Highlights
-
•
A PDCoV strain, CH/XJYN/2016, was successfully isolated and its biological characteristics were determined.
-
•
Pathogenicity of CH/XJYN/2016 in suckling piglets and conventional weaned pigs were determined.
-
•
An inactivated cell-adapted CH/XJYN/2016-based vaccine candidate was developed and its efficacy was evaluated.
Keywords: Porcine deltacoronavirus, Characterization, Pathogenicity, Protective efficacy, Pigs
Abstract
Porcine deltacoronavirus (PDCoV) is a novel swine enteropathogenic coronavirus that causes acute diarrhea, vomiting, dehydration and mortality in neonatal piglets, resulting in significant economic losses to the pig industry. However, there is currently little information on vaccine studies and commercially available vaccines for PDCoV. Hence, herein, a PDCoV strain, CH/XJYN/2016, was successfully isolated and serially propagated in vitro, and its biological characteristics were determined. Compared to that of previously reported and recently isolated PDCoV strains from China and the United States, the S gene of the CH/XJYN/2016 strain contains novel mutations. Infection studies revealed that CH/XJYN/2016 is pathogenic to suckling piglets and conventional weaned pigs. In addition, the median pig diarrhea dose (PDD50) of PDCoV in conventional weaned pigs was determined (2.0 log10PDD50/3 mL). Furthermore, an inactivated cell-adapted CH/XJYN/2016-based vaccine candidate was developed with different adjuvants. Compared with nonvaccinated pigs, conventional weaned pigs given the inactivated vaccine developed a potent humoral immune response and showed no clinical signs or viral shedding after challenge, indicating a potent protective effect of the vaccine against PDCoV infection. Therefore, the PDCoV vaccine developed in this study is a promising vaccine candidate that can be used for the control of PDCoV infection in pigs.
1. Introduction
Porcine deltacoronavirus (PDCoV) is an enveloped, single-stranded, positive-sense RNA virus belonging to the Deltacoronavirus genus of the Coronaviridae family (Hu et al., 2015). PDCoV was first reported in pigs in Hong Kong in 2012 (Woo et al., 2017) and was subsequently detected in swine herds in the United States and isolated from clinical cases of diarrhea in young pigs in 2014 (Hu et al., 2015; Marthaler et al., 2014; Wang et al., 2014). To date, PDCoV has been successively detected in Canada (Marthaler et al., 2014), South Korea (Lee and Lee, 2014), Mainland China (Wang et al., 2015), Mexico (Lee, 2015), Thailand (Janetanakit et al., 2016; Madapong et al., 2016), Vietnam and Lao PDR (Saeng-Chuto et al., 2017). Hence, PDCoV has recently become prevalent in pigs worldwide and has caused serious economic losses for the pig industry.
The complete genome of PDCoV is approximately 25 kb long and contains the 5′-untranslated region, open reading frame (ORFs) organized in the order ORF1a/1b, spike (S) glycoprotein gene, envelope (E) gene, membrane (M) gene, non-structural protein 6 (Nsp6) gene, nucleoprotein (N) gene, Nsp7 gene, and the 3′-UTR (Lee and Lee, 2014; Zhang, 2016). ORF1a/b encodes two overlapping viral replicase polyproteins that are processed into mature nonstructural proteins. Although the general characteristics of the structural and nonstructural proteins of PDCoV are similar to those of other swine coronaviruses, the detailed functions and roles of the structural and nonstructural PDCoV proteins in host cells are largely unknown (Jung et al., 2016). Among these structural proteins, the N protein is known to be the most abundant and multifunctional viral component (Lee and Lee, 2015).
Typical clinical symptoms of PDCoV infection include diarrhea, dehydration, variable vomiting and mortality in nursing piglets. However, compared with those of other swine coronaviruses, such as porcine epidemic diarrhea virus (PEDV) and transmissible gastroenteritis virus (TGEV), the clinical symptoms of PDCoV infection are milder and the mortality rates are lower in affected nursing pigs (Chen et al., 2015; Jung et al., 2016; Zhang, 2016). Previous reports have shown that PDCoV mortality rates range from 40 % to more than 80 % among neonatal pigs (2014; Song et al., 2015). Similar to that of PEDV and TGEV, the strongest tissue tropism of PDCoV is in villous enterocytes of the small and large intestines, leading to marked villous atrophy in the small intestine but not in the large intestine (Chen et al., 2015; Hu et al., 2015; Jung et al., 2016).
PDCoV has been successfully isolated and serially propagated in LLC porcine kidney (LLC-PK, ATCC No: CL-101) and swine testicular (ST, ATCC No: CRL1746) cells supplemented with trypsin or pancreatin (Dong et al., 2016; Hu et al., 2015; Jang et al., 2018; Zhang et al., 2019b). In addition, the porcine enterocyte cell line, IPEC-J2, is also susceptible to PDCoV infection but apoptosis may not be induced in the infected cells (Jung et al., 2018). As is the case for PEDV, trypsin contributes to a significant increase in PDCoV growth after several passages in LLC-PK cells, but not in ST cells (Hu et al., 2015). In addition, PDCoV can also be propagated in LLC-PK cells without supplemental trypsin; however, it does not produce cytopathic effects (CPEs) and the viral titer is reduced (Hu et al., 2015; Jung et al., 2016). Therefore, in general, LLC-PK and ST cells are suitable for the in vitro isolation and propagation of PDCoV field strains under optimal cell culture conditions.
Previous phylogenetic analysis showed that the Chinese PDCoV strain is more closely related to other PDCoV strains in China than to the strains from Southeast Asia, USA, Japan, and South Korea, suggesting the diversity of genetic relationships and regional and epidemic characteristics among these strains (Zhao et al., 2019). Additionally, the recent study shows that the Chinese PDCoV strains isolated from China had the same discontinuous amino acid deletions in Nsp2 and Nsp3 regions as Thailand, Vietnam and Laos, indicating that PDCoV may have undergone a high degree of variation since PDCoV was first detected in China (Sun et al., 2020). All of these studies show that the persistent occurrences of PDCoV infection in Asia and North America implies a potential risk of pandemic outbreaks. Therefore, effective vaccines are required to control and limit the spread of PDCoV. However, there are currently no vaccines available for PDCoV. Therefore, the main purpose of the present study was to isolate and serially propagate a PDCoV strain and to develop a vaccine candidate that can elicit a potent humoral immune response against PDCoV and effectively protect pigs against PDCoV infection. In this study, the PDCoV strain, CH/XJYN/2016 (GenBank No: MN064712), was successfully isolated and serially propagated using LLC-PK, and its biological characteristics and pathogenicity were determined. Notably, the infectious titer (the median pig diarrhea dose, PDD50) of PDCoV in conventional weaned pigs was determined for the first time. In addition, the immunoprotective effect of the cell-adapted strain CH/XJYN/2016-based inactivated vaccine candidate was evaluated. The results presented in this study indicated that the inactivated vaccine is a promising vaccine candidate for the prevention and control of PDCoV in China.
