Abstract
Transforming growth factor β (TGFβ) is an immunomodulatory cytokine which is able to modulate the host immune response eliciting an inefficient response against pathogens. In this sense, the role of this cytokine in porcine reproductive and respiratory syndrome (PRRS) has been poorly studied and the reported results are contradictory. Thus, in the present study, the expression of TGFβ was analysed both at tissue (lymphoid organs and lung) and serum level to study its correlation with the expression of PRRS virus (PRRSV). To carry out this study, 32 pigs were inoculated with the European PRRSV field isolate 2982 and sequentially killed from 0 dpi to the end of the study (24 dpi). Blood and tissue samples were collected to determine the expression of PRRSV and TGFβ. PRRSV was detected in inoculated animals from 3 dpi until the end of the study, however TGFβ was not detected in sera from inoculated animals. Contrary, an increase of TGFβ antigen was observed both in the lymphoid organs and in the lung of PRRSV-inoculated pigs when compared with control group. Since TGFβ play a role as an immunomodulatory cytokine of the immune response and also in the differentiation of regulatory T cells (Tregs), the upregulation of the TGFβ at tissue level may play a role in the impairment of the host immune response observed during PRRS, being observed a significant correlation between PRRSV and TGFβ expression at lung level.
Keywords: Porcine reproductive and respiratory syndrome, Tonsil, Lung, Serum, Transforming growth factor beta
1. Introduction
Transforming growth factor β (TGFβ) together with interleukin-10 (IL-10) are considered as immunomodulatory cytokines which are able to downregulate the host immune response (Letterio and Roberts, 1998). TGFβ1, one of the three isoforms of TGFβ (Javelaud and Mauviel, 2004), is able to inhibit macrophage activation by means of two mechanisms: (1) the inhibition of the synthesis of IFNγ, which activates macrophages and acts as an antiviral responder (Letterio and Roberts, 1998); and (2) the promotion of the production of IL-10 (Maeda et al., 1995).
Porcine Reproductive and Respiratory Syndrome (PRRS) is a worldwide spread pig disease caused by an arterivirus (Fauquet et al., 2005), known as PRRS virus (PRRSV), which induces an impairment of the host immune response favouring a prolonged viraemia and viral replication (Darwich et al., 2010). Few reports have been focused on the expression of TGFβ during PRRSV infection and the scarce results are controversial. Whereas no enhancement in mRNA or protein levels of TGFβ has been reported after infection or vaccination with European or type I PRRSV genotypes (Díaz et al., 2006, Silva-Campa et al., 2010), an increased mRNA and protein expression of TGFβ has been observed in infections with North American or type II genotypes (Silva-Campa et al., 2009, Renukaradhya et al., 2010).
Recently, our group has studied the serum and tissue expression of PRRSV and proinflammatory cytokines in PRRSV-infected pigs, showing a paracrine synthesis of these cytokines associated with a poor serum but a marked tissue expression (Gómez-Laguna et al., 2010a, Gómez-Laguna et al., 2010b, Barranco et al., 2011b). In the present study, samples from those previous experiments were used to analyse the expression of TGFβ both at serum and tissue level in an infection with a European isolate of PRRSV, in order to elucidate its potential role in the immunopathogenesis of PRRS.
2. Materials and methods
2.1. Virus, animals and experimental design
Twenty eight pathogen free, 5-week-old pigs from a PRRSV seronegative farm were randomly assigned to groups of four and inoculated by the intramuscular route with 1 ml of the third passage of PRRSV field isolate 2982 (with an ORF-5 homology of 93% with Lelystad virus; GenBank accession no. EF429108) at 103.0 TCID50. The inoculated animals were killed at 3, 7, 10, 14, 17, 21 and 24 days post-inoculation (dpi). Another group of four pigs were sham-inoculated controls, which were injected intramuscularly with 1 ml of sterile RPMI 1640 medium and killed at the end of the study (24 dpi). All animals were sedated with tiletamine-zolazepam (Zoletil™; Virbac; Barcelona, Spain) followed by a lethal dose of 5% sodium thiopental (Thiovet™; Vet Limited; Leyland, Lancashire, England). The experiment was carried out according to the guidelines of the European Union (Directive 86/609/EEC) and was approved by the local ethical committee of Centro de Investigación en Sanidad Animal (CISA-INIA; Valdeolmos, Madrid, Spain).
