Abstract
Community-acquired pneumonia (CAP) is a common illness, with the majority of patients treated out of the hospital, yet the greatest burden of the cost of care comes from inpatient management. In the past several years, the management of these patients has advanced, with new information about the natural history and prognosis of illness, the utility of serum markers to guide management, the use of appropriate clinical tools to guide the site-of-care decision, and the finding that guidelines can be developed in a way that improves patient outcome. The challenges to patient management include the emergence of new pathogens and the progression of antibiotic resistance in some of the common pathogens such as Streptococcus pneumoniae. Few new antimicrobial treatment options are available, and the utility of some new therapies has been limited by drug-related toxicity. Ancillary care for severe pneumonia with activated protein C and corticosteroids is being studied, but recently, inpatient care has been most affected by the development of evidence-based “core measures” for management that have been promoted by the Centers for Medicare and Medicaid Services, which form the basis for the public reporting of hospital performance in CAP care.
Key words: community-acquired pneumonia, drug resistance, methicilllin-resistant pneumonia, severe pneumonia, severity index, Staphylococcus aureus, Streptococcus pneumoniae
Abbreviations: APACHE, acute physiology and chronic health evaluation; CAP, community-acquired pneumonia; CMS, Centers for Medicare and Medicaid Services; CRP, C-reactive protein; CURB-65, confusion, elevated BUN level, elevated respiratory rate, low systolic or diastolic BP, and age > 65 years of age; DRSP, drug-resistant Streptococcus pneumoniae; HCAP, health-care-associated pneumonia; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio; PCT, procalcitonin; PSI, pneumonia severity index; SARS, severe acute respiratory syndrome
In the past several years, clinical advances in community-acquired pneumonia (CAP) have emerged in a number of areas that can aid in the care of both inpatients and outpatients. Major clinical issues for all CAP patients have been the changing spectrum of etiology, including drug-resistant Streptococcus pneumoniae (DRSP), methicillin-resistant Staphylococcus aureus (MRSA), and emerging viral pathogens (eg, severe acute respiratory syndrome [SARS] and avian influenza). In addition, there has been an interest in better understanding the natural history and prognosis of CAP by trying to define the role of prognostic scoring systems in guiding the decision about site of care (ie, inpatient, outpatient, or ICU) and by applying a number of serum markers (ie, C-reactive protein [CRP] and procalcitonin [PCT]) to prognosticate outcome. New antimicrobial agents have become available for both outpatients and inpatients, in several antibiotic classes, but the utility of some of these agents has been limited by new findings of toxicities that were not evident in registration trials of these medications (ie, gatifloxacin and telithromycin) prior to their approval for clinical use. In addition to new antimicrobial agents, paradigms for therapy have been advanced by a focus on better defining the optimal duration of therapy and on the role of adjunctive therapies for those with severe illness, including corticosteroids and activated protein C.
One of the major factors that has dominated the inpatient care of CAP in the United States has been the promulgation of “core measures,” or standards of care, which have been supported by the Centers for Medicare and Medicaid Services (CMS) and the Joint Commission on the Accreditation of Healthcare Organizations. Success in achieving these measures has been publicly reported for the performance of individual hospitals, and it seems possible that these data could serve in the future as the basis for “pay for performance,” thereby impacting the financial well-being of a specific health-care institution. Interest in these core measures has refocused attention on assuring that all patients receive evidence-based antibiotic choices, that they receive timely administration of antibiotics, that there is a proper use of blood cultures prior to antibiotic administration, and that each patient is current with pneumococcal and influenza vaccinations.
Understanding the Natural History and Prognosis of CAP
Most of the studies of CAP have examined the short-term outcomes of the illness, focusing on either 30-day or inpatient mortality. Kaplan and colleagues1 used a Medicare database to perform a matched case-control study to evaluate the long-term impact (ie, 1-year mortality rate) of older patients with CAP. The authors compared 158,960 CAP patients to 794,333 hospitalized control subjects (5 for each patient) matching for age, sex, and race. While the in-hospital mortality rate for CAP patients exceeded that of control subjects (11% vs 5.5%, respectively), the differences in the 1-year mortality rate were even more dramatic (40.9% vs 29.1%, respectively) [Fig 1 ]. The high mortality rate was impressive, and the differences could not be explained by the types of underlying disease; the findings persisted, even if only the hospital survivors were examined. These findings make it clear that CAP is much more than a self-limited illness for those who survive, and that the 1-year mortality rate of elderly patients with CAP is four times higher than the in-hospital mortality rate, with one in three survivors of CAP dying in the subsequent year, following hospital discharge. The exact cause of death was not examined in the study, but the population was generally elderly, with 85% being > 65 years of age; nursing home patients were included, and 70% had a comorbid medical illness. The findings expand on an older Scandinavian study2 that reported a lower 10-year survival rate in CAP patients > 60 years of age than in an age-matched population without CAP. In that study, the relative risk for death in CAP patients was 1.5 compared to those without CAP, and the 10-year survival rate was 39%, compared to 61% in the non-CAP population, with many of the deaths related to cardiovascular disease and subsequent pneumonia. All of these data make it very clear that CAP requiring hospital admission is a disease that should be prevented, whenever possible, in the elderly.
Figure 1.
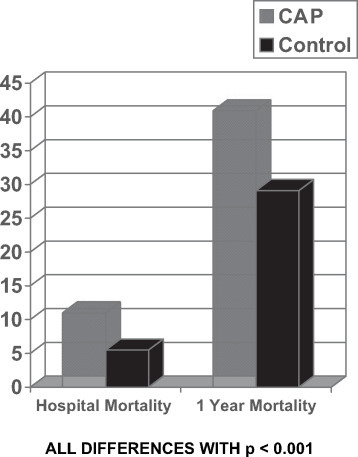
In this case-control study of Medicare patients with CAP, with five control subjects matched for age, sex, and race with each case, the in-hospital and 1-year mortality rates for patients with CAP were significantly higher than those for control subjects. From Kaplan et al.1
Prognostic Scoring Systems
The optimal management of CAP requires the prompt recognition of seriously ill patients to avoid such mistakes as the failure to use the hospital or ICU for patients who could benefit from care and observation in such settings. On the other hand, the major impact on the cost of CAP care is determined by whether or not a patient is admitted to the hospital.3 In the United States, < 20% of all CAP patients are admitted to the hospital, but the dollars spent on these patients account for > 90% of the total cost of care for this disease, emphasizing the impact of the hospital admission decision.3 For a number of years, prognostic scoring systems have been used to define not only the predicted mortality rate of CAP, but also, by inference, the site of care, reserving hospital admission for those with a high predicted mortality rate.