2. Materials and methods
2.1. Ethics statement
All pigs used in this study were well taken care of during the experiment and euthanized according laboratory procedure at the end of the experiment. All animal experiments were performed in biosecurity laboratory. Animal care and use protocols were reviewed and approved by the Institutional Animal Use and Care Committee of Lanzhou Veterinary Research Institute.
2.2. Samples, cells, and antibodies
In February 2016, twelve clinical samples, including porcine intestinal contents and feces, were collected from a small-scale pig farm (100 sows) with diarrhea outbreaks (more than half of the sucking piglets developed severe diarrhea and about one-third of piglets died) in Yining, Xinjiang Uygur Autonomous Region, China. The samples were stored at a low temperature with dry ice after collection. Subsequently, the samples were diluted 1:10 with serum-free Dulbecco's modified Eagle’s medium (DMEM, Invitrogen, USA) containing 1% penicillin-streptomycin (10,000 units/mL penicillin and 10,000 μg/mL streptomycin; Gibco, USA) and then centrifuged at 1500×g at 4 °C for 30 min. The supernatant was filtered using a 0.22-μm pore-size filter (Merck Millipore, Germany) to remove bacteria, and then the viral RNA was extracted using an RNeasy Mini kit (Qiagen, Germany) according to the operating instructions. The virus isolated from these samples was identified as PDCoV by N gene-based reverse transcription PCR (RT-PCR; developed by our lab). LLC-PK cells (ATCC No: CL-101) were purchased from ATCC and were cultured in Minimum Essential Medium (MEM, Gibco, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS, Gibco, Australia), 1% MEM nonessential amino acids (NEAA, Gibco, USA), 1% antibiotic-antimycotic (Gibco, USA) and 1% N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES, Gibco, USA). The cells were maintained at 37 °C in a humidified 5% CO2 incubator. Mouse anti-PDCoV N protein monoclonal antibodies (McAbs) were prepared and stored in our laboratory.
2.3. Isolation and passage of the virus
PDCoV isolation was performed using LLC-PK cells as described previously (Hu et al., 2015), with some modifications. In brief, 100 % confluent LLC-PK cell monolayers were prepared in a T-25 flask (Corning, USA) and washed three times with sterile phosphate-buffered saline (PBS; pH 7.2, Gibco, USA) to completely remove FBS. Then, the prepared supernatant of the PDCoV-positive samples was added as an inoculum for incubation. After incubation at 37 ℃ in 5 % CO2 for one hour, the inoculums were removed, and 2 mL of virus growth medium (MEM supplemented with 1 % NEAA, 1 % antibiotic-antimycotic and 1 % HEPES) was added. After that, the flasks were maintained at 37 °C in a humidified 5 % CO2 incubator and monitored daily until CPEs were observed in > 90 % of the cells. Then, the flask was subjected to three rounds of freezing and thawing, and the cell culture-harvested virions were collected as seed stock for the next serial passage.
2.4. Tissue culture infectious dose (TCID50) assay
The tissue culture infectious dose (TCID50) assay was performed using a procedure described previously with some modification (Liu et al., 2019). CPEs in LLC-PK cells were monitored for 72–96 h, and the viral titers were calculated according to the Reed and Muench method.
2.5. Immunofluorescence assay (IFA)
After forming a 100 % confluent cell monolayer in 6-well plates, LLC-PK cells were infected with the P10 of cell culture-adapted PDCoV strain CH/XJYN/2016 at a multiplicity of infection (MOI) of 0.01. The infected cells were subsequently cultured and were fixed using 4 % paraformaldehyde (Solarbio, China) at 4 °C for 30 min at 0, 12, 24 and 36 h post-infection, respectively, and then permeabilized with 0.25 % Triton X-100 (Solarbio, China) in PBS at room temperature (RT) for 10 min. After washing with PBS (Gibco™, USA), 5 % bovine serum albumin (BSA; Solarbio, China) was added for blocking at RT for 1 h. Then, the cells were incubated with PDCoV N protein McAbs #51 (prepared by our laboratory) and goat anti-mouse IgG conjugated to Alexa Fluor 488 (Abcam, UK) as primary and secondary antibodies, respectively. After washing, the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) for 5 min at RT, followed by observation with a fluorescence microscope (Olympus, Japan).
2.6. Electron microscopy assay
To image the virion particles of PDCoV, electron microscopy was used, and samples were prepared according to methods described in a previous study (Liu et al., 2019). Briefly, LLC-PK cells infected with PDCoV were harvested when CPEs were observed in more than 90 % of the cells. The harvested cell cultures were frozen and thawed three times and then centrifuged at 8500×g at 4 °C for 30 min. The cell debris in the supernatant was removed by filtration through a 0.22-mm filter, and then polyethylene glycol 8000 (PEG-8000; Solarbio, China) was incubated overnight at a 10 % final concentration. After that, the suspensions were ultracentrifuged at 12,000×g at 4 °C for 2 h to pellet the viral particles. The purified viral particles were resuspended using Tris buffered saline solution (TBS, Solarbio, China) and then negatively stained with 2% phosphotungstic acid. The image was observed with a transmission electron microscope (JEM-1200EX, Japan).
2.7. Sequencing and Phylogenetic Analysis of the Spike (S) gene
Viral RNA was extracted from the original fecal samples using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. Reverse transcription and PCR were performed with specific primers, which were designed and stored in our laboratory. Primer sequences: PDCoV-SF1: 5′- ATTATCTATGGTGATGATTCCA-3′, PDCoV-SR1:5′- ACAATTCAGCATGAGTGGTT-3′; PDCoV-SF2: 5′- CACCAGAATGCAGAGAGCTC-3′, PDCoV-SR2:5′- GACCGCTCTTGTAGATCATTAAT-3′; PDCoV-SF3: 5′-GCTACTCATTGCAGCCTCAACT-3′, PDCoV-SR3:5′- AACCAAGACGCGTCAGTAGTA-3′. The PCR-amplified target fragments were purified and recycled, and then were cloned into TOPO vector (Invitrogen, USA). The positive plasmids identified by restriction analysis were sent to Sangon Biotech (Shanghai, China) for sequencing. The full-length S gene were assembled and analyzed with DNAStar 7.0 software. Phylogenetic trees were constructed by the neighbor-joining method with bootstrap values calculated for each node from 1000 replicates. All phylogenetic tree figures were produced using Mega 4.0 software (Tamura et al., 2013).
