Abstract
Background
2009 pandemic influenza A(H1N1) has led to a global increase in severe respiratory illness. Little is known about kidney outcomes and dialytic requirements in critically ill patients infected with pandemic H1N1.
Study Design
Prospective observational study.
Setting & Participants
50 patients with pandemic H1N1 admitted to any of 7 intensive care units in Manitoba, Canada, were prospectively followed.
Outcome & Measurements
Outcomes were kidney injury and kidney failure defined using RIFLE (risk, injury, failure, loss, end-stage disease) criteria or need for dialysis therapy.
Results
The pandemic H1N1 group was composed of 50 critically ill patients with pandemic H1N1 with severe respiratory syndrome (47 confirmed cases, 3 probable). Kidney injury, kidney failure, and need for dialysis occurred in 66.7%, 66%, and 11% of patients, respectively. Mortality was 16%. Kidney failure was associated with increased death (OR, 11.29; 95% CI, 1.29-98.9), whereas the need for dialysis was associated with an increase in length of stay (RR, 2.38; 95% CI, 2.13-25.75).
Limitations
Small population studied from single Canadian province; thus, limited generalizability.
Conclusions
In critically ill patients with pandemic H1N1, kidney injury, kidney failure, and the need for dialysis are common and associated with an increase in mortality and length of intensive care unit stay.
Index Words: Acute kidney injury, intensive care unit, influenza A(H1N1)
Recent reports from Canada, Mexico, the United States, and Australia have illustrated the burden of 2009 pandemic influenza A(H1N1) infection on intensive care unit (ICU) resources, with an emphasis on respiratory failure, mechanical ventilation, and extracorporeal membrane oxygenation.1, 2, 3, 4, 5, 6, 7 Currently, little information exists about the impact of kidney injury and resource utilization in the form of dialytic support in critically ill patients with pandemic H1N1 infections.8, 9 Patients admitted to the ICU with acute kidney injury (AKI) are at increased risk of mortality, lengthened ICU and hospital stays, and the development of chronic kidney disease.10, 11, 12, 13, 14, 15, 16, 17, 18 It is unknown whether this observation can be extended to AKI in the setting of severe viral respiratory infections requiring ICU admission. During the severe adult respiratory syndrome coronavirus outbreak, kidney injury and the need for dialysis were uncommon; however, whether this holds true for pandemic H1N1 infection is unknown.17, 18
The objective of this study is to describe rates of kidney injury, kidney failure, and need for dialysis therapies in critically ill patients infected with pandemic H1N1.
Methods
Study Population and Design
The study population consisted of all patients (aged >8 years) admitted to any of the 7 ICUs serving the Province of Manitoba, Canada (population, 1,200,000). Admissions for patients with 2009 pandemic influenza A(H1N1) infection–related critical illness from April 1, 2009, to August 27, 2009, were included. Local research ethics board approval was obtained.
Data Collection
ICU data were collected prospectively for all patients with pandemic H1N1, as described elsewhere.6 Collected data include patient demographics, admitting and discharge diagnoses, laboratory values, physiologic variables, treatments, and in-hospital survival. Supplemental data and data validation for both cohorts were completed using independent retrospective chart review. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated at admission for all patients.19
Cohort Definitions
Infection with 2009 pandemic influenza A(H1N1) was classified according to the case definitions of the World Health Organization (WHO) and the Canadian National Microbiology Laboratory as follows. A confirmed case is defined as a person with an acute febrile respiratory illness with laboratory-confirmed influenza A(H1N1) virus (swine flu) infection (eg, at Cadham Provincial Laboratory/National Microbiology Laboratory) using 1 or more of the following tests: real-time reverse transcription–polymerase chain reaction (RT-PCR) or viral culture or pre/post antibody testing, and a probable case of infection is defined as a person with an acute febrile respiratory illness who is positive for influenza A, but negative for H1 and H3 using influenza RT-PCR or positive for influenza A using an influenza rapid test or an influenza immunofluorescence assay plus meets criteria for a suspected case.20 Only confirmed or probable cases were included in our study. Critical illness and ICU admission were determined by the requirement for invasive or noninvasive mechanical ventilation, need for a fraction of inspired oxygen ≥60%, or need for intravenous inotropes or vasopressor medications requiring ICU admission.6 Chronic kidney injury was defined as baseline creatinine level >140 μmol/L. Dialysis dependence was defined as the need for dialysis therapy before admission to the ICU.
