Graphical abstract
A series of l-arginine derivatives were designed, synthesized and assayed for their activities against amino-peptidase N (APN)/CD13 and metalloproteinase-2 (MMP-2). The results showed that most compounds exhibited high inhibitory activities against APN and low activities against MMP-2.

Keywords: Amino-peptidase N, Metalloproteinase-2, Inhibitors, l-Arginine derivatives, Synthesis
Abstract
A series of l-arginine derivatives were designed, synthesized and assayed for their activities against amino-peptidase N (APN)/CD13 and metalloproteinase-2 (MMP-2). The results showed that most compounds exhibited high inhibitory activities against APN and low activities against MMP-2. Within this series, two compounds 5q and 5s (IC50 = 5.3 and 5.1 μM) showed similar inhibitory activities compared with bestatin (IC50 = 3.8 μM), which could be used as novel lead compounds for the future APN inhibitors development as anticancer agents.
1. Introduction
Amino-peptidase N (APN/CD13; EC 3.4.11.2) is a zinc-dependent metalloproteinase that cleaves neutral or basic amino acids from the N-terminus of oligopeptides. Scientist found that outside the hematopoietic system, APN was widely expressed on various kinds of cells. For example, epithelial cells of the intestine and kidney, hepatocytes, osteoclasts, endometrial cells, fibroblasts, endothelial cells, bone marrow stromal cells and on neuronal synaptic membranes. APN is involved in many physiology and pathology processes such as hydrolysis of nutrients, inactivation of bioactive peptides, binding of corona viruses, mediating cytomegalovirus infection and antigen presentation. Furthermore, APN has been a target for anti-tumor agents due to its important functions in ECM degradation, tumor cells invasion and tumor angiogenesis.1, 2, 3
In the past two decades, several APN inhibitors have been found. For example, Bestatin, Probestin, Amastatin, Actinonin, Phebestin, Lapstatin, AHPA-Val, Leuhistin, Curcumin and Pasmmaplin A.4, 5, 6, 7, 8, 9, 10, 11, 12, 13 Among them, bestatin has been launched and is now widely employed clinically as an anti-tumor agent.14 In 2006, the 3D structure of APN has been studied according to the co-crystal complex of APN and bestatin by Kiyoshi.15 Our group has studied the binding site and catalytic domain of APN based on this co-crystal complex. The binding sites of APN with bestatin can be divided to three parts. Part 1 is a hydrophobic pocket (S1); part 2 is the zinc binding group (ZBG); part 3 is another hydrophobic pocket in the other side ().
With the help of computer-aided molecular design, in 2008, our group designed and synthesized a series of l-lysine derivatives and found that some compounds exhibited potential APN inhibitory activities16 (Fig. 1 ). Also in 2008, the crystal structures of Escherichia coli amino-peptidase N (e PepN) in complex with l-arginine, l-lysine, l-phenylalanine, l-tyrosine and l-tryptophan have been determined to understand the structural basis for APN’s hydrolysis pattern. The result showed that all these amino acids bind with their backbone atoms close to the active-site zinc ion and their side chain occupying the S1 pocket of e PepN. And the specificity of the S1-binding pocket suggest that the preferred amino acid is, in decreasing order, arginine, lysine, tyrosine and phenylalanine.17 Additionally, arginine is similar with lysine in structure except the guanidinium group and the longer carbon chain. According to the former reasons, we choose l-arginine as the starting material in order to get more efficient and potential APN inhibitors. The compounds designed are showed in Figure 1.
Figure 1.

The structure of l-lysine derivative and l-aginine derivative.
2. Chemistry
All the target compounds were designed and synthesized via the route shown in Scheme 1 . The guanidinium group of compound 1 was protected by nitro group to get compound 2. Compound 2 was then esterificated with methanol under HCl atmosphere to get compound 3. The acylation of compound 3 with acyl chloride, carboxylic acid or sulfochloride led to compounds 4a–w, 6a,b. Finally the ester groups of 4a–w, 6a,b were treated with NHOK in anhydrous methanol to get the target compounds 5a–w, 7a,b.
Scheme 1.

Reagents and conditions:(a) fuming nitric acid, fuming sulfuric acid; (b) MeOH, HCl; (c) Et3N, THF, 0 °C; (d) Et3N, TBTU, CH2Cl2; (e) NHOK, MeOH.
3. Results and discussion
All the inhibition results were listed in Table 1 . Similar to APN, MMP-2 is also a zinc-dependant metalloproteinase that involved in tumor invasion and metastasis. Thus the assay was performed on both of APN and MMP-2 so as to identify the compounds selectivity. Bestatin was used as the positive control.
Table 1.