The two commonly used tools for the purpose of predicting outcome in CAP patients have been the pneumonia severity index (PSI), which was developed in the United States, and the British Thoracic Society rule, which has recently been modified to the CURB-65 (referring to its assessment of the following five factors: confusion; elevated BUN level; elevated respiratory rate; low systolic or diastolic BP; and age > 65 years of age) rule.4 Each of these approaches has limitations, and it may be best to view them as complementary, ideally identifying patients at opposite ends of the disease spectrum.5 The PSI has been best validated as a way to identify patients with a low risk of mortality, but the scoring system can occasionally underestimate severity of illness, especially in young patients without comorbid illness because it heavily weights age and comorbidity, and does not measure CAP-specific disease severity.5 On the other hand, the CURB-65 approach may be ideal for identifying patients with a high risk of mortality with severe illness due to CAP, who might otherwise be overlooked without the formal assessment of subtle aberrations in key vital signs.5 However, one deficiency of the CURB-65 approach is that it does not generally account for comorbid illness and thus may not be easily applied in older patients who may still have a substantial mortality risk if even a mild form of CAP destabilizes a chronic, but compensated, disease process.
In one recent study4 that compared the PSI to the CURB-65 in 3,181 patients seen in an emergency department, both were determined to be good for predicting mortality and for identifying patients with a low risk of mortality. However, the PSI appeared to be more discriminating in identifying patients with a low risk of mortality, with 68% being defined by PSI to have a low risk (classes I to III), with a mortality rate of 1.4%, while 61% were defined by the CURB-65 to have a low risk (score of 0 to 1) with a mortality rate of 1.7%. However, the CURB-65 may have been more valuable at the severe disease end of the spectrum because it defined high-risk patients as those with a score of 2, 3, 4, or 5, each with a progressively increasing risk of death, while the PSI was less discriminating, defining only two groups as being severely ill. In another analysis,6 the CURB-65 score also appeared to identify, most accurately, those patients with CAP who were likely to benefit from treatment with drotrecogin alfa in the recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (or PROWESS) study. A reexamination of the data from that study demonstrated that a threshold CURB-65 score of ≥ 3 was associated with a decrease in the 28-day mortality rate in drotrecogin alfa-treated patients of 10.8% when compared to control subjects (p = 0.018) vs a decrease in mortality rate in treated patients in PSI classes IV and V of 9.7% compared to control subjects (p = 0.013).6
Capelastegui and colleagues7 used both the PSI and the CURB-65 approach to evaluate a large number of both inpatients and outpatients with CAP in Spain. They observed that the CURB-65 (and its simpler CRB-65 version, which excludes the measurement of BUN, and therefore can be used in outpatients) could accurately predict the 30-day mortality rate, the need for mechanical ventilation, and, to some extent, the need for hospitalization. In addition, the CURB-65 criteria correlated with the time to clinical stability, and thus a higher score was predictive of a longer duration of IV therapy and a longer length of hospital stay. The PSI also worked well to predict mortality in that study.
While both the PSI and CURB-65 are good for predicting mortality, neither can be used to define the site of care, without considering other clinical and social variables. A study at a public hospital in the United States, with many indigent patients, showed that the PSI could not define the need for hospital admission if patients were homeless or acutely intoxicated, or if they did not have a stable home environment that allowed them to be discharged from the hospital while receiving oral antibiotic therapy.8 In one recent commentary,5 the suggestion was made to combine both of these prognostic scoring tools, recognizing that neither approach can stand alone. Low-risk patients (ie, PSI classes I to III or CURB-65 score of 0 to 1) can be managed at home if serious vital sign abnormalities (in the case of PSI) or comorbidities (in the case of CURB-65) are absent, and if patients do not have social factors or other illnesses that are unstable and that necessitate hospitalization. Moderate-risk patients (ie, CURB-65 score of ≥ 2 or PSI classes IV and V) probably should be admitted to the hospital, and clinical assessment should be used to separate those who need ICU care from those who are likely to become clinically stable rapidly and who would then require only a short hospital stay.
Serum Markers To Predict CAP Outcomes
The two serum markers that have been most widely studied for this purpose are CRP and PCT. In general, both measures have been used to correlate with outcomes, but more data have recently been collected9 with PCT, and the most exciting finding has been that serial measures correlate not only with outcomes, but may also be useful for guiding the duration of therapy.
CRP was measured in one study of 201 patients with CAP who were compared to 84 healthy control subjects and 25 patients with suspected pneumonia, which was not confirmed on clinical follow-up, and the levels were highest in those with pneumonia.10 However, among those with proven CAP, the levels of CRP correlated with the clinical course, with the median level being higher in hospitalized patients than in outpatients (132.0 vs 76.9 mg/L, respectively; p < 0.001). These findings might in part be explained by the observation that CRP levels tended to be higher in those with pneumococcal and Legionella etiologies than in those with a viral or atypical pathogen pneumonia; possibly those with the bacterial illnesses were more severely ill, and thus more in need of hospitalization.
