Abstract
Trichothecenes are exquisitely toxic to the gastrointestinal (GI) tract and leukocytes and thus are likely to impair gut immunity. The purpose of this research was to test the hypothesis that the Type A trichothecene T-2 toxin interferes with the gut mucosal immune response to enteric reovirus infection. Mice were exposed i.p. first to 1.75 mg/kg bw T-2 and then 2 h later with 3 × 107 plaque-forming units of reovirus serotype 1, strain Lang (T1/L). As compared to vehicle-treated control, T-2-treated mice had dramatically elevated intestinal plaque-forming viral titers after 5 days and failed to completely clear the virus from intestine by 10 days. Levels of reovirus λ2 core spike (L2 gene) RNA in feces in T-2-treated mice were significantly higher at 1, 3, 5, and 7 days than controls. T-2 potentiated L2 mRNA expression in a dose-dependent manner with as little as 50 μg/kg of the toxin having a potentiative effect. T-2 exposure transiently suppressed induction of reovirus-specific IgA in feces (6 and 8 days) as well as specific IgA and IgG2a in serum (5 days). This suppression corresponded to decreased secretion of reovirus-specific IgA and IgG2a in Peyer's patch (PP) and lamina propria fragment cultures prepared 5 days after infection. T-2 suppressed IFN-γ responses in PP to reovirus at 3 and 7 days as compared to infected controls whereas IL-2 mRNA concentrations were unaffected. PP IL-6 mRNA levels were increased 2-fold 2 h after T-2 treatment, but no differences between infected T-2-exposed and infected vehicle-treated mice were detectable over the next 7 days. Overall, the results suggest that T-2 toxin increased both the extent of GI tract reovirus infection and fecal shedding which corresponded to both suppressed immunoglobulin and IFN-γ responses.
Keywords: T-2 toxin, Reovirus infection, IgA, IgG, Mucosal immune response, Real-time PCR, Cytokine
Introduction
Trichothecene mycotoxins produced by Fusarium species are frequently found in cereal grains and products intended for human and animal consumption. Although Type B trichothecenes, notably deoxynivalenol (DON), are most commonly encountered (Pestka and Smolinski, 2005), the more highly toxic Type A trichothecenes are also sometimes detectable (Schothorst and van Egmond, 2004). Type A trichothecenes have nearly 10 to 100 times greater toxicity in animal and in vitro models than the Type B group with T-2 toxin being of most concern (Ueno, 1984). T-2 is considered to be the principal etiologic agent in outbreaks of fatal human alimentary toxic aleukia (ATA) with clinical signs of this disease being gastrointestinal (GI) tract hemorrhage, bone marrow depletion, leukopenia, agranulocytosis, and sepsis (Joffe, 1978). This etiologic linkage is supported by studies in experimental animals (Lutsky et al., 1978).
T-2 adversely affects the immune response. In rodents, both i.p. and oral exposure to the toxin induces apoptosis of hematopoietic and lymphoid tissues, including bone marrow, Peyer's patches, spleen, and thymus (Ihara et al., 1997, Shinozuka et al., 1997a, Shinozuka et al., 1997b, Islam et al., 1998, Nagata et al., 2001). Experimental animals similarly treated with T-2 are more susceptible to systemic infections by Salmonella Typhimurium (Tai and Pestka, 1990, Ziprin and Elissalde, 1990, Kubena et al., 2001), Listeria monocytogenes (Ziprin and McMurray, 1988, Ziprin et al., 1987), Babesia microti (Corrier and Wagner, 1988), Mycobacterium (Kanai and Kondo, 1984, Ziprin and McMurray, 1988), and Herpes simplex virus (Friend et al., 1983). Although the intestine is a principal target in ATA, little is known of T-2′s capacity to suppress the gut mucosal immune response. Assessment of potential xenobiotic modulation of mucosal immune function has lagged behind well-established methods for measuring systemic immunotoxicity (Kawabata et al., 1995, Bondy and Pestka, 2005). In one exception, Cuff et al. (1998) successfully used enteric reovirus (respiratory enteric orphan virus) to assess the suppressive effects of cyclophosphamide on gastrointestinal immune system suggesting that this virus is a valuable model for investigating mucosal immunotoxicity.
Enteric reoviruses are double-stranded RNA viruses that have been isolated from the gastrointestinal and respiratory tracts of both humans and animals (Nibert et al., 1996) and that have been suspected to contribute to mild mucosal infections (Tai et al., 2005). In addition, reoviruses have been extremely useful models for studying many aspects of viral pathogenesis and host immunity (Tyler et al., 2001). Notably, reovirus exposure initiates a self-limited infection in mice in which the virus is cleared from gastrointestinal tract within 7 to 14 days after onset. Immune responses to enteric reovirus infection in murine gastrointestinal-associated lymphoid tissues (GALT) have been well-characterized relative to: (1) virus-specific intestinal IgA (London et al., 1987, Major and Cuff, 1996, Silvey et al., 2001), (2) serum IgG (Virgin et al., 1988, Major and Cuff, 1996), (3) cell-mediated immunity (London et al., 1987, Cuff et al., 1993), and (4) cytokine production (Fan et al., 1998, Mathers and Cuff, 2004).
We have recently observed that acute oral exposure of the mouse to the Type B trichothecene DON impairs reovirus clearance but enhances reovirus-specific immunoglobulin responses (Li et al., 2005). These effects correlated with suppressed Th1 and enhanced Th2 cytokine responses, respectively. The threshold for suppression by DON was 2 mg/kg bw. Given the greater reported toxicity of T-2 over DON (Ueno, 1984) and the inherent differences in the capacity of these two mycotoxins to modulate cytokine gene expression (Ouyang et al., 1995), exposure to even very low concentrations of T-2 might pose a significant hazard to human GI immune function. Here, we tested the hypothesis that T-2 interferes with the gut mucosal immune response to experimental enteric reovirus infection in the mouse. The results suggest that T-2 increased both the extent of GI tract reovirus infection and fecal shedding with as little as 50 μg/kg of the toxin being suppressive. Impaired gut resistance to reovirus by T-2 corresponded to suppressed immunoglobulin and IFN-γ responses.