2.8. Indirect enzyme-linked immunosorbent assay (i-ELISA)
An indirect ELISA based on PDCoV nucleocapsid (N) antigen, which was expressed as a recombinant protein in 293 cells in our laboratory, was developed to assess IgG antibody in pigs. In brief, a concentration of 0.25 ng/mL purified PDCoV N protein in 0.05 M NaHCO3 was used to coat ELISA plates (Corning, USA), which were then incubated with serum samples (1:400 dilution) at 37 °C for 1 h. After the plate was washed with PBST 3 times, an HRP-conjugated goat anti-pig IgG McAb (1:8000 dilution, Abcam, USA) was added and the plate was incubated at 37 °C for 30 min. Then, the plate was washed with PBST 3 times, 3, 3′, 5, 5′-tetramethylbenzidine (TMB) substrate was added and the plate was incubated at 37 °C for 10 min. Then, 50 μL of stop solution was added, and the absorbance was determined at 450 nm.
2.9. Suckling piglet infection experiments
To determine the pathogenicity of the isolated virus in suckling piglets, infection experiments were performed using 4-day-old conventional suckling piglets, as described in our previous study (Zhang et al., 2019a). Briefly, pregnant PDCoV-naïve sows that tested seronegative for PDCoV in the indirect ELISA were selected from a commercial pig farm with no previous herd history of PDCoV infection. After delivery, six piglets were randomly allotted into two groups: the infection group (n = 4) and the mock control group (n = 2). Each piglet was housed in an individual steel cage, and each group of pigs was housed in different rooms. All Piglets were artificially fed bovine milk and had free access to water. Piglets in the infection group and mock control group were separately inoculated with 3 mL of MEM containing 1.0 × 104 TCID50 of the cell culture-adapted PDCoV strain CH/XJYN/2016-P6. All piglets were observed daily for clinical signs after inoculation. Rectal swabs were collected daily from all piglets, tested by real-time PCR and scored for fecal consistency as follows: 0 = normal; 1 = pasty; 2 = semiliquid; and 3 = liquid (Zhang et al., 2019a). The piglets were euthanized when obvious clinical symptoms were observed and fecal viral RNA shedding was detected in the rectal swab samples. In addition, necropsy examinations of small intestinal tissues were also performed, as described previously (Zhang et al., 2019a). Intestinal samples were collected for hematoxylin and eosin (HE) staining and immunohistochemistry (IHC) examination.
2.10. Histopathology and immunohistochemistry (IHC) of the small intestine
Histopathology and IHC examinations were performed using a procedure described previously (Liu et al., 2019). After euthanization, the intestinal tissue specimens of each piglet were removed, fixed in 4% paraformaldehyde solution at RT for 48 h, and then processed and embedded in paraffin. The paraffin-embedded tissues were sectioned by microtome (Leica, Germany) and then deparaffinized and washed in ethanol. Subsequently, the intestinal tissue specimens were routinely stained with HE (Baso, China) for histopathology or subjected to IHC using PDCoV N-specific McAbs (prepared in our laboratory).
2.11. Determination of the infectious titer in conventional weaned pigs
To determine a standardized and validated dose for the subsequent pig challenge experiments, the median pig diarrhea dose (PDD50) of P6 of the cell culture-adapted PDCoV strain, CH/XJYN/2016, was determined by using conventional weaned pigs as described in our previous study (Zhang et al., 2019a). In detail, a total of twenty-five 45-day-old conventional weaned pigs were randomly divided into five experimental groups (G1-G5; Table 1 ). Each group of pigs was housed in a different room, and each piglet was housed in an individual steel cage. To ensure that all pigs were PDCoV-naïve, fecal and blood samples were collected before inoculation and then tested by real-time PCR (developed by our laboratory) and indirect ELISA. The primer and probe sequences used in real-time PCR were as follows: sense, 5′-ACGTCGTAAGACCCAGCATC-3′; antisense, 5′−CCCACCTGAAAGTTGCTCTC-3′; probe, 5′-FAM-GTATGGCTGATCCTCGCATCATGGC-BHQ1−3'. The PCR conditions were as follows: 42 °C for 5 min; 95 °C for 10 s, and followed by 40 cycles of 95 °C for 10 s and 57 °C for 20 s. In groups 1–4, pigs were separately inoculated orally with 3 mL of 10-fold serially diluted CH/XJYN/2016-P6 (diluted from 10° to 10−3; the original titer of virus stock: 4 log10 TCID50/mL) (Table 1). Pigs in group 5 were inoculated orally with 3 mL of PBS (Gibco, USA) as a mock control (Table 1). After inoculation, typical clinical signs were observed daily and scored for fecal consistency until 7 days post-infection (dpi). In addition, the intestinal faeces were collected daily using rectal swabs to detect viral RNA shedding by real-time PCR (above method). The PDD50 was defined as the reciprocal of the virus dilution at which 50 % of pigs developed serious diarrhea during 7 dpi using the Reed and Muench method (Liu et al., 2015).
Table 1.
Summary of pig groups, inoculum and diarrhea outcomes after infection with PDCoV inpigs.
| Groups | Pig numbers | Inoculuma | Calculated inoculum infectious titer (log10 TCID50/mL)c | Tested inoculum RNA titer (CT value)d | Diarrhea (percent)e | Fecal viral RNA shedding (CT value) |
|---|---|---|---|---|---|---|
| G1 | 5 | original P6b | 4.0 | 13.34 | 3 (5/5, 100) | 13.43−28.51 |
| G2 | 5 | 10−1 diluted P6 | 3.0 | 15.21 | 2−3 (5/5, 100) | 15.18−28.78 |
| G3 | 5 | 10−2 diluted P6 | 2.0 | 17.60 | 0−1 (0/5, 0) | - f |
| G4 | 5 | 10−3 diluted P6 | 1.0 | 23.61 | 0 (0/5, 0) | - f |
| G5 | 5 | PBS | – | – | 0 (0/5, 0) | - f |
All pigs were orally inoculated with 3 mL of inoculum.
G1 pigs were infected with undiluted cell culture-adapted CH/XJYN/2016 P6.
Titers were calculated based on the titer of the original P6 virus (original titer: 104.0 TCID50/mL) and dilution times.
CT value: the mean cycle threshold value. A cutoff point was set at 30; CT values greater than 30 were considered negative.
Fecal scores were determined during the 7 day postinfection (dpi) period for pigs in all groups. Scores for fecal consistency: 0 = normal; 1 = pasty; 2 = semiliquid; and 3 = liquid.
Negative or below the detection limit of real-time PCR.