| Compounds | R | IC50a (μM) |
IC50(MMP-2)/IC50(APN) | |
|---|---|---|---|---|
| APN | MMP-2 | |||
| 5a |  |
1662.6 ± 4.9 | 8.7 ± 3.9 | 0.05 |
| 5b |  |
331.8 ± 2.8 | 1642.9 ± 8.4 | 4.9 |
| 5c | 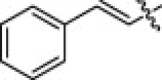 |
1008.0 ± 5.9 | 36.7 ± 1.6 | 0.04 |
| 5d | 80.6 ± 1.2 | 692.8 ± 5.7 | 8.6 | |
| 5e | 20.4 ± 1.1 | 149.6 ± 2.8 | 7.3 | |
| 5f | 21.9 ± 1.5 | 176.0 ± 2.4 | 8.0 | |
| 5g | 221.5 ± 2.6 | 2067.2 ± 23.5 | 9.3 | |
| 5h |  |
78.4 ± 2.5 | 253.2 ± 3.1 | 3.2 |
| 5i |  |
202.2 ± 2.9 | 89.2 ± 2.9 | 0.4 |
| 5j |  |
43.4 ± 1.3 | 101.6 ± 2.1 | 2.3 |
| 5k | 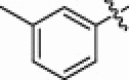 |
42.8 ± 1.9 | 623.1 ± 4.5 | 14.6 |
| 5l | 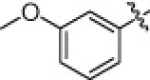 |
16.5 ± 2.2 | 150.8 ± 3.0 | 9.1 |
| 5m | 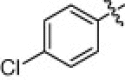 |
215.1 ± 3.3 | 237.6 ± 3.6 | 1.1 |
| 5n | 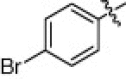 |
443.4 ± 2.7 | 483.6 ± 3.9 | 1.1 |
| 5o | 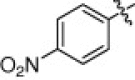 |
15.9 ± 2.1 | 203.6 ± 4.6 | 12.8 |
| 5p | 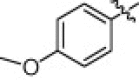 |
60.2 ± 2.2 | 322.5 ± 3.2 | 5.4 |
| 5q |  |
5.3 ± 1.2 | 348.5 ± 3.6 | 65.8 |
| 5r | 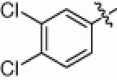 |
33.5 ± 1.7 | 193.6 ± 2.9 | 5.8 |
| 5s | 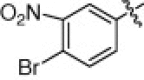 |
5.1 ± 0.6 | 236.3 ± 4.5 | 46.3 |
| 5t | 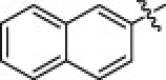 |
14.6 ± 0.8 | 85.5 ± 2.6 | 5.9 |
| 5u | 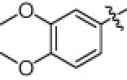 |
26.9 ± 1.3 | 345.5 ± 3.8 | 12.8 |
| 5v |  |
270.6 ± 3.4 | 41.1 ± 1.8 | 0.2 |
| 5w | 85.7 ± 2.1 | 226.4 ± 2.6 | 2.6 | |
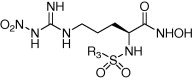 | ||||
| Compounds | R3 | IC50a (μM) | IC50(MMP-2)/IC50(APN) | |
| APN | MMP-2 | |||
| 7a | 60.2 ± 1.8 | 100.4 ± 3.9 | 1.7 | |
| 7b |  |
108.8 ± 2.3 | 28.9 ± 2.4 | 0.3 |
| Bestatin |  |
3.8 ± 0.1 | 162.0 ± 4.8 | 42.6 |
Mean value of three experiments and standard deviation are given.
Almost all the compounds except 5a, 5c, 5i, 5v and 7b showed better activities against APN than MMP-2. For example, 5q with an IC50 (MMP-2)/ IC50 (APN) ratio equals to 65.8, while 5s equals to 46.3. The result, to a certain extent, confirmed our strategy for designing APN inhibitors. This possibly descript from the differences between the structures of two enzymes, leading to different requirements for their respective inhibitors. APN is a membrane-bound zinc exopeptidase that catalyzed the removal of NH2-terminal amino acid from the peptide, while MMP-2 is a zinc-dependent endopeptidase that could cut the peptide to parts from the specific amino acid residue of the peptide. The former l-lysine derivatives and 3-phenylpropane-1, 2-diamine derivatives designed by our group to inhibit APN all showed better activities against MMP-2.16, 18 So l-arginine derivatives are more suitable for APN inhibitors.
Comparing 5a–d and 5e–w, we could find that the compounds contained substituted phenyl groups have better activities than those contained unsubstituted phenyl groups.
Among compounds 5e–w, we can conclude that the compounds contained two substituted groups on phenyl group have more potent activities than others. The most active compounds 5q and 5s both contain bi-substituted phenyl group. It is worth mentioning that the 2,4-dichlorobenzyl moiety of 5q was also effective in the precious l-lysine derivatives.16 The possible reason might be that the 2,4-dichloro substituted benzene ring could accommodate the hydrophobic site of APN suitably, suggesting it is a potential moiety for APN inhibitors.
From compounds 7a,b, we could confirm that the compounds contained sulfonyl groups showed equal activities against APN and MMP-2. That coincides with the fact that some sulfonyl groups containing compounds our group had synthesized before showed better MMP-2 inhibitory activities.19, 20
The most active compounds are 5q and 5s (IC50 = 5.3 and 5.1 μM), which have comparable activity with bestatin (IC50 = 3.8 μM). 5o, 5t and 5l also have considerable activities (IC50 = 15.9, 14.6 and 16.5 μM, respectively).