2.2. Clinical signs, viraemia and serum detection of TGFβ
The pigs were monitored daily for clinical signs, i.e. rectal temperature and a clinical respiratory score, as described previously (Gómez-Laguna et al., 2010a).
Blood samples were taken in EDTA-free tubes from eight animals at the different time-points (but the four remaining animals at 24 dpi), and were allowed to clot and centrifuged to obtain serum samples. Virus titration was carried out using an immunoperoxidase monolayer assay (IPMA) as previously reported (Wensvoort et al., 1991).
Serum samples were analysed for TGFβ expression by means of a commercial ELISA kit, following manufacturer's instructions (Swine TGFβ ELISA kit, Biosource). The ELISA kit was carried out using a non-species-specific monoclonal antibody and its sensitivity threshold was 15.6 pg/ml. All samples were analysed in duplicate. TGFβ concentration was calculated by using the linear-regression formula from optical densities of the cytokine standards provided by the manufacturer.
2.3. Histopathology and immunohistochemistry
Samples from the medial lobe of the right lung, mediastinal lymph node and tonsil were fixed in 10% neutral buffered formalin and in Bouin's solution, processed routinely and embedded in paraffin-wax. Four-μm sections of formalin-fixed tissue were stained with haematoxylin and eosin (HE) for histopathology examination.
The avidin-biotin-peroxidase complex technique (ABC) was used in Bouin-fixed samples for the immunohistochemical detection of PRRSV, and TGFβ proteins as described previously (Muñoz et al., 2009, Gómez-Laguna et al., 2010b). Primary antibodies monoclonal anti-PRRSV, clone SDOW-17/SR-30 (Rural Technologies Inc.), diluted 1 in 1000; and polyclonal chicken anti-recombinant human TGF-β1 (R&D Systems, Minneapolis, MN), diluted 1 in 100 were incubated overnight at 4 °C in a humid chamber. In each case, the corresponding biotinylated secondary antibody was incubated for 30 min at room temperature. An avidin-peroxidase complex (Vector Laboratories; Burlingame, CA, USA) was applied for 1 h at room temperature. Labelling was “visualized” by application of the NovaRED™ substrate kit (Vector Laboratories; Burlingame, CA, USA). For negative controls, the primary antibody was replaced by blocking solution, normal serum and isotype-matched reagents of irrelevant specificity.
2.4. Cell counting
The number of labelled cells were counted in 50 non-overlapping and consecutively selected high magnification fields of 0.20 mm2 (pulmonary parenchyma, paracortex and medulla of mediastinal lymph nodes, and lymphoreticular areas of tonsils) or 25 non overlapping consecutive selected structures (lymphoid follicles of mediastinal lymph nodes and tonsils) for each animal. Results are expressed as the number of cells per mm2. Immunolabelled cells were identified and counted morphologically as macrophages, lymphocytes, neutrophils or dendritic cells.
2.5. Statistical analysis
All the values are expressed as the mean ± SD. Since control animals were bled at 0, 7, 14, 21 and 24 dpi, blood values of inoculated animals at 3, 10 and 17 dpi were analysed with the mean value of the control animals at the prior- and post-time points. The values of all the studied parameters were evaluated for approximate normality of distribution by using Kolmogorov–Smirnov test. Differences between the means of control and inoculated animals were assessed by the Kruskal–Wallis test followed by the Mann–Whitney-U non-parametric test (GraphPad Instat 3.05; San Diego, CA, USA). Correlation between the expression of PRRSV and TGFβ antigens was assessed by the Spearman test (GraphPad Instat 3.05). P < 0.05 was considered to represent a statistically significant difference.