Outcome Definitions
The primary outcomes were AKI and acute kidney failure according to the RIFLE (risk, injury, failure, loss, end-stage disease) criteria and use of renal replacement therapy.21 Secondary outcomes were length of ICU stay and 28-day mortality. The RIFLE criteria for kidney injury are a validated tool for determining kidney injury based on the presence of either an increase in serum creatinine level from baseline or a decrease in urine output.12, 21, 22, 23, 24, 25, 26, 27 Severity is classified as risk of kidney injury and kidney failure. Dialytic therapies included the need for continuous replacement therapy and/or intermittent hemodialysis.
Data Analysis
Continuous variables of interest were summarized as mean or median with standard deviation or interquartile range, as appropriate. Between-groups comparisons in baseline admission characteristics, presence or absence of AKI, or need for dialytic therapies were made using independent-samples t test for continuous variables and χ2 or Fischer exact test for dichotomous variables. Laboratory, clinical, and physiologic variables and outcomes were assessed during ICU admission days 1, 3, 7, 14, and 28. Odds ratios for outcomes were determined using univariate logistic regression. The 95% confidence intervals (CIs) and P values were considered statistically significant for P ≤ 0.05. Statistical analyses were performed using SPSS, version 16 (www.spss.com).
Results
As of August 31, 2009, there were 221 hospitalized cases of pandemic H1N1 in the Province of Manitoba, Canada, with 11 confirmed deaths and 50 total admissions to the ICU for ventilator support.28 Of 50 patients, 47 (96%) were confirmed and 3 (4%) were probable by WHO criteria.
The average age of patients admitted to the ICU with pandemic H1N1 was 35.5 ± 15.8 years, and most were female (72%). Racial information was available for 66 of 82 (80%) of the pandemic H1N1 cohort, corresponding to 48% aboriginal, 36% white, and 14% other. Admission characteristics for pandemic H1N1 patients are listed in Table 1, Table 2. Average body mass index was high (34.8 ± 12.0 kg/m2), with the comorbid conditions of diabetes mellitus, cigarette smoking, asthma, and hypertension occurring in more than one-fifth of the cohort. Eight patients were pregnant at an average term of 25.8 ± 12.3 weeks. Mean APACHE II score and Glasgow Coma Scale were 19 ± 6 and 11.5 (interquartile range, 10-13), respectively. Most patients required vasopressor or inotropic blood pressure support medications (66%), and all required mechanical ventilation. Median creatine kinase value was increased at 325 μmol/L (interquartile range, 73-903).
Table 1.
Baseline Characteristics of Patients Admitted to the ICU With Pandemic H1N1 Infection
| Admissions | 50 |
| Demographics | |
| Age (y) | 35.5 ± 15.8 |
| Women | 36 (72) |
| Body mass index (kg/m2) | 34.8 ± 12.0 |
| Comorbid conditions | |
| Arrhythmia | 1 (2) |
| Asthma | 12 (24) |
| Coronary artery disease | 2 (4) |
| Cancer | 1 (2) |
| Chemotherapy | 1 (2) |
| Congestive heart failure | 3 (6) |
| Chronic obstructive pulmonary disease | 6 (12) |
| Diabetes | 17 (34) |
| Hypertension | 11 (22) |
| Immunosuppression | 8 (16) |
| Peripheral vascular disease | 3 (6) |
| Smoker | 16 (32) |
| Steroids | 11 (22) |
| Pregnancy | 8 (16) |
| Pregnancy (wk) | 25.8 ± 12.3 |
| Chronic kidney disease | 2 (4) |
Note: Values expressed as mean ± standard deviation, or number (percentage).