In order to investigate the interaction of our compounds with APN, the most active compound 5s was constructed with Sybyl/Sketch module and optimized using Powell’s method with the Tripos force field with convergence criterion set at 0.05 kcal/(Å mol), and assigned with Gasteiger–HÜckel method. The docking study performed using Sybyl/FlexX module, the residues in a radius of 7.0 Å around Bestatin in the co-crystal structure (PDB code: 2DQM) were selected as the active site. Other docking parameters implied in the program were kept default. From Figure 2 , we can see the backbone of 5s inserted to S1 pocket, the hydroxymate of 5s interacted with the zinc ion of APN and the 4-bromo-3-nitro benzamide side chain extended to pocket.
Figure 2.
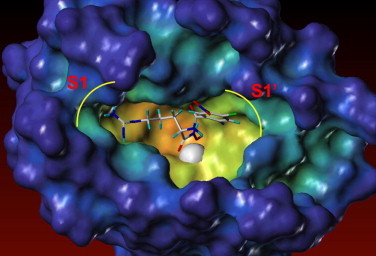
The docking mode of compound 5s with APN. Zinc ion is shown as pale sphere.
For a further and detail understanding of the binding mode of 5s with APN, a 2D picture was also created with the program ligplot. In Figure 3 , we can see the backbone of 5s could form hydrophobic contacts with Glu121, Met260 and Tyr376 of S1 pocket and form hydrogen bond with Glu121 by the imine of guanidinium group. The two oxygen atoms of hydroxymate chelated with the zinc ion of APN. The carbonyl of amide in R position could form hydrogen bond with Gly261 and Ala262 of pocket. The R substituted side chain of 5s could form hydrophobic contact with Gly261 of pocket. While, the nitro group at the aromatic ring could form hydrogen bonds with Arg783and Arg825.
Figure 3.

The docking result of 5s with APN showed by LIGPLOT. Compound 5s is shown in violet.
Although the computed information partially supported our assumption, the exact binding mode of the l-arginine derivatives with APN should be obtained from further X-ray crystal studies.
4. Conclusion
In all, we have synthesized a new series of l-aginine derivatives as APN inhibitors. Most of the compounds showed potent activity and selectivity against APN, in which 5q and 5s were comparable to bestatin and could be used as lead compounds for the development of future low molecular-weight peptidomimetic APN inhibitors as anticancer agents.
5. Experimental
5.1. Chemistry: general procedures
All the material were commercial available. All the solvents except fuming nitric acid and fuming sulfuric acid were distilled before use. All the reactions were monitored by thin-layer chromatography on 0.25 mm silica gel plates (60GF-254) and visualized with UV light or chloride ferric. 200–300 mesh silica gel was used in column chromatography. Proton NMR spectra were determined on a Brucker DRX spectrometer (300 MHz), δ in parts per million and J in hertz and TMS was used as an internal standard. Measurements were made in D2O solutions. ESI-MS were determined on an API 4000 spectrometer. Elemental analysis for compound was performed using an elementar vario EL III CN analyzer (Germany). Melting points were determined on an electrothermal melting point apparatus (uncorrected).
5.1.1. 2-Amino-5-(3-nitroguanidino)pentanoic acid (2)
The title compound was prepared as described by Hashimoto et al.21 from compound 1.
5.1.2. Methyl 2-amino-5-(3-nitroguanidino)pentanoate hydrochloride (3)
The title compound was prepared as described by Jordis22 from compound 2.
5.1.3. Methyl 5-(3-nitroguanidino)-2-(2-phenylacetamido)pentanoate (4a)
Phenylacetic acid (0.68 g, 5 mmol) and trimethylamine (3 equiv) were dissolved in 30 ml anhydrous dichloromethane (DCM). To this stirring solution was added TBTU (1.3 equiv) followed by compound 3. The resulting solution was stirred for 6 h and then washed with saturated Na2CO3, 10% citric acid and brine. Lying for a while, the white solid precipitated in DCM was filtered and washed with DCM, then dried in vacuum drying oven. Finally, 1.60 g product was obtained, yield 90.8%, mp 115–117 °C. ESI-MS m/z: 352.4 (M+H)+; 1H NMR (D2O): 1.58–1.65 (m, 2H), 1.71–1.76 (m, 2H), 3.13–3.14 (m, 2H), 3.47 (s, 2H), 3.62 (s, 3H), 4.22–4.26 (m, 1H), 7.20–7.30 (m, 5H).
Compounds 4c, 4e-n and 4r-w were synthesized following the procedure described above.
Methyl 2-cinnamamido-5-(3-nitroguanidino)pentanoate (4c): (1.46 g, 80.7%).
Methyl 2-(2-chlorobenzamido)-5-(3-nitroguanidino)pentanoate (4e): (1.67 g,89.9%).
Methyl 2-(2-iodobenzamido)-5-(3-nitroguanidino)pentanoate (4f): (2.02 g, 87.2%).
Methyl 2-(2-methylbenzamido)-5-(3-nitroguanidino)pentanoate (4g): (1.62 g, 92.3%).
Methyl 2-(2-methoxybenzamido)-5-(3-nitroguanidino)pentanoate (4h): (1.61 g, 87.7%).
Methyl 2-(3-chlorobenzamido)-5-(3-nitroguanidino)pentanoate (4i): (1.69 g, 91.0%).
Methyl 2-(3-nitrobenzamido)-5-(3-nitroguanidino)pentanoate (4j): (1.76 g, 92.1%).
