Summary
Background
Patients with COPD have frequent exacerbations. The role of respiratory viral infection is just emerging. We wished to determine prospectively the incidence of viral infection in exacerbated and stable COPD patients as well as smokers who do not have airways obstruction.
Methods
Stable and exacerbated COPD patients were recruited along with a group of patients who had smoked but who did not have any airways obstruction. Spirometry was performed and sputum specimens were tested for a range of 12 different respiratory viruses using PCR.
Results
One hundred and thirty-six patients with exacerbations of COPD, 68 stable COPD patients and 16 non-obstructed smokers were recruited. A respiratory virus was detected in 37% of exacerbations, 12% of stable COPD patients and 12% of non-obstructed smokers, p < 0.0005. Rhinovirus was most frequently detected. The symptom of fever was associated with virus detection, p < 0.05. Infection with more than one virus was only found in the exacerbated COPD patients.
Conclusion
Respiratory viral infection is associated with exacerbations of COPD. Rhinovirus was the most common infecting agent identified and in two cases human metapneumovirus was also detected. Dual infections were only seen amongst those patients admitted to hospital with acute exacerbations of COPD. Viruses were more commonly detected in those with more severe airways disease.
Keywords: COPD, Respiratory virus, Polymerase chain reaction, Metapneumovirus, rhinovirus
Introduction
Patients with COPD have frequent exacerbations which lead to increased airway inflammation and often subsequent hospitalization.1 Bacteria are associated with approximately 50% of exacerbations.2, 3 A number of studies have identified viral infections of the respiratory tract as precipitating agents. Studies using serology and viral culture identified respiratory viruses in 30% of patients during acute exacerbations of COPD.4 With the development of more sensitive molecular tests the role of viruses in COPD has been better defined. Walsh et al. identified several common respiratory viruses in a cohort of patients with COPD or congestive cardiac failure.5 Viruses were identified by serology and viral culture methods. Significant outcomes in terms of hospitalisation were seen for respiratory syncytial virus (RSV) and influenza A infected patients. Greenberg et al., using serology and viral culture, showed that in 23% of cases of hospitalisation of COPD patients a virus was detected and that the mean time to return to symptomatic baseline was 2 weeks.6 The most common viruses identified were of the picornavirus classification (rhinoviruses).
Several studies have used PCR for the diagnosis of viral infection. Initially, in 2000 Seemungal et al. examined 33 patients with COPD when stable and subsequently during an exacerbation.7 Ten of 43 exacerbations were associated with rhinovirus infection. Higher symptom scores and sputum interleukin-6 levels were seen with rhinovirus exacerbations. Seemungal et al. went on to show respiratory viral infection in 39% of exacerbations of COPD.8 Viral infection was associated with a more severe exacerbation, and in patients who had a higher frequency of exacerbations, i.e. those with the poorest lung function. Patients were reviewed in convalescence at 4–6 weeks. The majority (58%) of these viruses were rhinovirus (i.e. 23% of COPD exacerbations). Plasma IL-6 levels were elevated in all exacerbations, and levels were significantly increased in the virus positive group when comparison was made with those in whom no virus was detected.
Rohde et al. used PCR to detect viruses in patients with exacerbations of COPD. A respiratory virus was detected in 56% of cases in comparison to 19% of control subjects with stable COPD.9 The majority of viruses detected were picornaviruses. The most recent study from Beckham et al. combined specimens obtained for two previous studies for analysis by PCR.10 They detected a respiratory viral infection in 42% of acute respiratory illnesses. Respiratory viral infection in COPD patients during exacerbation has been associated with longer median symptom recovery time in comparison to exacerbations in which a viral agent was not identified (13 days versus 6 days respectively).8
The hypothesis tested in the present study was that acute respiratory viral infection is implicated in the pathogenesis of COPD exacerbations. The aims of the present study were to examine respiratory specimens for the presence of respiratory viruses in patients with COPD whilst stable and also during exacerbations.
Methods
Subjects
This study was approved by the Queen's University Belfast ethics committee and all patients gave written consent. The patient groups described in this paper have been described in other related publications. Patients hospitalised with exacerbations of COPD were recruited within 24 h of presentation over a 2-year period. A further group of stable COPD patients were also recruited. Stability was defined as no change in respiratory symptoms or alteration in therapy in the previous 8 weeks. Patient's symptoms were recorded in a binary format. Spirometry was performed, the best of 3 reproducible readings was taken (Vitalograph spirometer, Vitalograph, Buckingham, UK).11 Spirometry was repeated after nebulised beta agonist (salbutamol 2.5 mg). Any patients with significant improvement in FEV1 (>200 ml/15%) were excluded. Those patients with a history of bronchiectasis, neoplastic process or other serious concomitant disease were excluded.