3. Results
3.1. Clinical signs, viraemia and serum expression of TGFβ
Control animals displayed no clinical signs throughout the study. No differences were observed in the respiratory score between control and inoculated animals; however, from 3 dpi inoculated animals presented mild dullness and weight loss. The rectal temperature was mildly elevated at 3 and 10 dpi, but remained always between physiological ranges.
No virus was detected in control animals throughout the study. In inoculated animals, virus was first detected in blood samples at 3 dpi, showing maximum levels at 10 dpi and decreasing by the end of the study (Table 1 ). Virus was still detected in 2/4 animals at 24 dpi.
Table 1.
Virus titre (expressed as log 10) in PRRSV-inoculated pigs with the European 2982 PRRSV field isolate throughout the study.
| dpi | Viremic animals | Mean ± SD |
|---|---|---|
| 0 | 0/8 | 0.000 ± 0.000 |
| 3 | 4/8 | 1.362 ± 1.293 |
| 7 | 8/8 | 2.885 ± 0.659 |
| 10 | 8/8 | 3.280 ± 0.657 |
| 14 | 7/8 | 2.890 ± 1.416 |
| 17 | 6/8 | 2.308 ± 1.626 |
| 21 | 4/8 | 1.109 ± 1.220 |
| 24 | 2/4 | 1.068 ± 1.241 |
TGFβ serum concentration was below detection limit throughout the study in all the animals from both control and inoculated groups.
3.2. Histopathological examination
PRRSV-infected animals displayed the typical histopathological lesions of the disease with a marked thickening of the alveolar septa of the lung by mononuclear cell infiltration, together with a mild hypertrophy and hyperplasia of type 2 pneumocytes observed from 7 dpi onwards. A mild hypertrophy of germinal centres and apoptotic bodies were observed in the lymphoid follicles of the mediastinal lymph node and to a lesser extent in the tonsil from 7 dpi onwards. No significant lesions were observed in control animals.
3.3. Immunohistochemical expression of PRRSV and TGFβ antigens
PRRSV antigen was detected mainly in the cytoplasm of alveolar macrophages, and secondly in septal macrophages, in the lung (Fig. 1, Fig. 2 ), in the cytoplasm of macrophages in the medulla of the mediastinal lymph node (Fig. 1, Fig. 2) and in macrophages in the lymphoreticular areas of the tonsil (Fig. 1, Fig. 2). Occasionally scattered dendritic-like cells were observed both in the mediastinal lymph node and in the tonsil of inoculated animals. Viral expression in the lung and lymphoid organs of PRRSV-infected pigs was observed from 3 dpi until the end of the study, however, a different trend was observed in each examined organ. Whereas the expression of PRRSV reached a maximum at 7 dpi in the lung and mediastinal lymph node parenchyma decreasing onwards, a two-peak curve was observed in the tonsil, with a first peak at 3 dpi followed by a second peak at 14 dpi (Fig. 2).
Fig. 1.