In general, CRP, an acute-phase reactant that is synthesized in the liver, has not been as sensitive or specific for infection as PCT. PCT, the precursor of calcitonin, has no hormonal effects. Its value arises because serum levels are increased in severe bacterial infections, but not in viral illness. The release of PCT can be stimulated by microbial toxins (including lipopolysaccharide), cytokines (eg, tumor necrosis factor, interleukin-1, and interleukin-6), and by the cell-mediated immune response. Levels can be attenuated by virus-induced cytokines (interferon-γ). In a study11 of 185 patients who had PCT measured within 24 h of hospital admission for CAP, the levels correlated with PSI score (higher in classes III and V than in classes I and II) and the development of complications (higher with empyema, mechanical ventilation, and septic shock), and levels were also increased in those who died compared to those who did not. Interestingly, the levels were higher in patients with a low risk of mortality (ie, low PSI) with a bacterial etiology for CAP than in those without, but similar findings did not apply to those with more severe CAP. This may mean that low levels in outpatients could indicate that it is safe to withhold antibiotic therapy.
Another recent study9 supports the idea of using serial measurements of PCT levels to guide the need for antibiotic therapy and its duration. In this study, 302 patients were randomized to receive either standard care or therapy guided by serial measurements of levels of PCT, which were evaluated when the patient was first seen, 6 to 24 h later if antibiotics were withheld, and then at days 4, 6, and 8. Only 3% of all patients were not admitted to the hospital, making this primarily an inpatient study. With the use of PCT levels, 15% of patients had antibiotics withheld, compared to 1% of those receiving standard care. The use of PCT levels to guide therapy led to a significantly shorter duration of therapy that applied to all patients, regardless of PSI class. Most importantly, outcomes were similar in both groups, documenting the safety of looking for strategies to reduce antibiotic usage.
Serial measurements of PCT have also been used to define prognosis in patients with severe CAP. In one study12 of 110 patients who had only one measurement performed within 48 h of ICU admission, levels of PCT were higher in those with positive bacteriology results than in those with negative results, and in those with complications (eg, septic shock and organ dysfunction) and death than in those without. Bolstered by these findings, the same investigative group collected serial PCT levels in 100 ICU CAP patients on day 1 and day 3.13 In the study, survivors had a decrease in PCT levels, while nonsurvivors had an increase by day 3. Numerous clinical parameters, were also measured, as well as serial levels of CRP, but in the multivariate predictors of mortality, the relevant factors were as follows: need for mechanical ventilation (odds ratio [OR], 9.9); presence of multilobar infiltrates (OR, 5.6); increasing PCT levels (OR, 4.5); and worsening of a multiorgan failure score. Among mechanically ventilated patients, the PCT level on day 3 was highly predictive of mortality if it remained elevated. Serial measurements of CRP did not have predictive value in this study.
New Issues in the Pathogens Causing CAP
Drug-Resistant Pneumococcus
While the clinical relevance of DRSP continues to be debated, recent data14 have suggested that the frequency of some forms of drug resistance may be stabilizing or declining, while concerns still remain for other classes of antibiotics. Using data from 2002 to 2003, Doern et al14 studied 1,817 pneumococcal respiratory isolates from 44 US centers and observed that while penicillin resistance was present (34.2%), it was not occurring with an increased frequency. They found that 15.7% of isolates were intermediately sensitive and 18.5% were highly resistant to penicillin. On the other hand, macrolide resistance was increasing (although most was low-level, efflux pump-mediated), while trimethoprim-sulfa resistance was declining. Quinolone resistance rates were very low (< 1%), but 21% of the isolates had a first-step mutation (par C) that still permitted the antibiotics to be active. However, if a second mutation (gyr A) were acquired, these organisms could become quinolone-resistant, urging caution to observe trends in this type of mutation. In terms of reliable choices for suspected DRSP, quinolones remain effective, but ceftriaxone remained the most active β-lactam agent, with a 6.9% resistance rate. In clinical studies, ceftriaxone has been a reliable choice, even if DRSP is present, while, among the cephalosporins, cefuroxime is not a reliable choice since patients with bacteremia and in vitro resistance to this agent had a worse outcome than when organisms were sensitive to this agent.15 16
One of the clinical factors that is driving pneumococcal resistance is antibiotic use, and new data have shown that recent therapy, within the past 3 months, is a risk factor for pneumococcal resistance.17 In a remarkable study,17 the Toronto Bacterial Network evaluated data from patients in 3,339 cases of invasive pneumococcal infection, of whom 563 had a history of antibiotic therapy in the preceding 3 months and the identity of the therapy was known. In the study, recent therapy with penicillin, macrolides, trimethoprim-sulfa, and quinolones (but not cephalosporins) was associated with a higher frequency of resistance to that same agent. Among all of the classes of antibiotics, the one with the greatest effect of recent therapy on subsequent resistance (ie, highest OR of an effect) was quinolones. This latter finding is consistent with case reports of lack of response to quinolones in CAP patients that documented recent quinolone therapy as a major risk factor.18 All of these data lend further support to the idea of “patient-specific antibiotic rotation” in CAP, making sure that among all acceptable therapeutic alternatives the clinician takes a history of recent antibiotic use and chooses an agent that differs from what the patient had recently received.
Community-Acquired MRSA
MRSA has always been a nosocomial pathogen and a common cause of ventilator-associated pneumonia. In the past several years, MRSA has been reported as a cause of sporadic cases of severe CAP, especially following a preceding viral infection.19 This pathogen is not the same as its nosocomial counterpart, having a different genetic makeup, different host susceptibility, and different virulence and antibiotic sensitivity. The community-acquired strain generally belongs to a single pulse-field gel electrophoresis type, the USA 300 strain. In addition, it carries the genes for the production of a necrotizing toxin, the Panton-Valentine leukocidin, and it confers resistance to methicillin through the carriage of the type IV mecA gene, which is carried on the staphylococcal chromosomal cassette (type IV SCCmec element).19 The cases that have been reported have been severe necrotizing pneumonias, generally in previously healthy individuals following viral infection or documented influenza illness. The pneumonia is often rapidly progressive, bilateral, and with shock, cavitation of lung parenchyma, and pleural effusion. The organism is sensitive to a wide range of antibiotics, including vancomycin, clindamycin, trimethoprim-sulfa, and gentamicin, with variable sensitivity to quinolones.19 The optimal therapy is yet to be defined, but one case series20 reported failure with vancomycin alone, which was overcome by either the addition of clindamycin or the use of linezolid. These findings may relate to the fact that clindamycin and linezolid can inhibit toxin production, and thus successful therapy may require both an antibacterial and an antitoxin form of treatment.