Definitions
COPD and assessment of severity was classified according to the GOLD criteria.12 Exacerbation of COPD was classified according to the GOLD criteria with symptoms of increased dyspnoea, increased cough or increased sputum production.13 Stable COPD was classified according to the GOLD criteria without any symptoms of exacerbation or changes in treatment within the last 8 weeks.
Samples
Sputum samples were obtained either by spontaneous production or following nebulised hypertonic saline. Briefly, 4 ml of 3% saline was nebulised via an air driven nebuliser. Every 5 min spirometry was repeated to measure FEV1, and nebulisation continued if FEV1 had not fallen by more than 20%. This was continued up to 20 min. All sputum expectorated was collected. All samples were processed within 2 h. Specimens were mixed with 4 volumes of 0.1% dithiothreitol (Sigma, Poole, UK) and shaken in an orbital incubator (Gallenkamp, Loughborough, UK) for 15 min at 37 °C followed by the addition of 4 volumes of phosphate buffered saline and shaken for a further 5 min. The resulting suspension was then filtered through 50 μm Nylon Gauze (Lockertex, Warrington, UK) and spun down at 1000 × g for 10 min. After removing the supernatant the cell pellet was resuspended in Lysis Buffer (Qiagen, Crawley, UK). Total nucleic acid extraction was performed on 200 μl of sputum sample suspended in Lysis Buffer (QIAamp DNA Blood Mini Kit).
Polymerase chain reaction
Extracted samples were screened for common respiratory viruses (rhinovirus, human metapneumovirus, influenza A H1 and H3, influenza B, RSV A and B, coronavirus 229E, adenovirus and parainfluenza 1, 2 and 3) using an eight-well multiplexed, nested PCR system (Fig. 1 ).14 A positive control was used for each set of patient's tests. Mastermix and PCR cycling conditions are listed in the Supplementary material(appendix A1 and A2 respectively). Tests results were accepted as being valid when the positive control tested positive with correct sized product(s). A positive result was noted when second (or first and second) round products were noted and confirmed as being the correct size. Indeterminate test results were repeated. This method allowed rapid testing of clinical specimens for multiple viruses with sensitive and specific assays.
Figure 1.
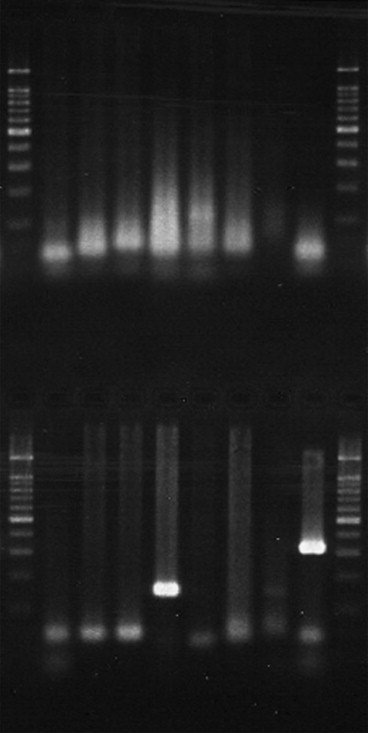
Nested PCR for respiratory virus screen with 1st and 2nd round products. Specimen positive for influenza A H3 (lane 4), with positive control of parainfluenza 1 (lane 8). Molecular weight markers flank specimens.
Statistical analysis
A power calculation was performed to determine study group sizes. Assuming a respiratory virus detection rate of 30% amongst COPD patients during exacerbations and 10% detection when stable a power calculation was performed using EpiInfo™ version 6. Using a confidence value of 95% with a power of 80% and a 2:1 ratio of group sizes, 111 patients with COPD exacerbations and 56 stable COPD subjects were required.
Normally distributed data were presented as mean ± standard deviation, whilst non-parametric data were expressed as median and inter quartile ranges. Normally distributed continuous variables were compared by t-test; otherwise the differences were assessed by the Mann–Whitney U-test. For discrete variables frequencies and percentages were reported and groups compared using the chi squared test. A significance level of 5% was chosen. Statistical analysis was carried out using SPSS (Chicago, IL) version 10.0.