Materials and methods
Chemicals
All chemicals including T-2 and tissue culture components were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise noted.
Mice
Animal studies were conducted in accordance with NIH guidelines and were approved by the Michigan State University (MSU) Committee on Animal Use and Care. Female B6C3F1 mice aged 7–10 weeks were obtained from Charles River (Portage, MI) and acclimated for 1 week prior to experiments. Mice were housed under negative pressure in a BSL 2 room with humidity and temperature control at the MSU University Research Containment Facility.
Virus
Reovirus serotype 1, strain Lang (T1/L) was grown in L929 fibroblast cells at 34 °C in DMEM medium with 5% (v/v) fetal bovine serum (FBS), 100 U/ml of penicillin, 100 μg/ml of streptomycin, and 0.25 μg/ml of amphotericin B (Gibco). A third-passage plaque-purified virion (Furlong et al., 1988) was used for mouse infection and prepared by 1,1,2-trichloro-1,2,2-trifluoroethane extraction and discontinuous CsCl gradient centrifugation (Silvey et al., 2001). Titers of purified viruses were determined by standard plaque assay (Cuff et al., 1990).
Experimental design and sample collection
Mice were injected i.p. once with 0.05 to 1.75 mg/kg bw T-2 with the highest dose representing approximately 1/2 the LD50 (Ueno, 1984, Li et al., 2005). After 2 h, mice were infected by oral gavage with 3 × 107 plaque-forming units (PFU) of reovirus in a total volume of 100 μl borate-buffered saline (pH 7.4) containing 0.3% (w/v) of gelatin (London et al., 1987). Fecal pellets were collected 0, 2, 4, 6, 8, and 10 days after viral infection and frozen at −20 °C. At experiment termination, mice were bled and euthanized. Intestines and spleens were procured from euthanized mice and processed as described below.
Lymphoid fragment cultures
A lymphoid fragment culture system (Logan et al., 1991) as modified by Cuff et al. (1998) was used to study immunoglobulin secretion. Briefly, seven PP per mouse were removed from intestine, pooled and washed three times in HBSS with 5 μg/ml of gentamicin and twice with tissue culture medium (TCM) consisting of RPMI 1640 medium supplemented with 10% (v/v) FBS, 2 mM l-glutamine, 0.5 μM of 2-mercaptoethanol, 5 μg/ml of gentamicin, 100 unit/ml penicillin, and 100 μg/ml streptomycin. Individual patches were cut in half and incubated collectively in 2 ml of TCM for 5 days at 37 °C under 5% CO2 without additional stimulation. Lamina propria cultures were prepared, after removing PP, by splitting intestine longitudinally and cutting it into pieces 2–3 cm long. The fragments were washed at least 3 times with HBSS with 0.2% (w/v) NaHCO3, 0.1 M HEPES, and 5 μg/ml gentamicin. One half of the fragments were used for viral titer detection, and the rest were incubated twice for 30 min in 5 mM EDTA in HBSS to remove epithelial cells. The fragments without epithelial cells were washed twice more and then incubated in 3 ml of TCM for 5 days at 37 °C under 5% CO2. Culture supernatants were analyzed for specific antibodies by ELISA.
Virus titration
For viral plaque counts of GI tract (Major and Cuff, 1997), intestinal segments were placed in 2 ml of sterile phosphate-buffered saline, freeze-thawed three times, and sonicated. Tissues were homogenized in 3 ml saline containing 0.3% gelatin. Serial dilutions (100 μl) in TCM were incubated on monolayers of L-929 fibroblasts in 12-well tissue culture plates for 45 min at 34 °C and thereafter overlaid with 3 ml of 1% (w/v) agar in Medium 199 and cultured at 34 °C. Plaques were counted after 7 days incubation.
Real-time PCR for reovirus
Total RNA was extracted from 200 μl of supernatant of 10% (w/v) fecal suspension using TRIZOL (Invitrogen) and real-time PCR for reovirus L2 gene was performed as reported previously (Li et al., 2005). PCR primers for λ2 core spike (L2 gene) of reovirus T1/L were forward, 5′ ctg acg tcg atc agg tcg ttg 3′ and reverse, 5′ gat gtg gca tgc atg cat gag 3′. Purified reoviruses were added into 10% (w/w) of fecal pellet suspension from vehicle mice at concentrations of 0, 101, 102, 103, 104, 105 106, and 107 PFU/ml for standard curve and sample quantification.
ELISA
Serum was separated from clotted blood samples and stored at 4 °C. Fecal pellets were suspended at 0.1 g/ml in phosphate-buffered saline, held on ice for 2 h, and then sonicated for 15 s. Solutions were cleared by high speed centrifugation for 10 min at 4 °C and stored at − 20 °C. Serum, culture fluid, and fecal supernatant were assayed for virus-specific IgA and IgG2A antibody by the ELISA of Major and Cuff (1997) as modified by Li et al. (2005). Absorbance was used as endpoint for fecal and fragment cultures. For sera, geometric mean antibody titers were determined from the highest serum dilutions to yield absorbances of 0.2 or higher.
Real-time PCR for cytokines
For cytokine PCR, PPs were collected and immediately put into RNA later (Ambion) at 4 °C and total RNA isolated with TRIZOL reagent (Invitrogen). RNA (1 μg) was denatured by incubation at 70 °C for 10 min with 250 pM Oligo (dT)12–15 primer (Invitrogen). Real-time PCR was performed as previously described (Kinser et al., 2004) on an ABI Prism 7700 Sequence Detector with ABI SYBR Green PCR core kit (Applied Biosystem). Primer sequences (Table 1 ) were based on published cytokine mRNA sequences (Samuel and Knutson, 1983, Overbergh et al., 2003). Reactions contained a total volume of 12.5 μl: 1 μM primer pair 3 mM Mg+2, 0.8 mM dNTP mixture, 6 ng cDNA template, 0.075 μl Taq Gold DNA polymerase (ABI). Reactions were started by incubation at 95 °C for 5 min and followed by 40 two-step thermal cycles of each 15 s denaturation at 95 °C, 60 s primer annealing, and extension at 60 °C. Real-time threshold cycle (C t) value for each sample was determined. Negative control and positive samples ranging from 101 to 108 copies/ml of target gene were used for standard curve and sample quantification.