2.12. Pig vaccination and challenge experiment
The experimental inactivated vaccines were prepared according to our previous study (Liu et al., 2019), with some modifications. In brief, the P30 of the cell culture-adapted CH/XJYN/2016 (106 TCID50/mL) strain was chemically inactivated using binary ethyleneimine (BEI). After three rounds of freezing and thawing, the supernatant of the viral cultures was collected by centrifugation and then inactivated by the addition of 0.2 M BEI to a final concentration of 2 mM at 30 °C for 24 h. Then, 20 % sodium thiosulfate was added to neutralize the remaining BEI. The effect of inactivation was assessed by the absence of viral growth in LLC-PK cell cultures. Subsequently, the inactivated vaccines were prepared by mixing BEI-inactivated cell culture-adapted CH/XJYN/2016 P30 with 206 adjuvant (China Agricultural Vet. Bio. Science) and Imject™ Alum Adjuvant (Thermo, USA) and were then stored at 4 °C until use.
Thirteen two-week-old PDCoV-naïve piglets that tested seronegative for PDCoV-specific antibodies by indirect ELISA were obtained from a commercial pig farm with no previous herd history of PDCoV outbreaks. All of the piglets were raised in the laboratory animal facility at the Lanzhou Veterinary Research Institute. Each group was housed in separate rooms for the duration of the experiment. The animal use protocols were approved by the Institute. All piglets were randomly allocated to two experimental groups (n = 5 per group) and one mock control group (n = 3). The two experimental groups were immunized with different formulations of the inactivated vaccine as follows: group 1, inactivated virus with Imject™ Alum Adjuvant (n = 5); group 2, inactivated virus with 206 adjuvant (n = 5) (Table 2 ). Piglets in the two experimental groups were immunized intramuscularly at 0 days postvaccination (dpv) with 2 mL of the prepared inactivated vaccines and were given two booster immunizations at 14 and 28 dpv. The mock control group received only PBS (n = 3). Blood samples were collected at 0, 7, 14, 21, 28, 35 and 42 dpv for antibody detection. All of pigs in three groups were challenged orally with 3 mL of 1000 PDD50 cell cultured CH/XJYN/2016-P6 at 42 dpv. After challenge, typical clinical symptoms of PDCoV infection were observed daily until 7 dpi, and scores of fecal consistency were rated according to our previous study (Liu et al., 2019). Samples of the intestinal faeces were collected daily to monitor viral shedding. Pigs will be identified as PDCoV infection-positive when the scores of fecal consistency were ≥ 2 and virus shedding could be detected in fecal samples at the same time.
Table 2.
Summary of the clinical scores and fecal viral shedding of pigs after challenge with the 1000 median PDD50 cell culture-adapted CH/XJYN/2016 P6.
| dpia | Group 1b (n = 5) |
Group 2c (n = 5) |
Mock control groupd (n = 3) |
|||
|---|---|---|---|---|---|---|
| NPe | CSf | NP | CS | NP | CS | |
| 0 | 0/5 | 0 | 0/5 | 0 | 0/3 | 0 |
| 1 | 0/5 | 0 | 0/5 | 1 | 0/3 | 1−3 |
| 2 | 0/5 | 0 | 0/5 | 1 | 3/3 | 2−3 |
| 3 | 0/5 | 1 | 0/5 | 0 | 3/3 | 2−3 |
| 4 | 0/5 | 1 | 0/5 | 0 | 3/3 | 2 |
| 5 | 0/5 | 0 | 0/5 | 0 | 3/3 | 1−2 |
| 6 | 0/5 | 0 | 0/5 | 1 | 0/3 | 0 |
| 7 | 0/5 | 0 | 0/5 | 0 | 0/3 | 0 |
Days postinoculation.
Pigs were vaccinated with the inactivated vaccine with Imject™ Alum Adjuvant.
Pigs were vaccinated with the inactivated vaccine with 206 adjuvant.
Piglets in the mock control group were inoculated orally with 3 mL of PBS.
Number of PDCoV-positive piglets (NP), determined by RT-PCR.
Clinical score for fecal consistency, as follows: 0 = normal; 1 = pasty; 2 = semiliquid; and 3 = liquid.
2.13. Virus neutralization (VN) test
Neutralizing antibody responses induced by the experimental inactivated vaccines were assessed by VN test, as described previously (Liu et al., 2019). The diluted virus supernatant of twenty passages (P20) of the cell culture-adapted CH/XJYN/2016 strain (200 TCID50/0.1 ml) was used as virus stock for incubation. Neutralizing antibody titers were calculated as the reciprocal of the highest serum dilution that inhibited CPEs.
2.14. Statistical analysis
Statistical analyses of the data in this study were performed using SPSS 16.0 software. One-way ANOVA with Tukey’s multiple comparisons test was used to determine statistical significance among the different groups. Differences between groups were considered significant when the P value was less than 0.05.
3. Results
3.1. Isolation and biological characteristics of the CH/XJYN/2016 strain
After centrifugation and filtration, the supernatant of the diluted clinical fecal samples containing one PDCoV strain, named CH/XJYN/2016, was used to inoculate LLC-PK cells for a series of passages. Obvious CPEs were first observed at the fifth passage at 36 h postinoculation (hpi), and CPEs were observed in more than 90 % of cells at 96 hpi. Compared with the uninoculated cells, the PDCoV-inoculated cells were characterized as enlarged, rounded, and clustered at the early stage, and then the infected cells showed signs of lysis and detachment from the cell monolayer at the late stage (data not shown).
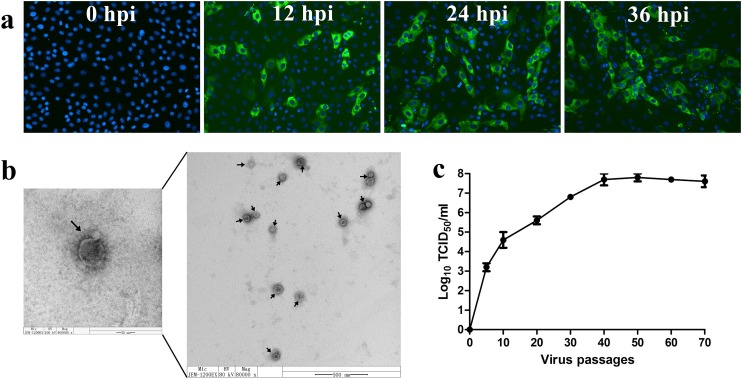
The propagation of the 10th passage of strain CH/XJYN/2016 was confirmed by detecting PDCoV antigens with IFA using a PDCoV N-specific McAbs and DAPI. The results showed that distinct green signals were observed in the PDCoV strain CH/XJYN/2016-infected LLC-PK cells starting at 12 hpi, and the signal intensity tended to increase significantly from 12 to 36 hpi (Fig. 1 a).
Fig. 1.