Viruses
Over the past several years, there has been a renewed interest in epidemic viral illness with the emergence of SARS, and recent concerns about avian influenza. These experiences have emphasized the epidemic nature of illness and the rapidity of patient-to-patient spread. In the case of SARS, the risk to health-care workers was evident. Very little is known about the frequency of viral infection in routine CAP, and thus a Spanish study21 of this topic is of interest. The investigators evaluated 338 patients with paired serologies for respiratory viruses in the setting of CAP, and classified patients as having pure viral, mixed viral, and bacterial or pneumococcal CAP.21
The viruses investigated included influenza, parainfluenza, respiratory syncytial virus, and adenovirus. Viruses were detected in 18% of patients, and in half of those patients viruses were the only pathogen present. Influenza was the most common infection, being present in 64% of patients with viral infection. The only clinical correlates of pure viral pneumonia, compared to pneumococcal pneumonia, were the presence of heart failure and the absence of expectoration. Only 8% of the pure viral pneumonia patients needed visits to the ICU, but 58% were in PSI classes IV and V. Interestingly, despite the high mortality risk of these patients (defined by PSI class), none died. Given the importance of influenza and viral respiratory infection in general, and the role of these infections in predisposing the patient to MRSA CAP, these data highlight the relatively common occurrence and importance of viral pneumonia in the community.
Aspiration Pneumonia
The bacteriology of aspiration pneumonia arising in the community setting has been confusing, and the exact role of anaerobes is uncertain. In a study22 of 95 patients, > 65 years of age, who were admitted to the ICU from a long-term care facility with presumed aspiration pneumonia, the bacteriology of infection was studied using a protected BAL fluid sample that had been collected within 4 h of ICU admission. Aspiration was presumed to be present because patients had known risk factors such as intestinal or swallowing disorders, neurologic disease, and anatomic abnormalities that could lead to aspiration. The data demonstrated that Gram-negative pathogens were the dominant type of pathogen, and that anaerobes were present in only 11 of the 95 patients; in only 5 patients were anaerobes the only pathogens present22 (Fig 2 ). In another study of lung abscess,23 which is a disease that is commonly attributed to aspiration and anaerobic infection, 90 patients were evaluated with an “uncontaminated” specimen including transthoracic needle aspirate, pleural fluid, blood cultures, and specimens from a surgical sample, but not bronchoscopy samples alone. In this group, pure anaerobic infection was present in only 18 patients, and 10 others had mixed infection. However, aerobic Gram-negative pathogens were present in 37 patients, with Klebsiella pneumoniae recovered from 28 patients. Thus, the level of involvement of enteric Gram-negative pathogens in these two aspiration-related illnesses is quite high and must be considered when selecting therapy.
Figure 2.

Results of nonbronchoscopic BAL fluid cultures collected within 4 h of ICU admission in 95 elderly nursing-home patients with aspiration pneumonia admitted to the ICU. The dominant organism group was enteric Gram-negative pathogens, and anaerobes were less common and often part of a mixed infection. From El-Solh et al.22
New Approaches to Therapy
Guidelines for CAP have stressed the approach of empiric therapy, recognizing the difficulty of obtaining pathogen-specific data that allow the early focusing of initial therapy choices. One recent study24 found that when therapy was given according to guidelines, it led to patients becoming clinically stable sooner than if other therapy had been used. However, the value of empiric therapy was evaluated directly in a study from the Netherlands25 that used a prospective, randomized, open study design to compare empiric therapy with pathogen-directed therapy in 262 patients with clinical and radiographically proven CAP. All patients had undergone extensive diagnostic testing, but the empiric therapy group received therapy with a β-lactam/β-lactamase inhibitor combined with erythromycin when not in the ICU or ceftazidime plus erythromycin when in the ICU. The pathogen-directed group had Gram stains performed on sputum samples and underwent urinary antigen testing, along with a clinical evaluation to define the suspected pathogen; then penicillin was used for the treatment of pneumococcus, erythromycin for atypical pathogens, amoxicillin/clavulanate for mixed infection, and flucloxacillin with optional gentamicin for therapy after influenza infection. There were no differences in either group for length of stay, early or late clinical failure, and 30-day mortality rate (Fig 3 ). However, empiric therapy patients did have a higher mortality if they were admitted to the ICU, and the empiric therapy group had more adverse events, which may have been related to the use of IV erythromycin rather than a newer macrolide with fewer IV side effects. While the study established the safety of empiric therapy, it did not test other benefits of diagnostic testing, such as the long-term control of antibiotic use and the avoidance of resistance.
Figure 3.
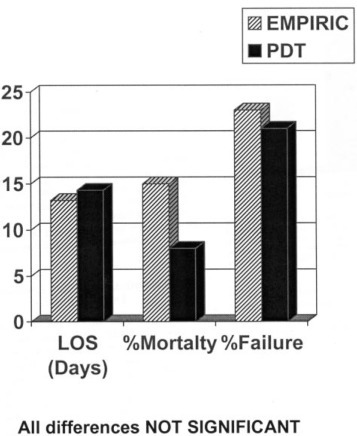
A randomized trial of pathogen-directed therapy (PDT) compared with empiric therapy in 262 adults with CAP found no significant differences in length of stay (LOS), mortality rate, or rate of therapeutic failure. From Van der Eerden et al.25
New Therapies and Toxicities
In the past several years, new therapies have been approved and new data have been collected about these agents. Moxifloxacin, a fluoroquinolone, has been used to treat CAP, and an inpatient trial26 27 in elderly hospitalized patients has demonstrated cardiac safety equivalent to levofloxacin, with a statistically significantly more rapid rate of clinical improvement at days 3 to 5 during therapy. The high bioavailability of quinolone agents may allow oral therapy to replace IV therapy, thus keeping some patients with CAP out of the hospital. Using oral levofloxacin, along with a cluster-randomized protocol design, Loeb et al28 documented the safety of this approach in nursing home patients who had CAP, but were able to eat and drink, had a pulse of ≤ 100 beats/min, a respiratory rate < 30 breaths/min, a systolic BP of ≥ 90 mm Hg, and an oxygen saturation of ≥ 92%.