Methyl 2-(3-methylbenzamido)-5-(3-nitroguanidino)pentanoate (4k): (1.61 g, 91.5%).
Methyl 2-(3-methoxybenzamido)-5-(3-nitroguanidino)pentanoate (4l): (1.65 g, 89.7%).
Methyl 2-(4-chlorobenzamido)-5-(3-nitroguanidino)pentanoate (4m): (1.69 g, 91.1%).
Methyl 2-(4-bromobenzamido)-5-(3-nitroguanidino)pentanoate (4n): (1.87 g, 90.0%).
Methyl 2-(3,4-dichlorobenzamido)-5-(3-nitroguanidino)pentanoate (4r): (1.78 g, 87.5%).
Methyl 2-(4-bromo-3-nitrobenzamido)-5-(3-nitroguanidino)pentanoate (4s): (1.85 g, 80.1%).
Methyl 2-(2-naphthamido)-5-(3-nitroguanidino)pentanoate (4t): (1.61 g, 83.4%).
Methyl 2-(3,4-dimethoxybenzamido)-5-(3-nitroguanidino)pentanoate (4u): (1.67 g,84.4%).
Methyl 5-(3-nitroguanidino)-2-(3,4,5-trimethoxybenzamido)pentanoate (4v): (1.80 g, 84.3%).
Methyl 5-(3-nitroguanidino)-2-(thiophene-2-carboxamido)pentanoate (4w): (1.44 g, 84.0%).
5.1.4. Methyl 5-(3-nitroguanidino)-2-(3-phenylpropanamido)pentanoate (4b)
Compound 3 (1.35 g, 5 mmol) and triethylamine (1 equiv) were mixed in 30 ml anhydrous DCM under ice-bath. 3-Phenylpropionyl chloride (1.2 equiv) dissolved in DCM was added dropwise. The resulting solution was stirred for 2 h and then washed with saturated Na2CO3, 10% citric acid and brine. After lying for a while, white solid would precipitate in DCM. The solid was filtered and washed with DCM, then dried in vacuum drying oven. Finally 1.69 g product was obtained, yield 92.7%.
Compounds 4d, 4o, 4p and 4q were synthesized following the procedure described above.
Methyl 2-benzamido-5-(3-nitroguanidino)pentanoate (4d): (1.43 g, 85.1%).
Methyl 2-(4-nitrobenzamido)-5-(3-nitroguanidino)pentanoate (4o): (1.74 g, 91.3%).
Methyl 2-(4-methoxybenzamido)-5-(3-nitroguanidino)pentanoate (4p): (1.65 g, 89.9%).
Methyl 2-(2,4-dichlorobenzamido)-5-(3-nitroguanidino)pentanoate (4q): (1.68 g, 90.3%).
5.1.5. Methyl 2-(4-methylphenylsulfonamido)-5-(3-nitroguanidino)pentanoate (6a)
Compound 6a was synthesized following the procedure of compound 4b. Finally, 1.72 g product was got, yield 88.9%.
Compound 6b was synthesized following the procedure of compound 6a.
Methyl 5-(3-nitroguanidino)-2-(phenylsulfonamido)pentanoate (6b): (1.62 g, 87.0%).
5.1.6. N-Hydroxy-5-(3-nitroguanidino)-2-(2-phenylacetamido)pentanamide (5a)
To a solution of compound 4a (0.71 g, 2 mmol) in 7 ml anhydrous methanol at room temperature was added dropwise a solution of NHOK (6 mmol) in methanol (3.4 ml). The mixture was stirred for 12 h and the solvent was evaporated in vacuum. The residue was purified by column chromatography (dichloromethane/methanol = 20/1–5/1). Finally, 059 g 5a was obtained. White solid, yield 83.4%, mp 76–79 °C. ESI-MS m/z: 353.5 (M+H)+; 1H NMR (D2O): 1.51–1.77 (m, 4H), 3.00–3.13 (m, 2H), 3.41–3.50 (m, 2H), 4.09–4.17 (m, 1H), 7.18–7.32 (m, 5H). Anal. Calcd for C14H20N6O5: C, 47.72; H, 5.72; N, 23.85. Found: C, 47.62; H, 5.65; N, 23.75.
Compounds 5b–w and 7a,b were synthesized following the procedure described above.
5.1.6.1. N-Hydroxy-5-(3-nitroguanidino)-2-(3-phenylpropanamido)pentanamide (5b)
White solid, yield 78.8%, mp 161–162 °C. ESI-MS m/z: 367.3 (M+H)+; 1H NMR (D2O):1.31–1.62 (m, 4H), 2.42–2.50 (m, 2H), 2.76–2.79 (t, J = 7.5 Hz, 2H), 3.12–3.16 (m, 2H), 4.12–4.18 (m, 1H), 7.16–7.29 (m, 5H). Anal. Calcd for C15H22N6O5: C, 49.19; H, 6.05; N, 22.94. Found: C, 49.01; H, 5.99; N, 22.88.