(a) Immunofluorescence detection results for the tenth passage (P10) of the cell culture-adapted PDCoV strain CH/XJYN/2016 in infected LLC-PK cells at 0, 12, 24 and 36 h after inoculation (400 ×). PDCoV antigens and nuclei were detected with mouse anti-PDCoV N protein monoclonal antibodies (McAbs) and 4′,6-diamidino-2-phenylindole (DAPI), respectively. LLC-PK cells were fixed and strained with mouse anti-PDCoV N and FITC-goat anti-mouse IgG at 12, 24 and 36 h post-infection (green), nuclei were counterstained with DAPI (blue) ; (b) Electron micrograph of PDCoV virions in cell culture media of infected LLC-PK cells. The arrow indicates the crown-shaped projections on the virus surface; (c) Viral titers of the PDCoV strain CH/XJYN/2016 propagated in LLC-PK cells after serial passage. All the results of a representative experiment performed with triplicate samples are shown.
Viral particles of PDCoV strain CH/XJYN/2016 purified from infected LLC-PK cell cultures were imaged with transmission electron microscopy (TEM). TEM observations revealed that the viral particles were the typical crown-shaped coronavirus particles, approximately 80–160 nm in diameter and with spiky surface projections, which were morphologically indistinguishable from other coronaviruses (Fig. 1b).
The viral titers of the serially passaged PDCoV CH/XJYN/2016 strain were determined as the TCID50 at 10-passage intervals. The infectious titers of the cell-adapted virus from P5 to P70 ranged from 103.2 to 107.8 TCID50/mL (Fig. 1c).
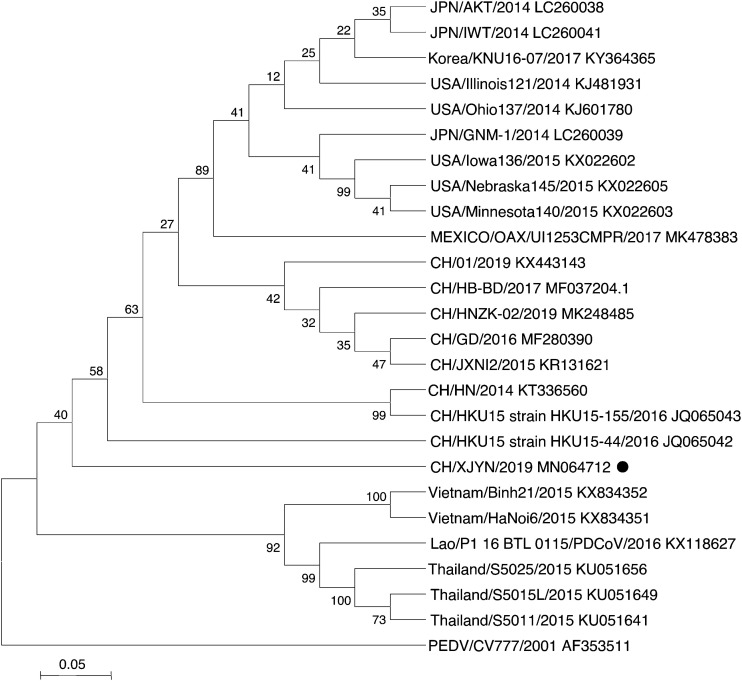
The results of the sequence analysis indicated that compared with that of other reported PDCoVs, the CH/XJYN/2016 strain S gene contained some new mutations. Details, thirty-seven single nucleotide substitutions and a 3-nt insertion were observed in S gene of the CH/XJYN/2016 strain. The complete S genes of strain CH/XJYN/2016 shares 98.74 %–97.70 % nucleotide identity with the other 100 PDCoV reference strains in GenBank. In addition, phylogenetic analyses based on the full-length S gene demonstrated that strain CH/XJYN/2016 clusters with other PDCoV strains isolated from China, the United States, Japan and South Korea, whereas the strains isolated from Thailand, Vietnam and Lao clustered into a large clade (Fig. 2 ). However, compared with that of other reported Chinese strains, the genetic distance of the CH/XJYN/2016 strain is closer to the original Hong Kong strain HKU15-44 (JQ065042) (Woo et al., 2012), sharing 98.74 % nucleotide identity. These results suggest that the US, Japan and South Korea clade and the Chinese clade might share a common parent.
Fig. 2.
Phylogenetic analysis based on the S gene sequence of the PDCoV strain CH/XJYN/2016. The tree was constructed with the neighbor-joining method, and bootstrap values from 1000 resamplings are shown for each node.
3.2. Pathogenicity of the CH/XJYN/2016 strain in suckling piglets
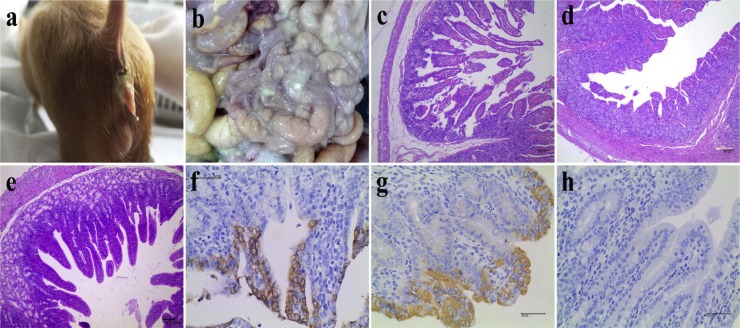
To determine the pathogenicity of the CH/XJYN/2016 strain, 4-day-old conventional suckling piglets were orally inoculated with P6 of the cell culture-adapted virus. Before inoculation, all piglets were lively, showed no clinical symptoms, had normal fecal consistency, and no viral RNA was detected in their fecal samples by real-time PCR. All the pigs from the infection group (n = 4) developed severe diarrhea with high levels of fecal viral RNA shedding from 1−2 dpi, and the symptoms persisted until the piglets were euthanized (Fig. 3 a). All the pigs from the infection group with severe diarrhea were underweight, depressed and vomiting. The pigs (n = 2) from the mock control group were active and fleshy during the experiment and negative for fecal PDCoV RNA shedding. At necropsy, typical macroscopic intestinal lesions were found, especially in the small intestine (Fig. 3b). In detail, the intestinal tract was distended, transparent, filled with yellow fluid, and mesenteric congestion was present (Fig. 3b). Further histopathological examination of all the pigs from the infection group showed viral enteritis, such as vacuolar degeneration in the enterocytes and shortening, fusion and sloughing of the villi (Fig. 3c and d). In addition, immunohistochemical examinations revealed that PDCoV antigens were present mainly in the villous enterocytes of the small intestine (Fig. 3f and g). The piglets in mock control group exhibited normal intestinal histopathology and no PEDV antigens were present in the small intestines (Fig. 3e and h).