Telithromycin, the first ketolide, is similar to a macrolide in terms of antimicrobial spectrum but is active against macrolide-resistant pneumococci. It has demonstrated a tendency to reduce the need for hospitalization when it has been used as an oral outpatient therapy for CAP, compared to clarithromycin.29 However, the drug is not optimally active against Haemophilus influenzae, and toxicity issues (see below) have limited its widespread use. Linezolid has also been shown to be effective against drug-resistant pneumococcus, but it is not considered to be a drug for empiric therapy of CAP, since it is being used as an agent against MRSA arising in both the hospital and the community.
A concern with these new agents is to define their role in CAP management. Safety has been a major consideration with the quinolone class of antibiotics, and gatifloxacin has recently been documented30 to cause hypoglycemia and hyperglycemia, limiting its ability to be used safely in diabetic patients. Quinolones have caused QT prolongation and cardiac arrhythmias, and this has limited the use of agents such as sparfloxacin. As mentioned before, a randomized, double-blinded comparative study of levofloxacin and moxifloxacin, using clinical evaluation and Holter monitoring, found no difference in the frequency of clinically significant cardiac events between the two agents.27 Telithromycin has recently been associated31 with infrequent cases of drug-induced liver necrosis, and awareness of this potential complication is essential if this drug is used.
Duration of Therapy
The optimal duration of therapy for CAP is not known, but several recent developments have pushed for shorter durations, especially in outpatients. A new formulation of azithromycin allows for the administration of a full course of therapy with a single 2-g dose in an outpatient population.32 Telithromycin has been used for 5 days in outpatients with CAP, and levofloxacin, 750 mg, is as effective when given for 5 days to inpatients with CAP as when given for 10 days at a dose of 500 mg.29 33 A recent study34 compared 3 days of therapy with amoxicillin to 8 days of therapy in hospitalized patients and showed the short-duration therapy to be comparable to longer duration therapy in terms of clinical success. However, the study included only patients with mild-to-moderate illness, and patients were eligible for short-duration therapy only if their conditions had improved substantially with IV therapy by day 3. One correlate of these findings is that a hospitalized patient who becomes clinically stable with IV therapy could be safely discharged from the hospital without continued inpatient observation. A recent Medicare database study35 compared CAP patients who were not observed, and were discharged on the same day as the switch to oral therapy, to those observed for a day after the switch. There were no differences in the 14-day readmission rate and the 30-day mortality rate between the groups, emphasizing the safety of not keeping the patient in the hospital for observation after the switch from IV therapy.
Adjunctive Therapy of Severe CAP
The care of patients with severe pneumonia has focused on the early identification of these patients and on prompt therapy with multiple antibiotics. Current guidelines for these patients recommend against monotherapy with any agent, including quinolones, and a recent randomized study36 of levofloxacin monotherapy, compared to combination therapy, in CAP patients admitted to the ICU supports these recommendations (Table 1 ). The study evaluated 398 patients admitted to the ICU and found that monotherapy was not as effective as combination therapy for those persons needing mechanical ventilation. Since the trial also excluded those patients who were in septic shock, the authors concluded that monotherapy could not be recommended for CAP patients who were in septic shock or for those receiving mechanical ventilation, which are conditions that represent the majority of individuals admitted to the ICU.
Table 1.
Current CAP Core Measures for Admitted Patients
| First dose of antibiotics within 4 h of arrival to hospital* |
| Oxygenation assessment within 24 h of hospital admission |
| Correct antibiotic for admitted patients |
| Non-ICU |
| ICU |
| Includes no monotherapy |
| Blood cultures within 24 h for all patients admitted to ICU in first 24 h |
| Blood for cultures drawn prior to antibiotics administration for those drawn in ED |
| Evaluation and offering of pneumococcal and influenza vaccination |
| Smoking cessation advice |
ED = emergency department.
Two adjunctive therapies, activated protein C and systemic corticosteroids, have been studied in patients with severe CAP.6 37 A retrospective analysis6 of the PROWESS study of activated protein C (drotrecogin-α) identified that 35.6% of those patients studied had CAP, and that approximately a quarter of them were infected with pneumococcus. Patients with CAP who received activated protein C had a survival benefit if they had an acute physiology and chronic health evaluation (APACHE) II score of > 25, or pneumococcal infection, a PSI class of IV or V, or a CURB-65 score of at least 3. For unclear reasons, patients who received adequate therapy had a small drop in mortality rate from 37 to 33%, while the benefit was much greater in those who received inadequate therapy with the mortality rate dropping from 65.2 to 47.1%. While the mortality rate reduction was 28% at 28 days, it fell to 14% at 90 days. These data are interesting and suggest a benefit for this expensive therapy, but ideally a randomized trial of patients with severe CAP, rather than a subset analysis alone, would be more convincing. In addition, the limited benefit for those who received adequate therapy, and the falloff in the 3-month survival benefit detract from the cost-effectiveness of this therapy.
Therapy with systemic corticosteroids has been demonstrated to be useful for patients who are in septic shock and have relative adrenal insufficiency. However, in a new study,37 therapy with systemic corticosteroids has been tested in patients with severe CAP, based on the idea that adverse outcomes are mediated by the inflammatory response to infection rather than by uncontrolled infection. In a small (48 patients), multicenter, randomized, blinded trial,37 therapy with a continuous infusion of hydrocortisone was compared to therapy with placebo. Although patients had severe CAP, not all of them were treated in an ICU. Steroid therapy led to significantly lower mortality, shorter length of ICU stay, and shorter duration of mechanical ventilation. In addition, steroid therapy led to fewer late complications. Although the data are impressive, confirmation in a larger study is needed. Nonetheless, the findings do suggest that steroid therapy is not dangerous, even for patients with a severe infection such as CAP.