Abbreviation: ICU, intensive care unit.
Table 2.
Selected Physiologic and Laboratory Characteristics of Patients on Admission to the ICU With Pandemic H1N1
| Mean APACHE II score | 19.2 ± 6.5 |
| Mean arterial pressure (mm Hg) | 74.1 (62.3-83.1) |
| Glasgow Coma Scale | 11.5 (10.0-13.0) |
| Inotropes or vasopressor medications | 33 (66) |
| Mechanical ventilation | 50 (100) |
| Central venous pressure (mm Hg) | 11.0 (7.5-16.0) |
| Positive end-expiratory pressure (mm Hg) | 10.0 (8.0-14.5) |
| White blood cell count (×109/L) | 6.9 (3.7-12.4) |
| Platelets (×109/L) | 172 (123-228) |
| Creatine kinase (U/L) | 325 (73-903) |
| Troponin T (μg/L) | 0.03 (0.01-0.22) |
| Alanine transaminase (U/L) | 35.5 (21.0-70.5) |
| Aspartate aminotransferase (U/L) | 67.0 (33.5-127.3) |
| Creatinine (μmol/L) | 72 (48.0-111) |
Note: Values expressed as number, mean ± standard deviation, number (interquartile range), or number (percentage). Conversion factor for creatinine in μmol/L to mg/dL, ×0.0113; no conversion necessary for troponin T in μg/L and ng/mL or white blood cell count and platelets in 109/L and 103/μL.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ICU, intensive care unit.
Patient outcomes are listed in Table 3. AKI occurred in 66.7%; acute renal failure, in 66%; and the requirement for dialysis, in 22% of patients with pandemic H1N1. Of 11 patients requiring dialysis after admission to the ICU, 2 (22%) died and 1 (11%) required ongoing dialysis therapy upon discharge. Median length of stay was 16.0 days (interquartile range, 9-38), with mortality of 16%.
Table 3.
Outcomes of Patients With Pandemic H1N1 Influenza A Admitted to the ICU
| Kidney injury | 32/48 (66.7) |
| By urine output criteria | 29/48 (60.4) |
| By creatinine criteria | 12/48 (25) |
| On day 1 | 23/48 (48) |
| Kidney failure | 33/50 (66) |
| By urine output criteriaa | 20/50 (40) |
| By creatinine criteria | 13/50 (26) |
| On day 1 | 14/50 (28) |
| Length of ICU stay (d) | 16.0 (9.0-38.0) |
| Dialysis at discharge | 1 (2) |
| Dialysis | 11 (22) |
| Death | 8 (16) |
Note: Values expressed as number (percentage) or number (interquartile range). By RIFLE criteria, kidney injury is defined as doubling of serum creatinine level or urine output <0.5 mL/kg/h for 12 hours. Kidney failure is defined as tripling of serum creatinine level or serum creatinine level ≥353.6 μmol/L with an acute increase >44 μmol/L or urine output <0.3 mL/kg/h for 24 hours or anuria for 12 hours.21 For the kidney injury cohort, patients with chronic kidney disease (n = 2) were excluded.
Abbreviations: ICU, intensive care unit; RIFLE, risk, injury, failure, loss, end-stage disease.
We examined the association between kidney disease and length of stay and mortality in patients with pandemic H1N1 (Table 4). Because of our small sample size, only univariate associations were explored. As expected, the odds of death was substantially higher in those who experienced kidney failure (odds ratio, 11.29; 95% CI, 1.29-98.8) and length of stay was increased in all patients who required dialysis (relative risk, 2.38; 95% CI, 2.13-25.75; data not shown).
Table 4.