Fig. 3.
Clinical symptoms, necropsy examinations, histopathology and IHC of the small intestines of piglets inoculated with P6 of the PDCoV strain CH/XJYN/2016 (104.0 TCID50/mL). (a) The infected pigs had severe diarrhea; (b) Necropsy examinations of the CH/ XJYN /2016-infected piglets. Severe hyperemia, swelling and transparency were present in the intestinal tissues, especially in the small intestine. (c-d) HE-stained tissue jejunum sections from CH/XJYN/2016-infected piglets (100 × magnification); (c) Severe villous ablation was observed in the infected piglets; (d) Severe villous atrophy and fusion, degeneration and necrosis of mucosal epithelial cells were observed in the infected piglets; (f-g) Detection of PDCoV antigen by IHC analysis of small intestine sections from CH/XJYN/2016-infected piglets (400 × magnification). PDCoV antigen signals appeared brown and were detected in jejunal epithelial cell samples from CH/XJYN/2016-infected piglets; (e) The normalvillous epithelium of the jejunum from mock control piglets; (h) No PEDV antigen was detected in jejunum from mock control piglets.
3.3. The median pig diarrhea dose (PDD50) in conventional weaned pigs
A total of twenty-five 45-day-old conventional weaned pigs were used to determine the PDD50 of the CH/XJYN/2016 strain. Before inoculation, all pigs were healthy and had no viral RNA shedding detected in fecal samples by real-time PCR. During the 7 dpi, obvious clinical symptoms, including watery diarrhea, vomiting, weight loss and depression, were observed in pigs from G1 and G2 (original and 10−1 diluted P6 virus), whereas none of the pigs in G3-G5 developed diarrhea, and all of their rectal swab samples were negative for PDCoV (Table 1). By 7 dpi, viral RNA fecal shedding in rectal swab samples became positive in G1 and G2 pigs, with cycle threshold (CT) values ranging from 13.43 to 28.78 (Table 1). Hence, based on the clinical observation and viral RNA fecal shedding results, 100 % (5/5) of pigs in G1 and G2 and no pigs (0/5) in G3, G4 and G5 (Table 1) were infected. The cutoff time point was set as 7 dpi for determination of the PDD50, which was 2.0 log10PDD50/3 mL, equivalent to 100 TCID50. This result indicates that the CH/XJYN/2016 strain is pathogenic in conventional weaned pigs, which is very useful for further pig challenge studies.
3.4. Immunogenicity of the inactivated vaccine candidates
Virus inactivation was verified by the absence of viral growth in LLC-PK cell cultures, and no obvious CPEs were observed in cell cultures within 96 h after inoculation (data not shown). These findings indicate that the virus was completely inactivated, and no live virus was present in the prepared vaccine. In addition, pig fecal rectal swabs collected 5 days postvaccination were negative for PDCoV, further confirming that no infectious PDCoV was present in the vaccine (data not shown).
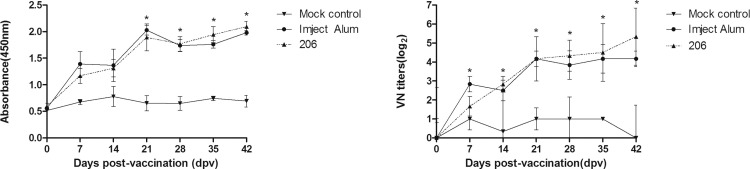
All pigs in the study were confirmed to be seronegative for PDCoV antibodies at day 0 by an indirect ELISA (data not shown). As shown in Fig. 4 a, compared with the mock control, two experimental inactivated vaccines induced significant PDCoV-specific serum IgG responses in pigs (P < 0.05). The levels of IgG antibodies in the two vaccine groups increased continuously after two booster immunizations at 14 and 28 dpv (Fig. 4a), indicating that the two experimental inactivated vaccines could induce a strong PDCoV -specific IgG response in pigs. The VN antibody titers also increased continuously in the two vaccine groups from 7 dpv and were significantly higher than those in the mock control group (P < 0.05, Fig. 4b). These results suggest that both of the experimental inactivated vaccines could induce a strong humoral immune response in pigs.
Fig. 4.
The levels of PDCoV-specific IgG and virus-neutralizing (VN) antibodies in pigs. Pigs were vaccinated at 0 days postvaccination (dpv) and were given two booster immunization at 14 and 28 dpv, respectively. Subsequently, the pigs were challenged orally with 3 mL of 1000 PDD50 cell culture-adapted CH/XJYN/2016 P6 at 42 dpv. Serum samples were collected at 0, 7, 14, 21, 28, 35 and 42 dpv, respectively. (a) The levels of PDCoV-specific IgG antibodies in vaccinated pigs; (b) The levels of PDCoV-specific VN antibodies in vaccinated pigs.
3.5. Protective efficacy of the inactivated vaccine candidates
All of the pigs in the three groups were apparently healthy and had no clinical symptoms before challenge. At 42 dpv, all pigs were challenged orally with 3 mL of 1000 PDD50 cell culture-adapted CH/XJYN/2016 P6. Upon challenge, the clinical signs and viral shedding were monitored closely. As shown in Table 2, in the mock control group (n = 3), serious diarrhea was observed, and viral shedding was detected in all 3 pigs after 2 dpi. In the two experimental inactivated vaccine groups, all of the pigs developed no obvious diarrhea, and no virus shedding was detected during the 7 dpi timeframe (Table 2), indicating that the experimental vaccines provide potent protection against PDCoV infection in pigs.
4. Discussion
In general, vaccination plays a significant role in the prevention and control of various emerging infectious diseases. Recent outbreaks of PDCoV in pig herds in multiple countries have caused significant economic losses for the pig industry (Dong et al., 2016; Janetanakit et al., 2016; Jang et al., 2018; Saeng-Chuto et al., 2017; Wang et al., 2014). Like those of PEDV and TGEV, PDCoV infections cause similar severe clinical disease and lesions, but the lack of an effective preventive vaccine for PDCoV has impeded the prevention and control of PDCoV in pig farms. Notably, reported data suggest that PDCoV has existed in mainland China for at least 11 years (Dong et al., 2015) and results in an increased mortality rate (more than 80 %) among suckling piglets (Song et al., 2015). In addition, because PDCoV is an emerging virus, the lack of a comprehensive understanding of the pathogenic characteristics of have PDCoV also deterred the development of prevention and control methods (Jung et al., 2016). Therefore, the infectious titer (PDD50) of PDCoV in 45-day-old conventional pigs and the protection efficacity of an inactivated vaccine based on the cell-adapted strain CH/XJYN/2016 were determined for the first time in the present study. This information will be helpful for future pig infection experiments and vaccine studies.