Core Measures for Inpatient Care
Since 1998, the CMS, in conjunction with the Joint Commission on the Accreditation of Healthcare Organizations, has promoted standards of care for CAP patients that have been shown to improve outcomes, with the expectation that hospitals will meet these standards whenever possible (Table 1). The pressure to achieve a high compliance rate with these measures has increased with the move to collect data on compliance and to publicly report the information. The evidence that supports these core measures is generally strong, but it may not be correct to try to achieve these measures for all patients in all clinical situations, and a reasonable goal may be 80 to 85% compliance, with a variety of unintended adverse consequences occurring if rates are higher.38
The current evidence-based standards (with most being based on retrospective database analysis) are as follows: to administer the first dose of antibiotics within 4 h of the patient's arrival at the hospital; to select one of the recommended antibiotic therapies for admitted patients, with different choices for those in the ICU and those on the medical ward; to make sure that if blood cultures are performed, they are collected prior to antibiotic administration; to provide smoking cessation advice to appropriate patients; and to evaluate the need and to offer to those who meet the criteria both pneumococcal and influenza vaccines. Several areas have been problematic, and new data are available to guide the clinician about the recommendations to administer therapy within 4 h, the recommendation not to use monotherapy for ICU-admitted CAP, the value of blood cultures, and the safety of repeat pneumococcal vaccinations.
One important change in the application of core measures is the recognition that some patients who are admitted to the hospital with pneumonia have health-care-associated pneumonia (HCAP), and that these patients are at risk for infection with multi-drug-resistant Gram-negative pathogens and MRSA, and thus need a different approach to therapy than the usual CAP patient.39 HCAP was included in the 2005 guidelines for nosocomial pneumonia as a form of nosocomial infection; thus, since July 2005 patients who have been identified as having HCAP have been excluded from the CMS core measure of CAP antibiotic choices. This is justified by data showing that HCAP has a different natural history than CAP, that the bacteriology is also different from CAP, and that, presumably, the therapy should not be the same.40
Controversy about the administration of antibiotics within 4 h of a patient's arrival at the hospital has been vigorous, and there is concern that even if large-scale databases show a reduced mortality rate with therapy given in this time interval, several unintended consequences can follow.38 These include the indiscriminate use of antibiotics in any patient with respiratory symptoms in the emergency department, even before the diagnosis is certain, and the temptation to prioritize pneumonia patients ahead of other sick individuals in a busy emergency department. Two recent studies41 42 have added to the controversy. The first study41 confirmed that when therapy is provided in 4 h, mortality is reduced, but the predictors of increased time to antibiotic administration were altered mental status, absence of fever, absence of hypoxia, and increasing age. When these factors were controlled for, the timing of therapy had no impact on mortality, and thus the authors concluded that time to the administration of antibiotics is not a good quality measure. In support of these findings was another study42 that found that 22% of 86 Medicare patients with CAP presented with atypical clinical features that led to diagnostic uncertainty, which could appropriately lead to a delay in the administration of antibiotics. In an editorial accompanying these articles, the observation was made that time to the administration of antibiotics has only been demonstrated to affect mortality in patients > 65 year of age, and that the findings of these studies support the idea that 100% compliance with the standard would not necessarily mean good medical care; thus, the goal should be a lower number of patients given antibiotics within 4 h.38 The standard of antibiotic administration within 4 h is likely to change in 2007.
Blood cultures have not been shown to favorably alter the outcomes of CAP patients, and thus some have argued against collecting them routinely. While all patients admitted to the hospital may not need this testing, it may be wise to still collect blood cultures in those patients with signs of severe illness, and it is important to collect the cultures prior to antibiotic administration. Metersky et al43 studied 13,043 Medicare patients with CAP who had been admitted to the hospital to define the predictors of bacteremia. They found that certain populations (especially those who had received prior antibiotic therapy) were unlikely to have true positive culture findings; thus, a large percentage of positive results in these patients would be false-positive results and could lead to mistakes in management. The predictors of bacteremia were as follows: prior antibiotic therapy (OR, 0.5); comorbid liver disease (OR, 2.3); systolic BP < 90 mm Hg (OR, 1.7); fever < 35°C or > 40°C (OR, 1.9); pulse > 125 beats/min (OR, 1.9); BUN level of > 30 mg/dL (OR, 2.0); serum Na level of < 130 mEq/L (OR, 1.6); and WBC count of < 5,000 cells/μL or > 20,000 cells/μL (OR, 1.7). The most common pathogen found in blood cultures was pneumococcus, but 643 of 886 pathogens were contaminants. The authors suggested that patients who have received prior antibiotic therapy and have none of the severity/comorbidity risk factors listed above should not have blood cultures performed, since only 3% of patients had true-positive findings for bacteremias. Those without prior antibiotic therapy and no predictors, or those with prior antibiotic therapy and one predictor should have one blood culture performed since the incidence of true-positive results was 5%. Finally, the yield is high in those patients with two predictors (16% bacteremia) or in those with one predictor and no prior antibiotic therapy (9% bacteremia); these patients should have two blood cultures performed.
One concern with the core measure emphasis on pneumococcal vaccination is the possibility that patients will receive repeated vaccination in less than the recommended 5-year interval because of the absence of a reliable history of vaccination, especially in those patients who have been repeatedly hospitalized or in those patients who have been treated in nursing homes. One way to deal with this is to vaccinate all patients if there is any uncertainty about the history of vaccination, and that may be justified by the proven efficacy of the vaccine and the long-term consequences if pneumonia does develop in a patient. If this approach is used, it is reassuring to know that it is generally safe to administer repeat pneumococcal vaccinations more frequently than the recommended 5-year interval. A study44 evaluated 179 patients who had received at least three vaccinations and compared their clinical courses to those of 181 patients who had received either one or two doses. Even though 54.6% of those patients who were revaccinated received their repeat vaccinations in < 6 years, there was only one patient with an adverse reaction, which was described as tachycardia and arm redness. Thus, it appears to be safe to administer a vaccination, and although this is not to be done indiscriminately, if it is done, the benefits are likely to far outweigh any associated risks.
