
Effects of signaling pathways on establishing the persistence of Vero E6 cells. SB203580 as a p38 MAPK inhibitor, PD098059 as a MEK inhibitor, and SP600125 as a JNK inhibitor were purchased from Calbiochem (San Diego, CA, USA). LY294002 as a PI3K inhibitor was purchased from Cell Signaling Technology Inc. (Beverly, MA, USA). All reagents were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 or 20 mM. All test wells, including mock-treated controls, were treated with DMSO. Vero E6 cells were pre-treated with SB203580, PD98059, SP600125, and LY294002 at concentrations from 10 to 50 μM for 1 h, and then inoculated with SARS-CoV at 2 m.o.i. The plates were fixed and stained at 20 days p.i.
Fig. 7.
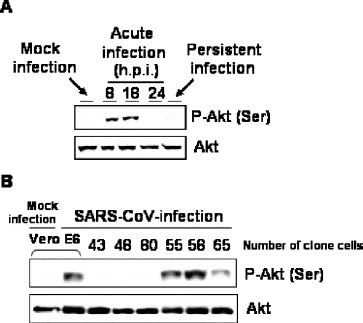
Phosphorylation status of Akt in viral infected cells. Western blot of acute infection (8, 18, and 24 h.p.i.) and persistent infection (passage 7) were performed using anti-phospho Akt antibody. The rabbit anti-phospho Akt (Ser473) antibody was purchased from Cell Signaling Technology Inc. and used at a dilution of 1:1000. SARS-CoV was infected to cloned Vero E6 cells at 10 m.o.i. The Western blot of the protein samples at 20 h.p.i. was performed using anti-phospho Akt antibody.
The present study showed that Vero E6 cells persistently infected with SARS-CoV were established and maintained after multiple passages. A population of cells produced from parental Vero E6 cells may have the potential to support persistent infection. The present study also showed that JNK or PI3K/Akt activation during SARS-CoV infection was necessary for the establishment of persistence, or for surviving the cells. Both JNK and PI3K/Akt are known to be involved in anti-apoptotic signaling pathways. Especially, our previous study indicated that the activation of Akt signaling pathways is important for anti-apoptosis in SARS-CoV-infected Vero E6 cells [14]. Pro-apoptotic signals, including p38 MAPK, induced cells to undergo apoptosis [13]. In some cell population in Vero E6 cells, anti-apoptotic signals may be stronger than pro-apoptotic signals, resulting in survival.
In the majority of patients infected with hepatitis C virus (HCV), acute infection results in persistent viral replication and the establishment of chronic infection. One viral protein, NS5A, which interacts with receptor binding protein 2 (Grb2), inhibits the activation of ERK1/2 by epidermal growth factor (EGF) [19]. Moreover, NS5A interacts with PI3K (p85), resulting in the promotion of the PI3K/Akt anti-apoptotic signaling pathway [8]. This mechanism may lead to persistent HCV infection in vivo. Tumor necrosis factor-α and interleukin-1β expression, as well as the activities of JNK and activator protein-1 (AP-1), were increased in transgenic mice constitutively expressing HCV core protein [20]. The alternation of cytokine expression and the activation of signaling pathways by core protein may contribute to hepatocarcinogenesis in persistent HCV infection. Thus, viral proteins involve in the activation or inactivation of signaling pathways. Recently, the expression of the nucleocapsid (N) protein of SARS-CoV was shown to induce the up-regulation of AP-1 [7], p38, and JNK, and the down-regulation of ERK and Akt [18]. Although these reports included results obtained in unnatural hosts in vitro, the N protein of SARS-CoV is a candidate for interaction with anti- and pro-apoptotic proteins of host cells. Thus, N protein may also play an important role in the establishment of persistent infection, even though a further experiment is necessary to confirm this hypothesis.
In this paper, we showed a possible mechanism of the establishment of SARS-CoV persistent infection The activation of JNK and PI3K/Akt signaling pathways helps a minor cell population with the potential for persistent infection, to establish persistence. Host gene expression and/or signaling pathways could play important roles in the establishment of persistence. Further investigations are needed to determine the up- or down-regulation of host mRNAs under signaling pathways of JNK and PI3K/Akt in SARS-CoV-infected Vero E6 cells.
Acknowledgements
We thank Drs. Y. Goto (The University of Tokyo, Japan), T. Kenri, Y. Sasaki, F. Taguchi (National Institute of Infectious Diseases, Japan), and O. Inanami (Hokkaido University, Japan) for helpful suggestions. We also thank Ms. M. Ogata (National Institute of Infectious Diseases, Japan) for her assistance. This work was supported, in part, by a grant-in-aid from the Ministry of Health, Labor, and Welfare of Japan and the Japan Health Science Foundation, Tokyo, Japan.