5.1.6.2. 2-Cinnamamido-N-hydroxy-5-(3-nitroguanidino)pentanamide (5c)
White solid, yield 70.4%, mp 296–298 °C. ESI-MS m/z: 365.3 (M+H)+; 1H NMR (D2O):1.53–1.57 (m, 2H), 1.73–1.77 (m, 2H), 3.28–3.35 (m, 2H), 4.01–4.04 (m, 1H), 6.76–6.84 (d, J = 16 Hz, 1H), 7.38–7.48 (m, 4H), 7.52–7.58 (m, 2H). Anal. Calcd for C15H20N6O5: C, 49.45; H, 5.53; N, 23.07. Found: C, 49.26; H, 5.49; N, 23.15.
5.1.6.3. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5d)
White solid, yield 73.5%, mp 76–79 °C. ESI-MS m/z: 339.5 (M+H)+; 1H NMR (D2O): 1.54–1.58 (m, 2H), 1.66–1.75 (m, 2H), 3.15–3.18 (m, 2H), 4.31–4.39 (m, 1H), 7.44–7.56 (m, 5H). Anal. Calcd for C13H18N6O5: C, 46.15; H, 5.36; N, 24.84. Found: C, 46.03; H, 5.22; N, 24.68.
5.1.6.4. 2-Chloro-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5e)
White solid, yield 85.8%, mp 105–108 °C. ESI-MS m/z: 373.3 (M+H)+; 1H NMR (D2O): 1.52–1.69 (m, 4H), 3.14–3.17 (m, 2H), 4.30–4.34 (m, 1H), 7.38–7.47 (m, 4H). Anal. Calcd for C13H17ClN6O5: C, 41.89; H, 4.60; N, 22.55. Found: C, 41.72; H, 4.49; N, 22.63.
5.1.6.5. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-2-iodobenzamide (5f)
Jacinth solid, yield 79.8%, mp 76–80 °C. ESI-MS m/z: 465.3 (M+H)+; 1H NMR (D2O): 1.54–1.69 (m, 4H), 3.15–3.19 (m, 2H), 4.27–4.30 (m, 1H), 7.13–7.46 (m, 4H). Anal. Calcd for C13H17IN6O5: C,33.64; H, 3.69; N, 18.10. Found: C, 33.48; H, 3.66; N, 18.02.
5.1.6.6. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-2-methylbenzamide (5g)
White solid, yield 89.9%, mp 201–203 °C. ESI-MS m/z: 353.5 (M+H)+; 1H NMR (D2O): 1.48–1.52 (m, 2H), 1.68–1.73 (m, 2H), 2.27 (s, 3H), 3.16–3.17 (d, J = 5.4 Hz, 2H), 4.12–4.18 (m, 1H), 7.22–7.33 (m, 4H). Anal. Calcd for C14H20N6O5: C, 47.72; H, 5.72; N, 23.85. Found: C, 47.51; H, 5.60; N, 23.81.
5.1.6.7. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-2-methoxybenzamide (5h)
Jacinth solid, yield 85.3%, mp 173–175 °C. ESI-MS m/z: 369.3 (M+H)+; 1H NMR (D2O): 1.52–1.63 (m, 2H), 1.67–1.76 (m, 2H), 3.14–3.22 (m, 2H), 3.92 (S, 3H), 4.38–4.45(m, 1H), 7.04–7.08 (t, J = 7.2 Hz, 1H), 7.17–7.20 (d, J = 7.8 Hz, 1H),7.48–7.54 (m, 1H), 7.82–7.85 (d, J = 7.8 Hz, 1H). Anal. Calcd for C14H20N6O6: C, 45.65; H, 5.47; N, 22.82. Found: C, 45.48; H, 5.43; N, 22.67.
5.1.6.8. 3-Chloro-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5i)
White solid, Yield 80.0%, mp 77–79 °C. ESI-MS m/z: 373.2 (M+H)+; 1H NMR (D2O): 1.55–1.76 (m, 4H), 3.15–3.17 (m, 2H), 4.32–4.34 (m, 1H), 7.47–7.96 (m, 4H). Anal. Calcd for C13H17ClN6O5: C, 41.89; H, 4.60; N, 22.55. Found: C, 41.63; H, 4.53; N, 22.47.
5.1.6.9. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3-nitrobenzamide (5j)
Yellow solid, yield 83.1%, mp 149–151 °C. ESI-MS m/z: 384.3 (M+H)+; 1H NMR (D2O): 1.52–1.54 (m, 2H), 1.73–1.75 (m, 2H), 3.15–3.17 (m, 2H), 4.35–4.37 (m, 1H), 7.72–7.77 (t, J = 7.8 Hz, 1H), 8.32–8.37 (m, 2H), 8.68 (s, 1H). Anal. Calcd for C13H17N7O7: C, 40.73; H, 4.47; N, 25.58. Found: C, 40.55; H, 4.53; N, 25.61.
5.1.6.10. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3-methylbenzamide (5k)
White solid, yield 86.5%, mp 176–179 °C. ESI-MS m/z: 353.5 (M+H)+; 1H NMR (D2O):1.49–1.54 (m, 2H), 1.71–1.75 (m, 2H), 2.36 (S, 3H), 3.16–3.18 (d, J = 5.4 Hz, 2H), 4.30–4.38 (m, 1H), 7.33–7.35 (d, J = 4.8 Hz, 2H), 7.66–7.71 (m, 2H). Anal. Calcd for C14H20N6O5: C, 47.72; H, 5.72; N, 23.85. Found: C, 47.55; H, 5.78; N, 23.66.