Table 1.
Primer sequences for real-time PCR amplification
| Gene | Genbank no. | Forward primer | Reverse primer | PCR product length (bp) |
|---|---|---|---|---|
| IFN-γ | K00083 | 5′ gag gaa ctg gca aaa gga tgg tga 3′ | 5′ tgt tgt tgc tga tgg cct gat tgt 3′ | 97 |
| IL-2 | X01772 | 5′ cct gag cag gat gga gaa tta ca 3′ | 5′ tcc aga aca tgc cgc aga g 3′ | 141 |
| IL-4 | M25892 | 5′ aca gga gaa ggg acg cca t 3′ | 5′ gaa gcc cta cag acg agc tca 3′ | 95 |
| IL-6 | X45452 | 5′ gag gat acc act ccc aac aga cc 3′ | 5′ aag tgc atc atc gtt gtt cat aca 3′ | 141 |
| IL-10 | M37897 | 5′ tgt gaa aat aag agc aag gca gtg 3′ | 5′ cat tca tgg cct tgt aga cac c 3′ | 85 |
Statistics
Data were analyzed using Sigma Stat software (Jandel Scientific, San Rafael, CA). For comparisons of two groups of data, Student's t test was performed. For comparison of multiple groups of data, a Kruskal–Wallis one-way ANOVA on ranks and SNK post hoc test were used. Data sets were considered significantly different when P < 0.05.
Results
The capacity of T-2-treated mice to modulate reovirus intestinal infection was initially assessed by plaque-forming assay. Mice that received vehicle alone and then infected with reovirus 2 h later contained approximately 104 PFU/intestine at 5 days with less than 101 PFU/intestine by 10 days (Fig. 1 ). However, mice treated i.p. 2 h prior to infection with T-2 (1.75 mg/kg bw) contained 6- and 500-fold more reovirus per intestine at 5 and 10 days, respectively (P < 0.05). Virus was undetectable in spleens of untreated or T-2-treated reovirus-infected mice (data not shown). The results suggest that T-2 increased the intestinal reovirus burden and prevented its completed clearance during the interval studied; however, the toxin did not facilitate breaching of the virus into the systemic compartment.
Fig. 1.
T-2 suppresses intestinal clearance of reovirus. Mice were treated with 0 or 1.75 mg/kg T-2 i.p. After 2 or 12 h, mice were infected with 3 × 107 PFU of reovirus by oral gavage. Five and ten days after infection, virus titers were determined by plaque assay. Data are means ± SEM (n = 9). Bars with asterisk are significantly different from infected control at same time point (P < 0.05).
Reovirus is shed in feces during intestinal infection and thus can be continuously monitored by real-time PCR of the L2 gene (Li et al., 2005). In control mice exposed to reovirus, approximately 100 to 200 copies of L2 RNA/g feces were detectable at 1 and 3 days (Fig. 2 ). The marker was undetectable at 5 and 7 days which is consistent with its clearance from intestinal tissue by 10 days. However, in T-2-treated mice, fecal L2 RNA copy numbers at 1, 3, 5, and 7 days were approximately 102-, 105-, 103-, and 101-fold greater than control mice (P < 0.05). Thus, T-2 increased both the amount of fecal reovirus and duration of its shedding.
Fig. 2.
T-2 increases reovirus shedding in feces. Mice were treated with 0 (closed circles) or 1.75 (open circles) mg/kg T-2 i.p. and then 2 h later infected with 3 × 107 PFU of reovirus p.o. Fecal pellets were collected at intervals and homogenized. Total RNAs were isolated and L2 gene detected by real-time PCR. Data are means ± SEM (n = 9) of viral L2 gene copies per gram fecal pellet. Points without same letter are significantly different (P < 0.05).
The dose response effects of T-2 were assessed using fecal L2 RNA copy number at 4 days. T-2 potentiated L-2 expression in a dose-dependent manner with as low as 50 μg/kg of the toxin causing significant potentiation (P < 0.05) (Fig. 3 ). L2 RNA copy number reached a plateau at 0.5 mg/kg T-2 and did not differ from 1 or 2 mg/kg (P > 0.05).
Fig. 3.
Dose response effects of T-2 on reovirus L2 RNA in feces. Mice were treated with various doses of T-2 i.p. and then 2 h later with 3 × 107 reovirus p.o. After 4 days, total fecal pellet and PP RNA were purified, and L-2 RNA quantified by real-time PCR. Data are mean ± SEM (n = 6). Bars without the same letter differ significantly (P < 0.05).
Reovirus induces a specific mucosal IgA response which likely contributes to clearance of the virus from the gut (London et al., 1987, Silvey et al., 2001). To assess mucosal IgA responses, reovirus-specific IgA in feces was compared over the 10 days course of infection in vehicle- and T-2-treated mice. Increased reovirus-specific IgA was detectable 6, 8, and 10 days after reovirus infection in vehicle-treated mice (Fig. 4 ). Induction of reovirus-specific IgA was significantly suppressed at 6 and 8 days in feces from T-2-treated mice (P < 0.05). Impaired IgA responses corresponded to suppression of reovirus-specific IgA secretion in fragment cultures of LP (Fig. 5A) and PP (Fig. 5B) obtained from T-2-treated mice 5 days after infection (P < 0.05).
Fig. 4.