Most of the reported Chinese strains were isolated from the eastern and central regions of China (Dong et al., 2016, 2015; Wang et al., 2015; Zhang et al., 2019c). Besides, PDCoV isolate, CHN-SC2015, was isolated from Sichuan Province in southwest China (Zhao et al., 2019). The PDCoV strain CH/XJYN/2016 in this study is a field strain that was isolated in early 2016 in Northwest China. Phylogenetic analyses based on the full-length S gene demonstrated that the CH/XJYN/2016 strain clusters with other PDCoV strains isolated from China, the United States, Japan and South Korea. However, compared with those of the other reported Chinese strains, the genetic distances of the CH/XJYN/2016 strain are closer to the original Hong Kong strain HKU15-44 (JQ065042) (Woo et al., 2012), sharing 98.74 % nucleotide identity. The sequence information of PDCoV strain CH/XJYN/2016 will contribute to the genetic and evolutionary characterization of PDCoV in China.
For the following several reasons, in this study, the CH/XJYN/2016 strain-P6 were used as challenge virus stocks. First, as we mentioned in results, the obvious CPEs of the CH/XJYN/2016 strain were first observed at the fifth passage at 36 h postinoculation (hpi). Therefore, in order to obtain enough virus stocks for next challenge study, the passage P6 was chosen. Second, in general, virus stocks for challenge study needs the passage as low as possible. Because the pathogenicity of the viruses will decrease with the passage numbers increases. Besides, the cell culture-adapted CH/XJYN/2016-P30 was used for activated vaccine is because it keeps the greatest of the antigen concentrations and the lowest of passage at same time.
Consistent with previous reports, all pigs from the infection group (n = 4) developed severe diarrhea with high levels of fecal viral RNA shedding from 1−2 dpi, and the symptoms persisted until the piglets were euthanized (Fig. 3a). Notably, no PDCoV-associated deaths were observed in this study. These results demonstrate that CH/XJYN/2016 is pathogenic to newborn piglets. Compared to that in 4-day-old neonatal piglets, the virus incubation period, time to shedding of fecal viral RNA and clinical disease onset (3–4 dpi) was increased in 45-day-old pigs. These results confirmed that nursing pigs have increased susceptibility to PDCoV infection, which is similar to the results of a previous study of PEDV infection (Annamalai et al., 2015).
Inactivated vaccines do not contain any live components; therefore, they are safe, with a reduced risk of inducing disease and are widely used for the prevention and control of various diseases, especially viral disease. For example, PEDV and TGEV, for which many inactivated vaccines have been developed and have been widely used in many countries, such as China, Korea, Japan and the United States (Gerdts and Zakhartchouk, 2017; Lee, 2015). In China, inactivated vaccines have been routinely used for PED. PDCoV infections, which have similar clinical symptoms as those of PEDV and TGEV, can also be prevented and controlled using inactivated vaccines. To provide a vaccine that could be used to control PDCoV transmission, we developed an inactivated vaccine based on the cell-adapted strain CH/XJYN/2016. The inactivated PDCoV experimental vaccines showed good safety and immunogenicity in pigs and significantly protected pigs against live virus challenge.
In the immune protection experiment, compared with the mock control, both inactivated vaccines induced significant PDCoV-specific humoral immune responses in pigs compared (P < 0.05). In addition, the levels of specific antibodies in the two vaccine groups increased continuously after two booster immunizations, indicating that a three immunization protocol is an effective immune procedure for PDCoV inactivated vaccines. Further animal challenge studies showed that the two inactivated vaccines both fully protected the pigs against 1000 PDD50 PDCoV infection; the pigs showed no obvious diarrhea, and no viral shedding was detected during the 7 dpi follow-up period. These results suggest that the inactivated PDCoV vaccine developed in this study could be used as a very promising vaccine candidate in the future.
In conclusion, we developed a PDCoV vaccine candidate that induces a potent humoral immune response in pigs and provides good protection against PDCoV infection. This vaccine candidate can potentially be used for the prevention and control of PDCoV in the future. In addition, this is the first report to describe the infectious titer (PDD50) of PDCoV in conventional weaned pigs, and the results will be helpful for future pig infection experiments and understanding the pathogenicity differences of PDCoV in pigs of different ages.
Author contributions
XL, YW and YZ conceived and designed the experiments. XL wrote the manuscript and analyzed the data. XL performed the sample collection. XL, XG, DZ, PZ, WL and LZ performed the experiments. All authors read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31602095), the National Key Research and Development Program (2016YFD0501505), the China Agriculture Research System (CARS-35) and the Central Public Interest Scientific Institution Basal Research Fund (Y2016CG23).
CRediT authorship contribution statement
Xiang Gao: Methodology, Investigation. Donghong Zhao: Methodology, Investigation. Peng Zhou: Methodology, Investigation. Liping Zhang: Methodology, Investigation. Mingxia Li: Methodology, Investigation. Weiyan Li: . Yongguang Zhang: Conceptualization. Yonglu Wang: Conceptualization. Xinsheng Liu: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.virusres.2020.197955.
Contributor Information
Xiang Gao, Email: 1075474953@qq.com.
Donghong Zhao, Email: zhaodonghong123@163.com.
Peng Zhou, Email: zhoupeng02@caas.cn.
Liping Zhang, Email: zhanglp2319@163.com.
Mingxia Li, Email: 1031082498@qq.com.
Weiyan Li, Email: liweiyan160129@163.com.
Yongguang Zhang, Email: zhangyongguang@caas.cn.
Yonglu Wang, Email: wangyonglumd@hotmail.com.