5.1.6.11. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3-methoxybenzamide (5l)
White solid, yield 85.6%, mp 68–71 °C. ESI-MS m/z: 369.3 (M+H)+; 1H NMR (D2O): 1.57–1.62 (m, 2H), 1.69–1.75 (m, 2H), 3.16–3.18 (m, 2H), 3.81 (s, 3H), 4.30–4.38 (m, 1H), 7.08–7.11 (m, 1H), 7.34–7.39 (m, 1H), 7.45–7.50 (m, 2H). Anal. Calcd for C14H20N6O6: C, 45.65; H, 5.47; N, 22.82. Found: C, 45.54; H, 5.38; N, 22.74.
5.1.6.12. 4-Chloro-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5m)
White solid, yield 82.1%, mp 67–72 °C. ESI-MS m/z: 373.3 (M+H)+; 1H NMR (D2O): 1.49–1.52 (m, 2H), 1.69–1.73 (m, 2H), 3.16–3.18 (d, J = 5.1 Hz, 2H), 4.29–4.36 (m, 1H), 7.53–7.56 (d, J = 8.4 Hz, 2H), 7.90–7.93 (d, J = 8.4 Hz, 2H). Anal. Calcd for C13H17ClN6O5: C, 41.89; H, 4.60; N, 22.55. Found: C, 41.48; H, 4.79; N, 22.56.
5.1.6.13. 4-Bromo-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5n)
Jacinth solid, yield 84.4%, mp 147–149 °C. ESI-MS m/z: 417.4 (M+H)+; 1H NMR (D2O):1.50–1.52 (m, 2H), 1.68–1.78 (m, 2H), 3.16–3.18 (m, 2H), 4.28–4.36 (m, 1H), 7.67–7.80 (d, J = 8.4 Hz, 2H), 7.82–7.85 (d, J = 8.4 Hz, 2H). Anal. Calcd for C13H17BrN6O5: C, 37.42; H, 4.11; N, 20.14. Found: C, 37.18; H, 4.24; N, 19.93.
5.1.6.14. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-4-nitrobenzamide (5o)
White solid, yield 91.3%, mp 148–150 °C. ESI-MS m/z: 384.3 (M+H)+; 1H NMR (D2O):1.51–1.54 (m, 2H), 1.69–1.76 (m, 2H), 3.14–3.16 (m, 2H), 4.31–4.36 (m, 1H), 8.09–8.11 (d, J = 8.4 Hz, 2H), 8.29–8.32 (d, J = 8.4 Hz, 2H). Anal. Calcd for C13H17N7O7: C, 40.73; H, 4.47; N, 25.58. Found: C, 40.46; H, 4.39; N, 25.61.
5.1.6.15. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-4-methoxybenzamide (5p)
White solid, yield 86.7%, mp 156–159 °C. ESI-MS m/z: 369.2 (M+H)+; 1H NMR (D2O):1.50–1.52 (m, 2H), 1.69–1.71 (m, 2H), 3.16–3.19 (m, 2H), 3.81(s, 3H), 4.31–4.33 (m, 1H), 6.97–7.00 (d, J = 8.7 Hz, 2H), 7.86–7.89 (d, J = 8.7 Hz, 2H). Anal. Calcd for C14H20N6O6: C, 45.65; H, 5.47; N, 22.82. Found: C, 45.55; H, 5.42; N, 22.80.
5.1.6.16. 2,4-Dichloro-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5q)
White solid, yield 83.4%, mp 154–156 °C. ESI-MS m/z: 407.4 (M+H)+; 1H NMR (D2O):1.61–1.64 (m, 2H), 1.73–1.77 (m, 2H), 3.16–3.18 (m, 2H), 4.26–4.33 (m, 1H), 7.43–7.51 (m, 2H), 7.67–7.68 (d, J = 1.8 Hz, 1H). Anal. Calcd for C13H16Cl2N6O5: C, 38.34; H, 3.96; N, 20.64. Found: C, 38.23; H, 4.04; N, 20.52.
5.1.6.17. 3,4-Dichloro-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)benzamide (5r)
White solid, yield 86.4%, mp 130–134 °C. ESI-MS m/z: 407.5 (M+H)+; 1H NMR (D2O):1.50–1.52 (m, 2H), 1.63–1.76 (m, 2H), 3.16–3.18 (d, J = 5.7 Hz, 2H), 4.29–4.36 (m, 1H), 7.75–7.78 (d, J = 8.4 Hz, 1H), 7.85–7.88 (dd, J 1 = 8.4 Hz, J 2 = 2.1 Hz, 1H), 8.17–8.18 (s, d, J = 2.1 Hz, 1H). Anal. Calcd for C13H16Cl2N6O5: C, 38.34; H, 3.96; N, 20.64. Found: C, 38.19; H, 3.94; N, 20.69.