In support of the recommendation to give pneumococcal vaccines more widely are the findings of two more recent studies.45 46 One study45 of the new heptavalent conjugated pneumococcal vaccine in children demonstrated a benefit in reducing invasive illness, not only for the population group immunized, but also to adults, particularly those > 65 years of age, who were not the target of the immunization efforts. The findings imply that the vaccination of a large segment of the at-risk population has a benefit for nonvaccinated patients by lowering the incidence and spread of invasive disease.45 Another database study46 evaluated the impact of prior pneumococcal vaccination on patients who had been hospitalized with CAP. Only 12% of 62,918 hospitalized CAP patients had received prior vaccinations, but this group was less likely to die from any cause, and had a lower risk of respiratory failure and other complications, as well as a reduced length of hospital stay, compared to patients who were not vaccinated.46
Conclusion
While the studies of CAP in the past several years have tackled a large number of important topics, the general direction of new developments, which have been discussed in this review, has been to describe ways to improve patient management and patient outcomes. Many of the findings have been incorporated into performance measures related to disease management, and the evidence base to support these recommendations is strong and continues to expand.
Footnotes
Dr. Niederman has been a speaker, consultant, or researcher for Schering, Johnson and Johnson, Aventis, Pfizer, Bayer, Merck, Elan, and Wyeth.
References
- 1.Kaplan V, Clermont G, Griffin MF. Pneumonia: still the old man’s friend? Arch Intern Med. 2003;163:317–323. doi: 10.1001/archinte.163.3.317. [DOI] [PubMed] [Google Scholar]
- 2.Koivula I, Sten M, Makela PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12 year follow-up study. Am J Med. 1999;159:1550–1555. doi: 10.1001/archinte.159.14.1550. [DOI] [PubMed] [Google Scholar]
- 3.Niederman MS, McCombs JI, Unger AN. The cost of treating community-acquired pneumonia. Clin Ther. 1998;20:820–837. doi: 10.1016/s0149-2918(98)80144-6. [DOI] [PubMed] [Google Scholar]
- 4.Aujesky D, Auble TE, Yealy DM. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. 2005;118:384–392. doi: 10.1016/j.amjmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Niederman MS, Feldman C, Richards GA. Combining information from prognostic scoring tools for CAP: an American view on how to get the best of all worlds. Eur Respir J. 2006;27:9–11. doi: 10.1183/09031936.06.00130305. [DOI] [PubMed] [Google Scholar]
- 6.Laterre PF, Garber G, Levy H. Severe community-acquired pneumonia as a cause of severe sepsis: data from the PROWESS study. Crit Care Med. 2005;33:952–961. doi: 10.1097/01.ccm.0000162381.24074.d7. [DOI] [PubMed] [Google Scholar]
- 7.Capelastegui A, Espana PP, Quintana JM. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151–157. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]
- 8.Goss CH, Rubenfeld GD, Park DR. Cost and incidence of social comorbidities in low-risk patients with community-acquired pneumonia admitted ot a public hospital. Chest. 2003;124:2148–2155. doi: 10.1378/chest.124.6.2148. [DOI] [PubMed] [Google Scholar]
- 9.Christ-Crain M, Stolz D, Bingisser R. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 10.Almirall J, Bolibar I, Toran P. Contribution of C-reactive protein to the diagnosis and assessment of severity of community-acquired pneumonia. Chest. 2004;125:1335–1342. doi: 10.1378/chest.125.4.1335. [DOI] [PubMed] [Google Scholar]
- 11.Masia M, Gutierrez F, Shum C. Usefulness of procalcitonin levels in community-acquired pneumonia according to the patients outcome research team pneumonia severity index. Chest. 2005;128:2223–2229. doi: 10.1378/chest.128.4.2223. [DOI] [PubMed] [Google Scholar]
- 12.Boussekey N, Leroy O, Georges H. Diagnostic and prognostic values of admission procalcitonin levels in community-acquired pneumonia in an intensive care unit. Infection. 2005;33:257–263. doi: 10.1007/s15010-005-4096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boussekey N, Leroy O, Alfandari S. Procalcitonin kinetics in the prognosis of severe community-acquired pneumonia. Intensive Care Med. 2006;32:469–472. doi: 10.1007/s00134-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 14.Doern GV, Richter SS, Miller A. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin Infect Dis. 2005;41:139–148. doi: 10.1086/430906. [DOI] [PubMed] [Google Scholar]
- 15.Lujan ML, Gallego M, Fontanals D. Prospective observational study of bacteremic pneumococcal pneumonia: effect of discordant therapy on mortality. Crit Care Med. 2004;32:625–631. doi: 10.1097/01.ccm.0000114817.58194.bf. [DOI] [PubMed] [Google Scholar]
- 16.Yu VL, Chiou CC, Feldman C. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin Infect Dis. 2003;37:230–237. doi: 10.1086/377534. [DOI] [PubMed] [Google Scholar]
- 17.Vanderkooi OG, Low DE, Green K. Predicting antimicrobial resistance in invasive pneumococcal infections. Clin Infect Dis. 2005;40:1288–1297. doi: 10.1086/429242. [DOI] [PubMed] [Google Scholar]
- 18.Fuller JD, Low DE. A review of Streptococcus pneumoniae infection treatment failures associated with fluoroquinolone resistance. Clin Infect Dis. 2005;41:118–121. doi: 10.1086/430829. [DOI] [PubMed] [Google Scholar]
- 19.Francis, JS, Doherty, MC, Lopatin, U, et al Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis 2005; 100-107 [DOI] [PubMed]
- 20.Micek ST, Dunne M, Kollef MH. Pleuropulmonary complications of Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus : importance of treatment with antimicrobials inhibiting exotoxin production. Chest. 2005;128:2732–2738. doi: 10.1378/chest.128.4.2732. [DOI] [PubMed] [Google Scholar]