Xinsheng Liu, Email: liuxinsheng@caas.cn.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Annamalai T., Saif L.J., Lu Z., Jung K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015;168(3-4):193–202. doi: 10.1016/j.vetimm.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Gauger P., Stafne M., Thomas J., Arruda P., Burrough E., Madson D., Brodie J., Magstadt D., Derscheid R., Welch M., Zhang J. Pathogenicity and pathogenesis of a United States porcine deltacoronavirus cell culture isolate in 5-day-old neonatal piglets. Virology. 2015;482:51–59. doi: 10.1016/j.virol.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Zeng S., Sun Q., Chen H., Xiao S. Porcine deltacoronavirus in mainland China. Emerg. Infect. Dis. 2015;21(12):2254–2255. doi: 10.3201/eid2112.150283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong N., Fang L., Yang H., Liu H., Du T., Fang P., Wang D., Chen H., Xiao S. Isolation, genomic characterization, and pathogenicity of a Chinese porcine deltacoronavirus strain CHN-HN-2014. Vet. Microbiol. 2016;196:98–106. doi: 10.1016/j.vetmic.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts V., Zakhartchouk A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017;206:45–51. doi: 10.1016/j.vetmic.2016.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H., Jung K., Vlasova A.N., Chepngeno J., Lu Z., Wang Q., Saif L.J. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J. Clin. Microbiol. 2015;53(5):1537–1548. doi: 10.1128/JCM.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetanakit T., Lumyai M., Bunpapong N., Boonyapisitsopa S., Chaiyawong S., Nonthabenjawan N., Kesdaengsakonwut S., Amonsin A. Porcine deltacoronavirus, Thailand, 2015. Emerg. Infect. Dis. 2016;22(4):757–759. doi: 10.3201/eid2204.151852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang G., Kim S.H., Lee Y.J., Kim S., Lee D.S., Lee K.K., Lee C. Isolation and characterization of Korean porcine deltacoronavirus strain KNU16-07. J. Vet. Sci. 2018;19(4):577–581. doi: 10.4142/jvs.2018.19.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Hu H., Saif L.J. Porcine deltacoronavirus infection: etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016;226:50–59. doi: 10.1016/j.virusres.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., Miyazaki A., Hu H., Saif L.J. Susceptibility of porcine IPEC-J2 intestinal epithelial cells to infection with porcine deltacoronavirus (PDCoV) and serum cytokine responses of gnotobiotic pigs to acute infection with IPEC-J2 cell culture-passaged PDCoV. Vet. Microbiol. 2018;221:49–58. doi: 10.1016/j.vetmic.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. Porcine epidemic diarrhea virus: an emerging and re-emerging epizootic swine virus. Virol. J. 2015;12:193. doi: 10.1186/s12985-015-0421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee C. Complete genome characterization of Korean porcine deltacoronavirus strain KOR/KNU14-04/2014. Genome Announc. 2014;2(6):e01191. doi: 10.1128/genomeA.01191-14. e01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Lee C. Functional characterization and proteomic analysis of the nucleocapsid protein of porcine deltacoronavirus. Virus Res. 2015;208:136–145. doi: 10.1016/j.virusres.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin C.M., Annamalai T., Gao X., Lu Z., Esseili M.A., Jung K., El-Tholoth M., Saif L.J., Wang Q. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Vet. Res. 2015;46:109. doi: 10.1186/s13567-015-0249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhang Q., Zhang L., Zhou P., Yang J., Fang Y., Dong Z., Zhao D., Li W., Feng J., Cui B., Zhang Y., Wang Y. A newly isolated Chinese virulent genotype GIIb porcine epidemic diarrhea virus strain: biological characteristics, pathogenicity and immune protective effects as an inactivated vaccine candidate. Virus Res. 2019;259:18–27. doi: 10.1016/j.virusres.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madapong A., Saeng-Chuto K., Lorsirigool A., Temeeyasen G., Srijangwad A., Tripipat T., Wegner M., Nilubol D. Complete genome sequence of porcine deltacoronavirus isolated in Thailand in 2015. Genome Announc. 2016;4(3):e00408–e00416. doi: 10.1128/genomeA.00408-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marthaler D., Raymond L., Jiang Y., Collins J., Rossow K., Rovira A. Rapid detection, complete genome sequencing, and phylogenetic analysis of porcine deltacoronavirus. Emerg. Infect. Dis. 2014;20(8):1347–1350. doi: 10.3201/eid2008.140526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeng-Chuto K., Lorsirigool A., Temeeyasen G., Vui D.T., Stott C.J., Madapong A., Tripipat T., Wegner M., Intrakamhaeng M., Chongcharoen W., Tantituvanont A., Kaewprommal P., Piriyapongsa J., Nilubol D. Different lineage of porcine deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound. Emerg. Dis. 2017;64(1):3–10. doi: 10.1111/tbed.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Zhou X., Peng Q., Chen Y., Zhang F., Huang T., Zhang T., Li A., Huang D., Wu Q., He H., Tang Y. Newly emerged porcine deltacoronavirus associated with diarrhoea in swine in China: identification, prevalence and full-length genome sequence analysis. Transbound. Emerg. Dis. 2015;62(6):575–580. doi: 10.1111/tbed.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W., Wang L., Huang H., Wang W., Cao L., Zhang J., Zheng M., Lu H. Genetic characterization and phylogenetic analysis of porcine deltacoronavirus (PDCoV) in Shandong Province, China. Virus research. 2020;278 doi: 10.1016/j.virusres.2020.197869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Byrum B., Zhang Y. Detection and genetic characterization of deltacoronavirus in pigs, Ohio, USA, 2014. Emerg. Infect. Dis. 2014;20(7):1227–1230. doi: 10.3201/eid2007.140296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.W., Yue H., Fang W., Huang Y.W. Complete genome sequence of porcine deltacoronavirus strain CH/Sichuan/S27/2012 from mainland China. Genome Announc. 2015;3(5):e00945. doi: 10.1128/genomeA.00945-15. e00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M., Zheng B.J., Chan K.H., Yuen K.Y. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86(7):3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C., Lau S.K., Tsang C.C., Lau C.C., Wong P.C., Chow F.W., Fong J.Y., Yuen K.Y. Coronavirus HKU15 in respiratory tract of pigs and first discovery of coronavirus quasispecies in 5’-untranslated region. Emerg. Microbes Infect. 2017;6(6):e53. doi: 10.1038/emi.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Porcine deltacoronavirus: overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84. doi: 10.1016/j.virusres.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu X., Zhang Q., Zhou P., Fang Y., Dong Z., Zhao D., Li W., Feng J., Zhang Y., Wang Y. Biological characterization and pathogenicity of a newly isolated Chinese highly virulent genotype GIIa porcine epidemic diarrhea virus strain. Arch. Virol. 2019;164(5):1287–1295. doi: 10.1007/s00705-019-04167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M.J., Liu D.J., Liu X.L., Ge X.Y., Jongkaewwattana A., He Q.G., Luo R. Genomic characterization and pathogenicity of porcine deltacoronavirus strain CHN-HG-2017 from China. Arch. Virol. 2019;164(2):413–425. doi: 10.1007/s00705-018-4081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Cheng Y., Xing G., Yu J., Liao A., Du L., Lei J., Lian X., Zhou J., Gu J. Detection and spike gene characterization in porcine deltacoronavirus in China during 2016-2018. Infect. Genet. Evol. 2019;73:151–158. doi: 10.1016/j.meegid.2019.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Qu H., Hu J., Fu J., Chen R., Li C., Cao S., Wen Y., Wu R., Zhao Q., Yan Q., Wen X., Huang X. Characterization and pathogenicity of the porcine deltacoronavirus isolated in Southwest China. Viruses. 2019;11(11) doi: 10.3390/v11111074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.