5.1.6.18. 4-Bromo-N-(1-(hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3-nitrobenzamide (5s)
Brown solid, yield 69.3%, mp 147–150 °C. ESI-MS m/z: 462.4 (M+H)+; 1H NMR (D2O):1.54–1.56 (m, 2H), 1.73–1.76 (m, 2H), 3.16–3.18 (m, 2H), 4.34–4.36 (m, 1H), 7.42–7.45 (d, J = 9 Hz, 1H), 7.93–7.96 (d, J = 9 Hz, 1H), 8.52 (s, 1H). Anal. Calcd for C13H16BrN7O7: C, 33.78; H, 3.49; N, 17.29. Found: C, 33.60; H, 3.42; N, 17.35.
5.1.6.19. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-2-naphthamide (5t)
Yellow solid, yield 75.4%, mp 72–74 °C. ESI-MS m/z: 389.4 (M+H)+; 1H NMR (D2O): 1.60–1.73 (m, 2H), 1.77–1.88 (m, 2H), 3.17–3.20 (m, 2H), 4.38–4.43 (m, 1H), 7.58–7.80 (m, 4H), 7.98–8.05 (m, 3H). Anal. Calcd for C17H20N6O5: C, 52.57; H, 5.19; N, 21.64. Found: C, 52.28; H, 5.07; N, 21.46.
5.1.6.20. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3,4-dimethoxybenzamide (5u)
White solid, yield 90.0%, mp 148–151 °C. ESI-MS m/z: 399.3 (M+H)+; 1H NMR (D2O):1.49–1.53 (m, 2H), 1.71–1.77 (m, 2H), 3.16–3.18 (m, 2H), 3.80 (s, 6H), 4.34–4.36 (m, 1H), 6.99–7.02 (d, J = 8.4 Hz, 1H), 7.48–7.54(m, 2H). Anal. Calcd for C15H22N6O7: C, 45.22; H, 5.57; N, 21.10. Found: C, 45.01; H, 5.49; N, 21.00.
5.1.6.21. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)-3,4,5-trimethoxybenzamide (5v)
White solid, yield 85.2%, mp 101–104 °C. ESI-MS m/z: 429.5 (M+H)+; 1H NMR (D2O):1.47–1.56 (m, 2H), 1.68–1.72 (m, 2H), 3.14–3.17 (m, 2H), 3.70 (s, 9H), 4.35–4.40 (m, 1H), 7.25 (s, 2H). Anal. Calcd for C16H24N6O8: C, 44.86; H, 5.65; N, 19.62. Found: C, 44.49; H,5.56; N, 19.46.
5.1.6.22. N-(1-(Hydroxyamino)-5-(3-nitroguanidino)-1-oxopentan-2-yl)thiophene-2-carboxamide (5w)
White solid, yield 78.6%, mp 160–162 °C. ESI-MS m/z: 345.4 (M+H)+; 1H NMR (D2O):1.50–1.69 (m, 2H), 1.71–1.80 (m, 2H), 3.16–3.18 (d, J = 5.4 Hz, 2H), 4.29–4.31 (m, 1H), 7.13–7.16 (t, J = 4.8 Hz, 1H), 7.76–7.77 (d, J = 5.4 Hz, 1H), 7.82–7.85 (d, J = 3.0 Hz, 1H). Anal. Calcd for C11H16N6O5S: C, 38.37; H, 4.68; N, 24.41. Found: C, 38.12; H, 4.62; N, 24.30.
5.1.6.23. N-Hydroxy-5-(3-nitroguanidino)-2-(phenylsulfonamido)pentanamide (7a)
White solid, yield 86.6%, mp 59–61 °C. ESI-MS m/z: 375.3 (M+H)+; 1H NMR (D2O): 1.15–1.23 (m, 2H), 1.30–1.49 (m, 2H), 2.98–3.08 (m, 2H), 3.52–3.57 (m, 1H), 7.54–7.61(m, 2H), 7.76–7.89 (m, 3H). Anal. Calcd for C12H18N6O6S: C, 38.50; H, 4.85; N, 22.45. Found: C, 38.29; H, 4.76; N, 22.63.
5.1.6.24. N-Hydroxy-2-(4-methylphenylsulfonamido)-5-(3-nitroguanidino)pentanamide (7b)
Orange solid, yield 82.2%, mp 64–66 °C. ESI-MS m/z: 389.3 (M+H)+; 1H NMR (D2O): 1.13–1.35 (m, 2H), 1.38–1.49 (m, 2H), 2.29–2.36 (m, 3H), 2.99–3.06 (q, J = 7.2 Hz, 2H), 3.50–3.54 (m, 1H), 7.11–7.13 (d, J = 8.1 Hz, 1H), 7.32–7.35 (d, J = 8.1 Hz, 1H),7.46–7.49 (d, J = 8.1 Hz, 1H), 7.63–7.66 (d, J = 8.1 Hz, 1H). Anal. Calcd for C13H20N6O6S: C, 40.20; H, 5.19; N, 21.64. Found: C, 40.06; H, 5.23; N, 21.58.