- 21.De Roux A, Marcos MA, Garcia E. Viral community-acquired pneumonia in nonimmunocompromised adults. Chest. 2004;125:1343–1351. doi: 10.1378/chest.125.4.1343. [DOI] [PubMed] [Google Scholar]
- 22.El Solh AA, Pietrantoni C, Bhat A. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167:1650–1654. doi: 10.1164/rccm.200212-1543OC. [DOI] [PubMed] [Google Scholar]
- 23.Wang JL, Chen KY, Fang CT. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis. 2005;40:915–922. doi: 10.1086/428574. [DOI] [PubMed] [Google Scholar]
- 24.Menendez R, Torres A, Zalacain R. Guidelines for the treatment of community-acquired pneumonia: predictors of adherence and outcome. Am J Respir Crit Care Med. 2005;172:757–762. [Google Scholar]
- 25.Van der Eerden MM, Vlaspolder F, de Graaff CS. Comparison between pathogen directed antibiotic treatment and empirical broad spectrum antibiotic treatment in patients with community acquired pneumonia: a prospective randomised study. Thorax. 2005;60:672–678. doi: 10.1136/thx.2004.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anzueto A, Niederman MS, Pearle J. Community-Acquired Pneumonia Recovery in the Elderly (CAPRIE): efficacy and safety of moxifloxacin therapy versus that of levofloxacin therapy. Clin Infect Dis. 2006;42:73–81. doi: 10.1086/498520. [DOI] [PubMed] [Google Scholar]
- 27.Morganroth J, Dimarco JP, Anzueto A. A randomized trial comparing the cardiac rhythm safety of moxifloxacin vs levofloxacin in elderly patients hospitalized with community-acquired pneumonia. Chest. 2005;128:3398–3406. doi: 10.1378/chest.128.5.3398. [DOI] [PubMed] [Google Scholar]
- 28.Loeb M, Carusone SC, Goeree R. Effect of a clinical pathway to reduce hospitalizations in nursing home residents with pneumonia: a randomized controlled trial. JAMA. 2006;295:2503–2510. doi: 10.1001/jama.295.21.2503. [DOI] [PubMed] [Google Scholar]
- 29.Lonks JR, Goldmann DA. Telithromycin: a ketolide antibiotic for treatment of respiratory tract infections. Clin Infect Dis. 2005;40:1657–1664. doi: 10.1086/430067. [DOI] [PubMed] [Google Scholar]
- 30.Park-Wyllie LY, Juurlink DN, Kopp A. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 31.Clay KD, Hanson JS, Pope SD. Brief communication: severe hepatotoxicity of telithromycin: three case reports and literature review. Ann Intern Med. 2006;4:415–420. doi: 10.7326/0003-4819-144-6-200503210-00121. [DOI] [PubMed] [Google Scholar]
- 32.Blasi F, Tarsia P. Value of short-course antimicrobial therapy in community-acquired pneumonia. Int J Antimicrob Agents. 2005;26:S148–S155. doi: 10.1016/s0924-8579(05)80321-8. [DOI] [PubMed] [Google Scholar]
- 33.Dunbar LM, Wunderink RG, Habib MP. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. 2003;37:752–760. doi: 10.1086/377539. [DOI] [PubMed] [Google Scholar]
- 34.El Moussaui R, de Borgie CA, van den Broek P. Effectiveness of discontinuing antibiotic treatment after three days versus eight days in mild to moderate-severe community acquired pneumonia: randomised, double blind study. BMJ. 2006;32:1355. doi: 10.1136/bmj.332.7554.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan RV, Rhew DC, Murray C. In-hospital observation after antibiotic switch in pneumonia: a national evaluation. Am J Med. 2006;119:e1–E7. doi: 10.1016/j.amjmed.2005.09.012. 512. [DOI] [PubMed] [Google Scholar]
- 36.Leroy O, Saux P, Bedos JP. Comparison of levofloxacin and cefotaxime combined with ofloxacin for ICU patients with community-acquired pneumonia who do not require vasopressors. Chest. 2005;128:172–183. doi: 10.1378/chest.128.1.172. [DOI] [PubMed] [Google Scholar]
- 37.Confalonieri M, Urbino R, Potena A. Hydrocortisone infusion for severe community-acquired pneumonia: a preliminary randomized study. Am J Respir Crit Care Med. 2005;171:242–248. doi: 10.1164/rccm.200406-808OC. [DOI] [PubMed] [Google Scholar]
- 38.Houck PM. Antibiotics and pneumonia: is timing everything or just a cause of more problems? Chest. 2006;130:1–3. doi: 10.1378/chest.130.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Hiramatsu K, Niederman MS. Health-care-associated pneumonia: a new therapeutic paradigm. Chest. 2005;128:3784–3787. doi: 10.1378/chest.128.6.3784. [DOI] [PubMed] [Google Scholar]
- 40.Kollef MH, Shorr A, Tabak YP. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture positive patients. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 41.Waterer GW, Kessler LA, Wunderink RG. Delayed administration of antibiotics and atypical presentation in community-acquired pneumonia. Chest. 2006;130:11–15. doi: 10.1378/chest.130.1.11. [DOI] [PubMed] [Google Scholar]
- 42.Metersky ML, Sweeney TA, Getzow MB. Antibiotic timing and diagnostic uncertainty in Medicare patients with pneumonia: is it reasonable to expect all patients to receive antibiotics within 4 hours? Chest. 2006;130:16–21. doi: 10.1378/chest.130.1.16. [DOI] [PubMed] [Google Scholar]
- 43.Metersky ML, Ma A, Bratzler DW. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342–347. doi: 10.1164/rccm.200309-1248OC. [DOI] [PubMed] [Google Scholar]
- 44.Walker FJ, Singleton RJ, Bulkow LR. Reactions after 3 or more doses of pneumococcal polysaccharide vaccine in adults in Alaska. Clin Infect Dis. 2005;40:1730–1735. doi: 10.1086/430305. [DOI] [PubMed] [Google Scholar]
- 45.Whitney CG, Farley MM, Hadler J. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–1764. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 46.Fisman DN, Abrutyn E, Spaude KA. Prior pneumococcal vaccination is associated with reduced death, complications and length of stay among hospitalized adults with community-acquired pneumonia. Clin Infect Dis. 2006;42:1093–1101. doi: 10.1086/501354. [DOI] [PubMed] [Google Scholar]