T-2 suppress reovirus-specific IgA response in feces. Mice were treated with 0 or 1.75 mg/kg of T-2 i.p. and then 2 h later orally infected with 3 × 107 PFU of reovirus. Fecal pellets were collected at 0, 2, 4, 6, 8, and 10 days after infection. Virus-specific IgA in fecal suspensions was determined by ELISA. Data are means ± SEM (n = 9). Asterisks indicate significant difference between control vehicle from groups given T-2 (P < 0.05).
Fig. 5.
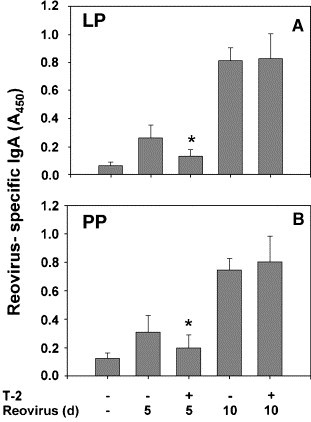
Ex vivo production of reovirus-specific IgA is suppressed in lamina propria (LP) and Peyer's patch (PP) cultures from T-2-treated mice. Mice were treated with 0 or 1.75 mg/kg of T-2 i.p. and then 2 h later infected with 3 × 107 PFU of reovirus. LP and PP fragment cultures were incubated for 5 days without additional stimulation. Reovirus-specific IgA in cultured supernatants was determined by ELISA. Data are means ± SEM (n = 9). Bars marked with asterisk are significantly different from corresponding reovirus-infected group treated with vehicle at same time point (P < 0.05).
T-2 significantly attenuated induction of reovirus-specific IgA in serum after 5 days but not after 10 days (P < 0.05) (Fig. 6A), suggesting that a corresponding and transient impact on the systemic humoral IgA response occurred. Similarly, the reovirus-specific IgG2A titer in serum from infected T-2-treated mice was 100 times less than that of infected vehicle controls at 5 days (Fig. 6B). IgG2A responses were also inhibited in tissue fragment cultures of spleen and PP from infected, T-2-treated mice at 5 days (P < 0.05) (Fig. 7 ). Reovirus-specific IgG2A was not detectable in fecal pellets and LP tissue cultures (data not shown).
Fig. 6.
T-2 suppresses reovirus-specific IgA and IgG2a response in serum. Mice were treated with 0 or 1.75 mg/kg of T-2 and then 2 h later orally infected with 3 × 107 PFU of reovirus. Five and ten days after infection, serum IgA and IgG2a titers were determined by ELISA. Data are means ± SEM (n = 9). Bars marked with an asterisk are significantly different from corresponding reovirus-infected group treated with vehicle at same time point (P < 0.05).
Fig. 7.
Ex vivo production of reovirus-specific IgG2a is suppressed in spleen and PP cultures of T-2-treated mice. Mice were treated with 0 or 1.75 mg/kg of T-2 i.p. and then 2 h later and then orally infected with 3 × 107 PFU of reovirus. Spleen and PP fragment cultures were incubated for 5 days without additional stimulation. Reovirus-specific IgG2A of cultured supernatants were determined by ELISA. Data are means ± SEM (n = 9). Bars marked with an asterisk are significantly different from corresponding infected control given vehicle at same time point (P < 0.05).
DON has been previously shown to skew the cytokine responses to reovirus infection in PP from Th1 to Th2 (Li et al., 2005). The effects of T-2 and reovirus on expression of Th1 (IFN-γ and IL-2) and Th2 (IL-4, IL-6, and IL-10) cytokine mRNAs in PP were therefore assessed over 7 days. Treatment with T-2 for 2 h without reovirus initially elevated IFN-γ (Fig. 8A) but suppressed IL-2 (Fig. 8B) mRNA levels. Reovirus markedly induced IFN-γ mRNA expression from 3 to 7 days but this was suppressed by T-2 (Fig. 8A). Reovirus also caused decreases in IL-2 (Fig. 8B) and IL-4 (Fig. 9A) mRNA which were not impacted by T-2. IL-6 mRNA was initially elevated by treatment with T-2 for 2 h without reovirus but did not differ from vehicle-treated controls during the course of reovirus infection (Fig. 9B). Reovirus caused decreases in IL-10 mRNA but this effect was abrogated by T-2 treatment (Fig. 8C). Thus, the primary effects of T-2 on cytokine gene expression in the PP were attenuation of reovirus-induced IFN-γ and interference with reovirus suppression of IL-10.
Fig. 8.
Kinetics of Th1 cytokine mRNA expression in Peyer's patches of T-2-treated mice. Mice were treated with 0 (closed circles) or 1.75 (open circles) mg/kg of T-2 i.p. and then 2 h later orally infected with 3 × 107 PFU of reovirus. After 0, 1, 3, 5, and 7 days, Peyer's patches (PP) RNAs were isolated and relative expression of IFN-γ and IL-2 mRNA measured by real-time PCR. Data are means ± SEM (n = 6). Asterisk indicates significant difference from corresponding infected control given vehicle (P < 0.05).
Fig. 9.
Kinetics of Th2 cytokine mRNA expression in Peyer's patches of T-2-treated mice. Mice were treated with vehicle (closed circles) or T-2 (open circles) as described in Fig. 8 and relative expression of IL-6, IL-10, and IL-4 mRNA determined by real-time PCR. Data are means ± SEM (n = 6). Asterisk indicates significant difference from corresponding infected control given vehicle (P < 0.05).
Discussion
Immune modulation by natural toxins and environmental toxicants has potential implications to human health relative to both mounting appropriate responses against infectious agents and generating untoward inflammatory or autoimmune responses (Germolec, 2004). Reovirus has widely been used to study viral pathogenesis (Tyler et al., 2001) and previous studies (Cuff et al., 1998, Li et al., 2005) suggest this virus to be a robust model for monitoring dysregulation of host resistance in the gut mucosal immune system. Here, a single T-2 exposure was found to suppress the host response to reovirus as evidenced by the inability to clear the virus from intestine as well as by increased fecal shedding of the virus. Rosen et al. (1960) described an outbreak of T1 reovirus infection among children over a 9-week period. Fecal shedding of the virus occurred for at least 1 week in 54% of the children and lasted at least 2 weeks in an additional 21%. All children with detectable virus in their stools exhibited elevated antibody titers to the virus. Thus, the murine model employed in this study appears to be relevant to human infection from the perspectives of both gut infection kinetics and seroconversion.