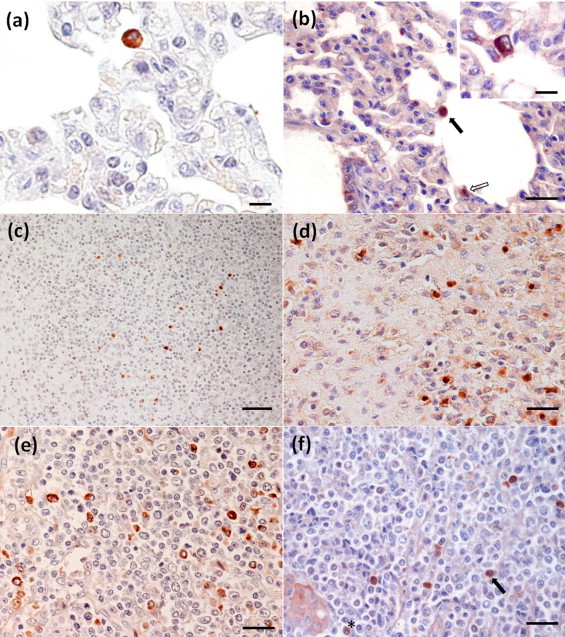
Immunohistochemical labelling against PRRSV (a,c,e) and TGFβ (b,d,f) antigens in inoculated animals. (a) Intracytoplasmic PRRSV immunolabelling in a PAM of a pig killed at 7 dpi. IHC. Bars, 10 μm. (b) Lung section with a moderate thickening of the alveolar septa showing a septal macrophage (empty arrow) and a PAM (black arrow) with intracytoplasmic immunostaining against TGFβ antigen in a pig killed at 24 dpi. IHC. Bars, 20 μm. Inset. A detail of the intracytoplasmic immunolabelling against TGFβ in a PAM a los 7 dpi. IHC. Bars, 10 μm. (c) PRRSV-positive macrophages in the medulla and paracortex of the mediastinal lymph node of a pig killed at 3 dpi. IHC. Bars, 40 μm. (d) Immunolabelling of numerous macrophages expressing TGFβ antigen in the medulla of the mediastinal lymph node of a PRRSV-inoculated pig killed at 3 dpi. IHC. Bars, 20 μm. (e) Numerous macrophages in the lymphoreticular tissue of the tonsil from a pig killed at 17 dpi showing an intense intracytoplasmic labelling against SDOW17/SR30 antibodies. IHC. Bars, 20 μm. (f) Several macrophages, and a lymphocyte (asterisk) and a neutrophil (arrow) showing positive immunolabelling against TGFβ antigen. IHC. Bars, 20 μm.
Fig. 2.
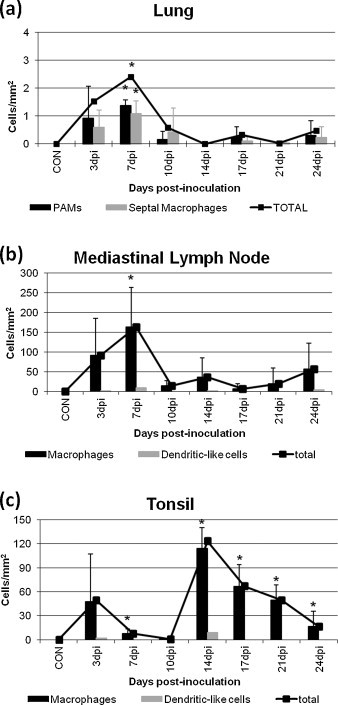
Frequency of PRRSV positive cells in the lung (a), mediastinal lymph node (b), and tonsil (c) of control and inoculated animals. * indicates significant differences (P < 0.05) with respect to the control group.
The pulmonary and mediastinal lymph node expression of TGFβ displayed a similar curve than the one observed for PRRSV antigen in the lung but peaking at 3 dpi and decreasing onwards (Fig. 3a and b). A significant correlation was observed between both parameters in the lung of PRRSV-infected pigs (r = 0.72; P < 0.05), but not in the mediastinal lymph node (r = 0.55; P = 0.17) (Fig. 4a and b, respectively). Moreover, TGFβ protein was chiefly observed in the cytoplasm of alveolar macrophages, and secondly in the cytoplasm of septal macrophages, neutrophils and lymphocytes (Fig. 1, Fig. 3). In the mediastinal lymph node, TGFβ protein was mainly observed in the cytoplasm of macrophages in the medulla and paracortex and secondly in the cytoplasm of lymphocytes from both structures, being observed at low frequencies in the cytoplasm of other cells, just as neutrophils or dendritic cells (Fig. 1, Fig. 3).
Fig. 3.