Univariate Impact of Kidney Injury, Kidney Failure, and Need for Dialytic Therapies on Mortality in the ICU
| OR for Mortality | 95% CI | |
|---|---|---|
| Dialysis (all) | 1.21 | 0.23-5.00 |
| Acute kidney injury | 5.67 | 0.65-49.62 |
| Acute kidney failure | 11.29 | 1.29-98.8 |
Abbreviations: OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
Key characteristics of patients who experienced a kidney outcome are listed in Table 5. AKI was more frequent in older patients (38.4 vs 28.7 years; P = 0.02), whereas kidney injury and failure were more common in patients with higher body mass index and a history of asthma. Again, the requirement for dialysis therapy was associated with an increase in length of ICU stay (P = 0.02).
Table 5.
Characteristics of Pandemic H1N1 Patients With and Without Kidney Injury, Kidney Failure, and Requirement for Dialysis
| Kidney Injury |
P | Kidney Failure |
P | Dialysis |
P | ||||
|---|---|---|---|---|---|---|---|---|---|
| No (n = 18) | Yes (n = 30) | No (n = 25) | Yes (n = 25) | No (n = 39) | Yes (n = 11) | ||||
| Age (y) | 28.7 ± 12.3 | 38.4 ± 16.5 | 0.02 | 34.1 ± 15.8 | 36.9 ± 16.1 | 0.5 | 35.4 ± 16.9 | 36.0 ± 11.8 | 0.9 |
| Female | 5/18 (28) | 9/30 (30) | 0.6 | 19/25 (76) | 8/25 (32) | 0.8 | 29/39 (74) | 7/11 (64) | 0.5 |
| Aboriginal | 6/18 (33) | 17/30 (57) | 0.1 | 10/25 (40) | 14/25 (56) | 0.4 | 20/39 (51) | 4/11 (36) | 0.5 |
| Body mass index (kg/m2) | 29.0 ± 2.4 | 38.5 ± 2.4 | 0.02 | 31.3 ± 2.3 | 39.1 ± 2.8 | 0.04 | 34.1 ± 2.2 | 37.2 ± 3.5 | 0.5 |
| Diabetes mellitus | 3/18 (17) | 13/30 (43) | 0.07 | 8/25 (32) | 9/25 (36) | 0.5 | 13/39 (33) | 4/11 (36) | 0.9 |
| Hypertension | 2/18 (11) | 9/30 (30) | 0.3 | 4/25 (16) | 7/25 (28) | 0.2 | 7/39 (18) | 4/11 (36) | 0.2 |
| Pregnancy | 4/18 (22) | 4/30 (13) | 0.5 | 5/25 (20) | 3/25 (12) | 0.4 | 6/39 (15) | 2/11 (18) | 0.9 |
| Asthma | 1/18 (6) | 11/30 (37) | 0.02 | 1/24 (4) | 11/25 (44) | <0.001 | 9/39 (23) | 3/11 (27) | 0.9 |
| Glasgow Coma Scale | 10.3 ± 1.3 | 10.6 ± 1.0 | 0.9 | 9.7 ± 1.2 | 10.7 ± 1.0 | 0.5 | 10.8 ± 0.8 | 7.9 ± 1.8 | 0.2 |
| APACHE II score | 16.3 ± 1.8 | 20.9 ± 1.1 | 0.04 | 17.5 ± 6.6 | 21.1 ± 6.0 | 0.06 | 18.9 ± 6.6 | 20.3 ± 6.4 | 0.5 |
| BP support agents | 9/18 (50) | 23/30 (77) | 0.1 | 13/25 (52) | 20/25 (80) | 0.07 | 24/39 (62) | 9/11 (82) | 0.3 |
| MAP (mm Hg) | 67.7 ± 15.1 | 78.5 ± 17.9 | 0.05 | 69.5 ± 15.5 | 78.7 ± 18.4 | 0.07 | 72.0 ± 15.9 | 80.1 ± 21.2 | 0.1 |