Relative to human safety assessment, the dose response experiment revealed that as little as 50 μg/kg bw of T-2 suppressed resistance to reovirus. This dose is approximately equivalent to a daily consumption of 350 μg/kg T-2 in mouse diet. In relation, reported T-2 concentrations in European wheat, maize, barley, and oats have been reported to be as much as 250, 260, 310, and 530 μg/kg (Canady et al., 2001). The LOEL observed herein for the mouse reovirus model was consistent with findings of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) who identified the lowest observed effect level of 29 μg/kg bw per day based on changes in white and red blood cell counts identified in a 3-week dietary study in pigs (Canady et al., 2001). JECFA used this LOEL and a safety factor of 500 to derive a provisional maximal tolerable daily intake for T-2 of 60 ng/kg bw per day. It should be further noted that we utilized i.p. exposure in this study to minimize experimental variation. While T-2 distributes rapidly to lymphoid tissue regardless of exposure route (Canady et al., 2001), it will nevertheless be critical to understand how acute and chronic exposure by ingestion to low concentrations of T-2 might impact host resistance to reovirus.
Although incompletely understood, the host resistance mechanisms for reovirus and other enteric viruses are multi-factorial and include both innate and adaptive immune components. For viruses that invade via the mucosal route, both IgA and IgG can provide protection and mediate viral clearance (Kato et al., 2001, Whitton and Oldstone, 2001). Reovirus selectively adheres to apical surfaces of M cells of the PP enabling transepithelial transport (Wolf et al., 1981, Helander et al., 2003). Secretory IgA antibodies provide a protective first line via entrapment of virus particles in mucus as well as blocking epithelial cell binding and entry (Phalipon et al., 2002). IgG can also provide protection against reovirus by neutralization and mobilization of factors that mediate clearance (Whitton and Oldstone, 2001). Our observation that T-2 markedly suppressed reovirus-induced Ig responses is of general importance because antibodies provide protective immunity against most viruses (Klasse and Sattentau, 2002).
An equally important finding was that T-2 suppression of IFN-γ expression from 3 to 7 days post-infection was consistent with diminished clearance of reovirus infection. IFN-γ facilitates antiviral immunity by suppressing viral replication, activating macrophages, inducing nitric oxide synthase and stimulating specific cytotoxic immunity via cell-surface-bound antigen-associated MHC proteins (Shtrichman and Samuel, 2001). Suppression of one or more of these mechanisms could prolong reovirus replication and survival. Clarification is now needed on whether T-2 impairment of IFN-γ impacts reovirus-induced changes in macrophage, NK, dendritic, or T cell function as well as epithelial cell physiology, pIgR levels, or immune tolerance.
The specific mechanisms by which T-2 suppresses Ig and IFN-γ response are unclear but might be explainable by selective leukocyte death in the mucosal or systemic compartments. Exposure to T-2 has been previously shown to induce apoptotic cell death in PP, thymus, spleen, or bone marrow (Shinozuka et al., 1997a, Shinozuka et al., 1997b, Nagata et al., 2001). Notably, Islam et al. (1998) found that within 6 h after i.p. injection with T-2 doses as low as 0.87 mg/kg, marked DNA fragmentation is observed in the thymus. Using flow cytometry, these investigators further determined that when compared with vehicle controls, CD4+CD8+ double positive subsets were decreased significantly at 24, 48, and 72 h in thymuses of mice treated with 1.75 mg/kg T-2. Furthermore, a significant decrease in CD4+CD8− thymocytes was also observed at 72 h after T-2 exposure. Nagata et al. (2001) found similar effects in thymus mice orally exposed to T-2 and that these effects extended into the PP. Attenuation in CD4+CD8+ and CD4+CD8− cell could conceivably impact requisite T helper activity for mounting both IgA and IgG responses. Similarly, alterations in these populations might also contribute to decreased production of IFN-γ. In support of this contention, we previously observed that in vivo DON exposure suppresses reovirus-induced IFN-γ secretion by CD4+ cells in PP cultures (Li et al., 2005). Additional analysis of the relationship among T-2 dose, differential leukocyte cell death, and suppressed reovirus responses is warranted.
T-2′s effects on mucosal immune response to reovirus differed in several respects from that observed previously for DON (Li et al., 2005). Much lower doses of T-2 (0.05 to 1.75 mg/kg) than DON (2 to 25 mg/kg) impaired reovirus clearance. Furthermore, 10 days after treatment with T-2 at the highest dose, a large amount of residual virus remained in the intestine whereas it was completely cleared in mice at the highest DON dose. Thus, less efficient clearance after T-2 exposure might relate to suppression of the mucosal IgA response. While DON similarly impaired reovirus-induced IFN-γ and attenuated early mucosal and systemic IgA and IgG responses (1 to 5 days), it later potentiated reovirus-specific IgA in feces, serum, and fragment cultures during later stages of the infection (5 to 10 days). DON-exposed mice also exhibited markedly increased IL-4, IL-6, and IL-10 mRNA expression in PP which was suggestive of a skewed Th2 response. All three of these cytokines promote proliferation and terminal differentiation of Ig-secreting cells as well as downregulate Th1 responses (Yamamoto et al., 1996, Diehl and Rincon, 2002, Conti et al., 2003, Mathers and Cuff, 2004). The lack of a similarly robust effect on Th 2 cytokines by T-2 suggests inherent differences between these two toxins (and perhaps Type A and Type B trichothecenes) in their capacity to upregulate cytokines during viral infection and might explain the lack of IgA enhancing effect.