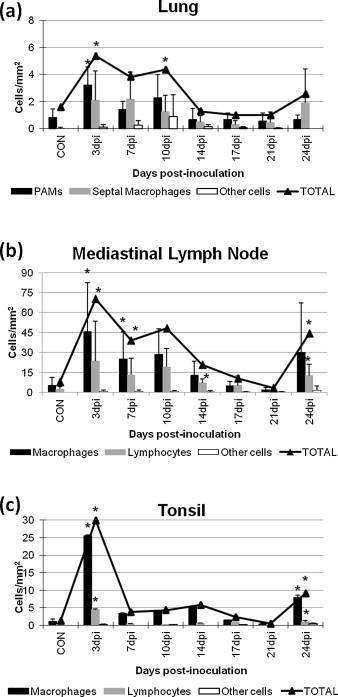
Frequency of TGFβ positive cells in the lung (a), mediastinal lymph node (b), and tonsil (c) of control and inoculated animals. * indicates significant differences (P < 0.05) with respect to the control group.
Fig. 4.
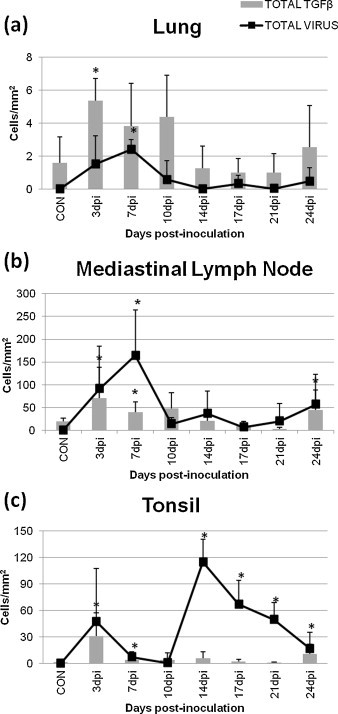
Counts for the total expression of the antigen of PRRSV (dot-line) and TGFβ (column) in the lung (a), mediastinal lymph node (b), and tonsil (c) of control and inoculated animals. * indicates significant differences (P < 0.05) with respect to the control group.
The expression of TGFβ in the tonsil of PRRSV-infected pigs also displayed a maximum at 3 dpi (P < 0.05) decreasing onwards (Fig. 3c), but no correlation was observed between the expression of this cytokine and PRRSV antigen in the tonsil (r = 0.12; P = 0.79) (Fig. 4c). Similarly to mediastinal lymph node, the expression of TGFβ was detected in the cytoplasm of macrophages and to a lesser extent in the cytoplasm of lymphocytes and neutrophils in the lymphoreticular areas of the tonsil (Fig. 1, Fig. 3). Additionally, the number of both PRRSV- and TGFβ-positive cells was significantly higher in lymphoid organs than in the lung of PRRSV-infected pigs (P < 0.05).
4. Discussion
The impairment of the immune response evoked during PRRSV infection is one of the major paradigms of the immunology in the modern research of porcine diseases. Several efforts are being conducted to elucidate the mechanisms used by the virus to evade the host immune response. In this sense, a significant role for IL-10 has been suggested in some reports (Díaz et al., 2005, Díaz et al., 2006, Gómez-Laguna et al., 2009, Gómez-Laguna et al., 2010b), being observed a correlation between the expression of PRRSV and IL-10 in the lung of PRRSV-infected animals (Gómez-Laguna et al., 2010b).
In our study, the lack of lesions and viraemia in control animals, and the evidence of both histopathological lesions and viraemia in infected animals, support the efficient infection of inoculated animals. However, no serum expression of TGFβ was detected throughout the study neither in control nor in infected animals, contrary to the results obtained from the immunohistochemical study. Recently, an enhancement in the expression of TGFβ has been reported for type II PRRSV genotypes (Silva-Campa et al., 2009, Renukaradhya et al., 2010) but not for type I genotypes (Díaz et al., 2005, Díaz et al., 2006, Silva-Campa et al., 2010). This finding may be related to the higher virulence attributed to type II genotypes of PRRSV (Martínez-Lobo et al., 2011), which usually trigger off a more accentuated cytokines cascade. In our study, the lack of serum expression of TGFβ together with the local expression of this cytokine at lymphoid organs and lung level, confirm this hypothesis, and point to a role for the local expression of cytokines in the modulation of the immune response in infections with European PRRSV isolates.