| WBC (×109/L) | 8.6 ± 2.0 | 9.0 ± 1.4 | 0.9 | 9.4 ± 1.5 | 11.7 ± 3.8 | 0.6 | 12.1 ± 2.5 | 5.3 ± 1.8 | 0.04 |
| Creatine kinase (μmol/L) | 667.5 ± 203.8 | 646.1 ± 186.3 | 0.9 | 680.9 ± 166.9 | 592.4 ± 219.3 | 0.8 | 567.3 ± 122.8 | 860.0 ± 428.4 | 0.4 |
| ICU length of stay (d) | 17.8 ± 3.7 | 25.5 ± 3.5 | 0.2 | 20.2 ± 3.2 | 24.7 ± 4.0 | 0.4 | 19.3 ± 2.6 | 33.3 ± 6.4 | 0.02 |
| Dialysis | 0/15 (0) | 11/18 (61) | 0.008 | 0/25 (0) | 11/25 (44) | <0.001 | NA | NA | NA |
| Death | 1/18 (6) | 7/30 (23) | 0.2 | 1/25 (4) | 8/25 (32) | 0.02 | 7/39 (18) | 2/11 (18) | 0.9 |
Note: Values expressed as mean ± standard deviation or number of patients (percentage). No conversion necessary for WBC count in 109/L and 103/μL.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BP, blood pressure; MAP, mean arterial pressure; NA, not applicable; WBC, white blood cell count.
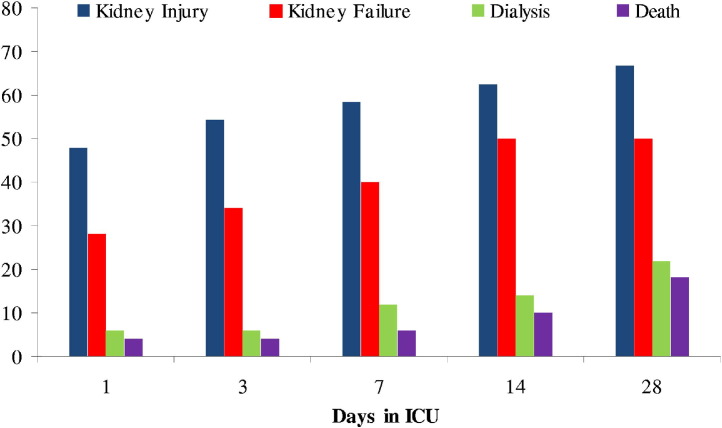
Time courses of AKI and kidney failure, need for dialysis, and death during the first 28 days of admission to the ICU are shown in Fig 1. AKI, kidney failure, and initiation of dialysis therapy occurred within 24 hours of ICU admission in 47.9%, 28%, and 6%, respectively. Most kidney injury and kidney failure occurred within the first 14 days (30 of 32 and 33 of 33 patients, respectively), whereas dialysis requirements peaked at day 28.
Figure 1.
Cumulative proportion of kidney injury, kidney failure, dialysis requirements, and death over time in 50 critically ill patients with pandemic H1N1. Cross-sectional data were obtained on days 1, 3, 7, 14, and 28 of intensive care unit (ICU) admission. Data presented as percentages when kidney injury and kidney failure were determined according to RIFLE (risk, injury, failure, loss, end-stage disease) criteria.
Discussion
Our present investigation of the impact of pandemic H1N1 on kidney injury includes a large cohort of critically ill patients and use of a standardized validated definition for kidney injury, with serial measurements performed prospectively during the patients' ICU stays. This allowed identification of the high number of patients affected by kidney injury and its subsequent impact on mortality and ICU resources. The major finding of our study is that kidney injury was common, occurred early in a high proportion of infected patients, and was associated with higher odds of death and longer length of stay.