Taken together, T-2 impaired clearance of the reovirus and increased its fecal shedding and these effects corresponded to suppression of both reovirus-induced Igs and IFN-γ. Elevated viral load in intestinal tissue is likely to increase inflammation and discomfort to the host during the infection process. Additionally, increased fecal shedding could enhance virus dissemination among individuals. While reovirus was used here primarily as a model, its pathogenic mechanisms and resultant host immune responses are shared with many enteric and respiratory viruses (Nibert et al., 1996). It might be speculated that trichothecene exposure could enable more virulent viruses to establish infections and cause serious diseases. Intriguingly, reovirus T1/L has been found to evoke acute respiratory distress in young mice (Majeski et al., 2003a, Majeski et al., 2003b, London et al., 2002). A human reovirus isolated from a severe acute respiratory syndrome (SARS) patient has been recently demonstrated to cause SARS-like symptoms in macaques and guinea pigs (He et al., 2005, Liang et al., 2005). Further insight is needed in reovirus and other viral models on the specific mechanisms by which T-2 and other trichothecenes interfere with cytokine- and Ig-regulated viral clearance and how these are impacted by dose, administration route, and duration of toxin exposure.
Acknowledgments
This study was supported by a Strategic Research Grant from the Michigan State University Foundation and by Public Health Service Grants ES03553 (JP) and AI034544 (CC) from the National Institutes for Health. We thank Dr. Norb Kaminski and Dr. Jack Harkema for advice on experiment design and Drs. Hui-Ren Zhou, Zahidul Islam, and Qunshan Jia for technical assistance.
References
- Bondy G.S., Pestka J.J. Gut mucosal immunotoxicology in rodents. In: Tryphonas H., Rournier M., Blakley B.R., Smits J.E.G., Brousseau P., editors. Investigative Immunotoxicology. Taylor and Francis; New York: 2005. pp. 197–210. [Google Scholar]
- Canady R.A., Coker R.D., Egan S.K., Krska R., Olsen M., Resnik S., Schlatter J. Safety Evaluation of Certain Mycotoxins in Food. World Health Organization; Geneva: 2001. T-2 and HT-2 toxins; pp. 557–680. [Google Scholar]
- Conti P., Kempuraj D., Kandere K., Di Gioacchino M., Barbacane R.C., Castellani M.L., Felaco M., Boucher W., Letourneau R., Theoharides T.C. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol. Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Corrier D.E., Wagner G.G. Comparison of the effect of T-2 toxin with that of dexamethasone or cyclophosphamide on resistance to Babesia microti infection in mice. Am. J. Vet. Res. 1988;49:2000–2003. [PubMed] [Google Scholar]
- Cuff C.F., Lavi E., Cebra C.K., Cebra J.J., Rubin D.H. Passive immunity to fatal reovirus serotype 3-induced meningoencephalitis mediated by both secretory and transplacental factors in neonatal mice. J. Virol. 1990;64:1256–1263. doi: 10.1128/jvi.64.3.1256-1263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuff C.F., Cebra C.K., Rubin D.H., Cebra J.J. Developmental relationship between cytotoxic alpha/beta T cell receptor-positive intraepithelial lymphocytes and Peyer's patch lymphocytes. Eur. J. Immunol. 1993;23:1333–1339. doi: 10.1002/eji.1830230622. [DOI] [PubMed] [Google Scholar]
- Cuff C.F., Fulton J.R., Barnett J.B., Boyce C.S. Enteric reovirus infection as a probe to study immunotoxicity of the gastrointestinal tract. Toxicol. Sci. 1998;42:99–108. doi: 10.1006/toxs.1998.2425. [DOI] [PubMed] [Google Scholar]
- Diehl S., Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002;39:531–536. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Fan J.Y., Boyce C.S., Cuff C.F. T-Helper 1 and T-helper 2 cytokine responses in gut-associated lymphoid tissue following enteric reovirus infection. Cell. Immunol. 1998;188:55–63. doi: 10.1006/cimm.1998.1350. [DOI] [PubMed] [Google Scholar]
- Friend S.C., Schiefer H.B., Babiuk L.A. The effects of dietary T-2 toxin on acute herpes simplex virus type 1 infection in mice. Vet. Pathol. 1983;20:737–760. doi: 10.1177/030098588302000609. [DOI] [PubMed] [Google Scholar]
- Furlong D.B., Nibert M.L., Fields B.N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J. Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germolec D.R. Sensitivity and predictivity in immunotoxicity testing: immune endpoints and disease resistance. Toxicol. Lett. 2004;149:109–114. doi: 10.1016/j.toxlet.2003.12.025. [DOI] [PubMed] [Google Scholar]
- He C., Pang W., Yong X., Zhu H., Lei M., Duan Q. Experimental infection of macaques with the human reovirus BYD1 strain: an animal model for the study of the severe acute respiratory syndrome. DNA Cell Biol. 2005;24:491–495. doi: 10.1089/dna.2005.24.491. [DOI] [PubMed] [Google Scholar]
- Helander A., Silvey K.J., Mantis N.J., Hutchings A.B., Chandran K., Lucas W.T., Nibert M.L., Neutra M.R. The viral sigma1 protein and glycoconjugates containing alpha2-3-linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J. Virol. 2003;77:7964–7977. doi: 10.1128/JVI.77.14.7964-7977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T., Sugamata M., Sekijima M., Okumura H., Yoshino N., Ueno Y. Apoptotic cellular damage in mice after T-2 toxin-induced acute toxicosis. Nat. Toxins. 1997;5:141–145. doi: 10.1002/1522-7189(1997)5:4<141::AID-NT3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Islam Z., Nagase M., Yoshizawa T., Yamauchi K., Sakato N. T-2 toxin induces thymic apoptosis in vivo in mice. Toxicol. Appl. Pharmacol. 1998;148:205–214. doi: 10.1006/taap.1997.8338. [DOI] [PubMed] [Google Scholar]