Results
Subjects
A total of 220 subjects were recruited in this study (Table 1 ); 136 were seen during an acute exacerbation, and 68 were recruited when stable. An additional 16 non-obstructed smokers with a FEV1 and FVC within the normal range were recruited. Patient characteristics are shown in Table 1, Table 2 ; the exacerbated and stable COPD groups were of similar age, sex, FEV1, tobacco use, baseline MRC score, inhaled steroid and body mass index (BMI) and pack years of tobacco use.
Table 1.
Patient demographics and results of investigations
| Measurement | Patient group |
||
|---|---|---|---|
| Exacerbated COPD | Stable COPD | Non-obstructed smokers | |
| No. patients | 136 | 68 | 16 |
| Sex (M/F) | 64/72 | 30/38 | 4/12 |
| Age | 70.2 ± 9.4 | 66.3 ± 9.4 | 52.2 ± 7.6 |
| FEV1, litres (% Pred) | 0.84 ± 0.47 (39 ± 20) | 1.00 ± 0.53 (48 ± 22) | 2.60 ± 0.56 (105 ± 11) |
| Tobacco (packs/year) | 48.0 ± 39.2a | 42.2 ± 26.0b | 44.0 ± 21.5 |
| C reactive protein | 14.8 (5.0–70.2)c | 5.0 (5.0–8.4) | 5.0 (5.0–5.0) |
| White cell count | 10.9 ± 3.7c | 8.7 ± 2.6 | 8.6 ± 2.7 |
| Neutrophil count | 7.9 ± 3.6c | 5.4 ± 2.3 | 4.9 ± 2.2 |
| BMI | 25.4 ± 6.1 | 26.0 ± 5.2 | 29.0 ± 5.3 |
Exacerbated versus stable; p = 0.21, 95% CI −3.3–14.9.
Stable COPD versus NOS; p = 0.66, 95% CI −15.6–10.1.
COPD exacerbation versus stable COPD; p < 0.0001.
Table 2.
Patient medication
| Measurement | Patient group |
||
|---|---|---|---|
| Exacerbated COPD | Stable COPD | Non-obstructed smokers | |
| No. patients | 136 | 68 | 16 |
| Short acting β2 agonist | 80 | 40 | 0 |
| Long acting β2 agonist | 57 | 42 | 0 |
| Inhaled steroid | 94 | 53 | 0 |
| Inhaled steroid (BDP) | 1040 ± 727 | 949 ± 588 | – |
| Oral theophylline | 45 | 23 | 0 |
| Theophylline (mg) | 482 ± 189 | 524 ± 198 | – |
| Nebulised therapy | 86 | 28 | 0 |
| Home oxygen | 39 | 11 | 0 |
| Maintenance oral steroids | 9 | 6 | 0 |
Blood investigations
CRP and white cell count (WCC) were significantly elevated during exacerbation when compared to stable COPD patients. CRP levels were 14.8 mg/dl (5.0–70.2 mg/dl) at exacerbation and 5.0 mg/dl (5.0–8.4 mg/dl) in stable patients (p < 0.0001). The WCC increased from 8.7 ± 2.6 to 10.9 ± 3.7 × 103/l in exacerbations of COPD (p < 0.0001). Similarly the neutrophil count increased from 5.4 ± 2.3 to 7.9 ± 3.6 during exacerbations (p < 0.0001). Duration of stay in hospital correlated (Pearson’s) with the CRP level on admission, r = 0.22, p < 0.05.
Patients’ symptoms
The majority of patients described symptoms of increased dyspnoea, cough along with increased sputum volume and purulence during exacerbations (Table 3 ). The presence of fever was associated with the identification in the same patient, p < 0.05. The majority of patients were MRC score 5 during exacerbations.
Table 3.
Patient symptoms during exacerbations
| Measurement | Recorded during an exacerbation |
|---|---|
| Total number of patients | 136 |
| Increased dyspnoea (%) | 134 (99) |
| Increased sputum volume (%) | 88 (65) |
| Increased sputum purulence (%) | 81 (60) |
| Increased cough (%) | 105 (77) |
| Nasal discharge/congestion (%) | 75 (55) |
| Wheeze (%) | 110 (81) |
| Sore throat (%) | 34 (25) |
| Fever (%)a | 38 (28) |
| Anthonisen scoreb | 1.8 ± 0.8 |
| Total symptom score | 4.6 ± 1.5 |
| MRC score | 5.0 (5.0–5.0) |
Symptom score is based on the sum value of binary coded respiratory symptoms: dyspnoea, increased sputum purulence, increased sputum volume, nasal discharge/congestion, wheeze, sore throat and cough.