On June 11, 2009, the WHO raised its pandemic alert level to the highest level, phase 6, indicating widespread community transmission on at least 2 continents.29 The second wave of the pandemic has strained health care resources in both hemispheres, with a large proportion of ICU occupancy dedicated solely to patients infected with pandemic H1N1.1, 2, 3, 4, 30, 31 Most admissions to the ICU are for respiratory failure and mechanical ventilation. Acute kidney failure is an important complication in critically ill patients and is associated with increased mortality, length of stay, and cost.10, 14, 15, 32 However, little is known about AKI5, 6, 7 in critically ill patients with pandemic H1N1. To our knowledge, ours is the first study to describe the impact of pandemic H1N1 on the time course of kidney injury, kidney failure, and anticipated need for dialysis therapy in critically ill patients.
AKI occurred frequently, with 66.7% of the pandemic H1N1 cohort affected during their ICU admission. Most experienced kidney injury (30 of 32 patients) within the first 24 hours of admission. Twenty-two percent of patients required dialysis; fortunately, most who survived (89%) did not require ongoing dialysis. We believe these findings are of importance for both prognostic and resource planning purposes in this important emerging pandemic.
Early reports of patients with pandemic H1N1 did not identify kidney failure or injury as a factor associated with death.1, 3 However, these reports were limited by the method used to identify kidney injury, which was nonstandardized and largely limited to identification of kidney disease as a pre-existing comorbid condition,30, 31 an average of serum creatinine measurements,5, 6 dialysis requirements only,4, 7 or not mentioned.2 In our study, we showed that kidney failure, defined according to validated and reproducible criteria, was associated with an odds ratio for death of 11.29 (95% CI, 1.29-98.9). Our cohort experienced an ICU mortality rate of 13%, which is consistent with other reports.1, 5, 6 In addition, patients requiring dialysis therapies had a longer ICU stay.16 Both these observations are consistent with the literature about the impact of AKI on patient outcomes in the ICU.10, 12, 13, 16, 23, 32, 33, 34, 35, 36
Most patients who experienced kidney failure with the need for dialysis recovered (10 of 11 patients; 89%), which is not surprising considering their young age and few comorbid conditions. Multiple large observational studies have shown an increased risk of chronic kidney failure, end-stage renal disease, and mortality in patients who experience AKI requiring temporary dialysis.10, 11, 13, 14, 15, 33, 37 It is unclear whether patients who experienced AKI with the need for dialysis therapy will be at a greater chance of progression to chronic kidney disease in the future, and periodic routine monitoring of kidney function in survivors would be advisable.
The pathophysiologic mechanism of the kidney injury likely is multifactorial acute tubular necrosis. Hypoperfusion, renal vasoconstriction, and rhabdomyolysis in the setting of a severe systemic inflammatory response with cytokine cascade likely are occurring concurrently and to varying degrees.38, 39 Although speculative, it is possible that pandemic H1N1 leads to a heightened inflammatory response in infected young patients, thereby leading to a greater degree of injury and subsequent mortality. Whether successive seroconversion to the pandemic H1N1 virus ameliorates or modifies the course of kidney injury is unknown.
Of note, large proportions of the infected population were female (72%) and aboriginal (48%), suggesting a possible genetic, hormonal, or environmental predisposition to infection or a disproportionate immune response. Numerous other studies have reported the increase in infection in female individuals and pregnant women.1, 5, 6, 40 In our study, 8 of 36 females were pregnant, with no detectable increase in adverse kidney outcomes.
Several limitations of the present study merit emphasis. Although our study included a large cohort (N = 50) of critically ill patients with pandemic H1N1, the sample size was too small to permit multivariate modeling or precise effect estimates. Multivariate analysis might have more accurately identified risk factors for kidney injury and failure in pandemic H1N1 and more accurately estimated the independent impact (ie, adjusted for confounding) of kidney failure on outcome in these patients. Although acute kidney failure was associated with mortality, the CI of the effect was wide (1.29-98.8), limiting its precision. Little information was available before ICU admission, and data for initial clinical presentation and type and timing of interventions might have shed further light on why kidney injury develops in some patients, but not others. Criteria for the initiation of dialysis therapy were not standardized; therefore, the incidence of dialysis may reflect local practice patterns to some degree, limiting generalizability. The study population is from a single region (Manitoba, Canada) and had a large proportion of First Nations (aboriginal) patients, factors that may limit generalizability to other centers and regions.