In our study the immunohistochemical study showed a significant correlation between the lung expression of PRRSV and TGFβ in infected animals, but no correlation was observed between these parameters neither in the mediastinal lymph node nor in the tonsil of infected animals. These results may point either to different roles of TGFβ in each infected organ or to different mechanisms involved in the synthesis of this cytokine in each organ during PRRS. Interestingly, similar findings were observed concerning the expression of PRRSV and IL-10 in the examined organs in parallel studies carried out by our research group (Gómez-Laguna et al., 2010b, Barranco et al., 2011a), which suggests a synergic action between the local expression of TGFβ and IL-10. The correlation observed between PRRSV and TGFβ antigens in the lung in the present study points to a direct induction of the production of TGFβ by PRRSV replication, whereas the different trend showed by these parameters in lymphoid organs suggests that either TGFβ may play a minor role in the modulation of the immune response in the mediastinal lymph node and in the tonsil of PRRSV-infected pigs or that an indirect mechanism might be involved in the expression of this cytokine in these organs during PRRSV infection.
Infections with other porcine viral or bacterial respiratory pathogens have showed also an enhancement on the expression of TGFβ. In this sense, Renukaradhya et al. (2010) reported a similar kinetics in the expression of TGFβ in the lung of PRRSV-infected pigs and in the lung of porcine respiratory coronavirus (PRCV)-infected pigs which trended to be higher in a co-infection PRRSV-PRCV model. This finding points to a role in the modulation of the immune response at lung level making easier secondary infections with either bacterial or other viral respiratory pathogens.
Additionally, an increase in the gene expression of TGFβ1 has also been reported in “fully resistant” compared to “susceptible” animals to Haemophilus parasuis infection (Wilkinson et al., 2010). Interestingly, “fully resistant” animals showed milder respiratory lesions which may be related to a role of this cytokine in controlling the onset of pulmonary lesions after respiratory infections. These findings agree with the hypothesis that the expression of immunomodulatory cytokines in PRRSV infection, as well as in other porcine respiratory diseases, helps to control a proinflammatory cytokines storm and to preserve the lung function.
In a previous study carried out by our group a marked expression of proinflammatory cytokines and interferons was observed in septal macrophages but no in PAMs in the lung of PRRSV-infected pigs (Gómez-Laguna et al., 2010b). Conversely, in the present study the expression of TGFβ was mainly observed in PAMs and secondly in other cells, such as septal macrophages, neutrophils and/or lymphocytes. Thus, taking into account the immunomodulatory role of TGFβ (Letterio and Roberts, 1998), the expression of this cytokine by PAMs may be involved in the lack of expression of IFNγ by these cells, and the subsequent lack of activation and impairment of PAMs, which favour secondary bacterial infections. In addition, comparing our results with parallel studies from our group (Barranco et al., 2011a) the enhancement on the expression of TGFβ observed in the present study coincided with a lower expression of proinflammatory cytokines in the lung and lymphoid organs of pigs inoculated with PRRSV field isolate 2982 (Gómez-Laguna et al., 2010a, Barranco et al., 2011a). All these findings suggest that the expression of TGFβ may play a significant role not only in the modulation of the immune response at local levels but also limiting the onset of an exacerbated inflammatory response.
Furthermore, it has been reported that TGFβ is able to downregulate the expression of CD163 (Pioli et al., 2004), a PRRSV receptor involved in viral uncoating (Van Gorp et al., 2008). Thus, the expression of TGFβ observed in our study in the lung and lymphoid organs of PRRSV-infected animals may be related with a regulation of receptor CD163, and indeed with a control of PRRSV replication in the examined organs.