References
- 1.Adler B., Adler H., Pfister H., Jungi T.W., Peterhans E. Macrophages infected with cytopathic bovine viral diarrhea virus release a factor(s) capable of priming uninfected macrophages for activation-induced apoptosis. J. Virol. 1997;71:3255–3258. doi: 10.1128/jvi.71.4.3255-3258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai Y., Liu Y., Yu D., Zhang X. Down-regulation of transcription of the proapoptotic gene BNip3 in cultured astrocytes by murine coronavirus infection. Virology. 2003;316:104–115. doi: 10.1016/j.virol.2003.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calain P., Roux L. Generation of measles virus defective interfering particles and their presence in a preparation of attenuated live-virus vaccine. J. Virol. 1988;62:2859–2866. doi: 10.1128/jvi.62.8.2859-2866.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan P.K., To K.F., Lo A.W., Cheung J.L., Chu I., Au F.W., Tong J.H., Tam J.S., Sung J.J., Ng H.K. Persistent infection of SARS coronavirus in colonic cells in vitro. J. Med. Virol. 2004;74:1–7. doi: 10.1002/jmv.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diaz M.O., Ziemin S., Le Beau M.M., Pitha P., Smith S.D., Chilcote R.R., Rowley J.D. Homozygous deletion of the a- and f31-interferon genes in human leukemia and derived cell lines. Proc. Natl. Acad. Sci. U. S. A. 1998;85:5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diderholm H., Dinter Z. Interference between strains of bovine virus diarrhea virus and their capacity to suppress interferon of a heterologous virus. Proc. Soc. Exp. Biol. 1966;121:976–980. doi: 10.3181/00379727-121-30940. [DOI] [PubMed] [Google Scholar]
- 7.He R., Leeson A., Andonov A., Li Y., Bastien N., Cao J., Osiowy C., Dobie F., Cutts T., Ballantine M., Li X. Activation of AP-1 signal transduction pathway by SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun. 2003;311:870–876. doi: 10.1016/j.bbrc.2003.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He Y., Nakao H., Tan S.L., Polyak S.J., Neddermann P., Vijaysri S., Jacobs B.L., Katze M.G. Subversion of cell signaling pathways by hepatitis C virus nonstructural 5A protein via interaction with Grb2 and P85 phosphatidylinositol 3-kinase. J. Virol. 2002;76:9207–9217. doi: 10.1128/JVI.76.18.9207-9217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maeda A., Hayashi M., Ishida K., Mizutani T., Watanabe T., Namioka S. Characterization of DBT cell clones derived from cells persistently infected with the JHM strain of mouse hepatitis virus. J. Vet. Med. Sci. 1995;57:813–817. doi: 10.1292/jvms.57.813. [DOI] [PubMed] [Google Scholar]
- 11.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani T., Inagaki H., Tada M., Hayasaka D., Murphy M., Fujisawa T., Hamada J., Kariwa H., Takashima I. The mechanism of actinomycin D-mediated increase of Borna disease virus (BDV) RNA in cells persistently infected by BDV. Microbiol. Immunol. 2000;44:597–603. doi: 10.1111/j.1348-0421.2000.tb02539.x. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani T., Fukush S., Saijo M., Kurane I., Morikawa S. Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun. 2004;319:1228–1234. doi: 10.1016/j.bbrc.2004.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S. Importance of Akt signaling pathway for apoptosis in SARS-CoV-infected Vero E6 cells. Virology. 2004;327:169–174. doi: 10.1016/j.virol.2004.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizutani T., Fukushi S., Murakami M., Hirano T., Saijo M., Kurane I., Morikawa S. Tyrosine dephosphorylation of STAT3 in SARS coronavirus-infected Vero E6 cells. FEBS Lett. 2004;577:187–192. doi: 10.1016/j.febslet.2004.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman S., Ries D. The astrocyte is a target cell in mice persistently infected with mouse hepatitis virus, strain JHM. Microb. Pathog. 1987;3:309–314. doi: 10.1016/0882-4010(87)90064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen- Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 18.Surjit M., Liu B., Jameel S., Chow V.T., Lal S.K. The SARS coronavirus nucleocapsid (N) protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J. 2004;383:13–18. doi: 10.1042/BJ20040984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan S.L., Nakao H., He Y., Vijaysri S., Neddermann P., Jacobs B.L., Mayer B.J., Katze M.G. NS5A, a nonstructural protein of hepatitis C virus, binds growth factor receptor-bound protein 2 adaptor protein in a Src homology 3 domain/ligand-dependent manner and perturbs mitogenic signaling. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5533–5538. doi: 10.1073/pnas.96.10.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsutsumi T., Suzuki T., Moriya K., Yotsuyanagi H., Shintani Y., Fujie H., Matsuura Y., Kimura S., Koike K., Miyamura T. Alteration of intrahepatic cytokine expression and AP-1 activation in transgenic mice expressing hepatitis C virus core protein. Virology. 2002;304:415–424. doi: 10.1006/viro.2002.1702. [DOI] [PubMed] [Google Scholar]