- Joffe A.Z. Fusarium poae and F. sporotichiodes as principal causal agents of alimentary toxic aleukia. In: Wyllie T.D., Morehouse L.G., editors. Mycotoxic Fungi, Mycotoxins, Mycotoxicoses: An Encylcopedic Handbook. Dekker; New York: 1978. pp. 21–86. [Google Scholar]
- Kanai K., Kondo E. Decreased resistance to mycobacterial infection in mice fed a trichothecene compound (T-2 toxin) Jpn. J. Med. Sci. Biol. 1984;37:97–104. doi: 10.7883/yoken1952.37.97. [DOI] [PubMed] [Google Scholar]
- Kato H., Kato R., Fujihashi K., McGhee J.R. Role of mucosal antibodies in viral infections. Curr. Top. Microbiol. Immunol. 2001;260:201–228. doi: 10.1007/978-3-662-05783-4_11. [DOI] [PubMed] [Google Scholar]
- Kawabata T.T., Burleson G.R., Ernst P.B., Ullrich S.E. Immunotoxicology of regional lymphoid tissue: the respiratory and gastrointestinal tracts and skin. Fundam. Appl. Toxicol. 1995;26:8–19. doi: 10.1006/faat.1995.1070. [DOI] [PubMed] [Google Scholar]
- Kinser S., Jia Q., Li M., Laughter A., Cornwell P., Corton J.C., Pestka J. Gene expression profiling in spleens of deoxynivalenol-exposed mice: immediate early genes as primary targets. J. Toxicol. Environ. Health, A. 2004;67:1423–1441. doi: 10.1080/15287390490483827. [DOI] [PubMed] [Google Scholar]
- Klasse P.J., Sattentau Q.J. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 2002;83:2091–2108. doi: 10.1099/0022-1317-83-9-2091. [DOI] [PubMed] [Google Scholar]
- Kubena L.F., Bailey R.H., Byrd J.A., Young C.R., Corrier D.E., Stanker L.H., Rottinghaust G.E. Cecal volatile fatty acids and broiler chick susceptibility to Salmonella Typhimurium colonization as affected by aflatoxins and T-2 toxin. Poult. Sci. 2001;80:411–417. doi: 10.1093/ps/80.4.411. [DOI] [PubMed] [Google Scholar]
- Li M., Cuff C.F., Pestka J. Modulation of murine host response to enteric reovirus infection by the trichothecene deoxynivalenol. Toxicol. Sci. 2005;87:134–145. doi: 10.1093/toxsci/kfi225. [DOI] [PubMed] [Google Scholar]
- Liang L., He C., Lei M., Li S., Hao Y., Zhu H., Duan Q. Pathology of guinea pigs experimentally infected with a novel reovirus and coronavirus isolated from SARS patients. DNA Cell Biol. 2005;24:485–490. doi: 10.1089/dna.2005.24.485. [DOI] [PubMed] [Google Scholar]
- Logan A.C., Chow K.P., George A., Weinstein P.D., Cebra J.J. Use of Peyer's patch and lymph node fragment cultures to compare local immune responses to Morganella morganii. Infect. Immun. 1991;59:1024–1031. doi: 10.1128/iai.59.3.1024-1031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London S.D., Rubin D.H., Cebra J.J. Gut mucosal immunization with reovirus serotype 1/L stimulates virus-specific cytotoxic T cell precursors as well as IgA memory cells in Peyer's patches. J. Exp. Med. 1987;165:830–847. doi: 10.1084/jem.165.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London L., Majeski E.I., Paintlia M.K., Harley R.A., London S.D. Respiratory reovirus 1/L induction of diffuse alveolar damage: a model of acute respiratory distress syndrome. Exp. Mol. Pathol. 2002;72:24–36. doi: 10.1006/exmp.2001.2414. [DOI] [PubMed] [Google Scholar]
- Lutsky I., Mor N., Yagen B., Joffe A.Z. The role of T-2 toxin in experimental alimentary toxic aleukia: a toxicity study in cats. Toxicol. Appl. Pharmacol. 1978;43:111–124. doi: 10.1016/s0041-008x(78)80036-2. [DOI] [PubMed] [Google Scholar]
- Majeski E.I., Paintlia M.K., Lopez A.D., Harley R.A., London S.D., London L. Respiratory reovirus 1/L induction of intraluminal fibrosis, a model of bronchiolitis obliterans organizing pneumonia, is dependent on T lymphocytes. Am. J. Pathol. 2003;163:1467–1479. doi: 10.1016/S0002-9440(10)63504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeski E.I., Harley R.A., Bellum S.C., London S.D., London L. Differential role for T cells in the development of fibrotic lesions associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia versus Acute Respiratory Distress Syndrome. Am. J. Respir. Cell Mol. Biol. 2003;28:208–217. doi: 10.1165/rcmb.4891. [DOI] [PubMed] [Google Scholar]
- Major A.S., Cuff C.F. Effects of the route of infection on immunoglobulin G subclasses and specificity of the reovirus-specific humoral immune response. J. Virol. 1996;70:5968–5974. doi: 10.1128/jvi.70.9.5968-5974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major A.S., Cuff C.F. Enhanced mucosal and systemic immune responses to intestinal reovirus infection in beta2-microglobulin-deficient mice. J. Virol. 1997;71:5782–5789. doi: 10.1128/jvi.71.8.5782-5789.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers A.R., Cuff C.F. Role of interleukin-4 (IL-4) and IL-10 in serum immunoglobulin G antibody responses following mucosal or systemic reovirus infection. J. Virol. 2004;78:3352–3360. doi: 10.1128/JVI.78.7.3352-3360.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Suzuki H., Ishigami N., Shinozuka J., Uetsuka K., Nakayama H., Doi K. Development of apoptosis and changes in lymphocyte subsets in thymus, mesenteric lymph nodes and Peyer's patches of mice orally inoculated with T-2 toxin. Exp. Toxicol. Pathol. 2001;53:309–315. doi: 10.1078/0940-2993-00196. [DOI] [PubMed] [Google Scholar]