Chi squared test, fever and respiratory virus detection, p < 0.01.
Anthonisen criteria.13
Respiratory virus detection
Respiratory viruses were detected in sputum and nasal/throat swabs in 50 of 136 (37%) patients during exacerbations of COPD and 8 of 68 (12%) stable COPD patients (p < 0.0001) (Table 4 ). The viruses detected (Table 5 ) were rhinovirus (57%), adenovirus (18%), parainfluenza 3 (9%), Influenza A H3 (5%), RSV B (3.5%), metapneumovirus (3.5%), coronavirus (2%) and RSV A (2%). There were dual infections in six cases however these were all confined to the exacerbated COPD group. A respiratory virus was more frequently detected during exacerbations in patients with more severe airways disease, p < 0.05 (Table 6 ). No associations were seen between viral infection and patient sex or medication (use of theophylline or inhaled steroid therapy).
Table 4.
Respiratory viral infection group comparisons
| Groups | Odds ratio (95% CI) | Relative risk (95% CI) | p value |
|---|---|---|---|
| Exacerbated vs stable | 4.4 (1.8–10.8) | 3.1 (1.6–6.2) | 0.0002 |
| Stable vs NOSa | 0.9 (0.2–7.2) | 0.9 (0.2–4.0) | 0.93 |
Non-obstructed smokers (NOS), patients who have smoked but who have normal spirometry.
Table 5.
Respiratory viral infection
| Variable | Patient group |
||
|---|---|---|---|
| Exacerbated COPD | Stable COPD | Non-obstructed smokers | |
| No. patients | 136 | 68 | 16 |
| Respiratory viral screen positive (%) | 50 (36.8)a | 8 (11.8) | 2 (12.5) |
| Rhinovirus | 32 | 3 | 0 |
| Adenovirus | 10 | 4 | 1 |
| Influenza A H1 | 0 | 0 | 0 |
| Influenza A H3 | 3 | 0 | 0 |
| Influenza B | 0 | 0 | 0 |
| Metapneumovirus | 2 | 0 | 0 |
| Parainfluenza 1 | 0 | 0 | 0 |
| Parainfluenza 2 | 0 | 0 | 0 |
| Parainfluenza 3 | 5 | 0 | 1 |
| Coronavirus | 1 | 1 | 0 |
| RSV A | 1 | 0 | 0 |
| RSV B | 2 | 0 | 0 |
| Dual infections | |||
| Flu A H3 and Adv | 2 | 0 | 0 |
| Rhinovirus and adenovirus | 2 | 0 | 0 |
| Rhinovirus and parainfluenza 3 | 2 | 0 | 0 |
p < 0.0005 (respiratory viral detection in exacerbated versus stable COPD).
Table 6.
Respiratory viral infection and COPD stage
| GOLD Stage | Respiratory virus screen status |
|
|---|---|---|
| Respiratory virus positive (%) | Respiratory virus negative | |
| 1 | 0 (0) | 1 |
| 2 | 11 (31) | 24 |
| 3 | 16 (31) | 35 |
| 4 | 23 (47)a | 26 |
Chi squared test, comparison of groups 1–3 with group 4, linear-by-linear association p < 0.05.
Discussion
The main findings of this study were that respiratory viruses were more frequently detected during acute exacerbations of COPD in patients admitted to hospital and that CRP levels correlated with exacerbation and duration of hospital stay. This study was performed over a 2-year period in order to avoid bias due to seasonality.
The detection rate of respiratory viruses during exacerbations of COPD in this study (37%) is comparable to results obtained by Seemungal et al. (39.2%)8 and Beckham et al. 41.8%)10 but less than the findings of Rohde et al. (56%).9 Lower detection rates may be related to time of sampling as patients presenting to hospital had developed symptoms for a median of 5 days prior to admission.
This is the first study to prospectively analyse this range of respiratory viruses in patients recruited during exacerbation of COPD. The most common infecting agent was rhinovirus in keeping with previous studies,8, 9, 10 however we also detected a high rate of adenoviral infection which has not previously been reported. This is in keeping with the findings of Coyle et al. who demonstrated that adenovirus is more commonly detected using PCR than conventional immunofluorescence and virus culture techniques.14 Previous investigators have related adenoviral infection to subsequent latent infection in the form of adenovirus E1A and that it may be important in the pathogenesis of COPD.15
Metapneumovirus was discovered in 2001 and initially detected in young children with a respiratory tract illness.16 It was subsequently found to play a role in community-acquired respiratory tract illness with 2.2% of patients presenting with ’influenza-like illness’ testing positive.17 Beckham et al. tested specimens from patients during exacerbations of COPD and when stable for metapneumovirus but all patients were negative.10 Rohde et al. found metapneumovirus to be present in 2.3% of patients during acute exacerbations of COPD and none of the stable COPD patients in keeping with the findings of this study.18 The detection of human metapneumovirus in this study confirms its role as a respiratory pathogen in adult patients with COPD.