5.2. APN inhibition assay
IC50 values against APN were determined by using l-Leu-p-nitroanilide as substrate and microsomal amino-peptidase from Porcine Kidney Microsomes (Sigma) as enzyme in 50 mM PBS, pH 7.2, at 37 °C. The hydrolysis of the substrate was monitored by following the change in the absorbance measured at 405 nm with the UV–vis spectrophotometer Pharmacia LKB, Biochrom 4060. All the solutions of the inhibitors were prepared in the assay buffer, and the pH was adjusted to 7.5 by the addition of 0.1 M HCl or 0.1 M NaOH. All the inhibitors were preincubated with APN for 30 min at room temperature. The assay mixture, which contained the inhibitor solution (concentration dependent on the inhibitor), the enzyme solution (4 μg/mL final concentration), and the assay buffer, was adjusted to 200 μL.
5.3. MMP-2 inhibition assay
Gelatinase A (MMP-2) and TNBS were purchased from Sigma, and the substance was synthesized as described by Vijaykumar et al. The gelatinase, substance and inhibitor were dissolved in sodium borate (pH 8.5, 50 mmol/L) and incubated for 30 min at 37 °C, and then 0.03% TNBS was added and incubated for another 20 min, the resulting solution was detected under 450 nm wavelength to gain absorption.
Acknowledgments
This work was supported by National High Technology Research and Development Program of China (863 Project, Grant No. 2007AA02Z314) and National Natural Science Foundation of China (Grant No. 30772654 and No. 90713041) and The Doctoral Foundation of Ministry of Education of the People’s Republic of China (Grant No. 20060422029).
References and notes
- 1.Nathalie L., Cynthia M.C., Emmanuel R., Ann B., Bernard P.R., Marie-Claude F.Z. Biochemistry. 1998;37:686. [Google Scholar]
- 2.Florence N., Nathalie L., Sophie D.N., René L.K., Laurent B., Hui X.C., Marie-Claude F.Z., Bernard P.R. FEBS Lett. 2000;467:81. [Google Scholar]
- 3.Xu W.F., Li Q.B. Curr. Med. Chem., Anti-Cancer Agents. 2005;5:281. doi: 10.2174/1568011053765949. [DOI] [PubMed] [Google Scholar]
- 4.Umezawa H., Aoyagi T., Suda H., Hamada M. Antibiotics. 1976;29:97. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 5.Aoyagi T., Yoshida S., Nakamura Y., Shigihara Y. Antibiotics. 1990;43:143. doi: 10.7164/antibiotics.43.143. [DOI] [PubMed] [Google Scholar]
- 6.Rich D.H., Moon B.J., Harbeson S. J. Med. Chem. 1984;27:417. doi: 10.1021/jm00370a001. [DOI] [PubMed] [Google Scholar]
- 7.Umezawa H., Aoyagi T., Tanaka T., Suda H. Antibiotics. 1985;38:1629. doi: 10.7164/antibiotics.38.1629. [DOI] [PubMed] [Google Scholar]
- 8.Nagai M., Kojima F., Naganawa H. Antibiotics. 1997;50:82. doi: 10.7164/antibiotics.50.82. [DOI] [PubMed] [Google Scholar]
- 9.Lampret B.R., Kidrie J., Kralj B. Arch. Microbiol. 1999;171:397. doi: 10.1007/s002030050726. [DOI] [PubMed] [Google Scholar]
- 10.Chung M.C., Lee H.J., Chun H.K. Biosci., Biotechnol., Biochem. 1996;60:898. doi: 10.1271/bbb.60.898. [DOI] [PubMed] [Google Scholar]
- 11.Aoyagi T., Yoshida S., Matsuda N. Antibiotics. 1991;44:573. doi: 10.7164/antibiotics.44.573. [DOI] [PubMed] [Google Scholar]
- 12.Egan M.E., Pearson M., Weiner S.A. Science. 2004;304:600. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]
- 13.Shim J.S., Lee H., Shin J. Cancer Lett. 2004;203:163. doi: 10.1016/j.canlet.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 14.Shimazawa R., Takayama H., Hashimoto Y. J. Enzyme Inhib. 1999;14:259. doi: 10.3109/14756369909030321. [DOI] [PubMed] [Google Scholar]
- 15.Ito K., Nakajima Y., Yoshimoto T. J. Biol. Chem. 2006;281:33664. doi: 10.1074/jbc.M605203200. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q., Chen M.Y., Zhu H.W., Zhang J., Fang H., Wang H.B., Xu W.F. Bioorg. Med. Chem. 2008;16:5473. doi: 10.1016/j.bmc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Addlagatta A., Gay L., Matthews B.W. Biochemistry. 2008;47:5303. doi: 10.1021/bi7022333. [DOI] [PubMed] [Google Scholar]
- 18.Shang L.Q., Wang Q., Fang H., Mu J.J., Wang X.J., Yuan Y.M., Wang B.H., Xu W.F. Bioorg. Med. Chem. 2008;16:6663. doi: 10.1016/j.bmc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Li Y.L., Xu W.F. Bioorg. Med. Chem. 2004;12:5171. doi: 10.1016/j.bmc.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Li Y.L., Xu W.F. Bioorg. Med. Chem. 2006;15:1287. doi: 10.1016/j.bmc.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto, S.; Okada, K.; Sakakibara, R.; Fujii, S. U.S. Patent [11] 3, 799, 988 March 26, 1974.
- 22.Jordis U., Sauter F., Siddiqi S.M. Indian J. Chem. Sec. B. 1989:294. [Google Scholar]