- Nibert M.L., Schiff L.A., Fields B.N. Reoviruses and their replication. In: Fields B.N., Knipe D.M., editors. Virology. 3rd ed. Raven Press; New York: 1996. pp. 691–722. [Google Scholar]
- Ouyang Y.L., Azcona-Olivera J.I., Pestka J.J. Effects of trichothecene structure on cytokine secretion and gene expression in murine CD4+ T-cells. Toxicology. 1995;104:187–202. doi: 10.1016/0300-483x(95)03147-8. [DOI] [PubMed] [Google Scholar]
- Overbergh L., Giulietti A., Valckx D., Decallonne R., Bouillon R., Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J. Biomol. Tech. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Pestka J.J., Smolinski A.T. Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health, B Crit. Rev. 2005;8:39–69. doi: 10.1080/10937400590889458. [DOI] [PubMed] [Google Scholar]
- Phalipon A., Cardona A., Kraehenbuhl J.P., Edelman L., Sansonetti P.J., Corthesy B. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- Rosen L., Hovis J.F., Mastrota F.M. An outbreak of infection with a type 1 reovirus among children in an institution. Am. J. Hyg. 1960;71:266–274. [Google Scholar]
- Samuel C.E., Knutson G.S. Mechanism of interferon action: human leukocyte and immune interferons regulate the expression of different genes and induce different antiviral states in human amnion U cells. Virology. 1983;130:474–484. doi: 10.1016/0042-6822(83)90101-0. [DOI] [PubMed] [Google Scholar]
- Schothorst R.C., van Egmond H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”, Subtask: trichothecenes. Toxicol. Lett. 2004;153:133–143. doi: 10.1016/j.toxlet.2004.04.045. [DOI] [PubMed] [Google Scholar]
- Shinozuka J., Guanmin L., Uetsuka K., Nakayama H., Doi K. Process of the development of T-2 toxin-induced apoptosis in the lymphoid organs of mice. Exp. Anim. 1997;46:117–126. doi: 10.1538/expanim.46.117. [DOI] [PubMed] [Google Scholar]
- Shinozuka J., Li G., Kiatipattanasakul W., Uetsuka K., Nakayama H., Doi K. T-2 toxin-induced apoptosis in lymphoid organs of mice. Exp. Toxicol. Pathol. 1997;49:387–392. doi: 10.1016/S0940-2993(97)80124-8. [DOI] [PubMed] [Google Scholar]
- Shtrichman R., Samuel C.E. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 2001;4:251–259. doi: 10.1016/s1369-5274(00)00199-5. [DOI] [PubMed] [Google Scholar]
- Silvey K.J., Hutchings A.B., Vajdy M., Petzke M.M., Neutra M.R. Role of immunoglobulin A in protection against reovirus entry into murine Peyer's patches. J. Virol. 2001;75:10870–10879. doi: 10.1128/JVI.75.22.10870-10879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai J.H., Pestka J.J. T-2 toxin impairment of murine response to Salmonella Typhimurium: a histopathologic assessment. Mycopathologia. 1990;109:149–155. doi: 10.1007/BF00436803. [DOI] [PubMed] [Google Scholar]
- Tai J.H., Williams J.V., Edwards K.M., Wright P.F., Crowe J.E., Jr., Dermody T.S. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J. Infect. Dis. 2005;191:1221–1224. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K.L., Clarke P., Debiasi R.L., Kominsky D., Poggioli G.J. Reoviruses and the host cell. Trends Microbiol. 2001;9:560–564. doi: 10.1016/s0966-842x(01)02103-5. [DOI] [PubMed] [Google Scholar]
- Ueno Y. Toxicological features of T-2 toxin and related trichothecenes. Fundam. Appl. Toxicol. 1984;4:S124–S132. doi: 10.1016/0272-0590(84)90144-1. [DOI] [PubMed] [Google Scholar]
- Virgin H.W., Bassel-Duby R., Fields B.N., Tyler K.L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J. Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton J.L., Oldstone M.B.A. The immune response to viruses. In: Knipe D.M., Howley P.M., editors. Fields Virology. 4th ed. Lippincott/The Williams and Wilkins Co; Philadelphia, PA: 2001. pp. 285–320. [Google Scholar]
- Wolf J.L., Rubin D.H., Finberg R., Kauffman R.S., Sharpe A.H., Trier J.S., Fields B.N. Intestinal M cells: a pathway for entry of reovirus into the host. Science. 1981;212:471–472. doi: 10.1126/science.6259737. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Vancott J.L., Okahashi N., Marinaro M., Kiyono H., Fujihashi K., Jackson R.J., Chatfield S.N., Bluethmann H., McGhee J.R. The role of Th1 and Th2 cells for mucosal IgA responses. Ann. N. Y. Acad. Sci. 1996;778:64–71. doi: 10.1111/j.1749-6632.1996.tb21115.x. [DOI] [PubMed] [Google Scholar]
- Ziprin R.L., Elissalde M.H. Effect of T-2 toxin on resistance to systemic Salmonella Typhimurium infection of newly hatched chickens. Am. J. Vet. Res. 1990;51:1869–1872. [PubMed] [Google Scholar]
- Ziprin R.L., McMurray D.N. Differential effect of T-2 toxin on murine host resistance to three facultative intracellular bacterial pathogens: Listeria monocytogenes, Salmonella Typhimurium, and Mycobacterium bovis. Am. J. Vet. Res. 1988;49:1188–1192. [PubMed] [Google Scholar]
- Ziprin R.L., Holt P.S., Mortensen R.F. T-2 toxin effects on the serum amyloid P-component (SAP) response of Listeria monocytogenes- and Salmonella Typhimurium-infected mice. Toxicol. Lett. 1987;39:177–184. doi: 10.1016/0378-4274(87)90230-x. [DOI] [PubMed] [Google Scholar]