The study showed relatively lower detection rates of influenza virus compared to other studies.8, 9, 10 This may be related to improved vaccination amongst this group of at risk individuals and it may also reflect that during recruitment for this study there was no influenza epidemic. There was also a lower incidence of RSV infection (3 cases). Previous investigators have suggested that it is more common. Some have suggested that it is present in low copy numbers. It is possible that a more sensitive real-time PCR assay is required in order to detect it.8 However a recent study suggests that RSV accounted for 11.4% of COPD admissions in hospitalised patients.19
This is the first study to also include a group of patients from the same community who do not have respiratory disease (non-obstructed smokers). There were similar detection rates of respiratory viruses in the stable COPD (11.8%) and NOS groups (12.5%) supporting previous findings which found respiratory viral infection to be present in asymptomatic individuals.6 The frequency of detection in these groups suggests that COPD patients do not have a higher carriage rate of viral infections to NOS but a larger study is required in order to confirm this.
One of the weaknesses of this study was that patients were recruited following admission to hospital. In some cases there may be several days between infection, development of symptoms and presentation. Depending on the duration of viral infection prior to seeking medical help there may be some cases in which patients present late and thus viral replication is decreasing, resulting in a reduced detection rate. This may lead to an underestimation of the prevalence of viral infection in these patients. Recent publications also suggest that RSV detection is increased with the use of real-time PCR assays.8 All respiratory viral assays in this study utilized nested PCR technology and not real-time PCR methods. Detection of respiratory viruses by PCR may in fact relate to airway colonization20 or viral persistence.21 However, several of the patients were seen at different time points during this study and the same virus was not detected by repeat sampling suggesting that those testing positive using the PCR screen were experiencing an acute viral infection.
Another possible confounding factor is that patients with COPD experience exacerbations in which no precipitating agent can be identified. These episodes may be related to the progressive nature of this disease process rather than an acute event. Thus the true role of respiratory viral infection in exacerbations may be under-estimated. A possible area of bias was that all patients recruited were hospitalized. Thus selection bias may have been imposed in that those patients admitted to hospital tended to have particular types of respiratory viral infection or that respiratory viral infection only precipitated an exacerbation and subsequent hospital admission in patients with severe COPD. Another potential source of bias is the seasonality of respiratory viral infection. We addressed this issue by recruiting patients during all months of the year over a 2-year period. Other potential sources of bias include patient selection; those patients most ill and requiring non-invasive ventilation were too ill to participate in the study.
Thompson et al. have published data linking influenza and RSV infection with increased mortality in the elderly.22 There are increased admissions in older people in winter. Fleming showed that acute respiratory infections are associated with hospitalisation and increased mortality.23 It reinforces the health service implications of winter infection and increased exacerbations of COPD. It is also noteworthy that the peak death rate is in patients diagnosed with acute respiratory disease.23 Influenza has been linked with increased health care use by the elderly and there is an excess mortality particularly in the elderly.24 This paper highlights the impact of respiratory viral infection on health care resources. It supports the use of influenza vaccination in patients with underlying respiratory disease in order to reduce exacerbations and hospital admissions.
In conclusion this study supports the hypothesis that respiratory viral infection is associated with exacerbations of COPD. Rhinovirus was the most common infecting agent identified and in two cases human metapneumovirus was also detected. Dual infections were only seen amongst those patients admitted to hospital with acute exacerbations of COPD. The development of multiplexed real-time PCR assays will enable this technology to be utilized in an acute diagnostic setting.
Acknowledgements
Staff and patients of the Mater Hospital Belfast. This study was supported by a unrestricted grants from AstraZeneca, Boehringer Ingelheim and the Mater hospital.
Footnotes
Supplementary information for this manuscript can be downloaded at doi:10.1016/j.rmed.2008.06.006.
Conflict of interest statement
No conflicts of interest declared.