Recently, a significant investment is being carried out to study the modulation of the immune response by regulatory T cells (Tregs). Nonetheless, few studies have been yet performed to determine the role of Tregs in PRRS. Thus, a TGFβ-dependent induction of Th3 cells by PRRSV-infected dendritic cells has been reported (Silva-Campa et al., 2009). In the present study we observed an enhancement in the expression of TGFβ in the lung and lymphoid organs of PRRSV-infected animals from 3 dpi onwards, which may be involved in the induction of Tregs observed by other authors. Further studies are being conducted to confirm the role of TGFβ in the induction of Tregs during PRRS.
In conclusion, in the present study we have shown that TGFβ is expressed locally in the lymphoid organs and lung of pigs infected with type I PRRSV genotypes. The expression of this cytokine may either favour or difficult the onset of an efficient host immune response. On the one hand, TGFβ may be able both to avoid overproduction of proinflammatory cytokines at tissue level and to difficult PRRSV replication by downregulating the expression of CD163. On the other hand, TGFβ may impair the host immune response by (1) inhibiting an efficient expression of antiviral cytokines, and/or (2) inducing the differentiation of Tregs.
Acknowledgements
We thank Dr. E. Mateu for his kind gift of PRRSV field isolate 2982 and G. Muñoz for her technical assistance. This work was supported financially by the Spanish Ministry of Education and Science, project number AGL2009-12438/GAN and EU funded NADIR project (FP7).
References
- Barranco I., Gómez-Laguna J., Rodríguez-Gómez I.M., García-Nicolás O., Quereda J.J., Salguero F.J., Ramis G., Pallarés F.J., Carrasco L. IL-10, IL-12 IFNα and IFNγ immunohistochemical expression in lymphoid organs of porcine reproductive and respiratory syndrome virus-infected pigs. Proceedings of the 3rd European Symposium of Porcine Health and Management; Espoo, Finlandia; 2011. p. 106. [Google Scholar]
- Barranco I., Gómez-Laguna J., Rodríguez-Gómez I.M., Salguero F.J., Pallarés F.J., Carrasco L. Differential expression of proinflammatory cytokines in the lymphoid organs of porcine reproductive and respiratory syndrome virus-infected pigs. Transbound. Emerg. Dis. 2011 doi: 10.1111/j.1865-1682.2011.01252.x. [DOI] [PubMed] [Google Scholar]
- Darwich L., Díaz I., Mateu E. Certainties, doubts and hypotheses in porcine reproductive and respiratory syndrome virus immunobiology. Virus Res. 2010;154:123–132. doi: 10.1016/j.virusres.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Díaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. Immune responses of pigs after experimental infection with a European strain of porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2005;86:1943–1951. doi: 10.1099/vir.0.80959-0. [DOI] [PubMed] [Google Scholar]
- Díaz I., Darwich L., Pappaterra G., Pujols J., Mateu E. Different European-type vaccines against porcine reproductive and respiratory syndrome virus have different immunological properties and confer different protection to pigs. Virology. 2006;351:249–259. doi: 10.1016/j.virol.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A. Virus taxonomy classification and nomenclature of viruses. In: Fauquet C.M., Mayo M.A., Maniloff J., Desselberger U., Ball L.A., editors. 8th ICTV Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press; London: 2005. p. 1259. [Google Scholar]