In conclusion, this study shows that kidney injury, kidney failure, and need for dialysis are common in critically ill patients with pandemic H1N1. Because of the strong association between kidney outcomes and both mortality and length of ICU stay, careful monitoring and characterization of kidney injury should be performed early in admission. These findings are of importance for clinicians in prognosticating their patients with suspected pandemic H1N1 infection, in addition to administrators planning for equipment and personnel training with this novel emerging infectious disease pandemic.
Acknowledgements
Support: Funding for this study was provided by the Public Health Agency of Canada and Hoffman-LaRoche Ltd. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Footnotes
Originally published online as doi:10.1053/j.ajkd.2010.01.011 on March 22, 2010.
References
- 1.The ANZIC Influenzae Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 2.New South Wales Public Health Network Progression and impact of the first winter wave of the 2009 pandemic H1N1 influenzae in New South Wales, Australia. Euro Surveill. 2009 Oct 22;14(42) doi: 10.2807/ese.14.42.19365-en. pii: 19365. [DOI] [PubMed] [Google Scholar]
- 3.Louie J.K., Acosta M., Winter K. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302(17):1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 4.The Australia and New Zealand Extracorporeal Membrane Oxygenation Influenza Investigators Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302(17):1888–1895. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez-Cherit G., Lapinsky S.E., Macias A.E. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A., Zarychanski R., Pinto R. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Intensive-care patients with severe novel influenza A (H1N1) virus infection—Michigan, June 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0710a1.htm Accessed July 10, 2009. [PubMed]
- 8.Wiebe C., Komenda P., Bueti J., Rigatto C., Sood M.M. Atypical clinical presentation, course and outcome of H1N1 influenza A infection in a dialysis patient. Lancet. 2009;374(9697):1300. doi: 10.1016/S0140-6736(09)61596-8. [DOI] [PubMed] [Google Scholar]
- 9.Marcelli D., Marelli C., Richards N. Influenza A(H1N1)v pandemic in the dialysis population: first wave results from an international survey. Nephrol Dial Transplant. 2009;24(12):3566–3572. doi: 10.1093/ndt/gfp557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw S., Laupland K., Doig C. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9(6):R700–R709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wald R., Quinn R.R., Luo J. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009;302(11):1179–1185. doi: 10.1001/jama.2009.1322. [DOI] [PubMed] [Google Scholar]
- 12.Ricci Z., Cruz D., Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2007;73(5):538–546. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 13.Barrantes F., Tian J., Vazquez R., Amoateng-Adjepong Y., Manthous C. Acute kidney injury criteria predict outcomes of critically ill patients. Crit Care Med. 2008;36(5):1397–1403. doi: 10.1097/CCM.0b013e318168fbe0. [DOI] [PubMed] [Google Scholar]
- 14.Coca S.G., Yusuf B., Shlipak M.G., Garg A.X., Parikh C.R. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53(6):961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lins R.L., Elseviers M.M., Daelemans R. Severity scoring and mortality 1 year after acute renal failure. Nephrol Dial Transplant. 2006;21(4):1066–1068. doi: 10.1093/ndt/gfk094. [DOI] [PubMed] [Google Scholar]
- 16.Metnitz P.G., Krenn C.G., Steltzer H. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Fowler R.A., Lapinsky S.E., Hallett D. Critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):367–373. doi: 10.1001/jama.290.3.367. [DOI] [PubMed] [Google Scholar]
- 18.Chu K.H., Tsang W.K., Tang C.S. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 2005;67(2):698–705. doi: 10.1111/j.1523-1755.2005.67130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 20.Public Health Agency of Canada Case definitions for national surveillance H1N1 flu virus. http://www.phac-aspc.gc.ca/alert-alerte/swine-porcine/hp-ps-info_definition-eng.php Accessed November 5, 2009.