Appendix. Supplementary data
References
- 1.Bhowmik A., Seemungal T.A., Sapsford R.J., Wedzicha J.A. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55(2):114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy T.F., Sethi S. Bacterial infection in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1992;146(4):1067–1083. doi: 10.1164/ajrccm/146.4.1067. [DOI] [PubMed] [Google Scholar]
- 3.Sethi S. Bacterial infection and the pathogenesis of COPD. Chest. 2000;117(5 Suppl. 1):S286–S291. doi: 10.1378/chest.117.5_suppl_1.286s. [DOI] [PubMed] [Google Scholar]
- 4.Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;117(5 Suppl. 2):S380–S385. doi: 10.1378/chest.117.5_suppl_2.380s. [DOI] [PubMed] [Google Scholar]
- 5.Walsh E.E., Falsey A.R., Hennessey P.A. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am J Respir Crit Care Med. 1999;160(3):791–795. doi: 10.1164/ajrccm.160.3.9901004. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg S.B., Allen M., Wilson J., Atmar R.L. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162(1):167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 7.Seemungal T.A., Harper-Owen R., Bhowmik A., Jeffries D.J., Wedzicha J.A. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur Respir J. 2000;16(4):677–683. doi: 10.1034/j.1399-3003.2000.16d19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seemungal T., Harper-Owen R., Bhowmik A., Moric I., Sanderson G., Message S. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(9):1618–1623. doi: 10.1164/ajrccm.164.9.2105011. [DOI] [PubMed] [Google Scholar]
- 9.Rohde G., Wiethege A., Borg I., Kauth M., Bauer T.T., Gillissen A. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case-control study. Thorax. 2003;58(1):37–42. doi: 10.1136/thorax.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckham J.D., Cadena A., Lin J., Piedra P.A., Glezen W.P., Greenberg S.B. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect. 2005;50(4):322–330. doi: 10.1016/j.jinf.2004.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brusasco V., Crapo R., Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005;26(1):1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels R.A., Buist A.S., Ma P., Jenkins C.R., Hurd S.S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46(8):798–825. [PubMed] [Google Scholar]
- 13.Anthonisen N.R., Manfreda J., Warren C.P., Hershfield E.S., Harding G.K., Nelson N.A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106(2):196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 14.Coyle P.V., Ong G.M., O'Neill H.J., McCaughey C., De Ornellas D., Mitchell F. A touchdown nucleic acid amplification protocol as an alternative to culture backup for immunofluorescence in the routine diagnosis of acute viral respiratory tract infections. BMC Microbiol. 2004;4(1):41. doi: 10.1186/1471-2180-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg J.C. Role of latent viral infections in chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2001;164(10 Pt 2):S71–S75. doi: 10.1164/ajrccm.164.supplement_2.2106063. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen B.G., de Jong J.C., Groen J., Kuiken T., de Groot R., Fouchier R.A. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7(6):719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockton J., Stephenson I., Fleming D., Zambon M. Human metapneumovirus as a cause of community-acquired respiratory illness. Emerg Infect Dis. 2002;8(9):897–901. doi: 10.3201/eid0809.020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rohde G., Borg I., Arinir U., Kronsbein J., Rausse R., Bauer T.T. Relevance of human metapneumovirus in exacerbations of COPD. Respir Res. 2005;6(1):150. doi: 10.1186/1465-9921-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falsey A.R., Hennessey P.A., Formica M.A., Cox C., Walsh E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 20.Macek V., Dakhama A., Hogg J.C., Green F.H., Rubin B.K., Hegele R.G. PCR detection of viral nucleic acid in fatal asthma: is the lower respiratory tract a reservoir for common viruses? Can Respir J. 1999;6(1):37–43. doi: 10.1155/1999/938049. [DOI] [PubMed] [Google Scholar]
- 21.Matsuse T., Hayashi S., Kuwano K., Keunecke H., Jefferies W.A., Hogg J.C. Latent adenoviral infection in the pathogenesis of chronic airways obstruction. Am Rev Respir Dis. 1992;146(1):177–184. doi: 10.1164/ajrccm/146.1.177. [DOI] [PubMed] [Google Scholar]
- 22.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 23.Fleming D.M. The contribution of influenza to combined acute respiratory infections, hospital admissions, and deaths in winter. Commun Dis Public Health. 2000;3(1):32–38. [PubMed] [Google Scholar]
- 24.Menec V.H., Black C., MacWilliam L., Aoki F.Y. The impact of influenza-associated respiratory illnesses on hospitalizations, physician visits, emergency room visits, and mortality. Can J Public Health. 2003;94(1):59–63. doi: 10.1007/BF03405054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