- Gómez-Laguna J., Salguero F.J., De Marco M.F., Pallarés F.J., Bernabé A., Carrasco L. Changes in lymphocyte subsets and cytokines during European porcine reproductive and respiratory syndrome: increased expression of IL-12 and IL-10 and proliferation of CD4(-)CD8(high) Viral Immunol. 2009;22:261–271. doi: 10.1089/vim.2009.0003. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., Pallarés F.J., Fernández de Marco M., Barranco I., Cerón J.J., Martínez-Subiela S., Van Reeth K., Carrasco L. Acute phase response in porcine reproductive and respiratory syndrome virus infection. Comp. Immunol. Microbiol. Infect. Dis. 2010;33:51–58. doi: 10.1016/j.cimid.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Gómez-Laguna J., Salguero F.J., Barranco I., Pallarés F.J., Rodríguez-Gómez I.M., Bernabé A., Carrasco L. Cytokine expression by macrophages in the lung of pigs infected with the porcine reproductive and respiratory syndrome virus. J. Comp. Pathol. 2010;142:51–60. doi: 10.1016/j.jcpa.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javelaud D., Mauviel A. Mammalian transforming growth factor-betas: Smad signaling and physio-pathological roles. Int. J. Biochem. Cell. Biol. 2004;36:1161–1165. doi: 10.1016/S1357-2725(03)00255-3. [DOI] [PubMed] [Google Scholar]
- Letterio J.J., Roberts A.B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- Maeda H., Kuwahara H., Ichimura Y., Ohtsuki M., Kurakata S., Shiraishi A. TGF-beta enhances macrophage ability to produce IL-10 in normal and tumor-bearing mice. J. Immunol. 1995;155(10):4926–4932. [PubMed] [Google Scholar]
- Martínez-Lobo F.J., Díez-Fuertes F., Segalés J., García-Artiga C., Simarro I., Castro J.M., Prieto C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet. Microbiol. 2011 doi: 10.1016/j.vetmic.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Muñoz M., Delgado L., Verna A., Benavides J., García-Pariente C., Fuertes M., Ferreras M.C., García-Marín J.F., Pérez V. Expression of transforming growth factor-beta 1 (TGF-beta1) in different types of granulomatous lesions in bovine and ovine paratuberculosis. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:239–252. doi: 10.1016/j.cimid.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Pioli P.A., Goonan K.E., Wardwell K., Guyre P.M. TGF-beta regulation of human macrophage scavenger receptor CD163 is Smad3-dependent. J. Leukoc. Biol. 2004;76:500–508. doi: 10.1189/jlb.1203617. [DOI] [PubMed] [Google Scholar]
- Renukaradhya G.J., Alekseev K., Jung K., Fang Y., Saif L.J. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral. Immunol. 2010;23(5):457–466. doi: 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Campa E., Flores-Mendoza L., Reséndiz M., Pinelli-Saavedra A., Mata-Haro V., Mwangi W., Hernández J. Induction of T helper 3 regulatory cells by dendritic cells infected with porcine reproductive and respiratory syndrome virus. Virology. 2009;387:373–379. doi: 10.1016/j.virol.2009.02.033. [DOI] [PubMed] [Google Scholar]
- Silva-Campa E., Cordoba L., Fraile L., Flores-Mendoza L., Montoya M., Hernández J. European genotype of porcine reproductive and respiratory syndrome (PRRSV) infects monocyte-derived dendritic cells but does not induce Treg cells. Virology. 2010;396:264–271. doi: 10.1016/j.virol.2009.10.024. [DOI] [PubMed] [Google Scholar]
- Van Gorp H., Van Breedam W., Delputte P.L., Nauwynck H.J. Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J. Gen. Virol. 2008;89:2943–2953. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- Wensvoort G., Terpstra C., Pol J.M., ter Laak E.A., Bloemraad M., de Kluyver E.P., Kragten C., van Buiten L., den Besten A., Wagenaar F. Mystery swine disease in The Netherlands: the isolation of Lelystad virus. Vet. Q. 1991;13:121–130. doi: 10.1080/01652176.1991.9694296. [DOI] [PubMed] [Google Scholar]
- Wilkinson J.M., Sargent C.A., Galina-Pantoja L., Tucker A.W. Gene expression profiling in the lungs of pigs with different susceptibilities to Glässer's disease. BMC Genomics. 2010;11:455. doi: 10.1186/1471-2164-11-455. [DOI] [PMC free article] [PubMed] [Google Scholar]