- 21.Bellomo R., Ronco C., Kellum J., Mehta R., Palevsky P., ADQI Workgroup Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricci Z., Ronco C. Kidney diseases beyond nephrology: intensive care. Nephrol Dial Transplant. 2008;23(3):820–826. doi: 10.1093/ndt/gfn044. [DOI] [PubMed] [Google Scholar]
- 23.Abosaif N.Y., Tolba Y.A., Heap M., Russell J., Nahas A.M.E. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis. 2005;46(6):1038–1048. doi: 10.1053/j.ajkd.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Maccariello E., Soares M., Valente C. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med. 2007;33(4):597–605. doi: 10.1007/s00134-007-0535-0. [DOI] [PubMed] [Google Scholar]
- 25.Ostermann M., Chang R.W.S. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35(8):1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 26.Uchino S., Bellomo R., Goldsmith D., Bates S., Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34(7):1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 27.Bagshaw S.M., George C., Bellomo R., ANZIC Database Management Committee A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(5):1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 28.Winnipeg Regional Health Authority Bulletin #31 H1N1 flu. http://news.gov.mb.ca/news/index.html?archive=&item=6216 Accessed July 10, 2009.
- 29.World Health Organization World now at the start of 2009 influenza pandemic. http://www.who.int.proxy2.lib.umanitoba.ca/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html Accessed June 11, 2009.
- 30.Oliveria W.K., Carmo E.H., Penna G.O. Pandemic H1N1 influenzae in Brazil: analysis of the first 34, 506 notified cases of influenzae-like illness with severe acute respiratory infection (SARI) Eurosurveillance. October 2009;14(42):1–6. doi: 10.2807/ese.14.42.19362-en. [DOI] [PubMed] [Google Scholar]
- 31.Jain S., Kamimoto L., Bramley A.M. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 2009;361(20):1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 32.Thakar C.V., Christianson A., Freyberg R., Almenoff P., Render M.L. Incidence and outcomes of acute kidney injury in intensive care units: a Veterans Administration study. Crit Care Med. 2009;37(9):2552–2558. doi: 10.1097/CCM.0b013e3181a5906f. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw S.M., George C., Dinu I., Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23(4):1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 34.Ostermann M., Chang R., Riyadh ICU Program Users Group Renal failure in the intensive care unit: acute kidney injury compared to end-stage renal failure. Crit Care. 2008;12(5):432–433. doi: 10.1186/cc7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocha E., Soares M., Valente C. Outcomes of critically ill patients with acute kidney injury and end-stage renal disease requiring renal replacement therapy: a case-control study. Nephrol Dial Transplant. 2009;24(6):1925–1930. doi: 10.1093/ndt/gfn750. [DOI] [PubMed] [Google Scholar]
- 36.Clermont G., Acker C.G., Angus D.C., Sirio C.A., Pinsky M.R., Johnson J.P. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62:986–996. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 37.Schiffl H. Renal recovery from acute tubular necrosis requiring renal replacement therapy: a prospective study in critically ill patients. Nephrol Dial Transplant. 2006;21(5):1248–1252. doi: 10.1093/ndt/gfk069. [DOI] [PubMed] [Google Scholar]
- 38.Abe M.H.T., Okada K., Kaizu K., Matsumoto K. Clinical study of influenza-associated rhabdomyolysis with acute renal failure. Clin Nephrol. 2006;66(3):166–170. doi: 10.5414/cnp66166. [DOI] [PubMed] [Google Scholar]
- 39.Ayala E., Kagawa F.T., Wehner J.H., Tam J., Upadhyay D. Rhabdomyolysis associated with 2009 influenza A(H1N1) JAMA. 2009;302(17):1863–1864. doi: 10.1001/jama.2009.1582. [DOI] [PubMed] [Google Scholar]
- 40.Jamieson D.J., Honein M.A., Rasmussen S.A. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374(9688):451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]